Abstract
This study estimated the long-term mortality hazards and disability risks associated with various combinations of smoking and physical inactivity measured over time in a sample of middle-aged adults. Data have been collected from a national sample of Swedish adults, originally interviewed in 1968 and followed until 2007 (N = 1,682). Smoking and physical activity status were measured at baseline and 13 years later (1981). Different patterns of change and stability in smoking and physical inactivity over this 13 year period were used as predictors of mortality through 2007. Also, associations between different patterns of these health behaviors and the odds of disability (measured in 2004) were estimated among survivors (n = 925). Results suggest that mortality rates were elevated among persistent (HR = 1.7; 95 % CI = 1.5–2.0) and new smokers (HR = 2.5; 95 % CI = 1.6–4.1), but not among discontinued smokers. However, mortality rates remained elevated among discontinued smokers who were also persistently inactive (HR = 1.9; 95 % CI = 1.3–2.6). Additional findings suggest that persistent physical inactivity during midlife was associated with increased odds of late life disability (OR = 1.8; 95 % CI = 1.1–2.7), but that smoking had no clear additive or multiplicative effects on disability. As such, these findings indicate that while persistent smoking during midlife primarily impacts subsequent mortality, persistent physical inactivity during midlife appears to counteract the survival benefits of smoking cessation, while also imposing a long-term risk on late life disability among those who do survive to old age.
Keywords: Smoking, Physical activity, Mortality, Disability, Midlife, Aging
Introduction
A mounting body of research suggests that certain personal behaviors—especially smoking and physical inactivity—are the leading root causes of death (Mokdad et al. 2004). As such, individuals who smoke or are physically inactive are less likely than those with healthier behaviors to achieve the gains in life expectancy that are currently being experienced by the population as a whole (Chipperfield 2008; Knoops et al. 2004). Studies also show that smokers and physically inactive individuals who survive into older ages are at increased risk for poor health and disability (Chakravarty et al. 2012; Lee et al. 2012).
However, it is now also becoming well-established that the health risks of these behaviors are mitigated substantially among adults who make positive behavioral changes. For example, data from 877,243 respondents to the U.S. Cancer Prevention Study II have shown that quitting smoking during midlife can increase life expectancy by as much as 8 years, with greater benefits among those who have quit at younger ages (Taylor et al. 2002). Similar findings have emerged from a study of close to 35,000 British Male doctors (Doll et al. 1994), as well as other smaller studies (Gerber et al. 2012). On the other hand, data from the Nurses’ Health Study suggest that quitting smoking, compared to continuing smoking, may have negligible benefit in terms of health-related quality of life (Sarna et al. 2008), perhaps indicating the presence of lingering impairments within former smokers that are not necessarily life threatening, but could diminish well-being.
Researchers have also found that the risks associated with physical inactivity can be mitigated as individuals transition out of inactive lifestyles. For example, in a population-based cohort study of 50-year-old Swedish men who were followed for over 35 years, researchers found that those who increased their levels of activity before age 60 exhibited a mortality risk that was equivalent to those who had maintained a high level of activity throughout this period (Byberg et al. 2009); in fact, the degree of improvement in survival reported in this study was on par with the effects of smoking cessation. A large multi-center prospective cohort study in the U.S. also indicated that increases in levels of physical activity among women aged 65 and older were associated with lower mortality rates relative to continually inactive women (Gregg et al. 2003). Risk of death was also found to be significantly reduced among adults ages 20–79 from the Copenhagen City Heart Study who had increased their levels of activity, with this association being most evident among those who were over 65 years of age (Schnohr et al. 2003). Positive changes in physical activity have also been found to postpone the onset of disability (Berk et al. 2006).
Taken together, these bodies of literature provide compelling evidence that the prospects for healthy aging can be improved considerably for middle-aged adults who quit smoking or become physically active. However, what is currently not well known is whether midlife patterns of smoking interact with midlife patterns of physical inactivity in determining mortality and health risks years later, during old age. Considering the combined impact of smoking and physical activity is consistent with a lifestyle approach to the study of behavior and health, in which the focus is on the combined effects of an individual’s collective pattern of multiple behaviors (Cockerham et al. 1997). Most studies adopting a lifestyle approach have done so by creating a combined health behavior index, representing a simple count of the number of risk behaviors in which an individual is currently engaged (e.g., Hubert et al. 2002; Khaw et al. 2008; Knoops et al. 2004; Kvaavik et al. 2010; Meng et al. 1999; Myint et al. 2009; Sabia et al. 2009; van Dam et al. 2008; Willcox et al. 2006). The findings from the vast majority of these studies suggest that risks of death, along with several other health outcomes, are proportional to the number of risk behaviors that a person has accumulated (Loef and Walach 2012). However, these studies have not been able to show how two specific risk behaviors, like smoking and physical inactivity, may interact with one another in affecting health in later life. Nor have they been able to account for the long-term health impact of changing, or failing to change, multiple risk behaviors during midlife.
