Abstract
Immunotherapy encourages the recipient’s own immune response to destroy cancer cells, and current evidence suggests that immunotherapies may be most beneficial in early metastatic castration-resistant prostate cancer (mCRPC). Sipuleucel-T is the first therapeutic cancer vaccine to be approved by both the US Food and Drug Administration and European Medicines Agency for the treatment of asymptomatic or minimally symptomatic mCRPC. Combining immunotherapy with other treatments may have potent anticancer effects; cytoreductive therapies can release tumor antigens and promote a proinflammatory environment that could augment immunotherapies. However, some cytoreductive agents or coadministered drugs may be immunosuppressive. Understanding these interactions between different mCRPC treatment modalities may offer further potential to improve patient outcomes.
Key words: Combination therapy, Prostate cancer, Sipuleucel-T
Immunotherapy has emerged as a powerful tool against prostate cancer, in addition to surgery, radiotherapy, hormone therapy, and chemotherapy. For 30 years, investigators tried to rebalance the compromised immune system in patients with urologic cancers using a number of different agents.1,2 In April 2010, the autologous cellular immunotherapy sipuleucel-T became the first therapeutic cancer vaccine to be approved by the US Food and Drug Administration (FDA).3 This therapy targets the prostatic acid phosphatase (PAP) and has been indicated for the treatment of asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC), based on results from three randomized, controlled, phase 3 studies.3–6 Recently, sipuleucel-T was also approved by the European Medicines Agency (EMA) for the treatment of asymptomatic or minimally symptomatic mCRPC in men in whom chemotherapy is not yet clinically indicated.7
Although this immunotherapy has been shown to extend overall survival (OS),5 sequencing or combining immunotherapy with other treatments for mCRPC has the potential to further improve outcomes.8,9 However, before immunotherapy-based combination regimens can be integrated into clinical practice, it is critical to have a better understanding of the interactions between these different modalities.
Evidence Acquisition
This article focuses primarily on sipuleucel-T, which is the only currently available immunotherapy for mCRPC. Manuscripts and conference abstracts that reported key sipuleucel-T clinical trials and subanalyses were identified. Other information on sipuleucel-T available through the FDA and EMA was searched. Additional publications relevant to immunotherapies in general and other specific immunotherapies were identified, and relevant Web sites such as clinicaltrials. gov were also searched.
Evidence Synthesis
This article presents clinical data on combining immunotherapies, particularly sipuleucel-T, with other treatments for prostate cancer. In the absence of clinical data, theoretical or other relevant information is included.
Immunotherapy
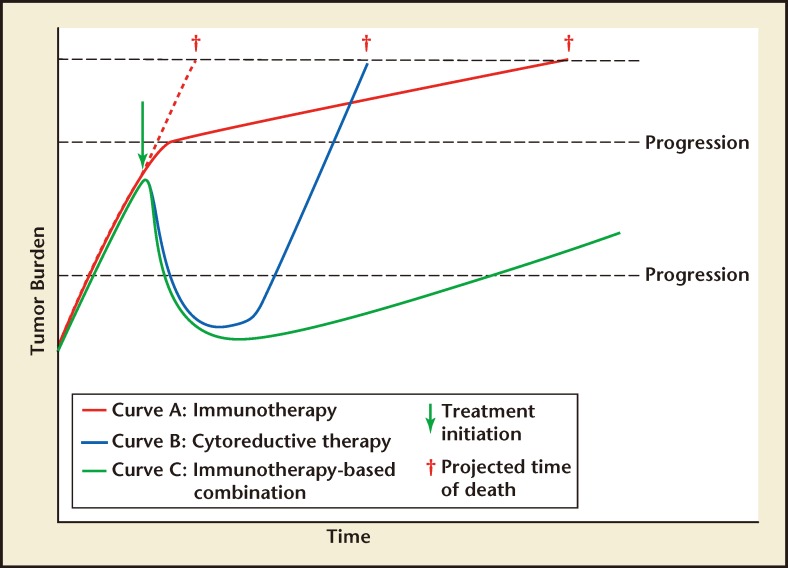
By definition, cancer immunotherapies enhance anticancer immune responses, helping the body to control cancerous cells. This mechanism of action is clearly distinct from that of traditional therapies such as radio- or chemotherapy. Indeed, a kinetic model of tumor growth rate has been proposed for patients receiving immunotherapy versus cytoreductive therapy (Figure 1).10,11 In this model, immunotherapies slow tumor growth rate over time without producing the marked short-term tumor shrinkage that is the hallmark of cytoreductive therapy. This may explain the improved OS observed after immunotherapy, without an increase in time to progression in advanced prostate cancer.
