Abstract
Background
The cost-effectiveness of the optimal use of hospital-based acute myocardial infarction (AMI) treatments and their potential impact on coronary heart disease (CHD) mortality in China is not well known.
Methods and Results
The effectiveness and costs of optimal use of hospital-based AMI treatments were estimated by the CHD Policy Model-China, a Markov-style computer simulation model. Changes in simulated AMI, CHD mortality, quality-adjusted life years, and total healthcare costs were the outcomes. The incremental cost-effectiveness ratio was used to assess projected cost-effectiveness. Optimal use of 4 oral drugs (aspirin, β-blockers, statins, and angiotensin-converting enzyme inhibitors) in all eligible patients with AMI or unfractionated heparin in non–ST-segment–elevation myocardial infarction was a highly cost-effective strategy (incremental cost-effectiveness ratios approximately US $3100 or less). Optimal use of reperfusion therapies in eligible patients with ST-segment–elevation myocardial infarction was moderately cost effective (incremental cost-effectiveness ratio ≤$10 700). Optimal use of clopidogrel for all eligible patients with AMI or primary percutaneous coronary intervention among high-risk patients with non–ST-segment– elevation myocardial infarction in tertiary hospitals alone was less cost effective. Use of all the selected hospital-based AMI treatment strategies together would be cost-effective and reduce the total CHD mortality rate in China by ≈9.6%.
Conclusions
Optimal use of most standard hospital-based AMI treatment strategies, especially combined strategies, would be cost effective in China. However, because so many AMI deaths occur outside of the hospital in China, the overall impact on preventing CHD deaths was projected to be modest.
Keywords: cost-benefit analysis, myocardial infarction, quality-adjusted life years, therapy
A cute myocardial infarction (AMI) is an increasing cause of death in China.1 Most Chinese patients with AMI die within the acute stage (30 days after onset), and of those who die, 75% die within the first 24 hours.2 Although reperfusion therapy and several standard oral medications (antiplatelet drugs, β-blockers, statins, and angiotensin-converting enzyme inhibitors) reduce AMI case fatality and are recommended by international and Chinese AMI management guidelines,3-6 nationwide Chinese hospital registries have found that at least half of eligible patients with AMI do not receive all standard oral medications. Fewer than 50% of patients with ST-segment–elevation myocardial infarction (STEMI) receive reperfusion therapy.7-9 Improved utilization of these acute treatment strategies is needed to reduce AMI deaths, but China’s limited healthcare resources require a careful estimate of their comparative effectiveness and cost-effectiveness in the Chinese population. Furthermore, because ≈62% of Chinese AMI deaths occur before hospital arrival,10 the potential impact of hospital-based AMI treatments on total coronary heart disease (CHD) mortality may be limited. This study aimed to estimate the effectiveness and cost-effectiveness of optimal use of key hospital-based AMI treatments, including their impact on total CHD deaths, using a computer-simulated, state-transition (Markov) model of CHD in China.
WHAT IS KNOWN
Acute myocardial infarction (AMI) is an increasing cause of death in China. Several nationwide registry studies found low utilization of key AMI acute treatments recommended by the guidelines in most Chinese hospitals.
Improved utilization of the hospital-based acute treatment strategies is needed to reduce AMI deaths, but China’s limited healthcare resources require rational optimization of limited medical resources.
Because about two thirds of Chinese AMI deaths occur before hospital arrival, the potential impact of hospital-based AMI treatments on total coronary heart disease mortality is not known.
WHAT THE STUDY ADDS
Most hospital-based AMI treatment strategies recommended by the guidelines would be highly or moderately cost effective in China.
Full and simultaneous improvements of all standard hospital-based AMI treatment strategies assessed in this study would only attribute to 9.6% reduction in the coronary heart disease mortality rate.
Given the trend toward higher absolute numbers and rates of coronary heart disease in China, prehospital emergency care, public education on symptoms of AMI, and available treatments for AMI should be improved.
Methods
CHD Policy Model-China and Its Parameters
The total number of hospitalized patients with AMI, deaths in hospitalized patients with AMI, and CHD deaths in 2013 based on the current AMI treatment status were estimated. Changes in total healthcare costs, quality-adjusted life years (QALYs), 30-day deaths of hospitalized patients with AMI, and yearly CHD deaths attributable to the optimal use of each treatment strategy were projected using the CHD Policy Model-China (Appendix Figure I in the Data Supplement).11 The Ethics Committees of both Beijing Anzhen Hospital (Beijing, China) and Columbia University Medical Center (New York, NY) informed that this simulation study need not get approval because no identifiable individual data were used.
The annual probability of first-ever hospitalized or out-of-hospital AMI, including sudden cardiac deaths, in the population without prior CHD was estimated from the China Multi-Provincial Cohort Study conditioned on the prevalence of major risk factors and relative risk of CHD associated with these risk factors.11 The yearly probability of repeat acute coronary events in patients with chronic CHD was estimated based on the prevalence of CHD and the risk of acute coronary events in patients with chronic CHD.12,13 Thirty-day deaths in hospitalized patients with AMI were projected based on the number of hospitalized patients with AMI and the 30-day case fatality rate observed in a study of AMI in Beijing.2 Total CHD deaths in 2013 comprised AMI deaths, sudden cardiac deaths, and chronic CHD deaths. To test the accuracy of model prediction over time, we used the model to compare predicted CHD mortality rates with the real mortality rates from 2000 to 2010 based on data from the World Health Organization (WHO) and National Health and Family Planning Commission of China (Appendix Figure II in the Data Supplement). A complete list of input parameter assumptions is listed in Appendix Table I in the Data Supplement.