Therefore, the purpose of the current study is to examine how patterns of stability or change in smoking and physical inactivity during midlife may combine with one another to influence both mortality rates throughout the ensuing two and a half decades, as well as risk for late life disability. While some other studies have examined interactions between these specific behaviors, these studies have typically measured the behaviors at just a single point in time. For instance, data from the Established Populations for Epidemiologic Studies of the Elderly (EPESE) have shown that lifestyles combining smoking and physical inactivity were associated with a particularly high risk for losses in active life expectancy (Ferrucci et al. 1999). A Swedish study has shown similar findings regarding the compounding effects of smoking and physical inactivity on health and mortality (Johansson and Sundquist 1999), while more recently, studies have shown that the health and mortality risks associated with smoking can be partially mitigated among those with moderate and high levels of physical activity (Ford et al. 2012; Garcia-Aymerich et al. 2007).
To the best of our knowledge, the joint effects of changes in smoking and physical inactivity during midlife on mortality and late life disability have not been previously examined. Examining these joint effects could reveal whether the benefits of a positive behavior change are moderated when another unhealthy behavior is maintained. Likewise, these joint effects could reveal whether the risk of remaining engaged in an unhealthy behavior over time is amplified in the presence of another unhealthy behavior, or mitigated in the presence of another healthy, or improving, behavior.
Methods
Data source and collection
Data have been collected from the level of living survey (LNU) and the Swedish panel study of living conditions of the oldest old (SWEOLD). LNU is a nationally representative study of the Swedish adult population, ages 18–75; it was first carried out in 1968, and subsequently in 1974, 1981, 1991, 2000, and 2010 (Fritzell and Lundberg 2007). Persons from the LNU sample who have passed the upper age limit of 75 years have been included in the SWEOLD study (Lundberg and Thorslund 1996), which has been carried out in 1992, 2002, 2004, and 2011. In the 2004 wave of SWEOLD, respondents ages 69 and older were interviewed. In both the LNU and SWEOLD, professional interviewers conducted structured interviews with participants in their homes. The interviews addressed questions about work life, family situation, health behaviors, economic conditions, living conditions, and health status.
The current study focuses on adults who were between the ages of 33 and 55 in 1968. The initial response rate in 1968 was 90 %. The analytic sample for the mortality analysis (n = 1,682) represents 80.2 % of those initial respondents, and includes only those who were interviewed in both the 1968 and 1981 LNU. To analyze associations between midlife behaviors and subsequent mortality, the 1968 and 1981 LNU data were merged with data on mortality status from the Swedish National Cause of Deaths Register. The mortality analysis sample was created after excluding all respondents who died within 1 year from their 1981 interview (n = 40), as their health behaviors during midlife may have been strongly influenced by their health status at that time. To analyze associations between midlife behaviors and disability status, the 1968 and 1981 LNU data were merged with health status data from the 2004 SWEOLD. As such, the disability analysis sample (n = 925) includes only those original respondents who survived to 2004.
Measures
Health behavior patterns
Each respondent’s smoking and physical inactivity behavior was measured in both 1968 and 1981. In both waves, smoking was measured dichotomously, distinguishing between current smokers and current non-smokers. The measure of physical inactivity used in both 1968 and 1981 was an index summarizing an individual’s participation in a variety of active behaviors, including gardening, hunting, dancing, and engaging in any type of sport during the past 12 months. Respondents engaging in any of these activities “sometimes” received one point; two points were given for activities that were conducted “often.” Two points were also given to respondents who engaged in a sport, independent of frequency. Persons with a summary score of less than two points were defined as being currently inactive.
Patterns of smoking and physical inactivity behavior over time were ascertained by combining each respondent’s status with respect to these behaviors in 1968 and 1981. Respondents who were non-smokers in both 1968 and 1981 were defined as “Non-smokers”; respondents who were smokers in 1968 and non-smokers in 1981 were defined as “Discontinued smokers”; those who were non-smokers in 1968 and smokers in 1981 were defined as “New smokers”; and, those who were smokers in both 1968 and 1981 were defined as “Persistent smokers.” The equivalent categories for physical inactivity were the “Active,” “Discontinued inactive,” “New inactive,” and “Persistent inactive” respondents.
We examined the joint effects of these behavioral patterns by cross-stratifying the smoking and physical inactivity patterns. To guard against prohibitively low cell sizes, we merged the “New smoker” and “Persistent smoker” categories, as well as the “New inactive” and “Persistent inactive” categories before cross-stratifying. The result was nine subgroups that represent nine distinct patterns of smoking and physical inactivity behavior over time, ranging from “Non-smoker, active” respondents to “Persistent/new smokers, persistent/new inactive” respondents.
Mortality
Follow-up on all-cause mortality was carried out from the time of interview in 1981 until April 2007. Date of death was obtained from the Swedish National Cause of Deaths Register. During the follow-up period, 875 individuals (52 %) died; those who were still alive at the end of the follow-up were censored.
Disability
Late life disability was measured in the 2004 SWEOLD with items that detect limitations with activities of daily living (ADL) and instrumental activities of daily living (IADL). ADL items included eating, dressing, toileting, transferring in and out of bed, and bathing; IADL items included house cleaning, shopping, and preparing food. Respondents who reported needing help with any of these activities were defined as being in a state of disability in 2004.
Control variables
The analyses in this study controlled for the effects of several sociodemographic characteristics, including age, gender, education (measured in years of completed schooling), and occupational status (categorized as unskilled blue-collar workers, skilled blue-collar workers, lower white-collar workers, and intermediate and higher white-collar workers) in 1968. Also, to account for the potential influence of poor health on smoking and physical inactivity behavior, all models controlled for health status in 1981, including measures of circulatory problems (chest pain; heart weakness; high blood pressure; breathlessness/shortness of breath; or dizziness), mobility problems (inability to walk 100 m fairly briskly without difficulties; inability to run 100 m without greater difficulties; or inability to go up and down stairs without difficulties), and psychological problems (anxiety, nervousness, anguish; general fatigue; sleeping problems; or depression).
