Abstract
Gliomas are the most common and deadly tumors in the central nervous system (CNS). In the course of studying the role of chemoattractant receptors in tumor growth and metastasis, we discovered that highly malignant human glioblastoma and anaplastic astrocytoma specimens were stained positively for the formylpeptide receptor (FPR), which is normally expressed in myeloid cells and accounts for their chemotaxis and activation induced responses to bacterial peptides. Screening of human glioma cell lines revealed that FPR was expressed selectively in glioma cell lines with a more highly malignant phenotype. FPR expressed in glioblastoma cell lines mediates cell chemotaxis, proliferation and production of an angiogenic factor, vascular endothelial growth factor (VEGF), in response to agonists released by necrotic tumor cells. Furthermore, FPR in glioblastoma cells activates the receptor for epidermal growth factor (EGFR) by increasing the phosphorylation of a selected tyrosine residue in the intracellular tail of EGFR. Thus, FPR hijacked by human glioblastoma cells exploits the function of EGFR to promote rapid tumor progression.
Keywords: glioma, formyl peptide receptor, chemotaxis, angiogenesis
1. Introduction
Glioma ranges in degree of aggressiveness from slowly growing low-grade tumors to rapidly growing high-grade tumors, such as anaplastic astrocytoma and glioblastoma multiforme (GBM). Approximately 17,500 primary central nervous system (CNS) tumors, which account for approximately 2% of all new cases of cancer, occur annually in the United States. The majority of these CNS tumors are malignant gliomas and high mortality rate converts this relatively infrequent malignancy into the third and fourth leading cause of cancer-related death among 15–54 year old men and women respectively [1, 2]. The mortality of GBM is especially high with a median survival time of 9–12 months despite multiple therapeutic regimens designed to optimize surgery, radiation and chemotherapy [3–6]. Like most malignancies, the cause of glioma has not been decoded until now. However, accumulating evidence suggests that malignant glioma cells have “acquired” the unique capability to sense growth signals present in the microenviroment and they also produce growth and angiogenesis factors for their own advantage. Many studies using established human glioma cell lines reveal that most of these cells express higher level of surface receptors important for cell survival and invasion. For instance, high levels of the receptor for epidermal growth factor (EGFR) [7–11] has been found on most glioma cells and is implicated as one of the most important growth-stimulating receptors in a great variety of malignant tumors including glioma [12]. In addition, all human glioma cell lines tested and cells isolated from surgically removed glioma specimens express platelet derived growth factor (PDGF) and its receptor that may form an autocrine growth stimulation loop [13].
More recently, the G protein coupled chemoattractant receptors (GPCR), which may mediate the metastasis of several malignant tumors, have also been shown to play a role in supporting glioma cell survival and promoting their production of angiogenic factors. One of such GPCRs is the chemokine receptor CXCR4 [14, 15], which by responding to its ligand CXCL12 (SDF1α) prolongs the survival of glioma cells in vitro, induces their directional migration and stimulates their production of the vascular endothelial growth factor (VEGF) [16] and CXCL8 (IL-8), two important angiogenic factors in malignant tumors [17]. Furthermore, another chemoattractant GPCR, the formylpeptide receptor FPR was detected in highly malignant human glioblastoma and anaplastic astrocytoma specimens [18, 19]. Studies of human glioma cell lines have revealed that FPR selectively “hijacked” by glioma cells exacerbates the tumor progression.
2. FPRs in phagocytic leukocytes
Human FPR was detected in 1976 on the surface of human neutrophils, and was cloned in 1990 from a myeloid leukemia-cell line [20, 21]. FPR binds to N-formyl-methionylleucyl-phenylalanine (fMLF), a product of the Gram− bacteria, with high affinity. Two related human genes, designated FPRL1 (FPR-like 1) and FPRL2 (FPR-like 2), were subsequently isolated [21–23] and shown to cluster with FPR on human chromosome 19q13.3 [22, 23]. FPRL1 is defined as a low-affinity fMLF receptor, based on its activation only by high concentrations of fMLF in vitro [24]. FPRL2 does not bind or respond to fMLF [24], but instead shares some non-formylated chemotactic peptide with FPRL1 [25, 26] and interacts more specifically with a heme binding protein fragment F2L [27, 28]. Both FPR and FPRL1 are expressed at high levels by human myeloid cells. On the other hand, FPRL2 is expressed in human monocytes, but not neutrophils [29], and by human mature dendritic cells (DCs) [30], which express reduced levels of FPR, and no FPRL1 [31, 32]. Both FPR and FPRL1 exhibit intriguing ligand promiscuity, suggesting their participation in multiple pathophysiological processes [33]. In the case of FPR, in addition to the bacterial fMLF, it also recognizes a variety of pathogen and host-derived peptide agonists. These include Gram+ bacterial products, peptide fragments from HIV envelope proteins [34], a neutrophil granule protein cathepsin G [35] and formylpeptides from mitochondria [36].