Selection of Key Treatment Strategies and Estimation of Current Utilization
Selected key hospital-based treatments recommended by both the Chinese and international guidelines for STEMI and non–STEMI (NSTEMI)3-6 were classified as follows: A1, optimal use of aspirin, β-blockers, statins, and angiotensin-converting enzyme inhibitors in patients with AMI during the first 30 days after onset; A2, optimal use of clopidogrel in patients with AMI during the first 30 days after onset; B, optimal use of unfractionated heparin in patients with NSTEMI; C1, optimal use of primary percutaneous coronary intervention (PCI) in tertiary hospitals and thrombolysis in secondary hospitals in patients with STEMI (with a consideration of the availability of PCI technology); C2, optimal use of primary PCI in all patients with STEMI; and C3, optimal use of primary PCI in high-risk patients with NSTEMI in tertiary hospitals. Only strategies C1 and C2 are mutually exclusive and cannot be combined for implementation. For each treatment strategy, model simulation assumed improvement from the current utilization level (base case) to optimal utilization; that is, utilization of the treatment in 100% of eligible patients without known contraindications to the treatment (100%–current utilization rate–contraindication rate). Patients with contraindications to one treatment could be eligible for other treatments.
The current utilization of the standard AMI treatments—alone or in combination with other treatments—was based on the Bridging the Gap in Coronary Heart Disease Secondary Prevention in China (BRIG) project.7 The BRIG project treatment utilization estimates were similar to those in the Clinical Pathways in Acute Coronary Syndromes in China (CPACS) study8 and the Chinese Registry of Acute Coronary Events (CRACE)9 (Appendix Table II in the Data Supplement). The prevalence of contraindications to treatments was obtained from clinical trials (Table 1).15,18-22,24,25,27,33,35
Table 1.
Main Assumptions for the Simulation of Optimal Use of Hospital-Based Acute Treatments for Patients With AMI in China
| Treatments | Target Patients |
Current Utilization, % |
Effectiveness, Relative Risk (95% CI) |
Costs in the Acute Stage, Average (Range) |
Contraindications Rates, % |
Rates of Adverse Events Attributable to the Treatment, % |
|---|---|---|---|---|---|---|
| Aspirin (75 mg daily, 30 d) | AMI | 93.7BRIG | 0.77 (0.70–0.89)14 | $0.04 ($0.039–$0.048)* | 0.7 (active bleeding)15 | 0.20 (intracranial hemorrhage†)16 |
| 0.30 (serious extracranial bleeding)17 |
||||||
| β-Blockers (atenolol 50 mg daily, 30 d) |
AMI | 71.1BRIG | 0.88 (0.80–0.98)14 | $0.49 ($0.44–$0.54)* | 22.3 (multiple‡)18–21 | — |
| ACEIs (Captopril 50 mg daily, 30 d) |
AMI | 75.1BRIG | 0.94 (0.89–0.98)14 | $1.09 ($0.98–$1.20)* | 0.7 (acute deterioration of glomerular filtration rate)22 |
0.30 (angioedema)22 |
| 3.3 (hyperkalemia)22 | ||||||
| Statins (simvastatin 40 mg daily, 30 d) |
AMI | 74.1BRIG | 0.77 (0.58–1.01)23 | $27.93 ($25.14–$30.72)* | 1.0 (liver disease)24,25 | 3.4×10−5 (rhabdomyolysis)26 |
| 1.0×10−5 (liver failure)26 | ||||||
| Clopidogrel (300 mg loading dose, 75 mg daily thereafter, 30 d) |
AMI | 49.4BRIG | 0.93 (0.87–0.99)16 | $103.50 ($93.15–$113.85)* | 0.7 (active bleeding)15 | 0.58 (cerebral and major noncerebral bleeding)16 |
| 1.6 (clopidogrel hypersensitivity)27 |
||||||
| Intravenous unfractionated heparin (1200 U hourly, 70-kg patient, 3 d) |
NSTEMI | 78.8BRIG | 0.84 (0.36–1.98)28 | $8.81 ($7.93–$9.70)* | 0.7 (active bleeding)15 | 0.20 (thrombocytopenia)29 |
| 0.80 (intracranial hemorrhage)30 | ||||||
| 2.20 (serious extracranial hemorrhage)31 |
||||||
| Thrombolysis with streptokinase |
STEMI | 21.7BRIG§ | 0.75 (0.71–0.79)14 | $2588 ($2329–$2846)32 | 6.80 (multipleǁ)33 | 0.31 (intracranial hemorrhage)34 |
| 0.41 (death attributable to thrombolysis)34 |
||||||
| PCI | STEMI | 27.8BRIG | 0.50 (0.35–0.71)14 | $5253 ($4728–$5779)¶ | 10.0 (ineligible patients)35 | 0.53 (death attributable to PCI)36 |
| PCI | NSTEMI | 25.0BRIG | 0.75 (0.63–0.90)37 | $5253 ($4728–$5779)¶ | 10.0 (ineligible patients)35 | 0.53 (death attributable to PCI)36 |
Ranges in parentheses were used in sensitivity analyses. ACEIs indicates angiotensin-converting enzyme inhibitors; AMI, acute myocardial infarction; BRIG, Bridging the Gap in Coronary Heart Disease Secondary Prevention in China Project; CI, confidence interval; NSTEMI, non–ST-segment–elevation myocardial infarction; PCI, percutaneous coronary interventions; and STEMI, ST-segment–elevation myocardial infarction.
Data from maximum retail price for each essential medicine set by the Evaluation Center of Drug Pricing, National Development and Reform Commission.
Case fatality rate of intracranial hemorrhage (40.7%) was from the average rate from 1999 to 2004 in the Sino-MONICA-Beijing Project.
Severe chronic obstructive pulmonary disease (1.8%), heart failure (pulmonary edema 1.0%, cardiogenic shock 1.0%), second- or third-degree heart block on the initial ECG (1.5%), and bradycardia-hypotension syndrome (17%).
Utilization rate of thrombolytic agents.
Hypertension (blood pressure >180/110 mm Hg), recent surgery or trauma, recent gastrointestinal or genitourinary bleeding, stroke, and bleeding diathesis.
Cost was estimated by the data from China’s Health Statistics Yearbook and Beijing Public Health Information Center.
Treatment Cost Estimation
Increased costs attributable to the optimization of acute treatment were calculated by multiplying the number of additional patients resulting from optimal utilization by the mean cost of the treatment. Total healthcare costs comprised acute treatment costs plus age- and sex-specific yearly per capita background healthcare costs as estimated from the Chinese National Health Services Survey38 and the China World Health Survey.39 All costs were estimated from a societal perspective and were inflated to 2013 using the average rate of inflation in China from 2008 to 2013 published by Trading Economics and then converted into US dollars ($) according to the exchange rate published by the Bank of China (1 Chinese yuan=$0.1611; accessed May 15, 2013).
