Abstract
Rationale
The metabotropic glutamate receptor subtype 5 (mGluR5) has been reported to be critically involved in drug reward and addiction. Because the mGluR5 negative allosteric modulators (NAMs) MPEP and MTEP significantly inhibit addictive-like behaviors of cocaine and other drugs of abuse in experimental animals, it has been suggested that mGluR5 NAMs may have translational potential for treatment of addiction in humans. However, neither MPEP nor MTEP have been evaluated in humans due to their off-target actions and rapid metabolism.
Objectives
Herein, we evaluate a potential candidate for translational addiction research: a new sulfate salt formulation of fenobam, a selective mGluR5 NAM that has been investigated in humans.
Results
In rats, fenobam sulfate had superior pharmacokinetics compared to the free base, with improved Cmax (maximal plasma concentration) and longer half life. Oral (p.o.) administration of fenobam sulfate (30 or 60 mg/kg) inhibited intravenous cocaine self-administration, cocaine-induced reinstatement of drug-seeking behavior and cocaine-associated cue-induced cocaine-seeking behavior in rats. Fenobam sulfate also inhibited oral sucrose self-administration and sucrose-induced reinstatement of sucrose-seeking behavior, but had no effect on locomotion.
Conclusions
This study provides additional support for the role of mGluR5 signaling in cocaine addiction and suggests that fenobam sulfate may have translational potential in medication development for the treatment of cocaine addiction in humans.
Keywords: Cocaine, fenobam, self-administration, reinstatement, cocaine-seeking behavior, incubation of cocaine craving, mGluR5
INTRODUCTION
Glutamate regulates neuronal activity via ionotropic and metabotropic glutamate receptor (mGluR) subtypes. Among mGluRs, members of the Group I family, mGluR1 and mGluR5, are coupled to intracellular phospholipase C via Gq protein (Schoepp and Conn 1993). mGluR5 is widely expressed in many regions of the brain, including critical mesolimbic structures such as the ventral tegmental area and the nucleus accumbens (Romano et al. 1996; Shigemoto et al. 1993). mGluR5 is mostly located postsynaptically (Mitrano and Smith 2007) and co-localized with adenosine A2A, dopamine D2 and NMDA receptors (Tebano et al. 2005).
mGluR5 became a major target of interest in the potential treatment of drug abuse in 2001 when it was reported that mice lacking mGluR5 do not self-administer cocaine and that pharmacological blockade of mGluR5 by the negative allosteric modulator (NAM) MPEP (2-methyl-6-(phenylethynyl)pyridine) inhibited cocaine self-administration (Chiamulera et al. 2001). Attenuation of mGluR5 signaling with MPEP or its analog MTEP (3-((2-methyl-1,3-thiazol-4-yl)ethynyl)pyridine) has been found to inhibit multiple addictive-like behaviors in animals, including cocaine self-administration (Kenny et al. 2005; Lee et al. 2005; Martin-Fardon et al. 2009; Paterson and Markou 2005; Tessari et al. 2004), cocaine-induced conditioned place preference (CPP) (Herzig and Schmidt 2004; McGeehan and Olive 2003), cocaine-induced hyperactivity (McGeehan et al. 2004), and cocaine-, cue- or stress-induced reinstatement of drug-seeking behavior (Backstrom and Hyytia 2006; Kumaresan et al. 2009; Lee et al. 2005; Martin-Fardon and Weiss 2012; Wang et al. 2012). These data strongly suggest that mGluR5 plays an important role in cocaine abuse and that mGluR5 NAMs may have potential for the treatment of cocaine addiction in humans (Heidbreder et al. 2003; Olive et al. 2005).
However, there are multiple reasons that MPEP and MTEP have not advanced to clinical trials (Lindsley and Emmitte 2009). MPEP blocks the norepinephrine transporter (Heidbreder et al. 2003), NMDA receptors (Movsesyan et al. 2001; O’Leary et al. 2000) and monoamine oxidase A (Lea and Faden 2006), while activating mGluR4 (Mathiesen et al. 2003). In addition, MTEP was reported to inhibit cytochrome P450 1A2 (Green et al. 2004), and both MPEP and MTEP are rapidly metabolized after injection. Therefore, extensive efforts have been undertaken to develop novel potent, selective, metabolically stable mGluR5 NAMs for use in humans (Emmitte 2011; Nicoletti et al. 2011; Rocher et al. 2011).
Fenobam, a brain-penetrant nonbenzodiazepine anxiolytic advanced to Phase II clinical trials in the early 1980s (Friedmann et al. 1980; Itil et al. 1978; Pecknold et al. 1982), has been identified as a selective mGluR5 NAM (Porter et al. 2005). Fenobam was granted orphan drug status by the FDA in 2006 and has been recently investigated as an experimental clinical treatment for Fragile X Syndrome (Berry-Kravis et al. 2009) and L-DOPA-induced dyskinesias (Rylander et al. 2010). Fenobam was recently reported to inhibit brain-stimulation reward (Cleva et al. 2012) and attenuate methamphetamine-seeking behavior in rats (Watterson et al. 2013). Considering these results, as well as the success of buprenorphine as an orphan drug approved for treating morphine dependence (Jaffe and O’Keeffe 2003), and fenobam’s superior mGluR5 selectivity compared to MPEP (Montana et al. 2009), fenobam is an intriguing clinical candidate for the treatment of psychostimulant dependence. However, it is unclear whether fenobam inhibits cocaine-taking and cocaine-seeking behaviors as has been demonstrated with MPEP and MTEP. In addition, clinical tests have utilized the free base monohydrate formulation of fenobam and variability in response to fenobam has been correlated with high variability in plasma levels of the drug after oral administration (Itil et al. 1978; Berry-Kravis et al. 2009). Therefore, development of new formulations of fenobam with improved oral bioavailability was deemed important for use in future clinical trials.
In this study, we first compared the pharmacokinetics and oral bioavailability of fenobam free base and fenobam sulfate, a new salt formulation of fenobam, and then evaluated the pharmacological effects of fenobam sulfate on intravenous cocaine self-administration, cocaine-induced reinstatement of drug-seeking behavior and cue-induced cocaine-seeking behavior in rats – three commonly used animal models to evaluate drug reward, craving and relapse (O’Brien and Gardner 2005). We also observed the effects of fenobam sulfate on oral sucrose self-administration and sucrose-induced reinstatement of sucrose-seeking behavior and locomotor activity. Overall, our aim was to evaluate fenobam sulfate in rat models of cocaine addiction and thus determine the potential for development of fenobam sulfate as a pharmacological treatment for cocaine addiction.
MATERIALS AND METHODS
Drugs
Fenobam free base and fenobam sulfate were provided by the Division of Pharmacotherapies and Medical Consequences of Drug Abuse (DPMCDA), NIDA. Cocaine HCl was provided by NIDA and dissolved in physiological saline., Since fenobam free base has low solubility in 40% hydroxypropyl-β-cyclodextrin (BCD), it was first dissolved in 10% DMSO and brought to final concentration in 40% BCD; fenobam sulfate was dissolved in 40% BCD. There are few reports that such a low concentration (10%) of DMSO alters the PK profile of drugs. Therefore, it is commonly used in animal pharmacokinetic studies to increase drug solubility. In all behavioral studies, fenobam sulfate was dissolved in 40% BCD and water for oral (p.o.) administration.