Figure 1.
Theoretical growth-moderating effect of (A) immunotherapy or (B) cytoreductive therapy as monotherapies, or (C) in combination. Adapted from Madan RA et al10 and Schlom J.11
Data for sipuleucel-T support this model. Sipuleucel-T is an autologous cellular immunotherapy, produced by culturing the patient’s purified peripheral blood mononuclear cells with a recombinant fusion protein of PAP coupled to granulocyte-macrophage colonystimulating factor (GM-CSF).3 These cells are then reinfused into the patient, where the product is designed to generate a prostate cell-specific immune response.3 Consistent with the kinetics of the immunotherapy model described above, the pivotal phase 3 sipuleucel-T study, Immunotherapy Prostate Adenocarcinoma Therapy (IMPACT), did not show a significant effect on time to disease progression, but rather demonstrated a statistically significant improvement in the primary endpoint of OS.5 There was a 22% relative reduction in the risk of death with sipuleucel-T versus the placebo group, which represents a survival increase of 4.1 months for the sipuleucel-T group (25.8 vs 21.7 months).5 The 36-month survival probabilities were 31.7% and 23.0% in the sipuleucel-T and placebo groups, respectively.5 These data are supported by an integrated analysis of two earlier phase 3 trials, D9901 and D9902A, which also demonstrated a survival benefit for patients treated with sipuleucel-T versus placebo.4 The survival benefit following sipuleucel-T treatment is associated with the development of immune responses,12 confirming the immunomodulatory effects of therapy. In addition, the concept that immunotherapy slows tumor growth is supported by the lengthening of prostate-specific antigen (PSA) doubling time with sipuleucel-T versus control that was noted in patients with androgen-dependent prostate cancer.13 Sipuleucel-T was generally well tolerated in the phase 3 trials, and most patients received all three of the scheduled infusions.4,5 Although traditional short-term markers of success, such as a reduction in PSA levels, are not associated with the OS benefit of sipuleucel-T treatment, the development of antigen-specific immune responses during therapy are associated with OS.12 Some of these immunologic effects, such as a transient increase in circulating eosinophil levels, may prove to be useful future markers of treatment success.14
Patients with a relatively low mCRPC disease burden appear to benefit most from immunotherapy with sipuleucel-T; those with the lowest PSA baseline levels had the greatest OS benefit compared with control treatment.5,15 A 49% reduction in the risk of death (hazard ratio [HR] 0.51; 95% confidence interval [CI], 0.31–0.85) and a median OS difference of 13.0 months was achieved by patients in the lowest baseline PSA quartile (< 22.1 ng/mL), compared with a 16% reduction in the risk of death (HR 0.84; 95% CI, 0.55–1.29) and a 2.8 month median OS difference for patients in the highest PSA quartile (> 134 ng/mL).15
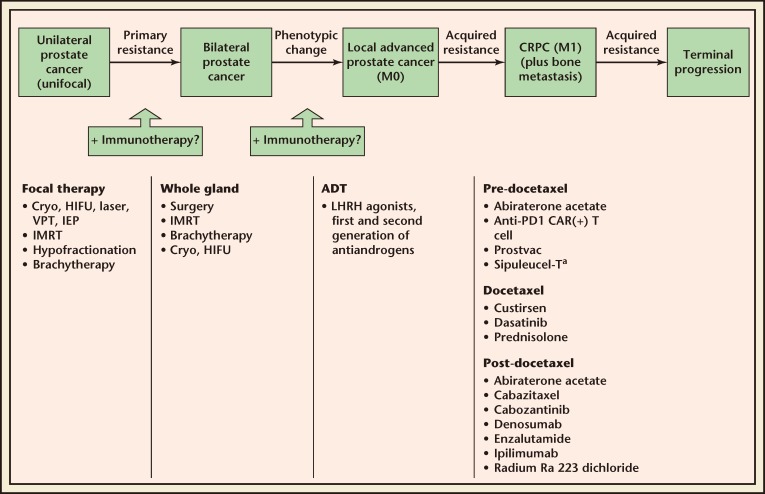
Indeed, patients treated early in mCRPC may be less immunocompromised, have more time for the immune system to respond, and have an increased opportunity for prolonged OS improvement.11,15 These findings suggest that, in terms of the current prostate cancer treatment paradigm (Figure 2), immunotherapy may be best placed early in the mCRPC treatment course.