Drug costs were obtained from the essential medicines maximum retail price list updated by the Evaluation Center of Drug Pricing, National Development and Reform Commission. The costs of reper-fusion therapies were estimated by the data from the China Health Statistics Yearbook, the Beijing Public Health Information Center, and health economics research.1,32 Detailed costs are listed in Table 1.
Effectiveness Estimation
Effectiveness of optimal use of the key AMI treatments was projected based on changes in QALYs gained, 30-day in-hospital AMI deaths prevented, and change in total CHD deaths. Patients with AMI who survived because of increased utilization of 1 of the treatment strategies would still be at risk for a chronic CHD death during the remainder of the year. The increased number of patients with AMI who survived to the end of the year because of optimized treatment utilization was equal to the total number of prevented CHD deaths.
The number of QALYs saved by optimal use of acute treatments was calculated by multiplying the number of life years gained from AMI survivors by the QALY weights of health states in both the first 30 days after AMI onset and AMI survivors in the chronic CHD state. QALY weights were calculated as 1–disability-adjusted life year weights estimated in the Global Burden of Disease 2010 Study.40 A QALY weight of 0.44 was applied for the first 30 days after a nonfatal AMI. The QALY weight for chronic AMI survivors was 0.90, considering that a proportion of survivors had symptoms of angina or heart failure.
The 30-day all-cause mortality benefits of selected treatments were mainly derived from the results of large meta-analyses or systematic reviews of randomized controlled clinical trials (Table 1).14,16,23,28,37 When multiple treatments were applied in combination, it was assumed that the effects were independent (Table 2).41,42
Table 2.
Utilization Rate and Relative Risk of Different Combinations of the Selected Drugs in Chinese AMI Inpatients
| Without Clopidogrel |
With Clopidogrel |
||||
|---|---|---|---|---|---|
| Combinations | Utilization Rate, %* |
Utilization Rate, % |
RR or Combined RR |
Utilization Rate, % |
RR or Combined RR |
| No drugs | 1.6 | 1.5 | 1.00 | 0.1 | 0.9316 |
| Aspirin | 3.5 | 2.7 | 0.7714 | 0.8 | 0.72 |
| β-Blockers | 0.5 | 0.4 | 0.8814 | 0.1 | 0.82 |
| Statins | 0.7 | 0.4 | 0.7723 | 0.3 | 0.72 |
| ACEIs/ARBs | 0.4 | 0.4 | 0.9414 | 0.0 | 0.87 |
| Aspirin+β-blockers | 3.8 | 2.8 | 0.68 | 1.0 | 0.63 |
| Aspirin+statins | 4.8 | 2.2 | 0.59 | 2.6 | 0.55 |
| Aspirin+ACEIs/ARBs | 4.1 | 3.3 | 0.72 | 0.8 | 0.67 |
| β-Blockers+statins | 0.4 | 0.1 | 0.68 | 0.3 | 0.63 |
| β-Blockers+ACEIs/ARBs | 1.1 | 0.9 | 0.83 | 0.2 | 0.77 |
| Statins+ACEIs/ARBs | 0.8 | 0.6 | 0.72 | 0.2 | 0.67 |
| Aspirin+β-blockers+statins | 9.8 | 4.9 | 0.52 | 4.9 | 0.48 |
| Aspirin+β-blockers+ACEIs/ARBs | 11.0 | 8.3 | 0.64 | 2.7 | 0.60 |
| Aspirin+statins+ACEIs/ARBs | 13.1 | 5.8 | 0.56 | 7.3 | 0.52 |
| β-Blockers+statins+ACEIs/ARBs | 0.9 | 0.4 | 0.64 | 0.5 | 0.60 |
| Aspirin+β-blockers+statins+ACEIs/ARBs | 43.6 | 16.0 | 0.49 | 27.6 | 0.46 |
ACEIs indicates angiotensin-converting enzyme inhibitors; AMI, acute myocardial infarction; ARBs, angiotensin receptor blockers; and RR, relative risk.
Different combinations of the use of aspirin, (β-blockers, ACEIs/ARBs, and statins.
For the proportion of treated patients estimated to experience treatment-related major adverse events during the first 30 days after AMI onset, QALY penalties were applied for the entire 30 days (0.39 for intracranial hemorrhage, 0.80 for extracranial bleeding, 0.91 for rhabdomyolysis, and 0.67 for transient liver failure).40,43,44 The incidence of treatment-related major severe adverse events was obtained from clinical trials (Table 1).16,17,22,26,29-31,34,36
Cost-Effectiveness Analysis
Incremental cost-effectiveness ratios (ICERs) were used to evaluate the cost-effectiveness of optimal use of the key treatments. ICERs were calculated by dividing the incremental change in total healthcare costs by the incremental change in QALYs.45 Cost-effectiveness was assessed both by comparing each individual or combined strategy with the current utilization (Table 3 and Appendix Table III in the Data Supplement, comparison with the base case) and by comparing each one of a succession of combination treatment strategies with prior simpler-to-implement strategies (incremental analysis, Table 4 and Figure). The metric recommended by WHO Choosing Interventions That Are Cost Effective (WHO-CHOICE) was used to assess the degree of cost-effectiveness (highly cost-effective, ICER less than the gross domestic product [GDP] per capita; moderately cost-effective, ICER of 1–3×GDP per capita; and not cost-effective, ICER of >3×GDP per capita).46 The GDP per capita of China in 2013 was estimated to be $5721 by the GDP per capita of China ($5445 in 2011) published by the World Bank, which was adjusted upward by the inflation rates in 2012 and 2013 published by Trading Economics.
Table 3.