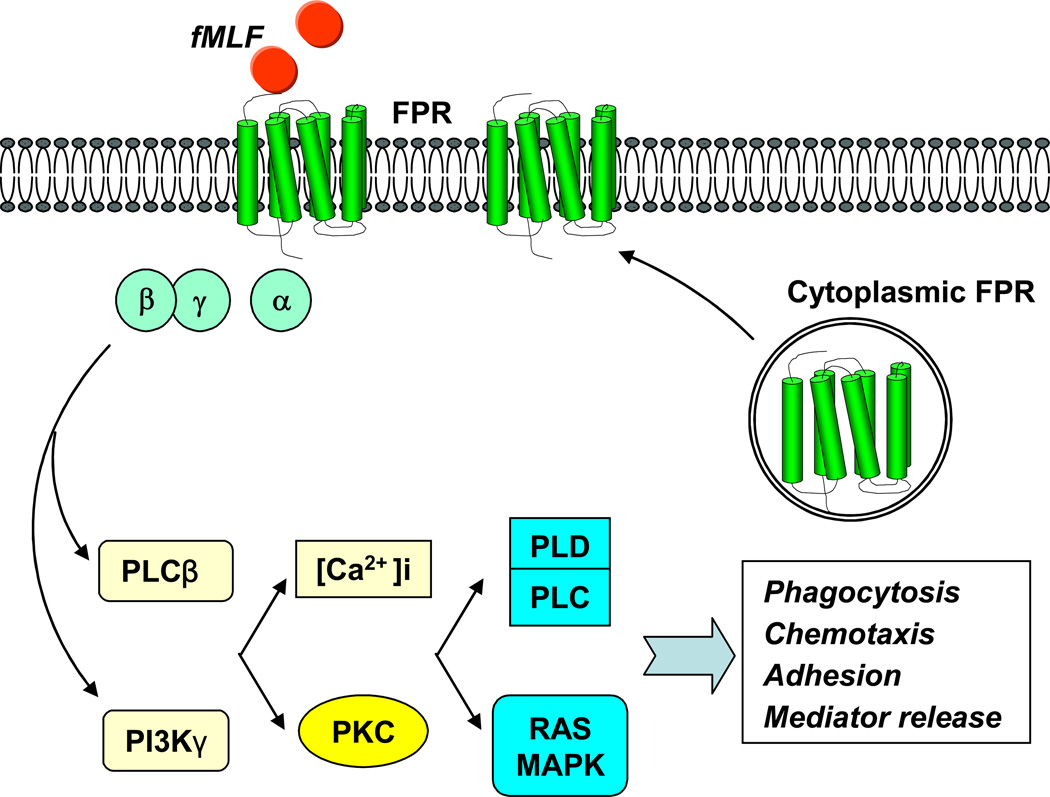
Activation of FPR by agonists may promote translocation of preexisting FPR in the cytoplasmic compartment to the cell membrane [37], and elicits a cascade of signal transduction pathways that are mediated by pertussis toxin-sensitive G proteins of the Gi subtype and closely controlled by phospholipase C and phosphoinositide (PI) 3 kinases [38–40]. Human myeloid cells activated by FPR agonist peptides undergo rapid shape change, showing increased adhesion, chemotaxis, phagocytosis and release of bactericidal and proinflammatory mediators (Figure 1). These functions of FPR render myeloid cells to manifest proinflammatory and anti-microbial phenotype. In fact, depletion of the human FPR counterpart mFPR1 from mice greatly decreased their ability to clear an infection by Listeria monocytogenes [41].
Figure 1. FPR signaling and function in phagocytes.