Data analysis
The main analyses employed Cox regression to compare the rates of mortality, between 1981 and 2007, for respondents with different patterns of smoking and physical inactivity during midlife. First, the independent mortality hazards associated with patterns of smoking, and patterns of physical inactivity, were assessed. Next, the joint effects of these patterns on mortality were assessed by entering the combined, cross-stratified, smoking and physical inactivity variables into the model. In supplementary analyses, we also tested for joint effects by specifying interactions between the smoking and physical inactivity variables. The proportional hazards assumptions were not violated, as assessed through examination of the Kaplan–Meier survival plots.
A similar set of analyses, examining both the independent and joint effects of midlife smoking and physical inactivity patterns, was carried out to assess associations between midlife behavior and late life disability among survivors to old age. Here, logistic regression was employed, with 2004 disability status as the outcome.
Results
Descriptive analyses
In 1968, 43.7 % of the mortality analysis sample were current smokers, and 32.6 % were physically inactive; in 1981, 32.2 % were smokers and 34.0 % were inactive. Individual behavioral patterns between 1968 and 1981 are characterized in Table 1. Over half (54.5 %) of the sample were non-smokers in both 1968 and 1981, while 13.3 % discontinued smoking, 1.8 % were new smokers, and close to one-third of the sample (30.4 %) were persistent smokers. Both gender and age varied significantly across these various smoking patterns. In addition, one-half (50.5 %) of the sample were active in both 1968 and 1981. The remainder of the sample was fairly equally distributed across the other patterns of physical inactivity. Significant variation with regard to gender, age, education, and occupational status was evident across these patterns of physical inactivity.
Table 1.
Characteristics associated with different smoking and physical inactivity profiles among Swedish adults ages 33–55 in 1968
| Sample size (%) | % Women | Mean age (SD) | Mean education years (SD) | % Unskilled blue collar | |
|---|---|---|---|---|---|
| Mortality analysis sample | 1,682 | 51.1 | 44.0 (6.6) | 8.1 (2.8) | 34.8 |
| Smoking in 1968/1981 | a | b | |||
| No/no (non-smoking) | 917 (54.5) | 63.1 | 44.5 (6.6) | 8.0 (2.8) | 36.5 |
| Yes/no (discontinued smoking) | 224 (13.3) | 26.8 | 44.4 (6.9) | 8.3 (2.9) | 29.0 |
| No/yes (new smoking) | 30 (1.8) | 50.0 | 41.5 (6.4) | 8.3 (2.5) | 20.0 |
| Yes/yes (persistent smoking) | 511 (30.4) | 40.1 | 43.2 (6.4) | 8.2 (2.6) | 35.4 |
| Inactivity in 1968/1981 | a | b | b | a | |
| No/no (active) | 850 (50.5) | 46.2 | 43.8 (6.5) | 8.5 (3.1) | 28.3 |
| Yes/no (discontinued inactivity) | 260 (15.5) | 47.7 | 42.7 (6.8) | 8.1 (2.4) | 34.2 |
| No/yes (new inactivity) | 283 (16.8) | 60.4 | 44.6 (6.4) | 7.9 (2.5) | 37.8 |
| Yes/yes (persistent inactivity) | 289 (17.2) | 59.2 | 45.4 (6.5) | 7.5 (2.3) | 51.6 |
| Disability analysis sample | 925 | 57.5 | 41.3 (6.0) | 8.6 (3.0) | 31.4 |
| Smoking in 1968/1981 | a | b | |||
| No/no (non-smoking) | 549 (59.4) | 66.5 | 42.0 (6.1) | 8.5 (3.0) | 33.3 |
| Yes/no (discontinued smoking) | 121 (13.1) | 33.1 | 41.3 (6.4) | 8.7 (3.1) | 27.3 |
| No/yes (new smoking) | 15 (1.6) | 46.7 | 38.5 (5.3) | 8.8 (3.1) | 33.3 |
| Yes/yes (persistent smoking) | 240 (25.9) | 50.0 | 40.0 (5.3) | 8.7 (3.0) | 28.7 |
| Inactivity in 1968/1981 | a | a | |||
| No/no (active) | 497 (53.7) | 53.5 | 41.4 (6.0) | 8.9 (3.3) | 27.4 |
| Yes/no (discontinued inactivity) | 153 (16.5) | 54.2 | 40.6 (6.2) | 8.3 (2.6) | 32.7 |
| No/yes (new inactivity) | 142 (15.4) | 66.9 | 41.2 (5.7) | 8.3 (2.6) | 31.0 |
| Yes/yes (persistent inactivity) | 133 (14.4) | 66.2 | 42.1 (6.0) | 8.0 (2.7) | 45.1 |
a p < 0.001 based on Chi square tests
b p < 0.001 based on F tests from ANOVA
The characteristics of the disability analysis sample are presented in the bottom portion of Table 1. Compared to the sample used in the mortality analysis, the reduced sample of survivors used in the disability analysis had a higher percentage of women, a lower mean age, a higher mean level of education, and a lower percentage of unskilled blue-collar workers.