Single Treatments Analysis: Simulated Cost and Effectiveness of Optimal Use of Selected Individual Hospital-Based Treatment Strategies for Patients With AMI in China
| In-Hospital AMI Deaths (in the Acute Stage) |
Prevented In- Hospital AMI Deaths |
In-Hospital AMI Case Fatality Rate, % |
Total No. of Annual CHD Deaths |
Prevented CHD Deaths |
CHD Mortality Rate (1/100 000) |
Increased QALYs (1000s) |
Increased Acute Treatment Costs (1000s) |
Increased Total Healthcare Costs (1000s) |
Cost-Effectiveness (Compared With Base Case) |
|---|---|---|---|---|---|---|---|---|---|
| Current utilization (base case) | |||||||||
| 255 600 | — | 26.8 | 649 200 | — | 95.7 | — | — | — | — |
| Strategy A1: the 4 oral drugs in patients with AMI* | |||||||||
| 245 800 | 9800 | 25.7 | 640 300 | 8900 | 94.4 | 22 | $34 952 | $67 764 | $3100 |
| Strategy A2: clopidogrel in patients with AMI | |||||||||
| 252 400 | 3200 | 26.4 | 646 300 | 3000 | 95.3 | 7 | $112 533 | $123 363 | $17 600 |
| Strategy B: unfractionated heparin in patients with NSTEMI | |||||||||
| 253 700 | 1900 | 26.6 | 647 500 | 1800 | 95.4 | 4 | $5011 | $11 370 | $2800 |
| Strategy C1: PCI in tertiary hospitals and thrombolysis with streptokinase in secondary hospitals in patients with STEMI | |||||||||
| 219 300 | 36300 | 23.0 | 616 000 | 33 200 | 90.8 | 81 | $610 268 | $731 779 | $9000 |
| Strategy C2: primary PCI in all patients with STEMI | |||||||||
| 202 000 | 53 600 | 21.2 | 600 100 | 49 100 | 88.5 | 120 | $1 099 239 | $1 278 357 | $10 700 |
| Strategy C3: primary PCI in high-risk patients with NSTEMI in tertiary hospitals | |||||||||
| 252 300 | 3300 | 26.4 | 646 200 | 3000 | 95.3 | 7 | $152 974 | $163 551 | $23 400 |
AMI indicates acute myocardial infarction; CHD, coronary heart disease; NSTEMI, non–ST-segment–elevation myocardial infarction; PCI, primary percutaneous coronary intervention; QALYs, quality-adjusted life years; and STEMI, ST-segment–elevation myocardial infarction.
Aspirin, β-blockers, angiotensin-converting enzyme inhibitors, and statins.
Table 4.
Incremental Analysis: Simulated Cost-Effectiveness of Combined Strategies for Optimal Use of Hospital-Based Treatment Strategies for Patients With AMI in China
| In-Hospital AMI Deaths Prevented |
QALYs (1000s) |
Annual Healthcare Costs (1000s) |
ICER* | 95% Uncertainty Interval† |
Comparator | Percent Moderately Cost Effective |
Percent Very Cost Effective |
|---|---|---|---|---|---|---|---|
| Current utilization (base case) | |||||||
| — | 674 058 | $583 613 093 | — | — | — | — | — |
| Strategy A1 (4 oral drugs in patients with AMI)‡ | |||||||
| 9800 | 674 080 | $583 680 857 | Dominated by A1 +B§ |
— | Base case | — | — |
| Strategy A1+B (unfractionated heparin in patients with NSTEMI) | |||||||
| 11 700 | 674 084 | $583 692 227 | $3000 | $1900–4400 | Base case | 99.4% | 97.7% |
| Strategy A1+B+A2 (clopidogrel in patients with AMI) | |||||||
| 14 900 | 674 091 | $583 815 590 | Dominated by A1+B+C1 |
— | A1+B | — | — |
| Strategy A1+B+C1 (PCI in tertiary hospitals and thrombolysis with streptokinase in secondary hospitals in patients with STEMI) | |||||||
| 48 000 | 674 166 | $584 424 005 | $8900 | $6400–19 300 | A1+B | 92.7% | 0.8% |
| Strategy A1+B+C1+C3 (primary PCI in high-risk patients with NSTEMI in tertiary hospitals) | |||||||
| 51 300 | 674 173 | $584 587 556 | $23 400 | $18 000–29 500 | A1+B+C1 | 1.6% | 0.0% |
AMI indicates acute myocardial infarction; ICERs, incremental cost-effectiveness ratios; NSTEMI, non–ST-segment–elevation myocardial infarction; PCI, primary percutaneous coronary intervention; QALYs, quality-adjusted life years; and STEMI, ST-segment–elevation myocardial infarction.
Each strategy was compared with the base case or prior most cost-effective strategy. ICERs were calculated as incremental change in total healthcare costs divided by incremental change in QALYs. Results were rounded to the nearest 100.
The 95% uncertainty interval of the ICERs from the result of probabilistic sensitivity.
Aspirin, β-blockers, angiotensin-converting enzyme inhibitors, and statins.
Dominated strategies are both less effective and more costly when compared with the extension. That is, although full implementation of the next strategy (row below in table) would need more investment than the dominated strategy, partial implementation of the next strategy would cost the same amount as but will be more effective than full implementation of the dominated strategy.
Figure.
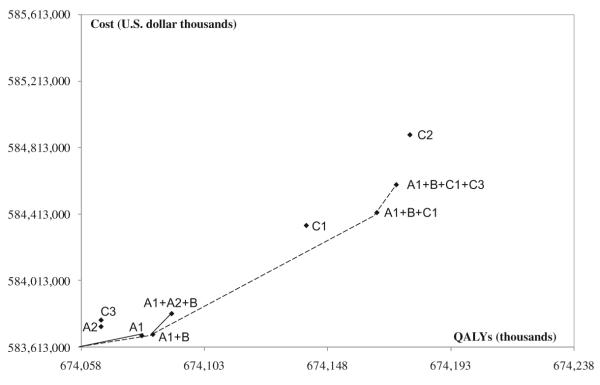
Effectiveness and costs of selected key hospital-based treatment strategies for patients with acute myocardial infarction (AMI) in China. The slope of lines in the figure represents the incremental cost-effectiveness ratios by comparing each 1 of a succession of combination treatment strategies to the prior simpler strategies. Shallower slopes are more cost-effective; steep slopes less cost-effective. A1, Four oral drugs in patients with AMI (aspirin, β-blockers, angiotensin-converting enzyme inhibitors, and statins). A2, Clopidogrel in patients with AMI. B, Unfractionated heparin in patients with non–ST-segment–elevation myocardial infarction (NSTEMI). C1, Primary percutaneous coronary intervention (PCI) in tertiary hospitals and thrombolysis with streptokinase in secondary hospitals in patients with ST-segment–elevation myocardial infarction (STEMI). C2, Primary PCI in all patients with STEMI. C3, Primary PCI in high-risk patients with NSTEMI in tertiary hospitals. QALYs indicates quality-adjusted life years.