Figure 2.
The position of sipuleucel-T treatment and other modalities in the natural course of prostate cancer. aApproved indication. ADT, androgen deprivation therapy; CAR, chimeric antigen receptor; Cryo, cryoablation; CRPC, castrate-resistant prostate cancer; HIFU, high-intensity focused ultrasound; IEP, irreversible electroporation; IMRT, intensity-modulated radiation therapy; LHRH, luteinizing hormone-releasing hormone; M0, nonmetastatic; M1, metastatic; PD, programmed death; VPT, vascular photodynamic therapy.
Another interesting aspect of immunotherapy that may help to explain its long-term benefits is antigen spread, which is also called epitope spreading or determinant spreading. This is a process in which the immune response, which initially targets defined antigenic peptides, begins to target additional antigens that are distinct from the initial target(s) and may be derived from completely different proteins.16 This process is facilitated by cellular toxicity or apoptosis; dead cells are taken up by activated antigen-presenting cells (APCs) that can then stimulate immune responses against different molecular targets from within the same tissues as the original target.16 Preliminary studies on immunotherapy suggest that there is a high frequency of antigen spreading in clinical responders, whereas nonresponders may not display reactivity to antigens other than those used for the original treatment.16 This phenomenon has been noted during sipuleucel-T treatment, which initially targets the immune response against PAP. Patients treated with sipuleucel-T consistently mounted elevated IgG antibody responses against a range of cancer antigens, whereas patients in the control arm did not.17 These responses were associated with improved OS.17
Although this article focuses on sipuleucel-T, as it is the only currently available immunotherapy for prostate cancer, other immunotherapies currently in development for the treatment of prostate cancer include PSA-TRICOM (recombinant viral therapy encoding PSA and the costimulatory molecules cluster of differentiation 80, intercellular adhesion molecule-1, and lymphocyte function-associated antigen-3),18,19 ipilimumab (monoclonal antibody that blocks the immunoregulatory molecule cytotoxic T-lymphocyte-associated protein 4 [CTLA-4]),19,20 GVAX (GM-CSF-transduced allogeneic prostate cancer cells),21,22 and modified T-cell therapy (anti-prostate-specific membrane antigen chimeric antigen receptor T cells).23
Potential Combination Therapies for Patients With mCRPC
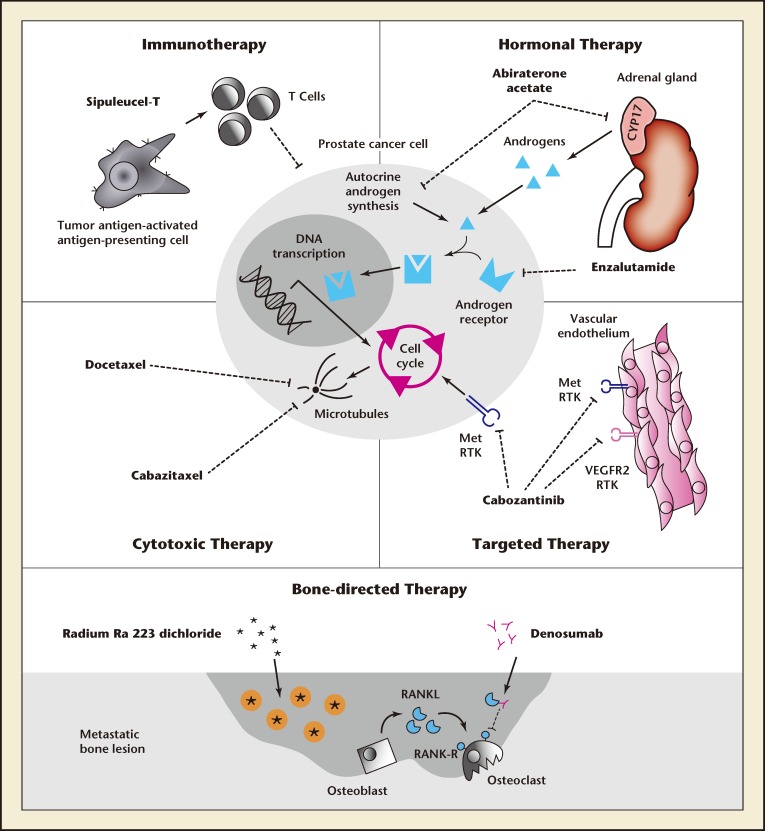
In addition to immunotherapy, recent advances in the treatment of mCRPC have also introduced novel, cytoreductive, nonchemotherapeutic targeted treat ments (Figure 3).24
Figure 3.