FPR activated by agonists elicits a cascade of signal transduction pathways involving G proteins, phospholipase C (PLC), phosphoinositide (PI) 3 kinases, protein kinase C, Ca2+, phospholipase D (PLD) and MAPKs to mediate phagocytosis, chemotaxis, adhesion and release of some mediators. The FPR agonists may also promote the translocation of preexisting FPR in the cytoplasm to cell membrane.
3. FPR in glioma
3.1. Expression by FPR in highly malignant human glioblastoma cells
As discussed above, FPR has been convincingly shown to be a GPCR that mediates host defense against bacterial infection phagocytic leukocytes [41]. However, during our investigation of the role of chemoattractant GPCRs in tumor growth and metastasis, we unexpectedly found that FPR was selectively expressed by more highly malignant human glioma specimens. For instance, 11 of 14 cases of grade III anaplastic astrocytoma and 6 of 6 grade IV GBM specimens strongly stained for FPR protein. In contrast, only two of 13 less aggressive grade II astrocytoma specimens showed positive FPR staining [18]. These findings prompted us to use established human glioma cell lines to investigate the relationship between FPR expression and the biological behavior of the tumor cells. For example, the U-87 cell line derived from a human GBM expresses a low level of glial fibrillar acidic protein (GFAP), a marker found in better differentiated astroglia. SHG44 cell line, established from a human astrocytoma specimen, in contrast contains higher levels of GFAP. Furthermore, U-87 cells are highly positive for astroglial precursor protein vimentin, which is weakly expressed by SHG44 cells. When injected to nude mice, U-87 cells formed more rapidly growing tumors as compared with SHG44 cells and interestingly, only U-87 cells expressed FPR transcripts [19]. Therefore, results obtained with cell lines corroborate the notion that FPR is selectively expressed by more highly malignant glioma cells.
3.2 FPR function in GBM cells
3.2.1 Tumor cell survival and production of VEGF
The function of FPR in glioblastoma cells was extensively examined by using the prototype agonist peptide, bacterial fMLF. In addition to mediating robust chemotaxis and calcium mobilization of glioblastoma cells in response to its agonist peptide, FPR exhibited several unique properties that are closely related to tumor progression. For instance, activation of FPR in glioblastoma cells under suboptimal culture conditions (i.e. at low FCS concentration) supports the survival of the tumor cells, in association with increased cellular levels of the anti-apoptotic proteins Bcl-2. In addition, FPR agonist peptide activated two important transcription factors NF-κB and STAT3 in glioblastoma cells. While increased NF-κB translocation has been also observed as a consequence of FPR signaling path way seen also in phagocytic leukocytes [42, 43], FPR in glioblastoma cells stimulated the phosphorylation of STAT3 at Ser-727 and Tyr-105 residues of which only Ser-727 was phosphorylated in human monocytes. Another transcription factor hypoxia inducible factor-1α (HIF-1α) was also activated by FPR agonist peptide in glioblastoma cells [18].
Since both STAT3 and HIF-1α are implicated in the transcriptional activation of the gene coding for VEGF [44–47], we investigated the capacity of activated FPR to increase the production of VEGF by tumor cells. In fact, supernatants from fMLF-stimulated glioblastoma cells induced migration and tubule formation of human vascular endothelial cells (EC) [18]. This property of the tumor cell supernatant was abolished by a neutralizing anti-human VEGF antibody [18], indicating that the activity of tumor supernatant on vascular EC was mediated by VEGF released by FPR agonist-stimulated glioblastoma cells.
The contribution of FPR to glioblastoma progression was then tested in vivo in nude mice in which tumor cells containing small interfering (si) RNA against FPR mRNA yielded tumors with markedly reduced rate of growth as compared to control cells transfected with random siRNA [18]. Thus the functional studies provide strong evidence for the involvement of FPR in supporting the rapid progression of glioblastoma.