Sensitivity Analyses
One-way sensitivity analyses were performed to explore the impact of assuming upper- or lower-limit bounds for the main analysis effectiveness and cost inputs on the results (ranges shown in Table 1). Monte Carlo probabilistic sensitivity analysis was used to simultaneously explore the uncertainty distributions of 2 key input assumptions: relative risk for all-cause mortality related to treatments compared with no treatment and treatment costs. Effectiveness and costs of each strategy were randomly drawn 1000 times from the effectiveness and cost uncertainty distributions and entered into simulations. The resulting 1000 pairs of total costs and QALYs were used to calculate the upper and lower 95% bounds of their ICERs and the proportion of ICERs under the predetermined thresholds for moderately cost-effective and very cost-effective.
Results
Main Analysis
Assuming the current level of risk factors and hospital-based AMI treatment utilization, the CHD Policy Model-China projected ≈1 534 700 acute coronary events, 954 800 in-hospital AMIs, and 649 200 CHD deaths in Chinese adults 35 to 84 years of age in 2013. The estimated crude CHD mortality rate was 95.7 per 100 000. Approximately 255 600 in-hospital AMI deaths (including patients with AMI who died in the emergency room) were predicted to occur within 30 days after AMI onset (Table 3).
When each single strategy was compared with the base case, optimal use of primary PCI for all eligible patients with STEMI in all hospitals (strategy C2) was the most effective treatment, potentially preventing ≈53 600 hospitalized AMI deaths in the acute stage and 49 100 CHD deaths in the whole year because 4500 patients died after 30 days to the end of 1 year. This would represent a 7.2% decrease in the total CHD mortality rate and add ≈120 000 QALYs in 2013 (Table 3). Optimal use of primary PCI in tertiary hospitals and thrombolysis with streptokinase in secondary hospitals in patients with STEMI (strategy C1) also seemed to be effective, potentially averting ≈36 300 AMI deaths in the acute stage and preventing ≈33 200 CHD deaths in the whole year, which would represent a 4.9% decline in the total CHD mortality rate and add ≈81 000 QALYs. As individual strategies, optimal use of neither clopidogrel for all patients with AMI nor primary PCI among eligible high-risk patients with NSTEMI in tertiary hospitals was cost-effective compared with the base case.
When the incremental cost-effectiveness of the treatment strategies was assessed, the combination of unfractionated heparin in all eligible patients with NSTEMI (strategy B) plus the 4 standard oral medications in all eligible patients with AMI (strategy A1) was a highly cost-effective strategy (ICER of $3000; Table 4 and Figure). Adding optimal use of primary PCI for STEMI in tertiary hospitals and thrombolysis with streptokinase for STEMI in secondary hospitals or primary PCI for patients with STEMI in all hospitals was moderately cost-effective (ICER of <$10 700; in the range of 1–3×GDP per capita). Strategies including clopidogrel for all AMI (strategy A1+A2+B) and adding primary PCI for NSTEMI (strategy A1+A2+B+C1+C3, data not shown) were both dominated by more cost-effective combination strategies that did not include these 2 treatments (Table 4).
Optimal use of each individual treatment was estimated to prevent 1800 to 49 100 CHD deaths, which would represent a 0.3% to 7.2% reduction in the CHD mortality rate. Optimal use of the 4 standard oral medications in all eligible hospitalized patients with AMI (strategy A1), which is the easiest strategy to implement rapidly, would reduce the total CHD mortality rate by only 1.3%. Implementing all strategies in combination (strategy A1+A2+B+C2+C3), regardless of cost-effectiveness, would decrease the CHD mortality rate by 9.6% at most.
Sensitivity Analyses
Varying cost inputs in 1-way sensitivity analyses led to little change in the ICERs (Appendix Table III in the Data Supplement), even at their extreme ranges. For example, compared with the base case, assuming higher costs of the 4 oral medications and heparin would lead to only a slightly higher ICER ($3100 per QALY gained), as would the upper range of the PCI cost for patients with STEMI (ICER of $11 500). Assuming a lower PCI cost did not substantially improve the cost-effectiveness of primary PCI for high-risk patients with NSTEMI (ICER of $21 300). In probabilistic sensitivity analyses, the strategy combining 4 standard oral medications for all AMI and heparin for NSTEMI was very cost-effective in 97.7% of simulations. The strategy that added PCI for STEMI in tertiary hospitals and thrombolysis in secondary hospitals was moderately cost-effective in 92.7% of simulations and rarely very cost-effective (0.8%). Adding primary PCI for NSTEMI to these strategies was moderately cost-effective in only 1.6% of simulations and never very cost-effective (Table 4).
Discussion
This study evaluated the cost-effectiveness of several AMI treatment strategies and their impact on total CHD mortality using the CHD Policy Model-China. The results suggest that optimal use of the 4 standard oral medications in all eligible patients with AMI , unfractionated heparin in patients with NSTEMI, or any of the STEMI reperfusion strategies (primary PCI alone or in combination with thrombolysis using streptokinase) would be cost-effective treatment strategies in China. Solely improving use of clopidogrel for patients with AMI or primary PCI among high-risk patients with NSTEMI in tertiary hospitals did not seem to be cost-effective strategies, at least according to the WHO-CHOICE standard.46 However, we also estimated that optimal use of all hospital-based AMI treatments in this study would decrease China’s total CHD mortality (inclusive of acute in- and out-of-hospital deaths) by 9.6% at most.