Experiment 1: Pharmacokinetic comparison of fenobam free base and fenobam sulfate
PK data were obtained under NIDA Contract N01DA-9-8883. Briefly, two groups of Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC, USA, n = 3 per sex, 6 per group, 12 animals total) were used. Intravenous (i.v.) catheterization of the right external jugular vein for blood sampling was performed under sodium pentobarbital (60 mg/kg, i.p.) anesthesia, utilizing standard aseptic surgical techniques as we reported previously. After recovery from surgery, each animal received a single oral (p.o.) dose of 30 mg/kg of fenobam free base or fenobam sulfate. The dose administered of each test material, fenobam free base or fenobam sulfate, was based on the anhydrous free base equivalents of fenobam. Blood samples were collected via jugular vein catheter prior to dosing and after dosing at different time points (0.25, 0.5, 1, 2, 3, 4, 8, 12 and 24 hrs after oral administration). Blood samples were centrifuged to obtain plasma and all samples were stored at −20° C until analysis. Fenobam concentrations in plasma samples were analyzed by a validated liquid chromatography/tandem mass spectrometry method with quantization limit of 5 ng/ml. Rat plasma samples (50 μl) were analyzed for fenobam with an LC/MS/MS procedure in a Sciex API 3000 system equipped with a Synergi Polar RP column (4.6 × 75 mm, 4 μm particle size), a mobile phase system consisting of acetonitrile : water : formic acid (42.5 : 57.5 : 0.01) (v:v:v) with 5 mM ammonium acetate and mass spectrometric detection with positive ionization by APCI (Atmospheric Pressure Chemical Ionization) and mass scanning by MRM (Multiple Reaction Monitoring) analysis. The transition ions for fenobam were m/z 267>114. Sample preparation consisted of addition of internal standard (bupropion), extraction of 50 μl of plasma with 1.00 ml of methyl t-butyl ether prior to separation by LC/MS/MS. The assay was validated from 5.00 ng/ml to 1000 ng/ml. The coefficients of variance and relative error for low, mid and high concentrations of quality controls were <15%.
The PK parameters of peak plasma concentration (Cmax), half-life (T1/2), and area under the curve (AUC) were calculated using WinNonlin software (Professional Edition, 5.2.1 ver., Pharsight Corp, CA, USA) in the noncompartmental mode. Cmax was defined as the highest level of plasma fenobam among 9 time-points detected. AUC was calculated by summation of plasma drug concentrations at all 9 time points. Cmax and AUC in both fenobam free base and fenobam sulfate groups were compared using unpaired, two-tailed t-tests.
Experiment 2: Cocaine self-administration
Animals
Male Long-Evans rats (250–300 g; Charles River Laboratories, Raleigh, NC, USA) were used for all experiments. They were individually housed in a climate-controlled room on a reverse light–dark cycle (lights on at 1900 hours, lights off at 0700 hours) with ad libitum access to food and water. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (US National Academy of Sciences), and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the US National Institutes of Health. The animal facility was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Surgery
Intravenous (i.v.) catheterization of the right external jugular vein was performed under sodium pentobarbital (60 mg/kg, i.p.) anesthesia, utilizing standard aseptic surgical techniques as we reported previously (Xi et al. 2006a; Xi et al. 2006b). To help prevent clogging, the catheters were flushed daily with a gentamicin–heparin–saline solution (0.1 mg/ml gentamicin, 30 IU/ml heparin; ICN Biochemicals, Cleveland, OH, USA).
Apparatus
Self-administration chambers from Med Associates Inc. (Saint Albans, VT, USA) were the same as used previously (Xi et al. 2006a; Xi et al. 2006b).
Self-administration training
After recovery from surgery, each rat was placed into a test chamber and allowed to lever-press for i.v. cocaine (1 mg/kg/infusion) on a fixed ratio 1 (FR1) reinforcement schedule. Each cocaine infusion delivered a volume of 0.08 ml/infusion over 4.65 sec and was paired with the simultaneous presentation of a stimulus light and tone (each lasting 4.65 sec). All self-administration sessions lasted 3 hours. FR1 reinforcement was used for six days to establish stable self-administration. Subjects were then allowed to continue cocaine (0.5 mg/kg/infusion) self-administration under FR2 reinforcement. To avoid cocaine overdose during the self-administration period, each animal was limited to a maximum of 50 cocaine injections per 3-h session. After stable self-administration was established on the FR2 schedule (less than 10% variation in total infusions over three consecutive days), the effects of fenobam sulfate (0, 30, 60 mg/kg, p.o.) on cocaine self-administration were determined in one group of rats (n = 11). Fenobam sulfate or vehicle was administered 30 minutes prior to self-administration testing. Each animal received three doses of fenobam sulfate over a week of testing, with the order of testing for various doses counterbalanced. After each drug test, animals continued daily cocaine self-administration until stable self-administration was re-established; typically, stable self-administration was re-established within 1–2 days. Each animal received a total of 3 injections corresponding to 3 drug doses in each group.
Multiple-dose cocaine self-administration training
To determine whether the pharmacological action of fenobam sulfate was also cocaine dose-dependent, we further studied the effects of fenobam sulfate on cocaine self-administration maintained by a full range of cocaine doses (0.03, 0.06, 0.125, 0.25, 0.5 mg/kg/infusion) in a single session (Mantsch et al., 2007; Hiranita et al., 2009; Song et al., 2012). The session consisted of five sequential 20-min components, each preceded by a 20-min time-out period for changing cocaine dose. The infusion volumes and durations in each component were identical except that cocaine concentrations for corresponding unit cocaine doses differed. There was a 30-min extinction period (0 mg/kg cocaine) before daily cocaine self-administration training. Training continued until: 1) a minimum of 5.0 mg/kg cocaine was self-administered within a session with less than 20% variation in the total number of cocaine injections compared with the previous session, 2) the dose of cocaine that maintained maximal response rates varied by no more than one-half log unit over two consecutive test sessions, and 3) maximal response rates were at least 5-fold higher than response rates maintained during extinction. Then, each rat randomly received either vehicle or 1 of 2 doses of fenobam sulfate (30, 60 mg/kg, p.o.) 30 min prior to the test session. Animals then received an additional 3–4 days of self-administration of cocaine alone until the baseline response rate was re-established prior to testing the next dose of fenobam sulfate. The order of testing for the various doses of drug or vehicle was counterbalanced.
Experiment 3: Sucrose self-administration
The procedures for sucrose self-administration were identical to the procedures for cocaine self-administration except for the following: 1) no surgery was performed on the animals; 2) active lever presses led to delivery of 0.1 mL of 5% sucrose solution into a liquid food tray on the operant chamber wall along with presentation of a stimulus light and tone; 3) 3-h FR1 training sessions were capped at 100 deliveries; 4) test sessions were 90-min in length and capped at 100 deliveries. After stable sucrose self-administration was established on the FR1 schedule (less than 10% variation in total deliveries over three consecutive days), the effects of fenobam sulfate (0, 30, 60 mg/kg, p.o.) on sucrose self-administration were determined in one group of rats (n = 7). Each animal received three doses of fenobam sulfate over a week of testing, with the order of testing for various doses counterbalanced. After each drug test, animals continued daily sucrose self-administration until stable self-administration was re-established; typically, stable self-administration was re-established within 1–2 days.