3.2.2 Transactivation of EGFR
Since tumor growth is a consequence of a complex combination of interweaving signal pathways of various cell surface receptors [48], we further investigated the potential role of FPR to act as a component of such a network. We found that human glioblastoma cell expressed high levels of EGFR [49], which is overexpressed on a variety of malignant tumors of human and mouse origin [50–57]. Upon stimulation with EGF, human glioblastoma cells underwent increased chemotaxis and rapid proliferation with phosphorylation of at least 4 tyrosine residues in the C-terminal domain of EGFR [49] . When tumor cells were stimulated with the FPR agonist fMLF, EGFR was also rapidly phosphorylated but with restriction to a single tyrosine residue Tyr992. This transactivation of EGFR by FPR agonist peptide accounted for approximately 40% of the biological activity of FPR in tumor cells and was dependent on a Src kinase pathway [49]. To investigate the significance of the cross-talk between FPR and EGFR in glioblastoma growth, we used siRNA to deplete both receptors. When implanted in nude mice, tumor cells depleted of either FPR or EGFR grew more slowly as compared with parental cells but depletion of both receptors completely abolished the tumorigenicity of the glioblastoma cells [49]. Thus, FPR aberrantly expressed in glioblastoma cells is capable of exploiting the function of EGFR to amplify tumor grow.
3.2.3 Interaction with native ligand
It should be noted that the function of FPR in glioblastoma cells was characterized mainly by using the bacterial peptide agonist fMLF. The question then arises whether the receptor is capable of recognizing host-derived agonists that may be present in the tumor microenviroment. We first tested glioblastoma cell responses to the neutrophil granule protein cathepsin G and found that this host-derived FPR agonist induced the migration of tumor cells expressing FPR [35]. Since mitochondrial peptides are also potential endogenous FPR agonist [36] and since glioblastomas frequently contain necrotic foci in the rapidly growing tumor mass that may release mitochondrial components, we examined the presence of this FPR agonist in supernatants of necrotic tumor cells.
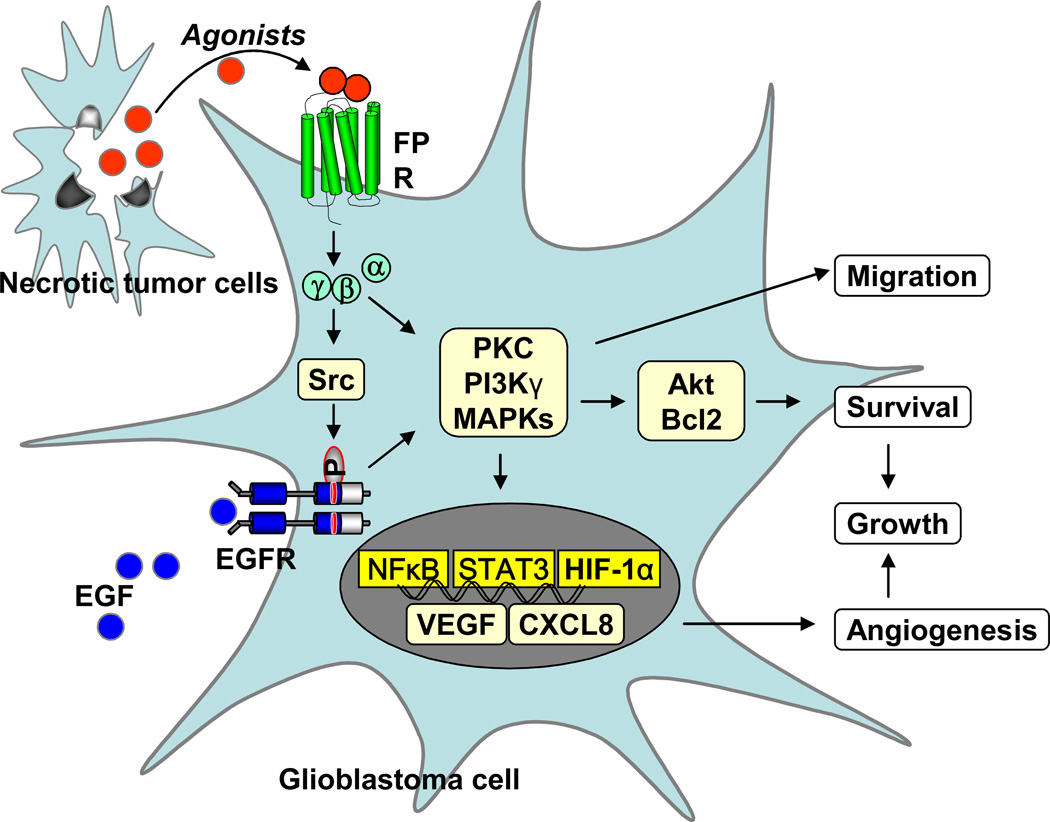
Supernatants of necrotic U-87 cell line and tumors formed by U-87 cells in athymic mice induced potent chemotaxis of live U-87 cells as well as a rat basophil leukemia cell line transfected to express human FPR (ETFR cells). The chemotactic activity released by necrotic GBM cells and tumor tissues was blocked by an anti-FPR antibody and by the FPR-specific antagonist tBoc-MLF [18]. The robust intracellular Ca2+ mobilization induced by necrotic glioblastoma cell supernatant attenuated subsequent cell response to fMLF, suggesting that agonist contained in the supernatants of necrotic tumor cells shares a receptor with fMLF [18, 58]. Further evidence to support the release of FPR agonist(s) by necrotic glioblastoma cells was provided by the observation that the tumor cell supernatant down-regulated FPR expressed on the surface of human monocytes and ETFR cells. These observations suggest that FPR expressed on glioblastoma cells is able to recognize agonist activity released in the tumor microenviroment thereby mediating tumor cell migration, growth and production of angiogenic factors in a paracrine and/or autocrine loop [18] (Figure 2).
