Abstract
Objective
Polyphosphate and heparin are anionic polymers released by activated mast cells and platelets that are known to stimulate the contact pathway of coagulation. These polymers promote both the autoactivation of factor XII and the assembly of complexes containing factor XI, prekallikrein, and high-molecular-weight kininogen. We are searching for salivary proteins from blood-feeding insects that counteract the effect of procoagulant and proinflammatory factors in the host, including elements of the contact pathway.
Approach and Results
Here, we evaluate the ability of the sand fly salivary proteins, PdSP15a and PdSP15b, to inhibit the contact pathway by disrupting binding of its components to anionic polymers. We attempt to demonstrate binding of the proteins to polyphosphate, heparin, and dextran sulfate. We also evaluate the effect of this binding on contact pathway reactions. We also set out to determine the x-ray crystal structure of PdSP15b and examine the determinants of relevant molecular interactions. Both proteins bind polyphosphate, heparin, and dextran sulfate with high affinity. Through this mechanism they inhibit the autoactivation of factor XII and factor XI, the reciprocal activation of factor XII and prekallikrein, the activation of factor XI by thrombin and factor XIIa, the cleavage of high-molecular-weight kininogen in plasma, and plasma extravasation induced by polyphosphate. The crystal structure of PdSP15b contains an amphipathic helix studded with basic side chains that forms the likely interaction surface.
Conclusions
The results of these studies indicate that the binding of anionic polymers by salivary proteins is used by blood feeders as an antihemostatic/anti-inflammatory mechanism.
Keywords: blood coagulation factor inhibitors, bradykinin, factor XI, factor XII, inflammation, kallikreins, leishmania
The contact pathway of coagulation in mammals is initiated by conversion of coagulation factor XII (FXII) to FXIIa in an autocleavage reaction that occurs when the zymogen binds to an anionic surface.1 FXIIa goes on to convert factor XI (FXI) to FXIa, which in turn activates factor IX (FIX), the protease component of the intrinsic factor Xase complex, leading to thrombin production and fibrin deposition.1,2 Although a deficiency in FXII leads to an increase in the clotting time in vitro, no significant bleeding disorder is associated with this.3 More recent studies with rodent models have shown that FXII deficiency protects against thrombosis after vascular injury, and additional studies have shown that inhibitors of this protease effectively reduce the generation and stability of thrombi in vivo, despite the lack of an effect on hemostasis.4,5 In addition to its role in coagulation, FXIIa converts prekallikrein to kallikrein, a serine protease closely related to FXI that is responsible for cleavage of the plasma protein high-molecular-weight kininogen (HK) to give the peptide bradykinin. Kallikrein also amplifies the contact pathway by converting FXII to FXIIa.1 When released from the kininogen, bradykinin causes a rapid increase in vascular permeability, edema, and an immediate sensation of pain.1 The activation of FXI and prekallikrein, as well as the cleavage of HK, is also enhanced in the presence of anionic surfaces, suggesting that surface molecules are an important component of multiple proteolytic processes.
A variety of negatively charged materials are known to act as surfaces in the auto activation of FXII. Untreated glass efficiently activates the pathway, and earth materials, such as kaolin, are used to initiate coagulation in the activated partial thromboplastin time test.6 Dextran sulfate (DS), a sulfated glycan, has long been used as an in vitro activator of FXII in laboratory studies.7 Endogenous anionic polymers, including polyP, heparin, and nucleic acids, activate FXII and are considered candidates to serve in this role in vivo.8–12 PolyP is released by platelets and mast cells, whereas heparin is released only by mast cells after activation by IgE-antigen complexes.13,14 On release, the highly sulfated glycosaminoglycan provides an anionic surface for the activation of FXII and has been shown to activate the contact pathway in vitro and in vivo.8 The binding of heparin by antithrombin gives it its familiar anticoagulant properties in the circulation, and as a consequence heparin-induced activation of the contact pathway leads only to bradykinin formation and not coagulation.12,15 PolyP chains are also potent activators of the contact pathway and have been shown to support the efficient autoactivation of FXII to FXIIa, its mature enzymatic form. Unlike heparin, however, this polymer is a potent endogenous cofactor for activation of FXI by thrombin and does not activate antithrombin. It therefore exhibits both procoagulant and proinflammatory activities in vivo and may be an important component of normal hemostasis.9,16–19
The saliva of blood-feeding arthropods contains a variety of peptides and small molecules that act to inhibit the hemostatic and inflammatory systems of the host. Proteins and peptides attacking various points in the coagulation cascade have been described, including targets in the contact pathway.20–23 Increases in bradykinin and histamine levels resulting from the activation of mast cells during blood feeding produce many of the symptoms associated with an insect bite, including itching, swelling, and pain. To a feeding insect these rapid responses in the skin are a significant impediment to the ingestion of blood. Sensations of pain and itching alert the host to the insect’s presence and may cause it to interrupt feeding, whereas edema may also make the physical process of blood intake more difficult.
Here, we show that the PdSP15 proteins, a group of salivary proteins from the African sand fly Phlebotomous duboscqi, a vector of parasitic Leishmania species, bind negatively charged surfaces, including polyP, heparin, and DS. By competing with FXII for binding sites they inhibit activation of the zymogen and consequently the processes of coagulation and bradykinin production in plasma. We have also determined the x-ray crystal structure of one of these proteins and found it to contain a positively charged surface dominated by a single α-helix studded with the side chains of basic amino acid residues along the length of its solvent-facing sides. This is the likely region for interaction with anionic surfaces.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
PdSP15a and b are closely related (86% amino acid identity) members of the insect odorant-binding protein family found in the saliva of P duboscqi.24 They are also very similar in sequence to the SP15 protein of the related sand fly P papatasi (≈65% amino acid identity; Figure I in the online-only Data Supplement).25 Proteomic analysis has shown this group to be the most abundant group of proteins in the saliva of P duboscqi.24
Recombinant PdSP15a and b were tested for inhibition of the clotting of normal plasma in the activated partial thromboplastin time assay, where coagulation is initiated by addition of an anionic colloidal silica suspension (Cephen) and calcium. In the presence of either of the salivary proteins, an ≈5-fold delay in clotting time was observed (Figure 1A and 1B). The degree of inhibition increased as a function of inhibitor concentration, up to the maximum tested concentration of 10 µmol/L. Both PdSP15 proteins produced nearly identical levels of inhibition suggesting that they act by the same mechanism. PdSP15a also inhibited coagulation in FIX, factor X, and FXI-deficient plasmas in a concentration-dependent manner (Figure 1D–1F), whereas clotting of FXII-deficient plasma was not inhibited (Figure 1C), suggesting a dependence of inhibition on the presence of FXII. In addition, we found that PdSP15a and b effectively inhibited the reciprocal activation of FXII and prekallikrein in the presence of DS (Figure 1G and 1H) further indicating that PdSP15 proteins prevent coagulation by interfering with the autoactivation or catalytic function of FXII/FXIIa.
Figure 1.