Health behavior patterns in midlife and mortality
Table 2 presents the results from a Cox regression model estimating the independent mortality hazard associated with smoking and physical inactivity patterns between 1968 and 1981. As the results from this model show, compared to persistent non-smokers, mortality rates were elevated among both new smokers (hazard ratio (HR) = 2.5; 95 % confidence interval (CI) = 1.6–4.1) and persistent smokers (HR = 1.7; 95 % CI = 1.5–2.0). Although the HR estimate for the new smokers was larger than that for the persistent smokers, the former group was quite small (n = 30), and the confidence intervals for these two groups overlap considerably. Also, compared to non-smokers, a greater percentage of discontinued smokers died during the follow-up period (46.2 vs. 55.8 %), but after adjusting for sociodemographic and 1981 health variables, the hazard ratio indicates a non-significant elevation in mortality hazard associated with discontinued smoking (HR = 1.1; 95 % CI = 0.9–1.4).
Table 2.
Mortality rates and hazard ratios for smoking and physical inactivity patterns measured in 1968 and 1981 (n = 1,682)
| N | % Died through 2007 | Mortality hazard ratio (CI)b | ||
|---|---|---|---|---|
| Smoking in 1968/1981 | a | |||
| Non-smoking | 917 | 46.2 | 1.0 | Reference |
| Discontinued smoking | 224 | 55.8 | 1.1 | (0.9–1.4) |
| New smoking | 30 | 60.0 | 2.5 | (1.6–4.1) |
| Persistent smoking | 511 | 60.3 | 1.7 | (1.5–2.0) |
| Inactivity in 1968/1981 | a | |||
| Active | 850 | 48.7 | 1.0 | Reference |
| Discontinued inactivity | 260 | 47.7 | 1.0 | (0.8–1.3) |
| New inactivity | 283 | 55.5 | 1.2 | (1.0–1.4) |
| Persistent inactivity | 289 | 62.3 | 1.2 | (1.0–1.5) |
a p < 0.001 for Chi square test of group differences in % died
bAdjusted for age, sex, education, social class, as well as health problems (circulatory, mobility, and psychological) in 1981
Regarding physical inactivity, the estimates from this model show that both newly inactive (HR = 1.2; 95 % CI = 1.0–1.4) and persistently inactive (HR = 1.2; 95 % CI = 1.0–1.5) adults exhibited only slight elevations in mortality hazard during the follow-up period. The mortality hazard associated with discontinued inactivity was not distinguishable from the mortality hazard of persistently active behavior.
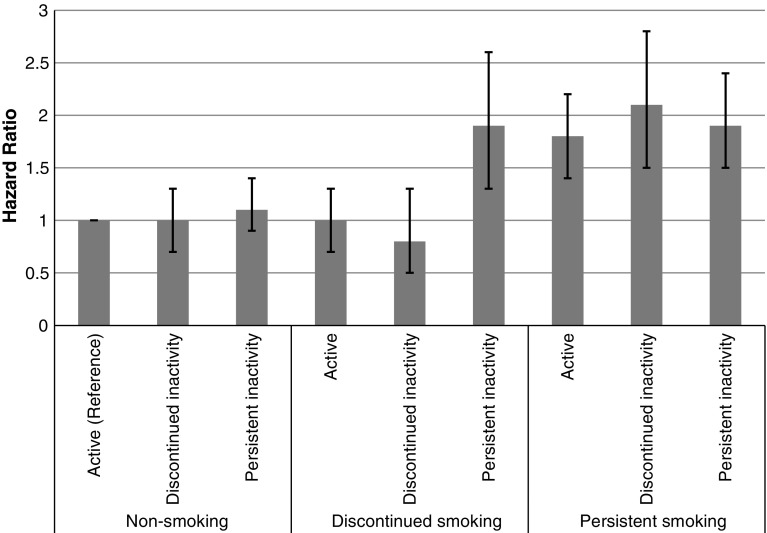
The joint effects of smoking and physical activity patterns on mortality are presented in Table 3. Compared to the reference group of persistently non-smoking, active individuals, all groups of persistent/new smokers exhibited elevated mortality hazards, regardless of the physical activity pattern. More specifically, the mortality hazard was elevated by close to twofold among persistent (or new) smokers who were active (HR = 1.8; 95 % CI = 1.4–2.2), among persistent/new smokers with a pattern of discontinued physical inactivity (HR = 2.1; 95 % CI = 1.5–2.8), as well as among persistent/new smokers who were also persistently, or newly, inactive (HR = 1.9; 95 % CI = 1.5–2.4). In contrast, almost none of the non-smoking or discontinued smoking subgroups exhibited elevated mortality hazards. The one exception involved individuals who were discontinued smokers in combination with persistent or new physical inactivity. These individuals exhibited a close to twofold elevation in mortality hazard compared to non-smoking, physically active individuals (HR = 1.9; 95 % CI = 1.3–2.6). This joint effect, in which the mortality hazard of discontinued smoking was elevated only among persistently inactive respondents, was also tested formally with an interaction term that was statistically significant at p = 0.008.
Table 3.