Figure 2. The role of FPR in glioblastoma progression.
FPR on glioblastoma cells is activated by agonists released by necrotic tumor cells. The signaling cascade coupled to FPR in tumor cells activates PI3 kinase, MAPKs, PLC, PLD, Akt/Bcl2 and transcription factors such as NFκB, STAT3 and HIF-1α, to enhance cell chemotaxis, growth and release of angiogenic factors. The FPR function in glioblastoma cells is partially mediated by EGFR through a Src-kinase dependent transactivation pathway.
4. Summary and perspectives
Malignant tumors take advantage of their microenvironment to favor their survival, growth, invasion, and metastasis [59]. For instance, tumor cells often produce aberrant levels of growth factors that stimulate cell surface receptors to increase cell proliferation in an autocrine manner. Tumor cells also produce high levels of VEGF constitutively or in response to stimulation that recruits endothelial cells and promotes endothelial cell proliferation and vascularization. In addition, malignant tumor cells express receptors that interact with agonists that are present in the vicinity of the tumor or produced by distant organs to increase tumor cell motility and thus to favor tumor cell invasion and metastasis. Chemokine receptors, such as CXCR4 and CCR7, have been implicated in promoting tumor metastasis, presumably by increasing the chemotaxis and extravasation of tumor cells in response to locally produced chemokine ligands [59–61]. The chemokine receptor CXCR4, expressed by a majority of glioma cell lines, mediates tumor cell migration and supports cell survival, presumably in response to the ligand SDF-1α (CXCL12) , which is present in tumor tissues [15]. However, because CXCR4 is also expressed in normal astrocytes, it may not represent a distinctive biomarker for differentiating normal astrocytes from malignant astrocytes. Nevertheless, recent studies have implicated CXCR4 as being expressed at higher levels in more aggressive glioma cells [14]. FPR is not widely expressed in glioma cell lines or in normal glial cells but, rather, is selectively expressed in more highly malignant glioblastoma cells and contributes to their tumorigenicity in vivo in immunocompromised mice. FPR is also detected in a majority of primary grade III anaplastic astrocytoma and grade IV GBM specimens. Identification of FPR agonist activity in the supernatants of necrotic tumor cells provides evidence that this receptor may interact with host-derived agonists produced in tumor lesions, presumably in the necrotic area frequently associated with highly malignant gliomas or in surrounding tissues that maybe compressed by growing tumors. It is thus plausible that FPR in live tumor cells may serve as a sensor for the agonists produced in a “ paracrine ” manner in the tumor microenvironment to promote cell migration, to support cell survival and proliferation, to activate transcription and the production of VEGF, and to transactivate EGFR, all of which exacerbate tumor growth.
Further study is required to more precisely define the relationship between the FPR expression and the progression of human primary gliomas and to identify the mechanistic basis for the control of FPR expression in highly malignant human glioma cells. In addition, the pathogenesis of human gliomas is likely to be complex, and FPR is probably not the sole factor that regulates the progression of malignant gliomas. Indeed, our observation that there were three FPR-protein-negative tumors of the 14 primary grade III anaplastic astrocytoma specimens examined and some GBM cell lines do not express functional FPR suggests that factors other than FPR also participate in the development of malignant human gliomas. Also, the relationship between FPR expression and the survival of glioma patients after treatment remains to be established. Nevertheless, the present study implicates the role of FPR in the rapid progression of highly malignant human gliomas and thus raises the possibility that FPR and its signaling pathway may be candidate molecular targets for developing novel therapeutics for gliomas.