PdSP15a and b delay coagulation of plasma and inhibit activation of the contact pathway. Coagulation of human plasma is inhibited by PdSP15a (A) and b (B) in the activated partial thromboplastin time (aPTT) assay. PdSP15a and b were preincubated with plasma and Cephen aPTT reagent (diluted 10×) before the addition of calcium chloride. Coagulation was detected as an increase in turbidity at 600 nm in a plate reader. Bars represent the mean (±SE) of 3 independent experiments. C to E, Inhibition of clotting in coagulation factor–deficient plasmas by PdSP15a as measured in the aPTT assay as described above. C, factor XII (FXII); (D) factor XI; (E) factor IX; and (F) factor X. Bars represent the mean (±SE) of 3 independent experiments. G and H, Inhibitory effect of PdSP15a and b on the reciprocal activation of FXII and prekallikrein (PK) in the presence of dextran sulfate (DS). FXII (final concentration, 0.2 nmol/L) was preincubated at 37°C with different concentrations of PdSP15a (G) or PdSP15b (H). After this, PK (10 nmol/L) and DS (0.2 µg/mL) were added to each well, and after an additional incubation period the chromogenic substrate S-2302 was added. Generation of kallikrein and FXIIa was detected by measuring the increase in absorbance at 405 nm as described in the Materials and Methods in the online-only Data Supplement. The reaction rates are expressed as percentages of the rate in the absence of inhibitor. The graphs show the mean (±SE) of 3 independent experiments.
We tested whether a block in the autoactivation of FXII was responsible for the results described above. FXII was activated by the addition of DS (1 µg/mL; molecular weight=500 000), polyP (P700; 52.5 µmol/L), or heparin (20 µg/mL) in the presence of 150 nmol/L FXII and the chromogenic substrate S-2302. The autoactivation of FXII in the presence of all 3 initiating polymers was reduced substantially in the presence of PdSP15a and b at concentrations of ≥500 nmol/L, with essentially complete inhibition being attained at concentrations of ≥1 µmol/L (Figure 2A–2F). The quantity of FXIIa produced in the presence of polyP was reduced ≈50% at a concentration of 250 nmol/L, 90% at 500 nmol/L, and 99% at 4 µmol/L (Figure 2G). Like FXII, FXI is autoactivated in the presence of DS in vitro although this pathway is not thought to be important in vivo as a means of initiating coagulation.26 When the DS-mediated activation of FXI was measured continuously as described above with FXII, PdSP15a was found to inhibit the process in a concentration-dependent manner (Figure 2H). Salivary gland homogenates produced similar activity to the recombinant proteins. Activation of FXII by polyP was inhibited ≈50% at a concentration of 5.6 ng/µL (ca. 0.6 salivary gland pair per 100 µL reaction) of salivary protein and 90% at a concentration of 15 ng/µL (Figure 2I). If the salivary gland extract was added after the coincubation of polyP and FXII, but before the addition of chromogenic substrate, essentially no inhibition was observed, suggesting that the inhibitor blocks the activation of FXII but not the amidolytic activity of FXIIa (Figure 2I).
Figure 2.
PdSP15a and b inhibit the autoactivation of factor XII (FXII) on surfaces of dextran sulfate (DS), polyphosphate (polyP), and heparin. A and B, Time course of autoactivation of FXII (140 nmol/L) in the presence of DS (1.0 µg/mL) as measured by hydrolysis of the substrate S-2302 at 37°C and 405 nm. Activation was measured continuously in the presence of 0, 0.5, 1.0, 2.0, 4.0, and 8.0 µmol/L PdSP15a (A) and PdSP15b (B) as indicated on each panel, and as described in the Materials and Methods in the online-only Data Supplement. C and D, Time course of activation of FXII by polyP (P700, 52.5 µmol/L, as PO3 monomer). Activation was measured as described above in the presence of 0, 0.5, 1.0, 2.0, 4.0, and 8.0 µmol/L PdSP15a (C) and PdSP15b (D). E and F, Time course of activation of FXII by heparin (10 µg/mL). Activation was measured as described above in the presence of 0, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 µmol/L PdSP15a (E) and PdSP15b (F). G, Quantification of FXIIa produced by polyP-mediated autoactivation in the presence of increasing concentrations of PdSP15b. After combining polyP (P700, 52.5 µmol/L), PdSP15b (250 nmol/L to 4 µmol/L), and FXII (140 nmol/L) as described in the Materials and Methods in the online-only Data Supplement, the mixtures were incubated for 1.5 hours at 37°C at which time the reactions were stopped with polybrene (6 µg/mL). Generated FXIIa was quantified by observing the cleavage of S-2302 at 405 nm and 37°C. Each data point (solid circles) represents the result of a single reaction, and 3 individual reactions were performed. H, Inhibition of the DS-mediated autoactivation of factor XI (FXI) in the presence of increasing concentrations of PdSP15a. The experiment was performed as in A, with exception that FXII was replaced by FXI at a concentration of 200 nmol/L, and the activity of FXIa was measured using the chromogenic substrate S-2366. PdSP15a concentrations (µmol/L) are shown on the graph. I, Inhibition of polyP-mediated autoactivation of FXII by Phlebotomous duboscqi salivary gland extract. Experiment was performed as in G with the recombinant protein replaced by gland extract at the concentrations indicated. Experiments containing salivary gland extract were performed in duplicate, except for the inhibitor-free treatment, which was performed in quadruplicate. All data points (solid circles) are shown. A second experiment (open circles) was performed in which the salivary gland extract was added after the coincubation of polyP and FXII but before the addition of the chromogenic substrate.
The results of autoactivation studies suggested that the PdSP15 proteins block the cleavage of FXII and FXI, either by binding with the proenzymes themselves or by blocking protease binding sites on anionic polymers. We evaluated PdSP15a as a direct inhibitor of FXIIa, kallikrein, or FXIa by measuring the cleavage of their respective chromogenic substrates in the presence and absence of PdSP15b. In the absence of anionic polymers, PdSP15b does not inhibit substrate cleavage by any of the 3 enzymes at concentrations ≤8 µmol/L of inhibitor, suggesting that PdSP15b does not bind these serine proteases or that formation of a complex does not block the access of chromogenic substrate to the catalytic site of the enzyme (Figure 3A–3C).
Figure 3.
PdSP15s do not inhibit the amidolytic activity of factor XIIa (FXIIa), factor XIa (FXIa), or kallikrein but inhibit the polyP-mediated activation of FXI by thrombin and the dextran sulfate (DS)–mediated activation of FXI by FXIIa. A to C, Hydrolysis of chromogenic substrates by FXIIa (A), FXIa (B), and kallikrein (C) in the presence of increasing concentrations of PdSP15b. The initial reaction rate for each inhibitor concentration was normalized to a value of 1.0 in the absence of inhibitor. FXIIa activity was measured using S-2302, whereas FXIa and kallikrein activities were measured using S-2366. D, Inhibition of FXI activation by thrombin in the presence of polyP (P700). The activation of FXI (30 nmol/L) by α-thrombin (5 nmol/L) in the presence of 2 µmol/L polyP was evaluated after 20 minutes incubation at 37°C. Reactions were performed in the presence of PdSP15a concentrations ranging from 0 to 500 nmol/L. The FXIa product was quantified using the substrate S-2366. E, An experiment similar to that shown in D performed in the absence of polyP, but at an α-thrombin concentration of 15 nmol/L. F, Inhibition of the activation of FXI by FXIIa in the presence of DS. The activation of FXI (20 nmol/L) by FXIIa (0.8 nmol/L) in the presence of 0.2 µg/mL DS was evaluated after 30 minutes incubation at 37°C. At the end of each incubation period, FXIIa was inhibited with corn trypsin inhibitor and the FXIa product was quantified using the substrate S-2366. This experiment was performed in the presence of increasing concentrations of PdSP15b between 0 and 2 µmol/L. G, The experiment in F was repeated in the absence of DS. FXIa was quantified after adding corn trypsin inhibitor and DS (0.2 µg/mL). For all assays the points represent the mean (±SE) of 3 replicates.