Potential modalities to be used as a combinatorial approach for castration-resistant prostate cancer. Reproduced with permission from Galsky MD et al.24 CYP17 indicates a cytochrome p450 complex involved in adrenal steroidal synthesis; Met RTK, Met receptor tyrosine kinase; VEGFR2, vascular endothelial growth factor receptor 2; RANKL, receptor activator of nuclear factor k-B ligand; RANK, receptor activator of nuclear factor k-B.
Cytoreductive therapy for mCRPC encompasses a broad spectrum of approaches, including systemic chemotherapy, radiation therapy, and secondary hormone therapies (eg, novel antiandrogens such as abiraterone acetate and enzalutamide). Cytoreductive therapies provide a transient decrease in tumor size, whereas immunotherapies slow the tumor growth rate and have a longterm effect (Figure 1). Therefore, theoretically, combining immunotherapy and cytoreductive therapy could produce both an immediate and long-lasting effect on tumor burden (curve C in Figure 1). This concept supports the use of combination therapy early in the mCRPC treatment paradigm, in order to maximize the long-term benefits. In addition, this combination has the potential to be synergistic; cytoreductive therapy initiates the release of large amounts of tumor antigens, which may facilitate the development of immune responses. However, some cytoreductive therapies may have immunosuppressive effects, so the timing of concurrent or sequential use of cytoreductive treatments with immunotherapies requires careful consideration and investigation. Several planned or ongoing clinical studies are currently investigating cytoreductive therapies in combination with sipuleucel-T (Table 1).
Table 1.
Ongoing and Completed Phase 2 Clinical Studies of Sipuleucel-T in Combination With Other Modalities for mCRPCa
| ClinicalTrials. gov Identifier | Combination Therapy | Planned or Estimated Enrollment (N) | Primary Endpoint | Study Status |
|---|---|---|---|---|
| Sipuleucel-T Combined With Nonimmunotherapies for mCRPC | ||||
| NCT01818986 | Sipuleucel-T and stereotactic ablative body radiation | 41 | To evaluate an improvement in time to progression compared with historical data for sipuleucel-T alone | Recruiting |
| NCT01807065 | Sipuleucel-T with or without radiotherapy | 50 | Proportion of patients able or willing to receive all three injections of sipuleucel-T | Recruiting |
| NCT01981122 (P12-2) | Concurrent vs sequential treatment with sipuleucel-T and enzalutamide | 100 | To evaluate T cell responses (proliferation) over the course of therapy | Recruiting |
| NCT01487863 (P11-3) | Concurrent vs sequential treatment with sipuleucel-T and abiraterone acetate | 60 | To evaluate cumulative CD54 upregulation over the course of sipuleucel-T therapy | Ongoing |
| NCT00027599 | Sipuleucel-T plus bevacizumab | 25 | To determine efficacy in terms of decline in PSA value and effect on PSA doubling time | Completed |
| Sipuleucel-T Combined With Other Immunotherapies for mCRPC | ||||
| NCT01706458 | Sipuleucel-T with or without pTVG-HP DNA booster vaccine | 30 | To measure immune responses | Recruiting |
| NCT01560923 | Sipuleucel-T with indoximod or placebo | 50 | To assess the augmentation of immune response with indoximod or placebo | Recruiting |
| NCT01420965 | Sipuleucel-T with or without CT-011 and cyclophosphamide | 57 | To test the effectiveness of combined treatment | Recruiting |
| NCT01804465 | Sipuleucel-T with immediate vs delayed ipilimumab | 66 | To assess safety and antibody responses | Ongoing |
Based on studies listed on www.clinicaltrials.gov on March 18, 2014.