Acknowledgements
The authors thank Dr. Joost J. Oppenheim for reviewing the manuscript and Mrs. Cheryl N. Magers and Mrs. Cheryl F. Lamb for secretarial assistance. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This project has been funded in part with federal funds from the National Cancer Institutes, National Institutes of Health, under Contract No. NO1-CO-12400. The research was also supported in part by the Intramural Research Program of the NCI, NIH.
NCI-Frederick is accredited by AAALAC international and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the ‘Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, D.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kesari S, Stiles CD. The bad seed: PDGF receptors link adult neural progenitors to glioma stem cells. Neuron. 2006;51:151–153. doi: 10.1016/j.neuron.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Boring CC, Squires TS, Tong T, et al. Cancer statistics, 1994. CA Cancer J Clin. 1994;44:7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Brada M, Sharpe G, Rajan B, et al. Modifying radical radiotherapy in high grade gliomas; shortening the treatment time through acceleration. Int J Radiat Oncol Biol Phys. 1999;43:287–292. doi: 10.1016/s0360-3016(98)00390-3. [DOI] [PubMed] [Google Scholar]
- 4.Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults. The prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989;71:487–493. doi: 10.3171/jns.1989.71.4.0487. [DOI] [PubMed] [Google Scholar]
- 5.Shibamoto Y, Yamashita J, Takahashi M, et al. Supratentorial malignant glioma: an analysis of radiation therapy in 178 cases. Radiother Oncol. 1990;18:9–17. doi: 10.1016/0167-8140(90)90018-r. [DOI] [PubMed] [Google Scholar]
- 6.Reni M, Cozzarini C, Ferreri AJ, et al. A retrospective analysis of postradiation chemotherapy in 133 patients with glioblastoma multiforme. Cancer Invest. 2000;18:510–515. doi: 10.3109/07357900009012189. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey PA, Wong AJ, Vogelstein B, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988;48:2231–2238. [PubMed] [Google Scholar]
- 8.Bigner SH, Humphrey PA, Wong AJ, et al. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50:8017–8022. [PubMed] [Google Scholar]
- 9.Huncharek M, Kupelnick B. Epidermal growth factor receptor gene amplification as a prognostic marker in glioblastoma multiforme: results of a meta-analysis. Oncol Res. 2000;12:107–112. doi: 10.3727/096504001108747576. [DOI] [PubMed] [Google Scholar]
- 10.Zawrocki A, Biernat W. Epidermal growth factor receptor in glioblastoma. Folia Neuropathol. 2005;43:123–132. [PubMed] [Google Scholar]
- 11.Haynik DM, Roma AA, Prayson RA. HER-2/neu expression in glioblastoma multiforme. Appl Immunohistochem Mol Morphol. 2007;15:56–58. doi: 10.1097/01.pai.0000213133.09160.da. [DOI] [PubMed] [Google Scholar]
- 12.Angers-Loustau A, Hering R, Werbowetski TE, et al. SRC regulates actin dynamics and invasion of malignant glial cells in three dimensions. Mol Cancer Res. 2004;2:595–605. [PubMed] [Google Scholar]
- 13.Lokker NA, Sullivan CM, Hollenbach SJ, et al. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62:3729–3735. [PubMed] [Google Scholar]
- 14.Bian XW, Yang SX, Chen JH, et al. Preferential expression of the chemokine receptor CXCR4 by more highly malignant human gliomas and association with poor patient survival. Neurosurgery. 2007;61:570–579. doi: 10.1227/01.NEU.0000290905.53685.A2. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Larsen PH, Hao C, et al. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 16.Yang SX, Chen JH, Jiang XF, et al. Activation of chemokine receptor CXCR4 in malignant glioma cells promotes the production of vascular endothelial growth factor. Biochem Biophys Res Commun. 2005;335:523–528. doi: 10.1016/j.bbrc.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 17.Oh JW, Drabik K, Kutsch O, et al. CXC chemokine receptor 4 expression and function in human astroglioma cells. J Immunol. 2001;166:2695–2704. doi: 10.4049/jimmunol.166.4.2695. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Bian X, Le Y, et al. Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. J Natl Cancer Inst. 2005;97:823–835. doi: 10.1093/jnci/dji142. [DOI] [PubMed] [Google Scholar]