The activation of FXI by thrombin is greatly enhanced in the presence of polyP, demonstrating that a ternary complex is necessary for optimal activation.9 We evaluated the effect of PdSP15a on this process in the presence of 2 µmol/L polyP (P700) or in its absence. In the presence of polyP, PdSP15a potently inhibited FXI activation by FXIIa, with an inhibitory concentration50 value of ≈75 nmol/L (Figure 3D). In the absence of polyP, the rate of FXI cleavage by thrombin production was greatly diminished and was not inhibited by PdSP15a at concentrations ≤1 µmol/L (Figure 3E). In a similar manner, the activation of FXI by FXIIa is known to be strongly enhanced by DS.27 We found that in the presence of 0.2 µg/mL DS, the activation of FXI by FXIIa was inhibited by PdSP15b with an approximate inhibitory concentration50 value of 250 nmol/L (Figure 3F). In the absence of DS, the rate of FXI activation was reduced and was not inhibited by PdSP15b (Figure 3G). Taken together, these results with DS and polyP suggest that PdSP15a and b act by inhibiting assembly of the thrombin or FXIIa complexes with FXI on the anionic polymer surface rather than by binding with the coagulation factors themselves.
The sequestration of anionic polymer–binding sites suggested that PdSP15 proteins would inhibit polyP-initiated coagulation by preventing autoactivation of FXII and other downstream reactions in plasma. We found that adding polyP (P700) to plasma at a concentration of 52.5 µmol/L produced a reduction in the clotting time of recalcified plasma from a mean of ≈640 s in the absence of polyP to a mean of 340 s (Figure 4A). Preincubation of plasma with PdSP15a before addition of polyP gave a concentration-dependent increase in the clotting time, producing values of ≈550 s at a concentration of 5 µmol/L and 600 s at a concentration of 20 µmol/L of inhibitor protein (Figure 4A).
Figure 4.
The inhibition of polyP-mediated coagulation in plasma and bradykinin generation in plasma and skin. A, Inhibition of polyP-mediated coagulation of human plasma by PdSP15a. Plasma was incubated with 52.5 µmol/L polyP (P700, as phosphate monomer) and concentrations of PdSP15a ranging from 0 to 20 µmol/L at 37°C, and the clotting times (solid circles) were measured in a coagulometer after addition of 25 mmol/L calcium chloride. The clotting time after addition of calcium chloride in the absence of polyP or PdSP15a is shown on the y axis (open diamond). B, Inhibition of plasma extravasation induced by polyP (P45) in mouse skin. Swiss mice intravenously injected with Evans blue dye were given 50 µL intradermal injections of PBS alone, PdSP15b in PBS, polyP alone in PBS, and PdSP15b plus polyP in PBS. After 30 minutes, the mice were euthanized and the skin was removed. Extravasation was indicated by a spot of Evans blue at the injection site. Evans blue in the tissue samples was quantified after extraction. The bars for each treatment indicate the mean (±SE) for 3 mice. The absorbance values were normalized with the PBS alone sample equaling 1.0 for each mouse. Mixture of PdSP15b with polyP before injection significantly reduced the extravasation of Evans blue (P<0.03; 2-tailed t test compared with treatment with polyP alone). C and D, Inhibition of high-molecular-weight kininogen (HK) peptide mimetic cleavage in human plasma by PdSP15a. Citrated human plasma diluted 1:20 in buffer (described in the Materials and Methods in the online-only Data Supplement) was supplemented with the HK peptide mimetic substrate Abz-GFSPFRSVTVQ-EDDnp (4 µmol/L) and DS (10 µg/mL). Assays with PdSP15a were performed using 2 different schemes. C, Citrated plasma was preincubated with FXII inhibitor before the addition of the contact activator DS. PdSP15a concentrations: 0 µmol/L (filled square), 2 µmol/L (open triangle), 5 µmol/L (filled inverted triangle), and 10 µmol/L (open diamond). The substrate was also tested in the absence of both DS and PdSP15a (filled circle). D, PdSP15a was added to the plasma together with DS. PdSP15a concentrations: 0 µmol/L (filled square), 10 µmol/L (open diamond). The substrate was also tested in the absence of DS or inhibitor (filled circle). In both cases hydrolysis was followed by measuring the fluorescence at λex=320 nm and λem=420 nm. The plots show the increase of fluorescence with time, reflecting substrate hydrolysis. The error bars in the figures represent the mean±SE of duplicate determinations performed within 1 representative experiment of 4.
To determine whether PdSP15a could block the cleavage of HK after activation by DS, we supplemented human plasma with Abz-GFSPFRSVTVQ-EDDnp, an intramolecularly quenched mouse HK–mimetic peptide.28 This substrate was synthesized under the assumption that DS would sequentially activate FXII and prekallikrein, generating kallikrein, which would in turn hydrolyze the C-terminal flanking sequence of bradykinin. As predicted, we found that the substrate was rapidly hydrolyzed in citrated human plasma supplemented with DS at a concentration of 10 µg/mL. As shown in Figure 4C, PdSP15a was not able to block the hydrolysis when tested at 2 and 5 µmol/L. However, at 10 µmol/L, PdSP15a prevented hydrolysis, reducing it to control levels (plasma plus substrate in the absence of DS; Figure 4C). We next repeated these assays without the preincubation step (ie, we mixed PdSP15a with DS shortly before addition to plasma). Again, we found that 10 µmol/L of the inhibitor abolished hydrolysis (Figure 4D). Additional controls showed that DS-induced hydrolysis was inhibited by the kallikrein inhibitor trans-4-aminomethylcy-clohexanecarbonyl-L-phenylalanyl–4-aminopheny l acetic acid (PKSI-527).29 We did not detect significant hydrolysis in FXII-deficient plasma treated with DS (data not shown). Together, these results suggest that PdSP15a is able to attenuate contact phase activation by DS in plasma.
Induction of bradykinin production in skin has been demonstrated previously after activation of the contact pathway by intradermal injection of heparin and polyP.11,12 We measured the effect of PdSP15b on the degree of polyP-induced plasma extravasation by observing plasma leakage in mice first injected with Evans blue dye. The dye allows visualization and quantification of plasma leakage in the skin surrounding an injection site. Injections of 1.5 mmol/L polyP (P45) produced extravasation surrounding the injection site as measured by spectrophotometry after extraction. This was significantly inhibited (P<0.03; 2-tailed t test) by mixing an equal quantity of polyP with PdSP15b at a concentration of 20 µmol/L before injection (Figure 4B).