CD, cluster of differentiation; mCRPC, metastatic castration-resistant prostate cancer; PSA, prostate-specific antigen.
Immunotherapy Plus Chemotherapy or Radiotherapy
The combination of immunotherapy and radiotherapy may also hold promise for patients with mCRPC. In addition to reducing tumor burden, radiotherapy can be immunostimulatory under certain circumstances.25 For example, a rare phenomenon is the abscopal effect in which radiotherapy leads to regression of tumors distant from the site to which it is administered, and is believed to occur through stimulation of antitumor immune responses. In fact, there is evidence that radiation-induced tumor cell death and related changes in antigen expression and inflammatory signals can affect lymphocyte and dendritic cell activation.26,27 It is therefore logical that the immunostimulatory effects of radiotherapy could be augmented by immunotherapy.
Two ongoing, phase 2 studies are evaluating sipuleucel-T and radiotherapy for patients with mCRPC, one in combination with external beam radiation therapy (EBRT) to a single site of metastasis, and one with stereotactic ablative body radiation to multiple metastatic sites (Table 1). The use of ipilimumab after low-dose, palliative radiation therapy is also being investigated in mCRPC (NCT00861614). Combinations that may be of additional future interest could include the administration of radium Ra 223 dichloride concurrently with, or prior to, sipuleucel-T treatment. Radium Ra 223 dichloride is an alpha particle- emitting radioactive therapeutic agent that was recently approved by the FDA and is indicated for the treatment of patients with mCRPC, symptomatic bone metastases, and no known visceral metastatic disease.28 Approval was based on a large, phase 3 study that showed improved OS with radium Ra 223 dichloride versus placebo.29
Although cytotoxic chemotherapy and the concomitant use of corticosteroids can be immunosuppressive, the immunostimulatory effects of radiotherapy may also be seen with chemotherapy.30 Indeed, there is evidence that combining cytotoxic chemotherapy with immunotherapy can be beneficial under certain circumstances. For example, in a murine model of prostate cancer, low-dose cyclophosphamide given prior to GVAX greatly enhanced immune responses, resulting in tumor regression.31 The dosage and timing of cyclophosphamide were critical in this study, with additive effects observed only when cyclophosphamide was given 1 day before immunotherapy and only with doses that did not result in T-cell depletion. Similarly, in preclinical models, chemotherapy converted the tumor into a site permissive for the activation of an adaptive immune response.32 In addition, regulatory T cells may be more sensitive to low-dose cyclophosphamide treatment than other cells,33 offering further insights into how this treatment could boost antitumor immune responses. Patients with high-risk, localized prostate cancer undergoing radical prostatectomy are currently being recruited for a neoadjuvant study evaluating the combination of GVAX, cyclophosphamide, and androgen ablation (NCT01696877). The study is expected to be completed in October 2015. Importantly, the sequential use of chemotherapy 6 or fewer months prior to sipuleucel-T treatment did not appear to have a deleterious effect on APCs in the phase 4 A Registry of Sipuleucel-T Therapy in Men with Advanced Prostate Cancer (PROCEED) study, and sipuleucel-T could be generated with similar product parameters as in patients who had not received previous docetaxel treatment.34 In addition, docetaxel after sipuleucel-T treatment was associated with a significantly higher median OS compared with docetaxel after placebo in the phase 3 D9901 and D9902A studies (P = .023).35
Immunotherapy Plus Novel Antiandrogens
Several planned or ongoing clinical studies are investigating novel antiandrogens in combination with sipuleucel-T. The ongoing, randomized, phase 2 P11-3 study is investigating abiraterone acetate and prednisone given concurrently with sipuleucel-T or approximately 6 weeks afterward in asymptomatic or minimally symptomatic mCRPC (Table 1).36 In preliminary findings, despite the known immunosuppressive action of prednisone, no significant differences were reported in immunologic parameters such as APC activation, total nucleated cell and APC count, or antigen-specific humoral and cellular immune responses between sequential or concurrent administration.36 In addition, the ongoing, randomized, phase 2 P12-2 trial is exploring concurrent or sequential administration of sipuleucel-T and the androgen receptor inhibitor enzalutamide (Table 1). Two ongoing phase 2 studies are also evaluating enzalutamide plus PSATRICOM versus enzalutamide alone in patients with chemotherapy-naive mCRPC or nonmetastatic prostate cancer (NCT01867333 and NCT01875250, respectively).