Our results suggest that several hospital-based AMI treatment strategies were effective and very cost-effective and should be promoted. However, full implementation of even the most cost-effective treatment strategies would face big challenges in China. First, some low-income patients with AMI cannot afford the high out-of-pocket payments required for some treatments. The CPACS study showed that 69% of patients were nonadherent to statins treatment because of high out-of-pocket costs.8 China’s basic medical insurance coverage had increased to 95% in 2011, and China’s 12th Five-Year Plan set a goal of lowering individual out-of-pocket payments to <30% of the share of total health expenditures.47 These 2 changes may lower the current obstacle of high out-of-pocket costs for individual patients with AMI. Second, there is currently no systematic program for overseeing the implementation of clinical practice guidelines in China. The American Heart Association Get With The Guidelines program has succeeded in lowering AMI case fatality by promoting and implementing practice guidelines, providing a blueprint for other countries.48
A nationwide investigation of 728 PCI-capable hospitals showed that the average numbers of PCI-capable hospitals were 40.1, 25.5, and 20.1 per 10 million of the population in the cities of Beijing, Tianjin, and Shanghai, respectively. In contrast, the national average was 8.1 per 10 million.49 The results show the geographic disparities in PCI capabilities in China. Improvements in PCI capabilities would require large investments: building catheterization laboratories, adding equipment, and training qualified medical personnel— none of which could be achieved in the short term. The National Health and Family Planning Commission enacted Cardiovascular Interventional Technology Practice Standards aimed at improving the quality of medical care, but these standards had a limited impact on the development of PCI, especially in secondary hospitals. These practical limitations led us to simulate a strategy to improve the use of primary PCI for STEMI in tertiary hospitals combined with thrombolysis for STEMI in secondary hospitals. This strategy seems to be moderately cost-effective, potentially preventing 36 300 AMI deaths, which would represent a 5.1% reduction in the CHD mortality rate. For all Chinese patients with STEMI, the logistical challenge of decreasing the door-to-balloon and door-to-needle time for reperfusion therapy must be urgently addressed. In a recent study of patients admitted to Beijing tertiary hospitals in which cardiac catheterization was available 24 hours a day, only 7% of patients treated with thrombolysis met the guidelines’ goal of a door-to-needle time of <30 minutes; 22% of patients underwent PCI in <90 minutes.50
Simultaneous and optimal use of all treatment strategies selected by this study was estimated to decrease the total CHD mortality by 9.6%, an impact that was limited by the large proportion of prehospital AMI deaths in China. A surveillance study covering the entire Beijing area showed that 62% of acute coronary events deaths occurred before arriving at the hospital in 2009.10 Meanwhile, 79% of prehospital deaths of acute coronary events occurred at home.10 One reason for this high out-of-hospital fraction may be a low awareness of the importance of obtaining timely medical care. A study in Beijing tertiary hospitals showed that the median time from symptom onset to admission of the patients with STEMI was 140 minutes.50 Another investigation showed that only 7.1% of patients with AMI called the emergency number immediately at the time of symptoms onset.51 The increasing trend in incidence of acute coronary events is yet another challenge for the ability of China’s healthcare system to reduce CHD deaths.52
Limitations of the Analysis
Similar to any computer simulation-based comparative effectiveness analysis, this study was limited by reliance on multiple assumptions and data inputs from diverse studies. We chose high-quality epidemiological data and China-specific, nationally representative data to construct the model. The medication use rates of BRIG participants used in this study were similar to those measured in the CPACS8 and the CRACE study.9 Twenty-four parameters involving treatment effectiveness, contraindications, and major severe adverse event rates in our model were based on American or European studies or large international meta-analyses, systematic reviews, or randomized controlled trials because no qualified parameters were available from studies conducted in China or among Chinese patients. However, we did not find enough evidence to indicate ethnic differences between Chinese patients with AMI and patients of other ethnicities among the parameters we used. As our sensitivity analyses suggested, our results were consistent across a range of all key model inputs. Urokinase is most widely used in fibrinolytic therapy for patients with STEMI in China, but few data on the clinical effectiveness of this medication have been published (ie, data on changes in 30-day all-cause mortality related to treatment). Assuming that the 2 thrombolytic agents have a similar effect on reducing 30-day all-cause mortality and similar adverse event profiles, the cost-effectiveness of a reperfusion strategy using urokinase for patients with STEMI would be similar to that of streptokinase (Appendix Table III in the Data Supplement).
This study evaluated the cost-effectiveness of optimizing the use of several hospital-based AMI treatment strategies. However, the evaluation did not include specific costs of system-level changes that might be required to achieve the assumed utilization rates (eg, infrastructure and workforce expansion, physician training, or quality improvement), so the costs may be underestimated. In this study, we assumed that the effectiveness of each treatment had an independent effect among the combinations of treatment strategies.
Conclusions
Using the WHO-CHOICE standard, most treatment strategies recommended by guidelines would be highly or moderately cost-effective in China. Improved primary PCI for patients with STEMI would prevent the largest number of hospitalized AMI deaths. Because so many AMI deaths occur outside of the hospital in China, full and simultaneous improvements of all standard hospital-based AMI treatment strategies assessed in this study would decrease CHD mortality by <10%. Improvement of the capacity of prehospital care for patients with AMI is urgently needed in China.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by grants from the National Program on Key Basic Research Project of China (contract No. 2012CB517806), the National Science & Technology Pillar Program during the 12th Five-Year Plan Period (contract Nos. 2011BAI09B01 and 2011BAI11B03), and the Capital Health Research and Development of Special (contract No. 2011-1005-01) to Drs Zhao and Liu and the Mentored Career Development Award (contract No. K08HL089675) from the National Heart, Lung, and Blood Institute of the U.S. National Institutes of Health to Dr Moran. These funding bodies had no role in the study design, data analysis, data interpretation, or writing of the manuscript.
Footnotes
The Data Supplement is available at http://circoutcomes.ahajournals.org/lookup/suppl/doi:10.1161/CIRCOUTCOMES.113.000674/-/DC1.
Disclosures
None.