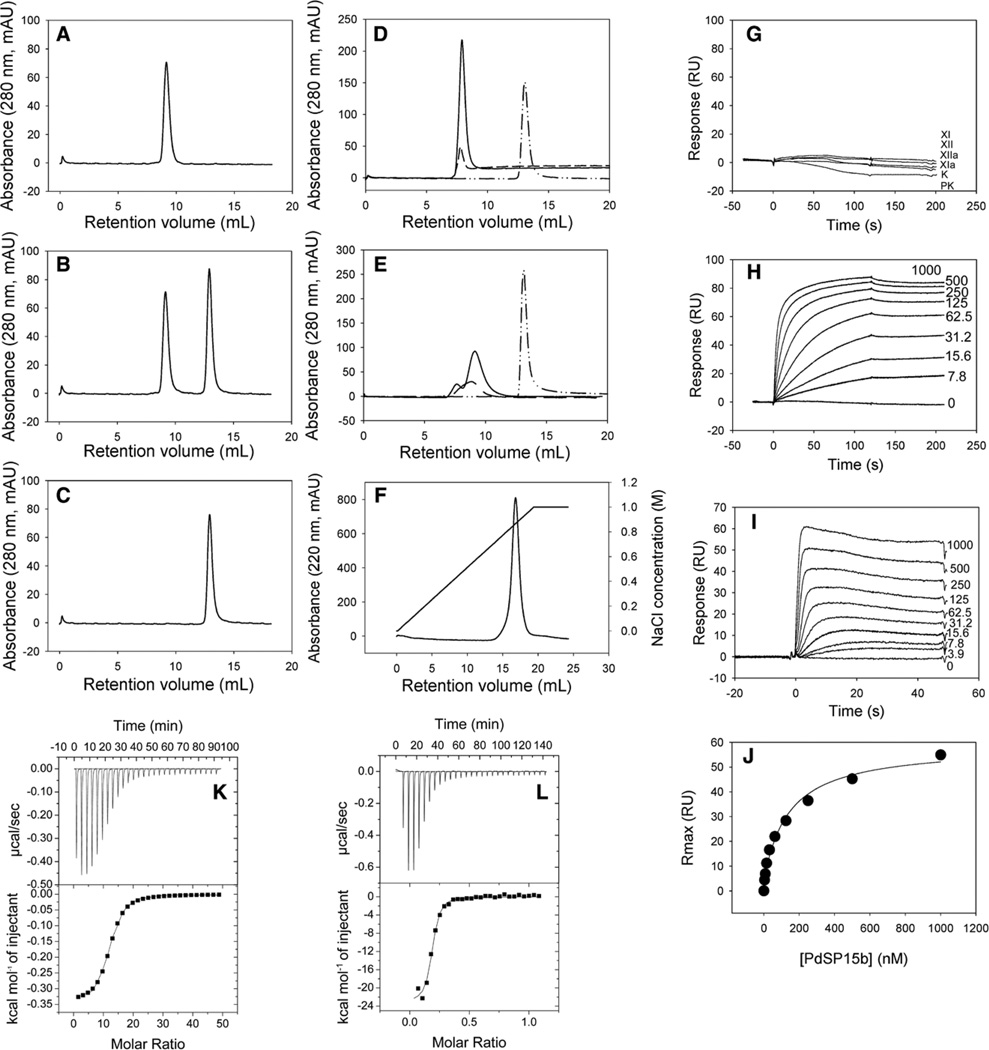
The results of the enzymatic assays described above suggested that PdSP15 proteins inhibit interaction of protein components of the pathway with anionic polymers. To measure this directly, we analyzed interactions of potential binding partners using gel filtration chromatography, isothermal titration calorimetry, and surface plasmon resonance. PdSP15b binding with FXII was evaluated directly by gel filtration chromatography where changes in the retention volume of individual components are taken as indicators of binding. FXII was mixed with PdSP15b at a sodium chloride concentration of 0.15 mol/L and subjected to gel filtration chromatography under the same buffer conditions. No change in the retention volume of either FXII or PdSP15b was observed in the mixture when compared with chromatograms of the individual components passed over the column separately (Figure 5A–5C). This indicates that PdSP15b does not form a high affinity complex with FXII that remains associated during chromatography. Accordingly, no significant interaction was observed between immobilized PdSP15b and FXII, FXIIa, FXI, FXIa, prekallikrein, or kallikrein in surface plasmon resonance experiments (Figure 5G).
Figure 5.
Binding interactions of PdSP15a and b with factor XII (FXII), dextran sulfate (DS), polyphosphate, and heparin. A to C, Analysis of FXII-PdSP15b interactions using gel filtration chromatography. FXII alone (57 µg; A), a mixture of FXII (57 µg) and PdSP15b (64 µg; B), or PdSP15b alone (64 µg; C) in 20 mmol/L Tris HCl, pH 8.0, 150 mmol/L NaCl were applied to a Superdex-75 column and eluted with the same buffer. The chromatograms show no significant change in the elution characteristics of the 2 individual proteins when they were mixed, indicating a lack of interaction. D, Elution of DS (dashed line), PdSP15b (dash dot dot line), and a mixture of the 2 (solid line) from a Superdex-75 column using the elution buffer described above. The PdSP15b peak disappeared completely when mixed with DS, indicating binding between the 2. E, An experiment performed as in D, with heparin (dashed line) substituted for DS. The peak for PdSP15b alone (dash dot dot line) disappears when the protein is mixed with heparin (solid line). F, Chromatography of PdSP15b on a heparin Sepharose column eluted with a sodium chloride gradient as indicated in the figure. The protein was eluted with ≈850 mmol/L NaCl as shown by the right-hand axis. G, Surface plasmon resonance analysis of coagulation factor binding to PdSP15b immobilized on a CM5 sensor chip using amine coupling chemistry. FXII, FXIIa, factor XI (FXI), FXIa, prekallikrein, and kallikrein (traces displayed in the order shown on the panel) each at a concentration of 200 nmol/L were passed over the surface in 10 mmol/L HEPES pH 7.4, 0.15 mol/L NaCl, 0.5% surfactant P20. None produced a significant resonance response. H, DS was passed over a surface of PdSP15b prepared in a similar manner to the surface in G. Concentrations of DS ranging from 0 to 500 nmol/L were tested and are indicated on the plot. I, PdSP15b was passed over a biotinylated heparin surface prepared as described in the Materials and Methods in the online-only Data Supplement. Concentrations of PdSP15b (0–1000 nmol/L) are shown on the plot. J, Equilibrium steady-state resonance levels for each concentration shown in I are plotted against the concentration of PdSP15b, yielding a dissociation constant (Kd) of 139 nmol/L. K, Isothermal titration calorimetry (ITC) analysis of PdSP15a binding with polyP (P45). The concentration of protein in the calorimeter cell was 20 µmol/L in 10 mmol/L sodium phosphate, pH 7.4, 150 mmol/L NaCl, and the syringe contained polyP at 4.5 mmol/L (as PO3 monomer) in the same buffer. Top, The raw heat data for each injection; bottom, the integrated injection enthalpies for with the heats of dilution subtracted. Fitting to a single site binding model yielded ΔH=−0.342±0.003 kcal/mol, TΔ S=5.9 kcal/mol, and an association equilibrium constant (Ka)=1.01±0.05×105 per mol/L. The calculated binding stoichiometry (mole of ligand bound per mole of protein) was 11.9. L, ITC analysis of PdSP15a binding with heparin. The concentration of protein in the calorimeter cell was 20 µmol/L in the buffer described in K, and the syringe contained heparin at a concentration of 200 µmol/L (based on an average molecular weight of 17 000) in the same buffer. Fitting to a single site binding model yielded ΔH=−23.2±0.6 kcal/mol, TΔS=−13.5 kcal/mol, and Ka=8.9±1.6×106/mol/L. The binding stoichiometry was 0.18.