- 19.Le Y, Hu J, Gong W, et al. Expression of functional formyl peptide receptors by human astrocytoma cell lines. J Neuroimmunol. 2000;111:102–108. doi: 10.1016/s0165-5728(00)00373-8. [DOI] [PubMed] [Google Scholar]
- 20.Boulay F, Tardif M, Brouchon L, et al. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem Biophys Res Commun. 1990;168:1103–1109. doi: 10.1016/0006-291x(90)91143-g. [DOI] [PubMed] [Google Scholar]
- 21.Boulay F, Tardif M, Brouchon L, et al. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- 22.Murphy PM, Ozcelik T, Kenney RT, et al. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J Biol Chem. 1992;267:7637–7643. [PubMed] [Google Scholar]
- 23.Bao L, Gerard NP, Eddy RL, Jr, et al. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics. 1992;13:437–440. doi: 10.1016/0888-7543(92)90265-t. [DOI] [PubMed] [Google Scholar]
- 24.Prossnitz ER, Ye RD. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 25.Christophe T, Karlsson A, Dugave C, et al. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem. 2001;276:21585–21593. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- 26.Betten A, Bylund J, Christophe T, et al. A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J Clin Invest. 2001;108:1221–1228. doi: 10.1172/JCI13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao JL, Guillabert A, Hu J, et al. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J Immunol. 2007;178:1450–1456. doi: 10.4049/jimmunol.178.3.1450. [DOI] [PubMed] [Google Scholar]
- 28.Migeotte I, Riboldi E, Franssen JD, et al. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durstin M, Gao JL, Tiffany HL, et al. Differential expression of members of the N-formylpeptide receptor gene cluster in human phagocytes. Biochem Biophys Res Commun. 1994;201:174–179. doi: 10.1006/bbrc.1994.1685. [DOI] [PubMed] [Google Scholar]
- 30.Yang D, Chen Q, Gertz B, et al. Human dendritic cells express functional formyl peptide receptor-like-2 (FPRL2) throughout maturation. J Leukoc Biol. 2002;72:598–607. [PubMed] [Google Scholar]
- 31.Yang D, Chen Q, Le Y, et al. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J Immunol. 2001;166:4092–4098. doi: 10.4049/jimmunol.166.6.4092. [DOI] [PubMed] [Google Scholar]
- 32.Braun MC, Wang JM, Lahey E, et al. Activation of the formyl peptide receptor by the HIV-derived peptide T-20 suppresses interleukin-12 p70 production by human monocytes. Blood. 2001;97:3531–3536. doi: 10.1182/blood.v97.11.3531. [DOI] [PubMed] [Google Scholar]
- 33.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- 35.Sun R, Iribarren P, Zhang N, et al. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J Immunol. 2004;173:428–436. doi: 10.4049/jimmunol.173.1.428. [DOI] [PubMed] [Google Scholar]
- 36.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 37.Xue M, Hsieh G, Raymond-Stintz MA, et al. Activated N-Formyl Peptide Receptor and High-Affinity IgE Receptor Occupy Common Domains for Signaling and Internalization. Mol Biol Cell. 2007;18:1410–1420. doi: 10.1091/mbc.E05-11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haribabu B, Zhelev DV, Pridgen BC, et al. Chemoattractant receptors activate distinct pathways for chemotaxis and secretion. Role of G-protein usage. J Biol Chem. 1999;274:37087–37092. doi: 10.1074/jbc.274.52.37087. [DOI] [PubMed] [Google Scholar]
- 39.Wenzel-Seifert K, Arthur JM, Liu HY, et al. Quantitative analysis of formyl peptide receptor coupling to g(i)alpha(1), g(i)alpha(2), and g(i)alpha(3) J Biol Chem. 1999;274:33259–33266. doi: 10.1074/jbc.274.47.33259. [DOI] [PubMed] [Google Scholar]
- 40.Pan ZK, Chen LY, Cochrane CG, et al. fMet-Leu-Phe stimulates proinflammatory cytokine gene expression in human peripheral blood monocytes: the role of phosphatidylinositol 3-kinase. J Immunol. 2000;164:404–411. doi: 10.4049/jimmunol.164.1.404. [DOI] [PubMed] [Google Scholar]
- 41.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Browning DD, Pan ZK, Prossnitz ER, et al. Cell type- and developmental stage-specific activation of NF-kappaB by fMet-Leu-Phe in myeloid cells. J Biol Chem. 1997;272:7995–8001. doi: 10.1074/jbc.272.12.7995. [DOI] [PubMed] [Google Scholar]