References
- 1.Ministry of Health . China Health Statistics Yearbook in 2011. China Union Medical University Press; Beijing, China: 2011. [Google Scholar]
- 2.Wang W, Zhao D, Yao L, Zhou M, Wu Z. Population-based epidemiological study for case fatality of acute coronary events in Beijing. Chin J Cardiol. 2000;28:228–230. [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 4.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 5.Cardiology Branch of Chinese Medical Association, Editor Committee of Chinese Journal of Cardiology Guidelines for the diagnosis and management of acute ST-segment elevation myocardial infarction. Chin J Cardiol. 2010;38:675–690. [Google Scholar]
- 6.Chinese Society of Cardiology of Chinese Medical Association Editorial Board of Chinese Journal of Cardiology Guideline for diagnosis and treatment of patients with chronic stable angina (no abstract) Chin J Cardiol. 2007;35:195–206. [PubMed] [Google Scholar]
- 7.Liu Q, Zhao D, Liu J, Wang W. Current clinical practice patterns and outcome for acute coronary syndromes in China: results of BRIG project. Chin J Cardiol. 2009;37:213–217. [PubMed] [Google Scholar]
- 8.Bi Y, Gao R, Patel A, Su S, Gao W, Hu D, Huang D, Kong L, Qi W, Wu Y, Yang Y, Turnbull F, CPACS Investigators Evidence-based medication use among Chinese patients with acute coronary syndromes at the time of hospital discharge and 1 year after hospitalization: results from the Clinical Pathways for Acute Coronary Syndromes in China (CPACS) study. Am Heart J. 2009;157:509–516. doi: 10.1016/j.ahj.2008.09.026. e1. [DOI] [PubMed] [Google Scholar]
- 9.Song XT, Chen YD, Pan WQ, Lü SZ. CRACE investigators. Gender based differences in patients with acute coronary syndrome: findings from Chinese Registry of Acute Coronary Events (CRACE) Chin Med J (Engl) 2007;120:1063–1067. [PubMed] [Google Scholar]
- 10.Gao YL, Su JT, Wei ZH, Liu JL, Wang J. Characteristics of out-of-hospital acute coronary heart disease deaths of Beijing permanent residents at the age of 25 or more from 2007 to 2009. Chin J Cardiol. 2012;40:199–203. [PubMed] [Google Scholar]
- 11.Moran A, Gu D, Zhao D, Coxson P, Wang YC, Chen CS, Liu J, Cheng J, Bibbins-Domingo K, Shen YM, He J, Goldman L. Future cardiovascular disease in China: Markov model and risk factor scenario projections from the coronary heart disease policy model-China. Circ Cardiovasc Qual Outcomes. 2010;3:243–252. doi: 10.1161/CIRCOUTCOMES.109.910711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosengren A, Wilhelmsen L, Hagman M, Wedel H. Natural history of myocardial infarction and angina pectoris in a general population sample of middle-aged men: a 16-year follow-up of the Primary Prevention Study, Göteborg, Sweden. J Intern Med. 1998;244:495–505. doi: 10.1111/j.1365-2796.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- 13.Lampe FC, Whincup PH, Wannamethee SG, Shaper AG, Walker M, Ebrahim S. The natural history of prevalent ischaemic heart disease in middle-aged men. Eur Heart J. 2000;21:1052–1062. doi: 10.1053/euhj.1999.1866. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich PA, McClellan M. Trends in treatment and outcomes for acute myocardial infarction: 1975-1995. Am J Med. 2001;110:165–174. doi: 10.1016/s0002-9343(00)00712-9. [DOI] [PubMed] [Google Scholar]
- 15.Frilling B, Schiele R, Gitt AK, Zahn R, Schneider S, Glunz HG, Gieseler U, Baumgärtel B, Asbeck F, Senges J, Maximum Individual Therapy in Acute Myocardial Infarction (MITRA); Myocardial Infarction Registry (MIR) Study Groups Characterization and clinical course of patients not receiving aspirin for acute myocardial infarction: results from the MITRA and MIR studies. Am Heart J. 2001;141:200–205. doi: 10.1067/mhj.2001.112681. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS, COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 17.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Graaf FR, Schuijf JD, van Velzen JE, Kroft LJ, de Roos A, Sieders A, Jukema JW, Schalij MJ, van der Wall EE, Bax JJ. Evaluation of contraindications and efficacy of oral Beta blockade before computed tomographic coronary angiography. Am J Cardiol. 2010;105:767–772. doi: 10.1016/j.amjcard.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 19.Killip T, III, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 20.Rathore SS, Gersh BJ, Berger PB, Weinfurt KP, Oetgen WJ, Schulman KA, Solomon AJ. Acute myocardial infarction complicated by heart block in the elderly: prevalence and outcomes. Am Heart J. 2001;141:47–54. doi: 10.1067/mhj.2001.111259. [DOI] [PubMed] [Google Scholar]
- 21.Chadda KD, Lichstein E, Gupta PK, Choy R. Bradycardia-hypotension syndrome in acute myocardial infarction: reappraisal of the overdrive effects of atropine. Am J Med. 1975;59:158–164. doi: 10.1016/0002-9343(75)90349-6. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 23.Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA, Kayikçioglu M, Arntz HR, den Hartog FR, Veeger NJ, Colivicchi F, Dupuis J, Okazaki S, Wright RS, Bucher HC, Nordmann AJ. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA. 2006;295:2046–2056. doi: 10.1001/jama.295.17.2046. [DOI] [PubMed] [Google Scholar]
- 24.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 25.Cohen DE, Anania FA, Chalasani N, National Lipid Association Statin Safety Task Force Liver Expert Panel An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Cheema AN, Mohammad A, Hong T, Jakubovic HR, Parmar GS, Sharieff W, Garvey MB, Kutryk MJ, Fam NP, Graham JJ, Chisholm RJ. Characterization of clopidogrel hypersensitivity reactions and management with oral steroids without clopidogrel discontinuation. J Am Coll Cardiol. 2011;58:1445–1454. doi: 10.1016/j.jacc.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Magee KD, Campbell SG, Moher D, Rowe BH. Heparin versus placebo for acute coronary syndromes. Cochrane Database Syst Rev. 2008:CD003462. doi: 10.1002/14651858.CD003462.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Warkentin TE, Eikelboom JW. Who is (still) getting HIT? Chest. 2007;131:1620–1622. doi: 10.1378/chest.07-0425. [DOI] [PubMed] [Google Scholar]