Gel filtration chromatography was also used to detect binding of PdSP15b with DS and heparin. PdSP15b was mixed with DS or heparin in the same buffer as described above. In both cases the peak attributable to the inhibitor disappears completely from the chromatogram, indicating that all PdSP15b is tightly bound to both DS and heparin (Figure 5D and 5E). Heparin binding was further indicated by the fact that PdSP15b bound tightly to a heparin Sepharose column (Figure 5F). The protein could be eluted with NaCl (≈0.8 mol/L) indicating that binding is electrostatic in nature. When PdSP15b was immobilized on a surface plasmon resonance sensor chip, and DS was passed over this surface, saturable, concentration-dependent binding was observed with negligible dissociation being observed after injection (Figure 5H). This behavior is consistent with the multiple-binding site interaction expected when passing high-molecular-weight DS over the immobilized protein. This surface could be completely regenerated by injection of 400 mmol/L NaCl, again indicating that binding was electrostatic in nature. When bio-tinylated heparin was immobilized and PdSP15b was passed over the surface, binding was observed, and a dissociation constant (Kd) of 139 nmol/L was obtained from fitting of the concentration dependence of the steady-state resonance value (Figure 5I and 5J).
PolyP and heparin binding with PdSP15a were quantified using isothermal titration calorimetry in an experiment where solutions of either ligand were injected into the calorimeter cell containing PdSP15a. In both cases, the binding reaction was exothermic, and fitting to a single site binding model yielded association equilibrium constants (Ka) of 1.0±0.05×105 per mol/L for polyP (P45) with concentration being expressed as PO3 monomer (Figure 5K) and 8.9±1.6×106 per mol/L for heparin (considering an average molecular weight of 17 000; Figure 5L). The binding stoichiometry of 12 binding sites per protein molecule for polyP suggests that a minimal binding site may include >10 phosphate units. The calculated stoichiometry of 0.18 for heparin suggests that at least 5 to 6 protein molecules bind with each molecule of heparin (molecular weight of ≈17 000).
The structure of PdSP15b was determined using x-ray diffraction methods to identify the probable structural determinants of anionic surface binding (Figure 6; Table 1). The structure consists of 6 α-helices designated A–F arranged in a bundle that is stabilized by 3 disulfide bonds (Figure 6A and 6B). One disulfide bond links Cys 7 of α-helix A with Cys 14 of α-helix B, a second disulfide links Cys 20 of α-helix B with Cys 88 of α-helix E, and a third disulfide links Cys 71 of α-helix D with Cys 97 of α-helix E.
Figure 6.
The crystal structure of PdSP15b. A, Ribbon diagram of the PdSP15b model with the α-helices labeled A–G based on correspondence with the α-helices of the mosquito protein D7R4. The side chains of basic residues (lysine and arginine) on α-helix E are shown as stick diagrams. B, The structure of A rotated 90° around the axis shown, putting the basic α-helix E in the foreground. C, α-Helix E shown in an expanded view oriented as shown in B with the basic residues shown as a stick diagram and labeled. D, Electrostatic surface model of PdSP15b oriented as in A. More positively charged parts of the surface are indicated as blue, more negatively charged parts of the surface are colored red, and neutral areas are colored white. Electrostatic calculations were performed using the program APBS. E, Electrostatic surface model oriented in the same way as the ribbon diagram in B. The model is colored as in D. F, Ribbon diagram of PdSP15b oriented to match D7R4 in G. The 6 α-helical elements are labeled A–G, highlighting the absence of α-helices B and H in D7R4 and the consequent absence of a small-molecule binding site. F, Ribbon diagram (green) of the mosquito serotonin-binding protein D7R4 with a bound serotonin molecule shown as a stick diagram (red). The α-helical elements are labeled A–H. The binding pocket for serotonin is formed largely from elements of α-helices B and H.
Table 1.
Data Collection, Phasing, and Refinement Statistics for PdSP15b and Its Selenomethionine Derivative
| Crystal | Selenomethionine | Wild Type |
|---|---|---|
| Resolution, Å | 20.0–3.3 | 48.6–2.60 |
| Beamline | 22-ID | 22-ID |
| Wavelength, Å | 0.97931 | 1.0000 |
| Completeness (total/high- resolution shell) |
99.2/98.6 | 99.0/90.1 |
| Average redundancy (total/high- resolution shell) |
8.1/8.4 | 8.4/4.6 |
| Rmerge (total/high-resolution shell)* | 11.5/18.4 | 16.1/59.5 |
| I/sigI (total/high-resolution shell) | 14.9/16.3 | 7.4/2.0 |
| Observed refections | 213 893 | 447 737 |
| Unique refections | 26 250 | 53 346 |
| Space group | P212121 | P212121 |
| Unit cell dimensions, Å | ||
| a | 74.91 | 74.96 |
| b | 97.45 | 97.12 |
| c | 235.44 | 236.07 |
| α, β,γ,° | 90 | 90 |
| Phasing statistics | ||
| No. of sites | 14 | |
| Contrast (SHELXE) | 0.86 | |
| No. of molecules in ASU | 7 | 7 |
| RMS deviations | ||
| Bond lengths, Å | 0.008 | |
| Bond angles, ° | 1.12 | |
| Ramachandran plot (favored/ allowed) |
92.3/100 | |
| Wilson temperature factor | 38.0 | |
| Rcryst/Rfree† | 19.7/23.8 |
Rmerge=Σh|Ih−<I>|/Σh<I>, where <I> is the mean intensity of all symmetry-related refections Ih.
Rcryst=Σ|Fobs−Fcalc|/ΣFobs. Rfree as for Rcryst using a 5% random subset of the data for calculation.
PdSP15a and b belong to the insect odorant-binding protein family, which consists mainly of ligand-binding proteins found in olfactory and gustatory sensory organs. The structure has some similarity to that of the biogenic amine/eicosanoid-binding D7 family from the salivary glands of mosquito species30,31 (Figure 6F and 6G). Superposition of PdSP15b with D7R4, a typical D7 protein from the mosquito Anopheles gambiae, shows that the 2 have related topology but differ in the positions and number of helical elements (Figure 6F and 6G). The D7 proteins characteristically contain 8 α-helices designated A–H that surround a central pocket forming the ligand-binding site. Comparison of the D7R4 and PdSP15b structures shows that PdSP15b lacks α-helices B and H of D7R4 (Figure 6F and 6G). The region of helix B is shortened to a disordered turn structure containing 4 residues, and the C-terminal end of the protein is truncated resulting in the loss of α-helix H. The positions of the remaining elements are somewhat similar with a superposition of PdSP15b and a model of D7R4 lacking α-helices B and H producing a root-mean-square deviation of 3.8 Å for 67 Cα atoms. Because these 2 elements are missing in the PdSP15 proteins, no well-formed binding pocket is present, and it is unlikely that these proteins serve as binders of small molecule ligands (Figure 6F and 6G).