Combining Multiple Immunotherapies
The immunotherapy repertoire is broadening, and early clinical studies have suggested that combining immunotherapies with a different but complementary mode of action may enhance immune responses.8 For example, in a phase 1 study in mCRPC, combined treatment with PSA-TRICOM and ipilimumab did not exacerbate the known immune-related adverse events associated with ipilimumab use, and many patients experienced a PSA decline from baseline. 19 Similarly, the combination of ipilimumab and GVAX resulted in substantial PSA declines for some mCRPC patients.37 Preclinical data have also suggested that combining agents that block CTLA-4 and programmed death-1 may boost tumor-specific immune responses.38 An overview of ongoing phase 2 clinical studies investigating sipuleucel-T combined with other immunotherapies for the treatment of mCRPC is shown in Table 1.
Future Development: Concepts for Combining Immunotherapies and Other Treatment Modalities in Earlier-Stage Prostate Cancer
Immunotherapy Plus Androgen Deprivation Therapy
Combining androgen deprivation therapy (ADT) and immunotherapy is an attractive therapeutic option, due to the acceptable toxicity profile of both agents, as well as the potential immunological action of ADT. ADT encourages T-cell trafficking to the prostate and decreases immune tolerance to self-antigens that are overexpressed on prostate cancer cells.39 ADT has also been shown to induce the thymus to produce naive T cells, which could then be activated by immunotherapy.40 With regard to timing, the most appropriate opportunity to use this combination may be at early biochemical recurrence after primary definitive therapy, when up to 40% of men present with slowly rising PSA and without any evidence of systemic progression.41 The phase 2 Sequencing of Sipuleucel-T and ADT in Men with Nonmetastatic Prostate Cancer (STAND) trial (NCT01431391) is evaluating sipuleucel-T either 2 weeks before or 3 months after the start of ADT in 68 men with biochemically recurrent prostate cancer at high risk for metastasis.42 Preliminary data suggest that tumor-specific immune responses are augmented when sipuleucel-T is administered after ADT.42 Similarly, an ongoing, open-label, crossover, phase 1 study is investigating type 1 dendritic cell-based immunotherapy in combination with androgen ablation for patients with nonmetastatic, hormone-sensitive prostate cancer (NCT00970203). These novel type-1 polarized dendritic cells are mature cells with an increased ability to stimulate T helper 1 type immune responses, which are proinflammatory and may mediate tumor elimination.2
Immunotherapy Plus Thermoab lation or Cryoablation
Cytore ductive therapies can result in necrotic cell death and release large amounts of tumor antigen, which can facilitate the development of an antitumor immune response. In a similar way, thermoablation has been shown to induce necrotic cell death in preclinical studies43 and cryoablation may also have immunostimulatory effects.44,45 Evidence suggests that combining an immunotherapy with thermo- or cryoablation may improve survival in patients with early-stage disease.45 There is some preclinical evidence that high-intensity focused ultrasound tumor ablation may also be immunostimulatory,46 potentially through similar mechanisms.