Mortality hazard ratios associated with different combinations of smoking and physical inactivity patterns in 1968 and 1981 (n = 1,682)
| Non-smoking | Discontinued smoking | Persistent/new smoking | |
|---|---|---|---|
| Active | 1.0 (Reference) n = 423 | 1.0 (0.7–1.3) n = 118 | 1.8 (1.4–2.2) n = 231 |
| Discontinued inactivity | 1.0 (0.7–1.3) n = 190 | 0.8 (0.5–1.3) n = 41 | 2.1 (1.5–2.8) n = 107 |
| Persistent/new inactivity | 1.1 (0.9–1.4) n = 304 | 1.9 (1.3–2.6) n = 65 | 1.9 (1.5–2.4) n = 203 |
Adjusted for age, sex, education, social class, as well as health problems (circulatory, mobility, and psychological) in 1981
The pattern of mortality hazards across all different combinations of smoking and physical inactivity behavior over time is presented in Fig. 1.
Fig. 1.
Mortality hazard ratios associated with different combinations of smoking and physical inactivity patterns in 1968 and 1981
Health behavior patterns in midlife and late life disability
Among survivors to old age, associations between midlife behavioral patterns and the odds of being disabled are presented in Table 4. This model shows no significant associations between midlife smoking patterns and late life disability. Furthermore, the model estimates indicate that discontinued inactivity in midlife was not associated with an increase in the odds of being disabled in old age. However, new inactivity in midlife [Odds Ratio (OR) = 1.6; 95 % CI = 1.0–2.4] and persistent inactivity in midlife (OR = 1.8; 95 % CI = 1.1–2.7) were both associated with elevated odds for being disabled in later life.
Table 4.
Odds ratios for disability in 2004, according to smoking and physical inactivity profiles in 1968–1981 (n = 925)
| N | % Disabled in 2004 | Disability odds ratio (CI)b | ||
|---|---|---|---|---|
| Smoking in 1968/1981 | a | |||
| Non-smoking | 549 | 35.0 | 1.0 | Reference |
| Discontinued smoking | 121 | 28.9 | 0.8 | (0.5–1.3) |
| New smoking | 15 | 26.7 | 1.0 | (0.3–3.6) |
| Persistent smoking | 240 | 30.4 | 1.0 | (0.7–1.5) |
| Inactivity in 1968/1981 | a | |||
| Active | 497 | 28.9 | 1.0 | Reference |
| Discontinued inactivity | 153 | 28.1 | 1.0 | (0.7–1.6) |
| New inactivity | 142 | 38.7 | 1.6 | (1.0–2.4) |
| Persistent inactivity | 133 | 46.7 | 1.8 | (1.1–2.7) |
a p < 0.001 for Chi square test of group differences in % died
bAdjusted for age, sex, education, social class, as well as health problems (circulatory, mobility, and psychological) in 1981
Table 5 presents findings from models estimating the joint effects of midlife smoking and physical inactivity patterns on the odds of late life disability. These findings provide no evidence of joint effects between smoking and physical inactivity during midlife on late life disability. The odds of being disabled in later life were elevated among those who exhibited persistent and new inactivity during midlife, but the strength of this association did not vary significantly across the various midlife smoking patterns. In particular, the odds of being disabled were elevated to a similar degree among non-smokers who were persistently inactive (OR = 1.7; 95 % CI = 1.1–2.6), and persistent smokers who were persistently inactive (OR = 2.1; 95 % CI = 1.2–3.7). None of the other combinations of smoking and physical inactivity were associated with elevated odds of disability. Supplemental tests of statistical interactions between the smoking and physical inactivity variables also found no evidence that these behaviors have multiplicative effects on the odds of late life disability.
Table 5.
Disability (2004) odds ratios associated with different combinations of smoking and physical inactivity patterns in 1968 and 1981 (n = 925)
| Non-smoking | Discontinued smoking | Persistent/new smoking | |
|---|---|---|---|
| Active | 1.0 (Reference) n = 264 | 1.2 (0.6–2.2) n = 64 | 0.8 (0.5–1.4) n = 117 |
| Discontinued inactivity | 1.2 (0.7–2.0) n = 121 | 0.5 (0.2–1.4) n = 30 | 1.2 (0.6–2.5) n = 54 |
| Persistent/new inactivity | 1.7 (1.1–2.6) n = 164 | 0.9 (0.3–2.5) n = 27 | 2.1 (1.2–3.7) n = 84 |
Adjusted for age, sex, education, social class, as well as health problems (circulatory, mobility, and psychological) in 1981
Discussion
By examining the mortality hazards and late life disability risks associated with different patterns of smoking and physical inactivity behavior during midlife, the current study reveals several important findings that have not previously emerged from studies assessing these health behaviors individually or at one point in time (Wong et al. 2008). In particular, our findings suggest that while midlife smoking is associated with long-term survival disadvantages in general, the extent of this disadvantage is regulated somewhat by both one’s level of persistence in smoking over time, as well as one’s pattern of physical activity during midlife. Furthermore, our findings suggest that physical inactivity in midlife is associated with risk for disability in late life. This association appears to be moderated only by one’s level of persistence in being physically inactive, and not by one’s smoking behavior.
Like several prior studies (Doll et al. 1994), our data showed that compared to persistent non-smokers, persistent and new (or more likely, relapsing) smokers during midlife exhibited increased mortality rates, while smokers who discontinued their habit during midlife did not experience elevated rates of death during the subsequent two-plus decades. At the same time, however, our findings advance our understanding of the long-term risk of midlife smoking by showing that among persistently inactive adults, quitting smoking during midlife may not alleviate mortality hazard. Indeed, according to our findings, the mortality rate of individuals who quit smoking while remaining persistently inactive was equivalent to the rate of those who were persistent smokers during midlife.