The electrostatic nature of anionic polymer binding by the PdSP15 proteins, as indicated by the salt sensitivity of interactions with heparin and dextran sulfate in chromatographic and surface plasmon resonance studies, suggests that a positively charged surface would be the most likely interaction region. In PdSP15b, the amphipathic α-helix D is lengthened relative to the D7s and studded with basic residues on its solvent-facing surface (Figure 6A–6E). Eight lysines and 3 arginines occur on the solvent-exposed side of this helix, whereas only 2 acidic residues, Asp 70 and Glu 78, are present (Figure 6C). Electrostatic calculations reveal a large, positively charged surface centered on α-helix D that lies at the edge of the overall protein structure, which is flattened and disk-like (Figure 6D and 6E). The highly basic nature of this region is conserved in the sequences of PdSP15a and b (Figure I in the online-only Data Supplement). Only Lys 81 in PdSP15a is replaced by threonine in PdSP15b, and Lys 74 in PdSP15b is replaced by glutamine in PdSP15a. Arrays of basic residues aligned along helical segments to give highly positively charged surface have been shown to be essential for glycosaminoglycan interactions in other proteins, including antithrombin-III and apolipoprotein E.32,33
Discussion
During feeding, salivary proteins of blood feeders limit host hemostatic and inflammatory responses to facilitate the intake of blood. Some of these proteins remove small-molecule mediators of mast cell and platelet activation from the area of a bite by binding them with high affinity. Mechanisms involving the sequestration of effectors and the coating of activating surfaces are commonly used by blood-feeding arthropods for inhibition of hemostasis and inflammation. Salivary proteins binding histamine, serotonin, norepinephrine, ADP, cysteinyl leukotrienes, leukotriene B4, thromboxane A2, and phosphatidylserine have been identified and shown to inhibit platelet activation, coagulation, and vasoconstriction in vitro and in vivo.34,35 Aegyptin, a mosquito protein that coats collagen surfaces, blocks the binding of von Willebrand factor and the interaction of collagen with glycoprotein VI, the major platelet collagen receptor.36 Many of these binding proteins have been crystallized in the presence of ligands, revealing sophisticated and highly selective ligand-binding sites.30,34,37,38 Here, we have extended the functional range of salivary scavengers to include the sequestration of binding sites for coagulation factors on heparin and polyP, 2 proven endogenous activators of coagulation and inflammation. We have demonstrated that PdSP15a and b bind these polymers and effectively inhibit the contact activation of FXII, the contact activation of FXI, the activation of FXI by thrombin and FXIIa, and the reciprocal activation of FXII and prekallikrein initiated by DS.
The amount of protein present in the salivary glands before feeding is small (ca. 1 µg), but the volume of blood ingested by a blood feeder is also very small (ca. 1 µL for a female sand fly), as is the area of skin damaged by the bite.39,40 Ligand-scavenging proteins pumped into the feeding space are thought to reach concentrations comparable with those of their target agonists because between 25% and 85% of salivary gland contents are expelled during a single feeding.40,41 Because scavenger proteins act by stoichiometrically binding relatively abundant agonist molecules, they tend to be among the most abundant in the saliva.40 Consistent with this, measures of transcript abundance and protein concentration have shown that PdSP15s are the most concentrated proteins in the salivary mixture by a factor of >2.24 We have shown that inhibition of polyP-mediated FXII activation in a manner consistent with an anionic polymer– binding mechanism can also be attained using salivary gland extracts at a protein concentration of 5 to 20 ng/µL, a level well below the protein concentration reached in the volume of the blood meal.
The PdSP15s seem to function in vivo as inhibitors of bradykinin formation because sand fly saliva contains a potent inhibitor of FXa (lufaxin and its relatives) that seems to act as the major anticoagulant.42 However, salivary mixtures are known to inhibit physiological processes (such as platelet activation) by attacking >1 activating pathway simultaneously.40 The PdSP15s may, therefore, also act redundantly in the inhibition of coagulation or thrombosis, particularly because the importance in normal hemostasis of processes such as the thrombin-mediated activation of FXI is still being established. Along with other anti-inflammatory proteins in the salivary mixture, PdSP15s most likely act primarily to delay the swelling and sensation of pain associated with the bite, thus avoiding defensive responses from the host.40 We also cannot rule out the possibility that PdSP15s play additional roles, and that anionic surface binding serves as a means of targeting the protein to specific locations, such as the glycosaminoglycan-covered surfaces of endothelial cells or the anionic phospholipid–containing surface of activated platelets.43
The importance of contact pathway inhibition in blood feeding is reflected by the fact that several recently described salivary inhibitors of FXIIa have shown efficacy as antithrombotics and anti-inflammatories in model systems. For example, infestin and Ir-CPI, from the insect Triatoma infestans and the tick Ixodes ricinus, respectively, bind FXIIa directly and inhibit thrombosis in vivo. Infestin is a Kazal-type serine protease inhibitor that shows specificity for FXIIa, whereas Ir-CPI is a Kunitz-type inhibitor that also inhibits the activity of FXIa and kallikrein.44,45 In both cases, occlusive thrombus formation was prevented by these proteins in animal models without an inhibitory effect on hemostasis. The efficacy of an anionic surface–binding mechanism of inhibition has been established more recently in studies where small molecule and macromolecular inhibitors of thrombosis are shown to specifically target anionic surfaces and prevent activation of the contact pathway. Various polycationic compounds, such as polybrene, polyamine dendrimers (eg. PAMAM G1-G5), polyethyleneimine, spermine, and protamine, bind polyP and nucleic acids with submicromolar affinity and effectively inhibit thrombosis and vascular leakage in vivo in rodent models.46,47 The same types of compounds have also been shown to inhibit inflammation by limiting the nucleic acid–mediated activation of toll-like receptors.48 The PdSP15 proteins are naturally occurring substances that inhibit hemostatic and inflammatory processes by a mechanism similar to these. The inhibition of coagulation initiated with the colloidal silica activated partial thromboplastin time reagent suggests that that they are relatively nonspecific and can block binding sites on a variety of potential activating polymers. The compact, allhelical PdSP15 proteins contain an amphipathic helical segment that contributes to a positively charged surface that is the most likely site of electrostatic interaction with all of these anionic materials.
The targeting of surfaces rather than specific proteins provides the PdSP15 proteins with the potential to inhibit multiple processes in hemostasis and inflammation. In addition to the activation of FXII, anionic surfaces serve to stabilize complexes of FXIIa with FXI, HK and prekallikrein, as well as the complex of thrombin with FXI. They are also known to inhibit the activity of tissue factor pathway inhibitor by stimulating the activation of FV,19 stabilize fibrin clots,19 and modulate the interaction between von Willebrand factor and the platelet receptor glycoprotein1b.49 PdSP15 proteins inhibit the activation of FXII and FXI, as well as the cleavage of FXI by FXIIa or thrombin, and would be expected to inhibit any anionic surface–mediated reaction. Mast cell proteases such as tryptase occur as heparin complexes, with heparin being essential for stabilization of the tetrameric structure of the enzyme. Perhaps these complexes could also be disrupted by heparin-binding proteins. The recognition of a variety of anionic substances, including glycosaminoglycans, and polyP, by the PdSP15 proteins suggests that they could be effective as both anticoagulants and anti-inflammatory molecules in both the circulation and the skin. The inhibition of coagulation, the cleavage of kininogen mimetic peptides in plasma, and plasma extravasation in skin show that the PdSP15 proteins are capable of inhibiting surface-mediated reactions in complex biological mixtures in vivo and may have properties that are useful therapeutically.