Immunotherapy Plus External Beam Radiation Therapy
In a small study of clinically localized prostate cancer, 36 patients were treated with EBRT plus a poxviral vector-based immunotherapy, and 7 patients were treated with EBRT alone.47 There were no significant differences between the treatment groups with or without immunotherapy in terms of OS and prostate cancer-specific survival. However, this was a very small study, and long-term immune responses were not generated, suggesting that the overall treatment regimen may not have been optimal.
Combined Immunotherapies
Although studies of combined immunotherapies for patients with early-stage prostate cancer are not ongoing, this is a potential combination strategy.
Conclusions
The treatment paradigm for mCRPC is evolving rapidly. Combining sipuleucel-T or future immunotherapies for mCRPC with other agents may offer substantial clinical benefits to patients. Combining a cytoreductive treatment with immunotherapy has the potential to rapidly diminish tumor burden and then slow cancer growth in the long term, extending OS. Furthermore, cytoreductive therapies can release tumor antigens and may promote a proinflammatory environment in and around the cancer lesion that could augment the action of immunotherapies. Preliminary evidence suggests that it may be possible to combine immunotherapy with radiotherapy, chemotherapy, or novel antiandrogens for mCRPC, but further clinical data are needed, as some of the cytoreductive agents, or coadministered drugs, can have immunosuppressive effects. Investigating the optimal timing and dosages will be essential to effectively combine or sequence these different therapeutic ap proaches. The use of immunotherapies with treatment modalities used in even earlier stages of prostate cancer is also under investigation.
Main Points.
The treatment paradigm for metastatic castration-resistant prostate cancer (mCRPC) is evolving rapidly. The first US Food and Drug Administration-approved vaccine for the treatment of asymptomatic or minimally symptomatic mCRPC, sipuleucel-T, or future immunotherapies, combined with other agents may offer substantial clinical benefit to patients.
Based on their success in decreasing tumor size, cytoreductive therapies can release tumor antigens and may promote a proinflammatory environment in and around the cancer lesion that could augment the action of immunotherapies.
The combination of immunotherapy with radiotherapy, chemotherapy, or novel antiandrogens for mCRPC holds promise, but further clinical data are needed, as some of the cytoreductive agents, or coadministered drugs, can have immunosuppressive effects.
Further studies on the interactions between different mCRPC treatment modalities, and investigation of the optimal timing and dosages are necessary to determine the most effective combinations and/or sequences of these approaches.
Footnotes
Medical writing assistance was provided by Katherine St. John and Joanne Swainston of Gardiner-Caldwell Communications, and funded by Dendreon Corporation. MV, NM, DA, RBS, and CMP have no conflicts of interest to disclose. RSC is/was a speaker for Dendreon Corporation. NDS is/was a consultant to and receives research support from the Dendreon Corporation. ME receives research support from the United Kingdom’s National Institute of Health Research via UCLH/ UCL Biomedical Research Centre, London UK. He is/was an investigator/advisor/speaker for GSK, Sanofi, Sonacare, Sophiris, and Nuada Medical. Dendreon Corporation supported the study design, data collection, and statistical analysis.
References
- 1.Kusmartsev S, Vieweg J. Enhancing the efficacy of cancer vaccines in urologic oncology: new directions. Nat Rev Urol. 2009;6:540–549. doi: 10.1038/nrurol.2009.177. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Butterfield LH, Tarhini AA, et al. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dendreon Corporation, authors. Sipuleucel-T prescribing information. [Accessed May 2014. 2014].
- 4.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 7.Dendreon UK Limited, authors. Sipuleucel-T summary of product characteristics. [Accessed May 2014].