Experiment 4: Sucrose-triggered reinstatement of sucrose-seeking behavior
Two additional group of rats (n = 11 total) were used to evaluate the effects of fenobam sulfate on sucrose-induced reinstatement of sucrose-seeking behavior. After stable sucrose self-administration was achieved, animals underwent extinction training until sucrose-seeking behavior was extinguished. To determine whether fenobam sulfate pretreatment inhibits sucrose-seeking behavior, extinguished animals were treated with fenobam sulfate or vehicle prior to the triggering of reinstatement via 5 non-contingent sucrose deliveries. The animals were divided into two dose groups. Group 1 (n = 6) randomly received vehicle or 30 mg/kg of fenobam sulfate and received the matched treatment six days later after two intervening extinction trials. Group 2 (n = 5) randomly received vehicle or 60 mg/kg of fenobam sulfate and received the matched treatment six days later after two intervening extinction trials. Lever presses during the reinstatement tests were recorded, but did not lead to either sucrose delivery or presentation of the conditioned cue-light and tone.
Experiment 5: Cocaine-primed reinstatement of cocaine-seeking behavior
After the completion of Experiment 1, animals continued cocaine self-administration for at least 3–5 days until stable self-administration was reestablished. Then, the animals underwent extinction training, during which cocaine was replaced by saline and the light and sound cues that previously accompanied cocaine infusions were turned off. After drug-seeking behavior was extinguished – defined as ≤10 active lever presses during each 3-h session for at least 3 consecutive days – the animals were divided into three dose groups (between-subjects design) to evaluate the effects of fenobam sulfate on cocaine-primed reinstatement of drug-seeking behavior (n = 9–10 per group). On the reinstatement test day, each group of animals received one dose (0, 30, or 60 mg/kg, p.o.) fenobam sulfate alone or 30 min prior to cocaine priming (10 mg/kg, i.p.). Then, the animals were placed into the operant chambers that were previously paired with cocaine self-administration. Reinstatement conditions were identical to those in the extinction sessions, i.e., active lever presses were recorded without drug infusions or accompanying cues for 3 h. Effects of fenobam sulfate on drug-seeking or cocaine-induced reinstatement were assessed by comparing the mean number of active lever presses per test session.
Experiment 6: Contextual cue-induced cocaine seeking
Two additional groups of rats were used in this experiment. One group of rats (n = 32) was used to evaluate contextual cue-induced incubation of cocaine craving by comparing extinction responding after 2 or 21 days (n = 16 rats in each subgroup) of withdrawal from cocaine self-administration. Another group of rats (n = 24) was used to examine the effects of fenobam sulfate (0, 30, 60 mg/kg, p.o., 30 min prior to testing) on contextual cue-induced incubation of cocaine craving (between-subjects design, n = 6–8 each subgroup). On the test day, rats were placed into the same self-administration chambers in which they were trained, and cocaine cue-induced drug-seeking behavior (i.e. extinction responding) was assessed under extinction conditions during which cocaine and cocaine-associated discrete cue light and tone were unavailable, and lever pressing resulted in no consequences. Each extinction session lasted 3 hs.
Experiment 7: Effects of fenobam sulfate on locomotor behavior
Finally, to determine whether the reduction in cocaine-taking and cocaine-seeking behavior was due to nonspecific locomotor impairment or sedative effects, we observed the effects of the same doses of fenobam sulfate on basal locomotor behavior in a separate group of rats (n = 8). Before testing, drug-naïve rats were habituated in a locomotor detection chamber (AccuScan, Columbus, OH, USA) for 1 h each day on two consecutive days. On each testing day, 1 h basal levels of locomotor activity were recorded prior to vehicle or fenobam sulfate administration; drug-induced changes in locomotor behavior were recorded for an additional 3 hs. Each animal was tested three times with different drug doses. The order of testing for various doses of fenobam sulfate was counterbalanced. The time intervals between testing were 2–3 days.
Data Analysis
All data are presented as means ± SEM. One-way ANOVA was used to determine the significance of the changes in reward-taking or reward-seeking behavior after drug administration. Whenever a significant main effect was found, individual group comparisons were carried out using pre-planned Bonferroni t-tests. Paired two-tailed t-tests were used to compare the differences in fenobam sulfate effects on sucrose reinstatement. Unpaired two-tailed t-tests were used to compare the differences in Cmax and AUC between fenobam free base and fenobam sulfate treatment groups.
RESULTS
Fenobam sulfate has superior absorption characteristics compared to fenobam free base
Figure 1 illustrates the comparative pharmacokinetic properties of fenobam free base and fenobam sulfate. Fenobam sulfate had a higher Cmax (Fig. 1a: p < 0.05) and longer average T1/2 [3.47 ± 2.57 (mean ± SD) versus 0.4 ± 0.15 hrs in female rats (n = 3); 2.27 ± 1.28 versus 1.37 ± 1.77 in male rats (n = 3)] than fenobam free base. Since the T1/2 data shows large variability across animals, we calculated the AUC of the plasma fenobam concentrations in each animal and pooled data from male and female rats together to increase the power for statistical analysis. We found that the AUC values in fenobam sulfate treated rats were significantly higher than those in fenobam free base treated rats (Fig 1b: p < 0.01). These data suggest that the sulfate formulation of fenobam has higher oral bioavailability than fenobam free base.
Figure 1.
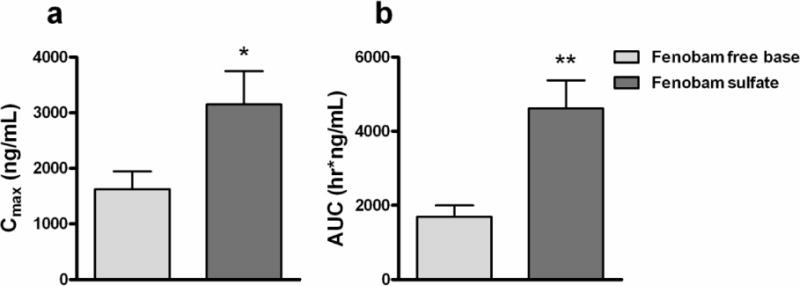
Blood fenobam concentrations following oral administration of 30 mg/kg fenobam free base or fenobam sulfate. (a) Cmax, maximal (peak) plasma concentration, and (b) AUC, Area Under the Curve of plasma fenobam concentrations over time (all 9 time points within 24 hrs) after oral administration of 30 mg/kg fenobam free base or fenobam sulfate. Free base, n = 6; sulfate, n = 6. * p < 0.05, ** p < 0.01.