P duboscqi transmits Leishmania parasites during feeding, and the inflammatory effects of salivary components have been shown to influence establishment of the parasite in the skin. Host delayed-type hypersensitivity responses to salivary proteins create a hostile environment for Leishmania, and some salivary proteins can serve as protective antigens.25,50 The anti-inflammatory effects of native salivary components could also affect parasite establishment, and with some pathogens arthropod salivas have been shown to enhance infectivity. The reasons for this enhancement are unclear in most cases and may be related to the functional properties of salivary proteins. Maxidilan, a vasodilator peptide from another sand fly species, exacerbates Leishmania infections and has been found to bind to the PAC1 receptor for the pituitary adenylate cyclase–activating polypeptide receptor and influence cytokine levels at the bite site.51–53 Modulation of the environment of the skin and circulation by the combined effects of many salivary proteins may play an important role in the establishment of vector-borne pathogens.
Supplementary Material
Significance.
Blood-feeding insects transmit a variety of parasitic, viral, and bacterial disease agents when they ingest blood from a mammalian host. Proteins in the saliva of these insects facilitate feeding by inhibiting physiological processes acting to prevent blood loss and inflammation. In this study, we show that 2 salivary proteins from the sand fly, Phlebotomous duboscqi, inhibit autoactivation of coagulation factor XII, a reaction that occurs when the enzyme precursor binds to anionic polymers. This is the initial step in the contact pathway of coagulation leading to activation of the coagulation cascade and formation of the proinflammatory peptide bradykinin. This pair of proteins has been given the name PdSP15 and shown to act by binding tightly with the polymers heparin and polyphosphate thereby preventing interaction with the coagulation factor. The likely physiological function of this mechanism would be the inhibition of the proinflammatory activities of bradykinin, particularly pain and vascular leakage.
Acknowledgments
We thank D. Garboczi and A. Gittis for discussions and R. Hearn for technical assistance. We also thank the staffs of the Southeast Regional Collaborative Access Team and the Structural Biology Center, Advanced Photon Source, Argonne National Laboratory, for assistance with x-ray data collection. We dedicate this article to the memory of Alexandre A. Peixoto.
Sources of Funding
This work was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. P.H. Alvarenga was supported, in part, by a grant from the Fundação de Amparo à Pesquisa do Rio de Janeiro Carlos Chagas Filho and Conselho Nacional de Desenvolvimento Científco e Tecnológico. Use of the Advanced Photon Source beamlines was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. W-31–109-Eng-38.
Nonstandard Abbreviations and Acronyms
- DS
dextran sulfate
- FXI
factor XI
- FXII
factor XII
- HK
high-molecular-weight kininogen
- polyp
polyphosphate
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.302482/-/DC1.
Disclosures
None.
References
- 1.Renné T, Schmaier AH, Nickel KF, Blombäck M, Maas C. In vivo roles of factor XII. Blood. 2012;120:4296–4303. doi: 10.1182/blood-2012-07-292094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115:2569–2577. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lämmle B, Wuillemin WA, Huber I, Krauskopf M, Zürcher C, Pflugshaupt R, Furlan M. Thromboembolism and bleeding tendency in congenital factor XII deficiency-a study on 74 subjects from 14 Swiss families. Thromb Haemost. 1991;65:117–121. [PubMed] [Google Scholar]
- 4.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renné T. The procoagulant and proinflammatory plasma contact system. Semin Immunopathol. 2012;34:31–41. doi: 10.1007/s00281-011-0288-2. [DOI] [PubMed] [Google Scholar]
- 7.Samuel M, Pixley RA, Villanueva MA, Colman RW, Villanueva GB. Human factor XII (Hageman factor) autoactivation by dextran sulfate Circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. J Biol Chem. 1992;267:19691–19697. [PubMed] [Google Scholar]
- 8.Brunnée T, Reddigari SR, Shibayama Y, Kaplan AP, Silverberg M. Mast cell derived heparin activates the contact system: a link to kinin generation in allergic reactions. Clin Exp Allergy. 1997;27:653–663. [PubMed] [Google Scholar]
- 9.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrissey JH, Choi SH, Smith SA. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012;119:5972–5979. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oschatz C, Maas C, Lecher B, Jansen T, Björkqvist J, Tradler T, Sedlmeier R, Burfeind P, Cichon S, Hammerschmidt S, Müller-Esterl W, Wuillemin WA, Nilsson G, Renné T. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34:258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287:28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 15.Smith SA, Morrissey JH. Heparin is procoagulant in the absence of antithrombin. Thromb Haemost. 2008;100:160–162. doi: 10.1160/TH08-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Rienstra CM, Reinstra CM, Morrissey JH. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112:2810–2816. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci USA. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isawa H, Orito Y, Iwanaga S, Jingushi N, Morita A, Chinzei Y, Yuda M. Identification and characterization of a new kallikrein-kinin system inhibitor from the salivary glands of the malaria vector mosquito Anopheles stephensi. Insect Biochem Mol Biol. 2007;37:466–477. doi: 10.1016/j.ibmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Isawa H, Orito Y, Jingushi N, Iwanaga S, Morita A, Chinzei Y, Yuda M. Identification and characterization of plasma kallikrein-kinin system inhibitors from salivary glands of the blood-sucking insect Triatoma infestans. FEBS J. 2007;274:4271–4286. doi: 10.1111/j.1742-4658.2007.05958.x. [DOI] [PubMed] [Google Scholar]
- 22.Isawa H, Yuda M, Orito Y, Chinzei Y. A mosquito salivary protein inhibits activation of the plasma contact system by binding to factor XII and high molecular weight kininogen. J Biol Chem. 2002;277:27651–27658. doi: 10.1074/jbc.M203505200. [DOI] [PubMed] [Google Scholar]
- 23.Kato N, Iwanaga S, Okayama T, Isawa H, Yuda M, Chinzei Y. Identification and characterization of the plasma kallikrein-kinin system inhibitor, haemaphysalin, from hard tick, Haemaphysalis longicornis. Thromb Haemost. 2005;93:359–367. doi: 10.1160/TH04-05-0319. [DOI] [PubMed] [Google Scholar]