- 8.Antonarakis ES. Combining active immunotherapy with immune checkpoint blockade for the treatment of advanced prostate cancer. Asian J Androl. 2012;14:520–521. doi: 10.1038/aja.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake CG. Combination immunotherapy approaches. Ann Oncol. 2012;23(suppl 8):viii41–viii46. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res. 2011;17:4558–4567. doi: 10.1158/1078-0432.CCR-10-3223. [DOI] [PubMed] [Google Scholar]
- 14.McNeel DG, Lin DW, Gardner T, et al. Correlation of increased eosinophil count following sipuleucel-T treatment with outcome in patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2012;30(4 suppl):abstract 4650. [Google Scholar]
- 15.Schellhammer PF, Chodak G, Whitmore JB, et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81:1297–1302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 16.Ribas A, Timmerman JM, Butterfield LH, Economou JS. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 2003;24:58–61. doi: 10.1016/s1471-4906(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 17.Guhathakurta D, Fan LQ, Vu T, et al. Immune response against non-targeted tumor antigens after treatment with sipuleucel-T and its association with improved clinical outcome. Eur J Cancer. 2013;49(suppl 4):S12. doi: 10.1158/1078-0432.CCR-14-2334. Abstract MC 13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 22.Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 23.Slovin SF, Wang X, Hullings M, et al. Chimeric antigen receptor (CAR+) modified T cells targeting prostate specific membrane antigen (PSMA) in patients with castrate metastatic prostate cancer. J Clin Oncol. 2013;31(suppl) abstract TPS3115. [Google Scholar]
- 24.Galsky MD, Small AC, Tsao CK, Oh WK. Clinical development of novel therapeutics for castration-resistant prostate cancer: historic challenges and recent successes. CA Cancer J Clin. 2012;62:299–308. doi: 10.3322/caac.21141. [DOI] [PubMed] [Google Scholar]
- 25.Röbel F, Frey B, Multhoff G, Gaipl U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation [published online ahead of print October 15, 2013] Cancer Lett. doi: 10.1016/j.canlet.2013.09.015. doi: 10.1016/j.canlet.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein SE, Timmerman R, McBride WH, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol. 2011;2011:439752. doi: 10.1155/2011/439752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayer Healthcare Pharmaceuticals, authors. Radium 223 prescribing information. [Accessed May 2014].
- 29.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Kepp O, Ghiringhelli F, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Wada S, Yoshimura K, Hipkiss EL, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ménard C, Martin F, Apetoh I, et al. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor activity. Cancer Immunol Immunother. 2008;57:1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higano CS, Armstrong AJ, Cooperberg MR, et al. Impact of prior docetaxel (D) on sipuleucel-T (sipuleucel-T) product parameters in PROCEED patients (pts) J Clin Oncol. 2013;31(suppl):abstract 5034. [Google Scholar]
- 35.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive sipuleucel-T (Provenge) followed by docetaxel derive greatest survival benefit. Presented at the Chemotherapy Symposium 14th Annual Meeting; November 8–11, 2006; New York, NY [Google Scholar]
- 36.Small EJ, Lance RS, Redfern CH, et al. A randomized phase II trial on sipuleucel-T with concurrent or sequential abiraterone acetate (AA) plus prednisone (P) in metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2013;31(suppl) doi: 10.1158/1078-0432.CCR-15-0079. abstract 5047. [DOI] [PubMed] [Google Scholar]
- 37.van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocytemacrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 38.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gannon PO, Poisson AO, Delvoye N, et al. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;71:496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonpavde G, Di Lorenzo G, Higano CS, et al. The role of sipuleucel-T in therapy for castration-resistant prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:639–647. doi: 10.1016/j.eururo.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 42.Antonarakis ES, Kibel AS, Adams G. et al. A randomized phase II study evaluating the optimal sequencing of sipuleucel-T and androgen deprivation therapy (ADT) in biochemically recurrent prostate cancer (BRPC): immune results. J Clin Oncol. 2013;31(suppl) abstract 5016. [Google Scholar]
- 43.Stern JM, Stanfield J, Kabbani W, et al. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urol. 2008;179:748–753. doi: 10.1016/j.juro.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 45.Sidana A, Chowdhury WH, Fuchs EJ, Rodriguez R. Cryoimmunotherapy in urologic oncology. Urology. 2010;75:1009–1014. doi: 10.1016/j.urology.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Xia JZ, Xie FL, Ran LF, et al. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol. 2012;38:1363–1371. doi: 10.1016/j.ultrasmedbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Kamrava M, Kesarwala AH, Madan RA, et al. Longterm follow-up of prostate cancer patients treated with vaccine and definitive radiation therapy. Prostate Cancer Prostatic Dis. 2012;15:289–295. doi: 10.1038/pcan.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]