Fenobam sulfate inhibits cocaine self-administration
Figure 2 illustrates the effects of fenobam sulfate on cocaine self-administration, demonstrating that oral administration of fenobam sulfate (30–60 mg/kg) significantly inhibited cocaine self-administration in a dose-dependent manner (Fig. 2a: F2,10 = 5.36, p < 0.05). Oral administration of fenobam sulfate also significantly reduced active lever responding in a dose-dependent manner (Fig. 2b: F2,10 = 8.05, p < 0.01). Individual group comparisons demonstrated that fenobam sulfate, at 60 mg/kg, significantly decreased cocaine infusions (t = 3.22, p < 0.05) and active lever presses (t = 4.01, p < 0.01). These effects lasted for less than 24 hrs as cocaine self-administration on the following day returned to previous stable levels. There were no significant differences in inactive lever responding across treatments (Fig. 2b).
Figure 2.
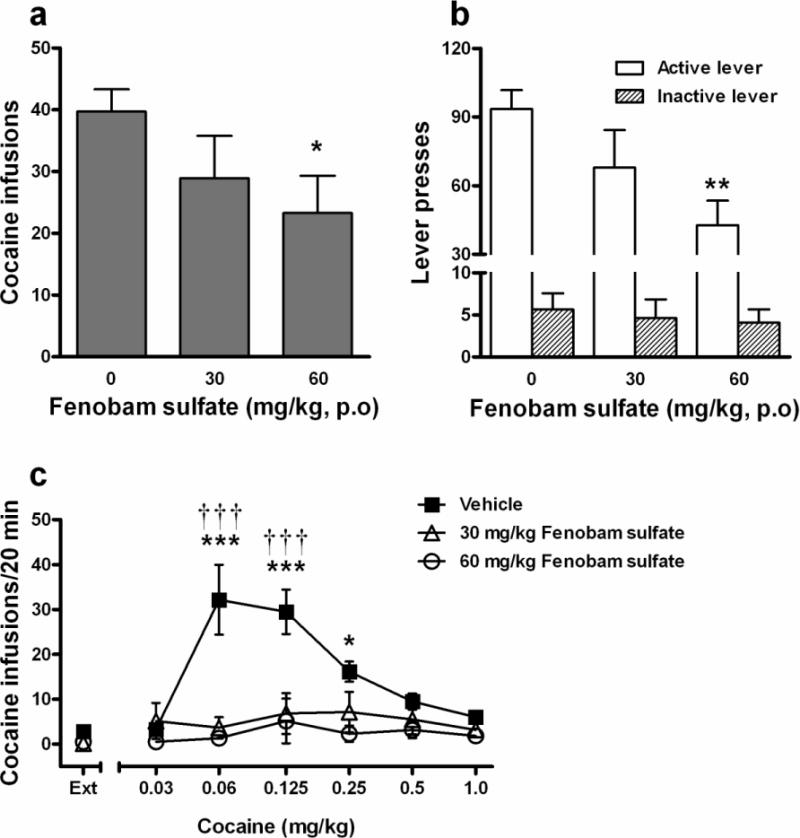
Effects of fenobam sulfate on intravenous cocaine self-administration. Oral (p.o) administration of fenobam sulfate significantly inhibited cocaine self-administration (a) and active lever responses (b) in rats. Within-subjects design, n = 11. * p < 0.05, ** p < 0.01 compared to vehicle. It also dose-dependently shifted cocaine dose-response self-administration curve downward (c; vehicle (■), 30 mg/kg (△), 60 mg/kg (○)).Within-subjects design, n = 6. 30 mg/kg fenobam vs. vehicle: ††† p < 0.001; 60 mg/kg fenobam vs. vehicle: * p < 0.05, *** p < 0.001.
Figure 2c illustrates that fenobam sulfate, at the same doses (30–60 mg/kg, p.o.), significantly and dose-dependently inhibited cocaine self-administration maintained by lower doses of cocaine (0.06, 0.125, 0.25 mg/kg/infusion) and shifted the cocaine dose-response self-administration curve downward. Two-way ANOVA with two-factor repeated measures over cocaine and fenobam sulfate doses revealed a significant treatment (vehicle vs. fenobam) main effect (F2,75 = 10.98, p < 0.01), significant time (cocaine dose) main effect (F5,75 = 7.69, p < 0.001) and a significant treatment ′ time interaction (F10,75 = 5.95, p < 0.001). Individual group comparisons revealed a statistically significant reduction in cocaine self-administration following 30 mg/kg fenobam treatment at cocaine doses of 0.06 (t = 5.98, p < 0.001) and 0.125 mg/kg/infusion cocaine (t = 4.76, p < 0.001) and following 60 mg/kg fenobam treatment at cocaine doses of 0.06 (t = 6.47, p < 0.001), 0.125 (t = 5.11, p < 0.001), and 0.25 mg/kg/infusion cocaine (t = 2.90, p < 0.05), when compared to the vehicle control group.
Fenobam sulfate inhibits oral sucrose –taking and sucrose-seeking behavior
Figure 3 illustrates the effects of fenobam sulfate on oral sucrose self-administration. Systemic administration of fenobam sulfate failed to alter the total number of sucrose deliveries (Fig. 3a: F2,6 = 1.14, p > 0.05) or the total numbers of active lever presses (Fig. 3b: F2,6 = 1.46, p > 0.05). Although one-way ANOVA revealed a statistically significant treatment main effect of fenobam on inactive lever responding (Fig. 3b: F2,6 = 4.34, p < 0.05), post-hoc individual group comparisons revealed no significant reduction in inactive lever responding after 30 mg/kg (t = 2.65, p > 0.05) or 60 mg/kg (t = 2.45, p > 0.05) fenobam sulfate when compared to vehicle control group.
Figure 3.
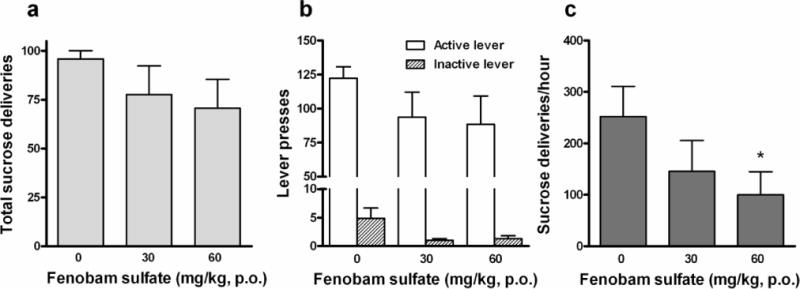
Effects of fenobam sulfate on oral sucrose self-administration. Oral (p.o) administration of fenobam sulfate failed to alter total numbers of sucrose deliveries (a) and active/inactive lever presses (b) within 90 min test duration. However, fenobam sulfate significantly reduced the rate of sucrose self-administration (sucrose deliveries per hour) (c) Within-subjects design, n = 7. * p < 0.05 compared to vehicle.
Since sucrose deliveries were limited to maximal 100 deliveries during 90-min testing and the majority of animals completed the maximal 100 deliveries in less time, we renormalized the data to the rate of sucrose deliveries per hour. The normalized data show that systemic administration of fenobam sulfate significantly and dose-dependently inhibited oral sucrose self-administration (Fig. 3c: F2,6 = 5.19, p < 0.05). Individual group comparisons revealed a significant reduction in the rate of sucrose delivery after 60 mg/kg fenobam sulfate (t = 3.14, p < 0.05).