Additionally, our findings regarding changes in physical inactivity indicate that when assessed independently, midlife patterns of physical inactivity have fairly negligible associations with subsequent mortality. Moreover, our findings indicate that, among non-smokers and persistent smokers, positive changes in physical activity during midlife offer relatively inconsequential advantages in terms of survival. Indeed, we found that active behavior was not able to counteract the mortality hazard imposed by persistent smoking, while a history of physical inactivity did not increase the mortality hazard among non-smokers. These findings are somewhat inconsistent with previous findings showing strong associations between midlife physical activity and mortality (Byberg et al. 2009). Our findings, however, suggest that when assessed in combination with smoking, the risks of inactivity may be overshadowed by the risks of smoking. Pampel and Rogers (2004) have proposed a similar hypothesis with respect to the combined effects of smoking and socioeconomic status, stating that the risks of smoking may be relatively small within the context of the harms brought on by disadvantaged socioeconomic circumstances. Though Pampel and Rogers (2004) failed to find support for this hypotheses, our study of physical inactivity and smoking does show some support for this general type of combined effect, by finding that the risk of inactivity is small or negligible among those who are already exposed to the risks of smoking. Only among discontinued smokers did the risks of persistent inactivity become apparent.
Regarding the impact of midlife behavioral patterns on adults who survive into late life, some prior research has suggested that ex-smokers tend not to enjoy the same health-related quality of life as compared to never smokers (Sarna et al. 2008; Strandberg et al. 2008). Our findings with respect to late life disability, however, show no evidence of increased risk among former smokers. Instead, we found that persistent physical inactivity during midlife was associated with risk of late life disability, and that this association was neither exacerbated, nor alleviated, by midlife smoking status. Prior studies of physical activity and smoking among older adults have similarly found that relatively recent patterns of physical activity are associated with disability risk among both smokers and non-smokers (Ferrucci et al. 1999); our findings suggest that older adults’ physical inactivity histories dating back more than three decades, to midlife, may continue to bare consequences for disability risk during late life. Smoking histories, however, do not seem to carry the same long-term consequences for disability, perhaps due to the selective mortality of smokers prior to reaching old age.
Each of these main findings build upon a growing number of studies that have examined how one’s prospects for healthy aging are influenced by the combined effects of engaging in multiple risk behaviors during midlife (e.g., Britton et al. 2008; Myint et al. 2011; Willcox et al. 2006). While most of these studies have supported the notion that multiple risk behaviors are associated with a cumulating, or compounding, of health risk, very few have attempted to account for behavior changes over time when assessing the potential joint effects of key risk behaviors in midlife. By revealing a previously undocumented influence of midlife physical inactivity on the survival of individuals who quit smoking in midlife, our findings highlight the value of considering midlife health lifestyles not as a series of unrelated and static behaviors, but rather as a dynamic and interactive collection of behaviors.
Still, further research is needed to uncover why discontinuing smoking while maintaining a physically inactive lifestyle during midlife seems to offer such little benefit when it comes to survival. Perhaps this type of joint effect is the direct result of physiological interactions caused by these two behaviors. For example, the cardiovascular benefits resulting from smoking cessation (Bakru and Erlinger 2005) may somehow be blocked, or negated, by the cardiovascular deterioration resulting from physical inactivity. Alternatively, perhaps this joint effect is an indication that one’s health lifestyle is more than just a direct accounting of risk behaviors observed at a given time, and instead is a proxy for less readily observable, yet potentially influential, ways in which individuals think about, and attempt to care for, their own health (Cockerham et al. 1997). For instance, perhaps lifestyles characterized by discontinued smoking plus regular physical activity in midlife are indicative of only minor lapses in otherwise healthy lifestyles, whereas lifestyle profiles characterized by discontinued smoking plus persistent inactivity are indicative of more chronic and extensive patterns of unhealthful living across the adult life course. In such cases, perhaps only a comprehensive change in lifestyle—involving multiple behavioral improvements—is sufficient to change one’s future health prospects significantly.
In assessing these findings, it is important to note that a particular strength of this study was its design, which allowed for the measurement of health behavior patterns at midlife, and the prospective assessment of mortality over the course of the subsequent three decades. The ability to use data on health behaviors collected during midlife helps to minimize the effects of selective attrition that frequently hamper studies focusing on the impact of health behaviors among aging adults. In particular, when selective attrition is not accounted for, the likelihood of finding associations between unhealthy lifestyles and health may be diminished because many individuals from the population that had unhealthy lifestyles are apt to have already died or to be too impaired to participate (Andel et al. 2008; Pluijm et al. 2007; Rovio et al. 2005; Sabia et al. 2009). Nevertheless, this type of selective attrition could not be accounted for in our disability analysis, which focused only on the subsample of respondents who survived until 2004. Therefore, the total scope of the long-term health impact of midlife smoking and physical inactivity behavior can only be fully appreciated by examining impacts not only on the health status of survivors to old age but also on mortality leading up to old age.
Furthermore, studies of associations between health behaviors and late life health typically are unable to account for the possibility that declining health has influenced one’s lifestyle choices (de Groot et al. 2004). However, by measuring individuals’ health behaviors at midlife in the current study, while also controlling for baseline (1981) health status, a more accurate representation of actual behavior patterns that existed before the onset of illness is likely to be reflected. This allows us to be more confident that the associations estimated reflect the influence of behavior on health, rather than the reverse. However, it remains possible that other uncontrolled for health problems at baseline may be partially responsible for some of the associations found in this study between midlife health behaviors and late life health.