- 24.Kato H, Anderson JM, Kamhawi S, Oliveira F, Lawyer PG, Pham VM, Sangare CS, Samake S, Sissoko I, Garfield M, Sigutova L, Volf P, Doumbia S, Valenzuela JG. High degree of conservancy among secreted salivary gland proteins from two geographically distant Phlebotomus duboscqi sandflies populations (Mali and Kenya) BMC Genomics. 2006;7:226. doi: 10.1186/1471-2164-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, Sacks DL, Ribeiro JM. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott CF, Colman RW. Fibrinogen blocks the autoactivation and thrombin-mediated activation of factor XI on dextran sulfate. Proc Natl Acad Sci USA. 1992;89:11189–11193. doi: 10.1073/pnas.89.23.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu W, Sinha D, Shikov S, Yip CK, Walz T, Billings PC, Lear JD, Walsh PN. Factor XI homodimer structure is essential for normal proteolytic activation by factor XIIa, thrombin, and factor XIa. J Biol Chem. 2008;283:18655–18664. doi: 10.1074/jbc.M802275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korkmaz B, Attucci S, Juliano MA, Kalupov T, Jourdan ML, Juliano L, Gauthier F. Measuring elastase, proteinase 3 and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrates. Nat Protoc. 2008;3:991–1000. doi: 10.1038/nprot.2008.63. [DOI] [PubMed] [Google Scholar]
- 29.Fukumizu A, Tsuda Y, Wanaka K, Tada M, Okamoto S, Hijikata-Okunomiya A, Okada Y. Amino acids, peptides. LIII Synthesis and biological activities of some pseudo-peptide analogs of PKSI-527, a plasma kallikrein selective inhibitor: the importance of the peptide backbone. Chem Pharm Bull (Tokyo) 1999;47:1141–1144. doi: 10.1248/cpb.47.1141. [DOI] [PubMed] [Google Scholar]
- 30.Calvo E, Mans BJ, Ribeiro JM, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci USA. 2009;106:3728–3733. doi: 10.1073/pnas.0813190106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mans BJ, Calvo E, Ribeiro JM, Andersen JF. The crystal structure of D7r4, a salivary biogenic amine-binding protein from the malaria mosquito Anopheles gambiae. J Biol Chem. 2007;282:36626–36633. doi: 10.1074/jbc.M706410200. [DOI] [PubMed] [Google Scholar]
- 32.Dong J, Peters-Libeu CA, Weisgraber KH, Segelke BW, Rupp B, Capila I, Hernáiz MJ, LeBrun LA, Linhardt RJ. Interaction of the N-terminal domain of apolipoprotein E4 with heparin. Biochemistry. 2001;40:2826–2834. doi: 10.1021/bi002417n. [DOI] [PubMed] [Google Scholar]
- 33.Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarenga PH, Francischetti IM, Calvo E, Sá-Nunes A, Ribeiro JM, Andersen JF. The function and three-dimensional structure of a thromboxane A2/cysteinyl leukotriene-binding protein from the saliva of a mosquito vector of the malaria parasite. PLoS Biol. 2010;8:e1000547. doi: 10.1371/journal.pbio.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francischetti IM, Ribeiro JM, Champagne D, Andersen J. Purification, cloning, expression, and mechanism of action of a novel platelet aggregation inhibitor from the salivary gland of the blood-sucking bug, Rhodnius prolixus. J Biol Chem. 2000;275:12639–12650. doi: 10.1074/jbc.275.17.12639. [DOI] [PubMed] [Google Scholar]
- 36.Calvo E, Tokumasu F, Marinotti O, Villeval JL, Ribeiro JM, Francischetti IM. Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin alpha2beta1, and von Willebrand factor. J Biol Chem. 2007;282:26928–26938. doi: 10.1074/jbc.M705669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Chang BW, Mans BJ, Ribeiro JM, Andersen JF. Structure and ligand-binding properties of the biogenic amine-binding protein from the saliva of a blood-feeding insect vector of Trypanosoma cruzi . Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 1):105–113. doi: 10.1107/S0907444912043326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Oliveira F, Chang BW, Collin N, Gomes R, Teixeira C, Reynoso D, My Pham V, Elnaiem DE, Kamhawi S, Ribeiro JM, Valenzuela JG, Andersen JF. Structure and function of a “yellow” protein from saliva of the sand fly Lutzomyia longipalpis that confers protective immunity against Leishmania major infection. J Biol Chem. 2011;286:32383–32393. doi: 10.1074/jbc.M111.268904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choumet V, Attout T, Chartier L, Khun H, Sautereau J, Robbe-Vincent A, Brey P, Huerre M, Bain O. Visualizing non infectious and infectious Anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS One. 2012;7:e50464. doi: 10.1371/journal.pone.0050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro JM, Arca B. From Sialomes to the Sialoverse: An Insight into Salivary Potion of Blood-Feeding Insects. Adv Insect Physiol. 2009;37:59–118. [Google Scholar]
- 41.Ribeiro JM, Modi GB, Tesh RB. Salivary apyrase activity of some old world phlebotomine sand flies. Insect Biochem Mol Biol. 1989;19:409–412. [Google Scholar]
- 42.Collin N, Assumpção TC, Mizurini DM, Gilmore DC, Dutra-Oliveira A, Kotsyfakis M, Sá-Nunes A, Teixeira C, Ribeiro JM, Monteiro RQ, Valenzuela JG, Francischetti IM. Lufaxin, a novel factor Xa inhibitor from the salivary gland of the sand fly Lutzomyia longipalpis blocks protease-activated receptor 2 activation and inhibits inflammation and thrombosis in vivo. Arterioscler Thromb Vasc Biol. 2012;32:2185–2198. doi: 10.1161/ATVBAHA.112.253906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen JF, Gudderra NP, Francischetti IM, Valenzuela JG, Ribeiro JM. Recognition of anionic phospholipid membranes by an antihe-mostatic protein from a blood-feeding insect. Biochemistry. 2004;43:6987–6994. doi: 10.1021/bi049655t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decrem Y, Rath G, Blasioli V, Cauchie P, Robert S, Beaufays J, Frère JM, Feron O, Dogné JM, Dessy C, Vanhamme L, Godfroid E. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206:2381–2395. doi: 10.1084/jem.20091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagedorn I, Schmidbauer S, Pleines I, Kleinschnitz C, Kronthaler U, Stoll G, Dickneite G, Nieswandt B. Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation. 2010;121:1510–1517. doi: 10.1161/CIRCULATIONAHA.109.924761. [DOI] [PubMed] [Google Scholar]
- 46.Jain S, Pitoc GA, Holl EK, Zhang Y, Borst L, Leong KW, Lee J, Sullenger BA. Nucleic acid scavengers inhibit thrombosis without increasing bleeding. Proc Natl Acad Sci USA. 2012;109:12938–12943. doi: 10.1073/pnas.1204928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SA, Choi SH, Collins JN, Travers RJ, Cooley BC, Morrissey JH. Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. Blood. 2012;120:5103–5110. doi: 10.1182/blood-2012-07-444935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Sohn JW, Zhang Y, Leong KW, Pisetsky D, Sullenger BA. Nucleic acid-binding polymers as anti-inflammatory agents. Proc Natl Acad Sci USA. 2011;108:14055–14060. doi: 10.1073/pnas.1105777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montilla M, Hernandez-Ruiz L, Garcia-Cozar FJ, Alvarez-Laderas I, Rodriguez-Martorell J, Ruiz A. Polyphosphate binds to human von Willebrand factor in vivo and modulates its interaction with Glycoprotein Ib. J Thromb Haemost. 2012;10:2315–2323. doi: 10.1111/jth.12004. [DOI] [PubMed] [Google Scholar]
- 50.Belkaid Y, Valenzuela JG, Kamhawi S, Rowton E, Sacks DL, Ribeiro JM. Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: An adaptive response induced by the fly? Proc Natl Acad Sci USA. 2000;97:6704–6709. doi: 10.1073/pnas.97.12.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lerner EA, Ribeiro JM, Nelson RJ, Lerner MR. Isolation of maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J Biol Chem. 1991;266:11234–11236. [PubMed] [Google Scholar]
- 52.Soares MB, Titus RG, Shoemaker CB, David JR, Bozza M. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J Immunol. 1998;160:1811–1816. [PubMed] [Google Scholar]
- 53.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.