Figure 4a shows the effects of fenobam sulfate on reinstatement of sucrose-seeking behavior. Pretreatment with 60 mg/kg (paired, two-tailed t-test: t = 3.22, p < 0.05), but not 30 mg/kg (t = 0.55, p > 0.05), fenobam sulfate significantly inhibited sucrose-induced reinstatement of sucrose-seeking behavior. There were no statistically significant variations in the total inactive lever responses across treatments.
Figure 4.
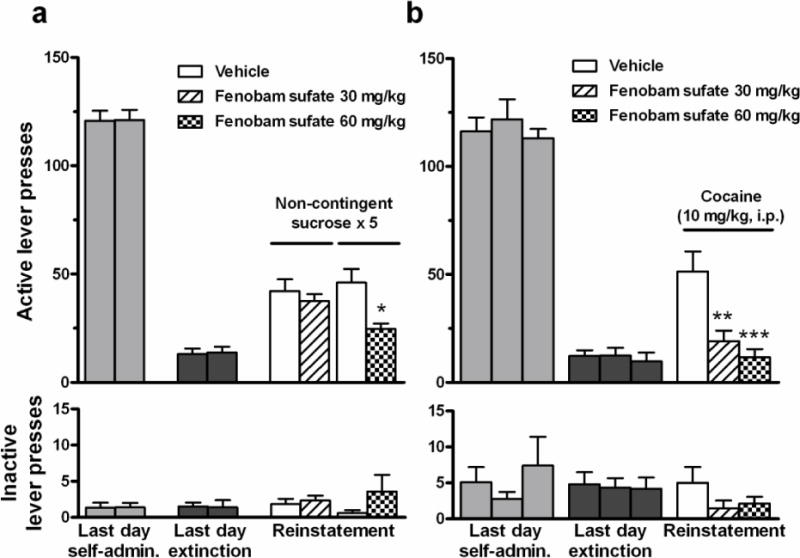
Effects of fenobam sulfate on sucrose-induced reinstatement of reward-seeking behavior and cocaine-primed reinstatement of drug-seeking behavior. (a) Two groups of animals (vehicle and 30 mg/kg, n = 6; vehicle and 60 mg/kg, n = 5) were trained to self-administer sucrose and then sucrose-taking/-seeking behavior was extinguished. Pretreatment with 60 mg/kg fenobam sulfate significantly inhibited reinstatement of extinguished sucrose-seeking behavior following non-contingent sucrose delivery. (b) Three groups of animals (vehicle, n = 10; 30 mg/kg fenobam sulfate, n = 9; 60 mg/kg fenobam sulfate, n = 9) were trained to self-administer cocaine and then cocaine-taking/-seeking behavior was extinguished. Pretreatment with fenobam sulfate (0, 30 or 60 mg/kg, p.o.) dose-dependently inhibited cocaine-induced reinstatement of extinguished drug-seeking behavior in rats. * p < 0.05, ** p < 0.01, *** p < 0.001, compared to vehicle.
Fenobam sulfate inhibits cocaine-primed reinstatement of cocaine-seeking behavior
Figure 4b illustrates the total number of active and inactive lever presses observed during the last session of cocaine self-administration, the last session of extinction, and the reinstatement test session in the three different fenobam sulfate dose groups. A single, non-contingent cocaine priming dose (10 mg/kg, i.p.) produced robust reinstatement of extinguished operant responding (i.e., active lever presses) in rats previously reinforced by i.v. cocaine infusions. One-way ANOVA revealed a statistically significant treatment main effect of fenobam sulfate (Fig. 4b: F2,25 = 10.23, p < 0.001) on active lever responding. Bonferroni individual group comparisons revealed a significant reduction in drug-seeking after 30 mg/kg (t = 3.42, p < 0.01) or 60 mg/kg (t = 4.23, p < 0.001) fenobam sulfate when compared to vehicle control group. There were no significant differences in inactive lever responding after any dose of fenobam sulfate pretreatment (Fig. 4b). Fenobam sulfate alone (60 mg/kg, p.o.) failed to evoke reinstatement of drug-seeking behavior (active lever responses: 16 ± 4.4 after fenobam sulfate, n=7, versus 21 ± 4.6 after vehicle, n=5, P>0.05).
Fenobam sulfate inhibits contextual cue-induced cocaine-seeking behavior
Figure 5a shows the total numbers of cocaine infusions, active lever responses and inactive lever responses during the initial 13 days of cocaine self-administration training, demonstrating that the majority of rats rapidly acquired stable cocaine self-administration behavior after 2 weeks of training. Fig. 5b illustrates contextual cue-induced incubation of cocaine craving – a time-dependent increase in cue-induced cocaine-seeking behavior (active lever responses) after 3 weeks of withdrawal from last cocaine self-administration compared with only 2 days of withdrawal (Fig. 5b, unpaired, two-tailed t-test: t = 6.12, p < 0.001). There was no significant difference in inactive lever responses after 2 or 21 days of withdrawal (Fig. 5b). Fig. 5c illustrates the effects of fenobam sulfate on incubation of cocaine craving. One-way ANOVA revealed a statistically significant treatment main effect of fenobam sulfate (Fig. 5c: F2,19 = 4.32, p < 0.05) on active lever presses. Individual group comparisons revealed significantly reduced incubation of cocaine craving after 60 mg/kg fenobam sulfate (t = 2.94, p < 0.05) when compared to vehicle control group. Pretreatment with fenobam sulfate did not significantly inhibit inactive lever responding in forced drug-abstinent rats (Fig. 5c: F2,19 = 1.63, p > 0.05).
Figure 5.
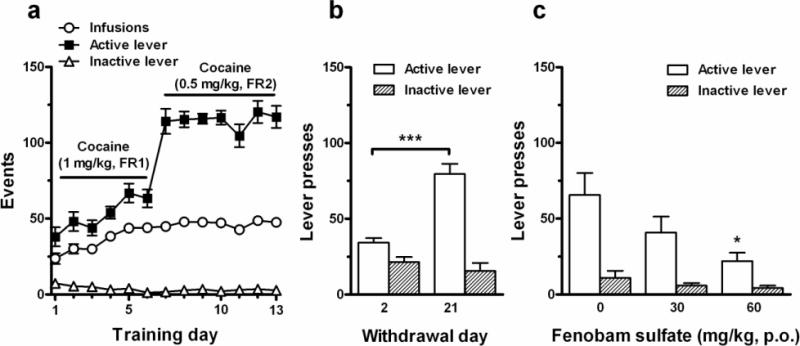
Effects of fenobam sulfate on contextual cue-induced incubation of cocaine craving. (a) Total numbers of cocaine infusions (○), active (■) and inactive lever (△) responses during initial 13 days of cocaine self-administration training. (b) Extinction responses after 2 or 21 days of withdrawal from cocaine self-administration (n = 16 rats in each subgroup), illustrating a progressive increase in cue-induced cocaine-seeking (i.e., active lever presses) over time of withdrawal. (c) Pretreatment with fenobam sulfate (0, 30 or 60 mg/kg, p.o.) inhibited cue-induced cocaine-seeking behavior. Fenobam sulfate test: vehicle, n = 6; 30 mg/kg, n = 8; 60 mg/kg, n = 8. * p < 0.05, *** p < 0.001.