Other limitations of the current study involve the measurement of health behaviors. First, our measure of smoking did not distinguish between levels of smoking (e.g., heavy vs. light) or smoking history, both of which may have important implications for mortality (Hart et al. 2010). Second, our data did not allow us to account for possible fluctuations in behavior that might have occurred between 1968 and 1981, or the precise timing of behavior changes during this period. Therefore, our measures of behavior change and stability are rather crude. Additionally, we did not account for changes in behavior that may have occurred beyond 1981. However, given that most prior studies of these combined effects have measured these behaviors at just a single point in time, being able to capture at least some information about the stability, or lack of stability, of these behaviors over time, represents an important advance. Third, because our definition of physical inactivity was based on the frequencies of various behaviors observed in 1968 and 1981, this definition does not necessarily equate to current public health guidelines for physical activity. Moreover, it is possible that the validity of our measure of physical inactivity is weaker than that of our smoking measure. This may have resulted in a relatively heterogeneous group of physically inactive individuals, and could partially explain why we found generally weaker effects for physical inactivity than for smoking with respect to mortality. And fourth, the prevalence and distribution of smoking and physical inactivity throughout the population of midlife adults observed in 1968–1981 are not necessarily accurate representations of the current prevalence and distribution of risk (Ahacic et al. 2008).
Despite these data limitations, the current study lends important insight into health behaviors and their implications for survival and health leading up to and during old age. Perhaps most importantly, these results suggest that smoking cessation in midlife offers no clear improvements in long-term survival prospects unless one is also physically active, or becomes active, during midlife. Persistent physical inactivity during midlife also appears to impose a long-term risk for late life disability, but any additional impact due to smoking patterns during midlife was not apparent among those who survived into old age. Future research in this area is needed in order to determine if the health effects of specific combinations of the health behaviors are due purely to the combined physiologic effects that the behaviors impose, or whether one’s specific health lifestyle profile might represent some more latent characteristic of an individual that is linked to other risk or protective factors existing in the social environment.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute on Aging, Grant R01 AG031109, “Health Behaviors and Lifestyles in Old Age in the United States and Japan” (BAS), and the Swedish Council for Working Life and Social Research, Grant 2010-0954, “Do health behaviors still matter for survival to advanced ages?”, and Grant 2010-1788, “Health and health behavior in a lifecourse perspective”.
Contributor Information
Benjamin A. Shaw, Phone: 518-402-0290, FAX: 518-402-0414, Email: bashaw@albany.edu
Neda Agahi, Email: Neda.agahi@ki.se.
References
- Ahacic K, Kennison R, Thorslund M. Trends in smoking in Sweden from 1968 to 2002: age, period, and cohort patterns. Prev Med. 2008;46(6):558–564. doi: 10.1016/j.ypmed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol Ser A. 2008;63(1):62–66. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- Bakru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Survey. PLoS Med. 2005;2(6):e160. doi: 10.1371/journal.pmed.0020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk DR, Hubert HB, Fries JF. Associations of changes in exercise level with wubsequent disability among seniors: a 16-year longitudinal study. J Gerontol Ser A. 2006;61(1):97–102. doi: 10.1093/gerona/61.1.97. [DOI] [PubMed] [Google Scholar]
- Britton A, Shipley M, Singh-Manoux A, Marmot MG. Successful aging: the contribution of early-life and midlife risk factors. J Amer Geriatr Soc. 2008;56(6):1098–1105. doi: 10.1111/j.1532-5415.2008.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byberg L, Melhus H, Gedeborg R, Sundstrom J, Ahlbom A, Zethelius B, Berglund LG, Wolk A, Michaelsson K. Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. BMJ. 2009;338:b688. doi: 10.1136/bmj.b688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty EF, Hubert HB, Krishnan E, Bruce BB, Lingala VB, Fries JF. Lifestyle risk factors predict disability and death in healthy aging adults. Am J Med. 2012;125(2):190–197. doi: 10.1016/j.amjmed.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield JG. Everyday physical activity as a predictor of late-life mortality. Gerontology. 2008;48(3):349–357. doi: 10.1093/geront/48.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerham WC, Rütten A, Abel T. Conceptualizing contemporary health lifestyles: moving beyond Weber. Sociol Q. 1997;38(2):321–342. doi: 10.1111/j.1533-8525.1997.tb00480.x. [DOI] [Google Scholar]
- de Groot LCPMG, Verheijden MW, de Henauw S, Schroll M, van Staveren WA. Lifestyle, Nutritional Status, Health, and Mortality in Elderly People Across Europe: a review of the longitudinal results of the SENECA study. J Gerontol Ser A. 2004;59(12):1277–1284. doi: 10.1093/gerona/59.12.1277. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309(6959):901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Izmirlian G, Leveille S, Phillips CL, Corti M-C, Brock DB, Guralnik JM. Smoking, physical activity, and active life expectancy. Am J Epidemiol. 1999;149(7):645–653. doi: 10.1093/oxfordjournals.aje.a009865. [DOI] [PubMed] [Google Scholar]