- 30.Sabatine MS, Morrow DA, Dalby A, Pfisterer M, Duris T, Lopez-Sendon J, Murphy SA, Gao R, Antman EM, Braunwald E. ExTRACT-TIMI 25 Investigators. Efficacy and safety of enoxaparin versus unfractionated heparin in patients with ST-segment elevation myocardial infarction also treated with clopidogrel. J Am Coll Cardiol. 2007;49:2256–2263. doi: 10.1016/j.jacc.2007.01.092. [DOI] [PubMed] [Google Scholar]
- 31.Blazing MA, de Lemos JA, White HD, Fox KA, Verheugt FW, Ardissino D, DiBattiste PM, Palmisano J, Bilheimer DW, Snapinn SM, Ramsey KE, Gardner LH, Hasselblad V, Pfeffer MA, Lewis EF, Braunwald E, Califf RM, “A to Z” Investigators Safety and efficacy of enoxaparin vs unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA. 2004;292:55–64. doi: 10.1001/jama.292.1.55. [DOI] [PubMed] [Google Scholar]
- 32.Jiang L. An economic evaluation of thrombolysis in myocardial infarction. China Prac Med. 2008;3:101–103. [Google Scholar]
- 33.French JK, Williams BF, Hart HH, Wyatt S, Poole JE, Ingram C, Ellis CJ, Williams MG, White HD. Prospective evaluation of eligibility for thrombolytic therapy in acute myocardial infarction. BMJ. 1996;312:1637–1641. doi: 10.1136/bmj.312.7047.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.TERIMA Group of Investigators TERIMA-2: national extension of thrombolytic treatment with recombinant streptokinase in acute myocardial infarct in Cuba. Thromb Haemost. 2000;84:949–954. [PubMed] [Google Scholar]
- 35.King SB, III, Yeh W, Holubkov R, Baim DS, Sopko G, Desvigne-Nickens P, Holmes DR, Jr, Cowley MJ, Bourassa MG, Margolis J, Detre KM. Balloon angioplasty versus new device intervention: clinical outcomes. A comparison of the NHLBI PTCA and NACI registries. J Am Coll Cardiol. 1998;31:558–566. doi: 10.1016/s0735-1097(97)10523-x. [DOI] [PubMed] [Google Scholar]
- 36.Malenka DJ, O’Rourke D, Miller MA, Hearne MJ, Shubrooks S, Kellett MA, Jr, Robb JF, O’Meara JR, VerLee P, Bradley WA, Wennberg D, Ryan T, Jr, Vaitkus PT, Hettleman B, Watkins MW, McGrath PD, O’Connor GT, The Northern New England Cardiovascular Disease Study Group Cause of in-hospital death in 12,232 consecutive patients undergoing percutaneous transluminal coronary angioplasty. Am Heart J. 1999;137::632–638. doi: 10.1016/s0002-8703(99)70215-2. 4 pt 1. [DOI] [PubMed] [Google Scholar]
- 37.Sarkees ML, Bavry AA. Acute coronary syndrome (unstable angina and non-ST elevation MI) Clin Evid (Online) 2009;1:209. [PMC free article] [PubMed] [Google Scholar]
- 38.Centre for Health Statistics and Information, Ministry of Health . An Analysis Report of National Health Services Survey in 2003. Peking Union Medical College Press; Beijing, China: 2004. [Google Scholar]
- 39.World Health Organization . China World Health Survey 2002. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 40.Moran AE, Forouzanfar MH, Roth G, Mensah GA, Ezzati M, Flaxman A, Murray CJL, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: The Global Burden of Disease 2010 study. Circulation. doi: 10.1161/CIRCULATIONAHA.113.004046. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hippisley-Cox J, Coupland C. Effect of combinations of drugs on all cause mortality in patients with ischaemic heart disease: nested case-control analysis. BMJ. 2005;330:1059–1063. doi: 10.1136/bmj.330.7499.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005;293:699–706. doi: 10.1001/jama.293.6.699. [DOI] [PubMed] [Google Scholar]
- 44.Mathers CD, Lopez AD, Murray CJL. The burden of disease and mortality by condition: data, methods, and results for 2001. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, editors. Global Burden of Disease and Risk Factors. Vol. 45. World Bank; Washington, DC: 2006. 240 pp. [PubMed] [Google Scholar]
- 45.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 46.Baltussen RMAT, Tan Torres T, Hutubessy RC, Acharya A, Evans DB, Murray CJ. Generalized Cost-Effectiveness Analysis: A Guide. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 47.Zhang L, Liu N. Health reform and out-of-pocket payments: lessons from China. Health Policy Plan. 2013 doi: 10.1093/heapol/czt006. Published ahead of print February 21, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Eagle KA, Montoye CK, Riba AL, DeFranco AC, Parrish R, Skorcz S, Baker PL, Faul J, Jani SM, Chen B, Roychoudhury C, Elma MA, Mitchell KR, Mehta RH, American College of Cardiology’s Guidelines Applied in Practice (GAP) Projects in Michigan; American College of Cardiology Foundation (Bethesda, MD) Guidelines Applied in Practice Steering committee Guideline-based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction: the American College of Cardiology’s Guidelines Applied in Practice (GAP) Projects in Michigan. J Am Coll Cardiol. 2005;46:1242–1248. doi: 10.1016/j.jacc.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 49.Yuan F, Song XT, Lu SZ. Percutaneous coronary intervention in mainland China in 2008: register results. Chin J Cardiol. 2010;38:629–632. [PubMed] [Google Scholar]
- 50.Zhang SY, Hu DY, Sun YH, Yang JG. Current management of patients with ST elevation myocardial infarction in metropolitan Beijing, China. Clin Invest Med. 2008;31:E189–E197. doi: 10.25011/cim.v31i4.4779. [DOI] [PubMed] [Google Scholar]
- 51.Yan L. The influence on medicine - taking behavior of patients with AMI by their relatives. J Commun Med. 2012;10:20–22. [Google Scholar]
- 52.Sun JY, Liu J, Xie XQ, Wei ZH, Wang W, Wang M, Qi Y, Guo MN, Zhang XY, Wan H, Zhao D. Surveillance on the incidence of acute coronary events in the permanent residents of Beijing aged 25 years and more from 2007 to 2009. Chin J Cardiol. 2012;40:194–198. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