Fenobam sulfate does not inhibit locomotion
Figure 6 illustrates locomotor behavior before and after oral fenobam sulfate (30, 60 mg/kg) administration, demonstrating a lack of an inhibitory effect on locomotor behavior. Two-way ANOVA for repeated measures over time revealed no statistically significant fenobam treatment main effect (F2,357 = 2.40, p > 0.05), a significant time main effect (F17,357 = 21.02, p < 0.001) and a significant treatment × time interaction (F34,357 = 1.77, p < 0.01). Individual group comparisons revealed a significant increase in locomotion at only the first 10-min time bin after 30 mg/kg fenobam sulfate (t = 4.88, p < 0.001) when compared to vehicle control group. The significant interaction between time and drug treatment was driven principally by the initial time-point; excluding the first 30 min after drug administration, to better match the 30-min fenobam sulfate pretreatment utilized in the self-administration and reinstatement tests, revealed no statistically significant effect of fenobam sulfate treatment (F2,294 = 1.85, p > 0.05), a significant effect of time (F17,294 = 1.82, p < 0.05) and no significant interaction between time and drug treatment (F34,294 = 0.89, p > 0.05).
Figure 6.
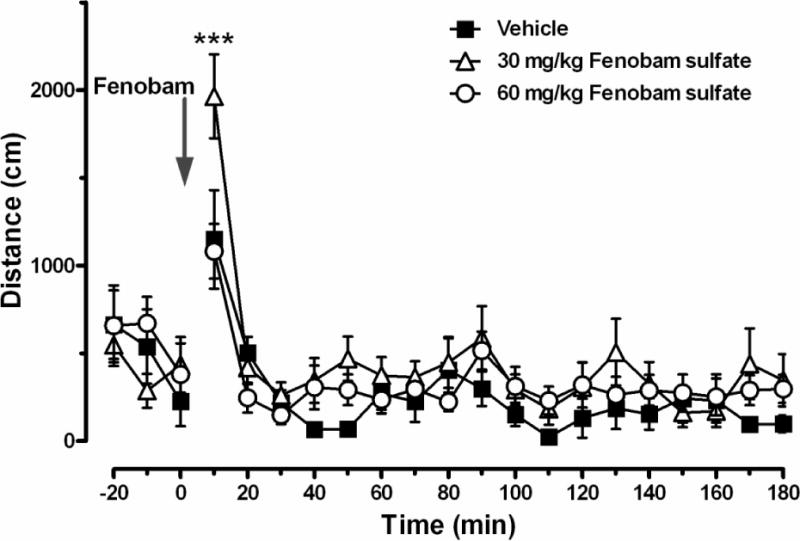
Effects of fenobam sulfate on locomotor activity. Compared to vehicle (■), fenobam sulfate, at 30 mg/kg (△), but not 60 mg/kg (○), produced a transient increase in locomotion within the initial 10 min, but not at any other time points following oral drug administration. Within-subjects design, n = 8. *** p < 0.001 compared to vehicle.
DISCUSSION
The present study evaluated the effects of fenobam sulfate in behavioral models of cocaine addiction and relapse. We found that oral administration of fenobam sulfate produced significant inhibitory effects on cocaine self-administration, cocaine-induced reinstatement of drug-seeking behavior, and contextual cue-induced cocaine seeking. In addition, fenobam sulfate also significantly inhibited oral sucrose self-administration and sucrose-induced reinstatement. The present findings, combined with the use of fenobam in human clinical studies, suggest that fenobam sulfate is a potential candidate for translational development in the treatment of cocaine addiction.
The behavioral inhibition in the above experiments was unlikely due to nonspecific sedation or locomotor impairment because fenobam sulfate did not inhibit open field locomotor activity. Indeed, there was a significant increase in locomotion within 10 min after oral fenobam sulfate administration. However, we do not think that this increase in locomotion contributed to the effects on cocaine- or sucrose-driven self-administration and reinstatement behaviors because the operant behavioral tests began 30 min after fenobam sulfate administration; no fenobam effect on locomotor activity was detectable after 30 minutes.
Drug addiction is characterized by compulsive drug-taking and drug-seeking behavior after abstinence (Gawin and Kleber 1986; Satel et al. 1991). Intravenous drug self-administration is one of the most commonly used animal models for studying a drug’s reinforcing effects (Panlilio and Goldberg 2007). The present finding that fenobam sulfate significantly inhibits cocaine self-administration is consistent with previous studies using the prototypic MPEP, in which it was found that MPEP significantly inhibits cocaine self-administration under fixed-ratio reinforcement in mice (Chiamulera et al. 2001) or under progressive-ratio reinforcement in rats (Paterson and Markou 2005), cocaine-induced CPP (Herzig and Schmidt 2004; McGeehan and Olive 2003), cocaine-induced hyperlocomotion (Herzig and Schmidt 2004), and cocaine-induced locomotor sensitization (Dravolina et al. 2006). The neural mechanisms underlying the antagonism of cocaine reward by mGluR5 NAMs are not fully understood. Several studies suggest that blockade of mGluR5 decreases brain reward functioning as measured by electrical intracranial self-stimulation (Cleva et al. 2012; Kenny et al. 2005), suggesting that a diminished rewarding response to cocaine may underlie the antagonism of cocaine self-administration after mGluR5 blockade.
Two animal models are commonly used to evaluate drug- or cue-induced relapse to drug-seeking behavior (Grimm et al. 2001; O’Brien and Gardner 2005). One is drug priming-induced reinstatement (relapse) of drug-seeking behavior, in which animals are first trained for cocaine self-administration and then drug-seeking behavior is extinguished in the absence of cocaine and cocaine-paired cues (light and tone). Rats are then tested for cocaine priming-induced reinstatement (relapse) of extinguished drug-seeking behavior (O’Brien and Gardner 2005). The second model is incubation of craving, in which animals are forcibly withdrawn from cocaine self-administration without behavioral extinction of the previously-reinforced operant responding. After varying periods of withdrawal time in their home cages, animals are re-exposed to the environmental context previously paired with drug self-administration, i.e., the self-administration chambers. Subsequent lever responding (i.e., extinction responding) leads to no consequence (no drug, no conditioned cues) or to only conditioned cues (Lu et al. 2004). Strikingly, there is a progressive increase in drug seeking over duration of withdrawal. Since the time-course of the increase in cocaine seeking is somewhat similar to that of the expression of psychostimulant sensitization after withdrawal and that of drug craving in humans during abstinence, it has been termed “cue-induced incubation of cocaine craving” (Grimm et al. 2001; Lu et al. 2004).