- Ford ES, Bergmann MM, Boeing H, Li C, Capewell S. Healthy lifestyle behaviors and all-cause mortality among adults in the United States. Prev Med. 2012;55(1):23–27. doi: 10.1016/j.ypmed.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzell J, Lundberg O. Health inequalities and welfare resources. Bristol: Policy Press; 2007. [Google Scholar]
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- Gerber Y, Myers V, Goldbourt U. Smoking reduction at midlife and lifetime mortality risk in men: a prospective cohort study. Am J Epidemiol. 2012;175(10):1006–1012. doi: 10.1093/aje/kwr466. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cauley JA, Stone K, Thompson TJ, Bauer DC, Cummings SR, Ensrud KE. Relationship of changes in physical activity and mortality among older women. JAMA. 2003;289(18):2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- Hart C, Davey Smith G, Gruer L, Watt G. The combined effect of smoking tobacco and drinking alcohol on cause-specific mortality: a 30 year cohort study. BMC Public Health. 2010;10(1):789. doi: 10.1186/1471-2458-10-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert HB, Bloch DA, Oehlert JW, Fries JF. Lifestyle habits and compression of morbidity. J Gerontol Ser A. 2002;57(6):M347–M351. doi: 10.1093/gerona/57.6.M347. [DOI] [PubMed] [Google Scholar]
- Johansson S-E, Sundquist J. Change in lifestyle factors and their influence on health status and all-cause mortality. Int J Epidemiol. 1999;28(6):1073–1080. doi: 10.1093/ije/28.6.1073. [DOI] [PubMed] [Google Scholar]
- Khaw K-T, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5(1):e12. doi: 10.1371/journal.pmed.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops KTB, de Groot LCPGM, Kromhout D, Perrin A-E, Moreiras-Varela O, Menotti A, van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women. J Am Med Assoc. 2004;292(12):1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med. 2010;170(8):711–718. doi: 10.1001/archinternmed.2010.76. [DOI] [PubMed] [Google Scholar]
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide; an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55:163–170. doi: 10.1016/j.ypmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Lundberg O, Thorslund M. Fieldwork and measurement considerations in surveys of the oldest old. Experiences from the Swedish level of living surveys. Soc Indic Res. 1996;37:165–189. doi: 10.1007/BF00315527. [DOI] [Google Scholar]
- Meng L, Maskarinec G, Lee J, Kolonel LN. Lifestyle factors and chronic diseases: application of a composite risk index. Prev Med. 1999;29(4):296–304. doi: 10.1006/pmed.1999.0538. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. J Am Med Assoc. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Myint PK, Luben RN, Wareham NJ, Bingham SA, Khaw K-T (2009) Combined effect of health behaviours and risk of first ever stroke in 20,040 men and women over 11 years’ follow-up in Norfolk cohort of European prospective investigation of cancer (EPIC Norfolk): prospective population study. BMJ 338: b349 [DOI] [PMC free article] [PubMed]
- Myint PK, Smith RD, Luben RN, Surtees PG, Wainwright NW, Wareham NJ, Khaw K-T. Lifestyle behaviours and quality-adjusted life years in middle and older age. Age Ageing. 2011;40(5):589–595. doi: 10.1093/ageing/afr058. [DOI] [PubMed] [Google Scholar]
- Pampel FC, Rogers RG. Socioeconomic status, smoking, and health: a test of competing theories of cumulative advantage. J Health Soc Behav. 2004;45(3):306–321. doi: 10.1177/002214650404500305. [DOI] [PubMed] [Google Scholar]
- Pluijm SM, Visser M, Puts MTE, Dik MG, Schalk BWM, van Schoor NM, Schaap LA, Bosscher RJ, Deeg DJH. Unhealthy lifestyles during the life course: association with physical decline in late life. Aging Clin Exp Res. 2007;19(1):75–83. doi: 10.1007/BF03325214. [DOI] [PubMed] [Google Scholar]
- Rovio S, Kåreholt I, Helkala E-L, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and alzheimer’s disease. Lancet Neurol. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Health behaviors from early to late midlife as predictors of cognitive function. Am J Epidemiol. 2009;170(4):428–437. doi: 10.1093/aje/kwp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna L, Bialous SA, Cooley ME, Jun H-J, Feskanich D. Impact of smoking and smoking cessation on health-related quality of life in women in the nurses’ health study. Qual Life Res. 2008;17(10):1217–1227. doi: 10.1007/s11136-008-9404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnohr P, Scharling H, Jensen JS. Changes in leisure-time physical activity and risk of death: an observational study of 7,000 men and women. Am J Epidemiol. 2003;158(7):639–644. doi: 10.1093/aje/kwg207. [DOI] [PubMed] [Google Scholar]
- Strandberg AY, Strandberg TE, Pitkala K, Salamaa VV, Tilvis RS, Miettinen TA. The effect of smoking in midlife on health-related quality of life in old age. Arch Intern Med. 2008;168(18):1968–1974. doi: 10.1001/archinte.168.18.1968. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92(6):990–996. doi: 10.2105/AJPH.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, He Q, Chen R, Yano K, Masaki KH, Grove JS, Donlon TA, Willcox DC, Curb JD. Midlife risk factors and healthy survival in men. J Am Med Assoc. 2006;296(19):2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- Wong R, Ofstedal M, Yount K, Agree E. Unhealthy lifestyles among older adults: exploring transitions in Mexico and the US. Eur J Ageing. 2008;5(4):311–326. doi: 10.1007/s10433-008-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]