- 43.Huang S, Chen LY, Zuraw BL, et al. Chemoattractant-stimulated NF-kappaB activation is dependent on the low molecular weight GTPase RhoA. J Biol Chem. 2001;276:40977–40981. doi: 10.1074/jbc.M105242200. [DOI] [PubMed] [Google Scholar]
- 44.Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 45.Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. Faseb J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 46.Wang M, Zhang W, Crisostomo P, et al. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42:1009–1015. doi: 10.1016/j.yjmcc.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P, Wang Y, Hui Y, et al. Inhibition of VEGF expression by targeting HIF-1 alpha with small interference RNA in human RPE cells. Ophthalmologica. 2007;221:411–417. doi: 10.1159/000107502. [DOI] [PubMed] [Google Scholar]
- 48.Srinivasan DM, Kapoor M, Kojima F, et al. Growth factor receptors: implications in tumor biology. Curr Opin Investig Drugs. 2005;6:1246–1249. [PubMed] [Google Scholar]
- 49.Huang J, Hu J, Bian X, et al. Transactivation of the epidermal growth factor receptor by formylpeptide receptor exacerbates the malignant behavior of human glioblastoma cells. Cancer Res. 2007;67:5906–5913. doi: 10.1158/0008-5472.CAN-07-0691. [DOI] [PubMed] [Google Scholar]
- 50.Dobashi Y, Takei N, Suzuki S, et al. Aberration of epidermal growth factor receptor expression in bone and soft-tissue tumors: protein overexpression, gene amplification and activation of downstream molecules. Mod Pathol. 2004;17:1497–1505. doi: 10.1038/modpathol.3800218. [DOI] [PubMed] [Google Scholar]
- 51.Layfield LJ, Willmore C, Tripp S, et al. Epidermal growth factor receptor gene amplification and protein expression in glioblastoma multiforme: prognostic significance and relationship to other prognostic factors. Appl Immunohistochem Mol Morphol. 2006;14:91–96. doi: 10.1097/01.pai.0000159772.73775.2e. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez G, Perry A, Tan BR, et al. Expression of epidermal growth factor receptor in squamous cell carcinomas of the anal canal is independent of gene amplification. Mod Pathol. 2006;19:942–949. doi: 10.1038/modpathol.3800608. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Zhang S, MacLennan GT, et al. Epidermal growth factor receptor protein expression and gene amplification in small cell carcinoma of the urinary bladder. Clin Cancer Res. 2007;13:953–957. doi: 10.1158/1078-0432.CCR-06-2167. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Jones TD, Zhang S, et al. Amplifications of EGFR gene and protein expression of EGFR, Her-2/neu, c-kit, and androgen receptor in phyllodes tumor of the prostate. Mod Pathol. 2007;20:175–182. doi: 10.1038/modpathol.3800724. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Chang A. Somatic mutations of the epidermal growth factor receptor and non-small-cell lung cancer. J Med Genet. 2007;44:166–172. doi: 10.1136/jmg.2006.046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoadley KA, Weigman VJ, Fan C, et al. EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics. 2007;8:258. doi: 10.1186/1471-2164-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyers MB, Merluzzi VJ, Spengler BA, et al. Epidermal growth factor receptor is increased in multidrug-resistant Chinese hamster and mouse tumor cells. Proc Natl Acad Sci U S A. 1986;83:5521–5525. doi: 10.1073/pnas.83.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le Y, Gong W, Li B, et al. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- 59.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 60.Wang JM, Deng X, Gong W, et al. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 61.Wang JM, Chertov O, Proost P, et al. Purification and identification of chemokines potentially involved in kidney-specific metastasis by a murine lymphoma variant: induction of migration and NFkappaB activation. Int J Cancer. 1998;75:900–907. doi: 10.1002/(sici)1097-0215(19980316)75:6<900::aid-ijc13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]