Because fenobam is a selective mGluR5 NAM (Porter et al. 2005), and has had some success in clinical trials for treatment of anxiety and fragile × syndrome (Friedmann et al. 1980; Pecknold et al. 1982; Berry-Kravis et al. 2009), it is an intriguing candidate for possible clinical development for the treatment of other neuropsychiatric disorders, including psychostimulant addiction (Carroll, 2008). However, previous clinical work utilizing fenobam free base monohydrate was limited by variable bioavailability (Itil et al. 1978; Berry-Kravis et al. 2009). The low water solubility of fenobam free base may have contributed to its inconsistent absorption profile. Whereas, fenobam sulfate has improved water solubility that may contribute to its greater overall bioavailability in rats compared to the free base as measured by Cmax, T1/2 and AUC.
An important finding in the present study is that systemic administration of fenobam sulfate significantly inhibited cocaine self-administration in a dose-dependent manner. This is consistent with previous reports using MPEP or MTEP (Kenny et al. 2005; Lee et al. 2005; Martin-Fardon et al. 2009; Paterson and Markou 2005). To determine whether such a reduction in drug-taking behavior was due to a reduction in cocaine’s rewarding strength, we observed the effects of fenobam sulfate on cocaine self-administration dose-response curve based upon a well-accepted view that a leftward or upward shift of a dose-response curve is usually interpreted as an increase in pharmacological action, and vice versa (Hiranita et al., 2009). We found that fenobam sulfate dose-dependently shifted the cocaine self-administration dose-response curve downward, suggesting a reduction in cocaine’s rewarding efficacy after mGluR5 blockade. We note that a within-session, not between-session, multiple-dose cocaine self-administration paradigm was used in the present study because it is less time-consuming, resource (drugs, animals)-efficient with fewer concerns about tolerance or sensitization caused by repeated drug administration used in between-session experiments (Bentzley et al., 2013). The major weaknesses include: 1) the cocaine dose-response curve observed in a within-session design may not precisely reveal the dose-response relationship because brain drug may not be completely cleaned up before the next cocaine dose testing, which may overlay with those by the subsequent dose injections; and 2) if the time course of the pharmacological action of a tested drug (for example, fenobam) is short than the duration (3 hrs) of multiple-dose self-administration testing, it may produce an artifact effect that the tested drug has no effect on higher doses of cocaine tested later in the paradigm. Given that very low doses (0.125 – 1.0 mg/kg/infusion) of cocaine were used in self-administration, suggesting that brain cocaine may be rapidly cleaned out after each testing, and that the pharmacological action of many testing drugs last at least 2–3 hrs, the within-session multiple-dose of cocaine self-administration paradigm is routinely used in this field (Mantsch et al., 2007; Hiranita et al., 2009; Song et al., 2012).
In addition, we also found that fenobam sulfate significantly and dose-dependently inhibited cocaine priming-induced reinstatement of drug-seeking behavior. This is consistent with previous reports that MPEP (Backstrom and Hyytia 2007; Lee et al. 2005) or MTEP (Kumaresan et al. 2009; Martin-Fardon et al. 2009; Martin-Fardon and Weiss 2012; Wang et al. 2012) inhibit cocaine-, cue-, or stress-induced reinstatement of drug-seeking behavior. In addition, the present study also for the first time demonstrated that blockade of mGluR5s by fenobam sulfate inhibited contextual cue-induced cocaine seeking in rats, supporting an important role of mGluR5 in relapse to drug-seeking behavior. The neural mechanisms underlying these actions are not fully understood. Previous studies suggests that cocaine priming, footshock stress or re-exposure to cocaine-associated cues evokes glutamate release within the VTA and NAc (McFarland et al. 2003; Miguens et al. 2008; Wang et al. 2005; Wang et al. 2007; Xi et al. 2006a; You et al. 2007), which has been shown to play an important role in relapse to drug-seeking behavior (Kalivas and Volkow 2011; Knackstedt and Kalivas 2009). Given that mGluR5s are mostly located on postsynaptic cells (Mitrano et al. 2008; Mitrano and Smith 2007) and co-localized with adenosine A2a, dopamine D2 and NMDA receptors (Tebano et al. 2005), it is plausible to hypothesize that blockade of postsynaptic mGluR5 may attenuate cocaine- or cue-induced increases in synaptic glutamate transmission within the VTA and NAc, therefore inhibiting cocaine- or cue-induced reinstatement of drug-seeking behavior. More studies are required to test this hypothesis and other possible mechanisms such as possible interactions between mGluR5 and NMDA or dopamine D2-like receptors.
In addition to inhibition of cocaine self-administration, fenobam sulfate also inhibited sucrose self-administration and sucrose-induced reinstatement. This is consistent with previous reports that MPEP or MTEP decrease food-taking behavior in rats and non-human primates (Paterson et al. 2005; Platt et al. 2008) and with a recent report that fenobam free base inhibits food- and sucrose-induced reinstatement of reward-seeking behavior (Watterson et al. 2013). We note, however, that in the present study fenobam sulfate appears to be more effective in inhibiting cocaine-taking and cocaine-seeking behavior compared to sucrose-taking and sucrose-seeking behavior (Figs. 2–4). For example, although fenobam sulfate (30–60 mg/kg, p.o.) significantly lowered the rate of sucrose delivery per hour, it failed to alter the total numbers of sucrose deliveries and active/inactive lever presses during the 90 min testing duration. In addition, fenobam sulfate, at 30 mg/kg, significantly inhibited cocaine-, but not sucrose-induced reinstatement. At 60 mg/kg, fenobam sulfate produced more than 80% reduction in cocaine-induced reinstatement, but ~50% reduction in sucrose-induced reinstatement. These data suggest that mGluR5 does regulate general appetite behavior as reported previously (Bradbury et al. 2005). However, within an appropriate therapeutic dose window, mGluR5 NAMs may selectively decrease cocaine-taking and cocaine-seeking behavior without significant effects on other appetitive behaviors.
Initially, research into the pharmacology of mGluR5s was focused on the identification and characterization of competitive glutamate analogs (Schoepp and Conn 1993), but success was very limited due to a high degree of homology between mGluRs at the orthosteric glutamate binding site located in a large bi-lobed N-terminal domain. Subsequently, substantial efforts have been taken to develop noncompetitive mGluR5 antagonists that target allosteric binding sites (Nicoletti et al. 2011) and demonstrate mGluR subtype selectivity. SIB–1757 was the first mGluR5-selective antagonist that inhibited glutamate-induced receptor activation in a non-competitive manner (Varney et al. 1999). Subsequent lead optimization of SIB–1757 led to the discovery of MPEP, a more potent, systemically active mGluR5 NAM (Gasparini et al. 1999). Since then, MPEP has served as an important research tool. However, off-target effects – notably NMDA receptor antagonism (Movsesyan et al. 2001; O’Leary et al. 2000) – precluded further clinical development (Lindsley and Emmitte 2009). MTEP is a second generation mGluR5 NAM that is both structurally and pharmacologically similar to MPEP (Cosford et al. 2003) but without substantial NMDA receptor activity. However, MTEP was not developed further due to potent inhibition of cytochrome P450 1A2 and a rapid metabolism (Smith et al. 2004). Therefore, several groups have explored the MPEP and MTEP pharmacophores in search of high-affinity mGluR5 ligands (Emmitte 2011; Rocher et al. 2011).
Fenobam is a selective mGluR5 NAM that has been administered to humans. A recent open-label clinical trial for the treatment of Fragile X Syndrome reported no adverse effects of fenobam, but some inconsistent measures of efficacy, possibly associated with large inter-individual variations in reported plasma levels of fenobam following oral administration (Berry-Kravis et al. 2009). In the present study, a new sulfate salt formulation of fenobam gave Cmax and AUC values approximately two- to three-fold higher than the free base. Orally active fenobam sulfate attenuated cocaine self-administration, cocaine-induced reinstatement of drug-seeking behavior, and cue-induced incubation of cocaine craving in rats and thus may be more effective than the free base in the clinic.
In summary, the present study reports that fenobam sulfate is efficacious in several preclinical models of cocaine addiction. Further, as fenobam has previously been approved for clinical studies, and more recently has been suggested for the treatment of Fragile X Syndrome and L-DOPA-induced dyskinesias (Berry-Kravis et al. 2009; Rylander et al. 2010), there appears to be significant potential for this new formulation for translational use as a treatment for cocaine abuse. These preclinical data provide strong support for designing human studies using fenobam sulfate as the first clinical candidate in this class to treat cocaine abuse and addiction.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. T.M.K. was supported by an NIH Postdoctoral Intramural Research Training Associate (IRTA) Fellowship. Pharmacokinetic data was provided under NIDA Contract, N01DA-9-8883. We thank Drs. Phil Skolnick and Amrat Patel, Division of Pharmacotherapies and Medical Consequences of Drug Abuse, National Institute on Drug Abuse, for providing the fenobam free base and fenobam sulfate, helpful discussions, and a critical reading of this manuscript.
Footnotes
Disclosure/conflict of interest: All authors hereby declare that, except for income received from their respective primary employers, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services. There are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–86. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology. 2013;226:113–25. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–71. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L, Cosford ND, Anderson J, Varney MA, Strack AM. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther. 2005;313:395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci. 2008;1141:221–32. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Cleva RM, Watterson LR, Johnson MA, Olive MF. Differential Modulation of Thresholds for Intracranial Self-Stimulation by mGlu5 Positive and Negative Allosteric Modulators: Implications for Effects on Drug Self-Administration. Front Pharmacol. 2012;2:93. doi: 10.3389/fphar.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46:204–6. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology. 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Emmitte KA. Recent advances in the design and development of novel negative allosteric modulators of mGlu(5) ACS Chem Neurosci. 2011;2:411–432. doi: 10.1021/cn2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann CTH, Davis LJ, Ciccone PE, Rubin RT. Phase II double blind controlled study of a new anxiolytic, fenobam (McN-3377) vs placebo. Current Therapeutic Research. 1980;27:144–151. [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–13. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Green MD, Jiang X, King CD. Inhibition of human hepatic CYP isoforms by mGluR5 antagonists. Life Sci. 2004;75:947–53. doi: 10.1016/j.lfs.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Bianchi M, Lacroix LP, Faedo S, Perdona E, Remelli R, Cavanni P, Crespi F. Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse. 2003;50:269–76. doi: 10.1002/syn.10261. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–84. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther. 2009;329:677–86. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itil T, Seaman B, Huque M, Mukhopadhyay S, Blasucci D, Nq K, Ciccone P. The clinical and quantitative EEG effects and plasma levels of fenobam (McN-3377) in subjects with anxiety: an open rising dose tolerance and efficacy study. Current Therapeutic Research. 1978;24:708–724. [Google Scholar]
- Jaffe JH, O’Keeffe C. From morphine clinics to buprenorphine: regulating opioid agonist treatment of addiction in the United States. Drug Alcohol Depend. 2003;70:S3–11. doi: 10.1016/s0376-8716(03)00055-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–86. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179:247–54. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–44. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–66. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–40. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Emmitte KA. Recent progress in the discovery and development of negative allosteric modulators of mGluR5. Curr Opin Drug Discov Devel. 2009;12:446–57. [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–26. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA, Yang Z. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology. 2007;192:581–91. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–90. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. (−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine (MTEP) similarly attenuate stress-induced reinstatement of cocaine seeking. Addict Biol. 2012;17:557–64. doi: 10.1111/j.1369-1600.2011.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–30. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Janak PH, Olive MF. Effect of the mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) on the acute locomotor stimulant properties of cocaine, D-amphetamine, and the dopamine reuptake inhibitor GBR12909 in mice. Psychopharmacology. 2004;174:266–73. doi: 10.1007/s00213-003-1733-2. [DOI] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–2. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology. 2008;196:303–13. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Arnold C, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocaine-treated rats. Neuroscience. 2008;154:653–66. doi: 10.1016/j.neuroscience.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RWt. The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity compared with the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J Pharmacol Exp Ther. 2009;330:834–43. doi: 10.1124/jpet.109.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan VA, O’Leary DM, Fan L, Bao W, Mullins PG, Knoblach SM, Faden AI. mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2001;296:41–7. [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–41. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- O’Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000;131:1429–37. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–55. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction (Abingdon, England) 2007;102:1863–70. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–61. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol. 1982;2:129–33. [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology. 2008;200:167–76. doi: 10.1007/s00213-008-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muhlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–21. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Rocher JP, Bonnet B, Bolea C, Lutjens R, Le Poul E, Poli S, Epping-Jordan M, Bessis AS, Ludwig B, Mutel V. mGluR5 negative allosteric modulators overview: a medicinal chemistry approach towards a series of novel therapeutic agents. Curr Top Med Chem. 2011;11:680–95. doi: 10.2174/1568026611109060680. [DOI] [PubMed] [Google Scholar]
- Romano C, van den Pol AN, O’Malley KL. Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: protein, mRNA splice variants, and regional distribution. J Comp Neurol. 1996;367:403–12. doi: 10.1002/(SICI)1096-9861(19960408)367:3<403::AID-CNE6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W, Bezard E, Cenci MA. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39:352–61. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Satel SL, Southwick SM, Gawin FH. Clinical features of cocaine-induced paranoia. Am J Psychiatry. 1991;148:495–8. doi: 10.1176/ajp.148.4.495. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Conn PJ. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993;14:13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–7. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Smith ND, Poon SF, Huang D, Green M, King C, Tehrani L, Roppe JR, Chung J, Chapman DP, Cramer M, Cosford ND. Discovery of highly potent, selective, orally bioavailable, metabotropic glutamate subtype 5 (mGlu5) receptor antagonists devoid of cytochrome P450 1A2 inhibitory activity. Bioorg Med Chem Lett. 2004;14:5481–4. doi: 10.1016/j.bmcl.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci USA. 2012;109:17675–80. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC, Schwarzschild MA, Chen JF, Cunha RA, Popoli P. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J Neurochem. 2005;95:1188–200. doi: 10.1111/j.1471-4159.2005.03455.x. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–33. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Varney MA, Cosford ND, Jachec C, Rao SP, Sacaan A, Lin FF, Bleicher L, Santori EM, Flor PJ, Allgeier H, Gasparini F, Kuhn R, Hess SD, Velicelebi G, Johnson EC. SIB-1757 and SIB-1893: selective, noncompetitive antagonists of metabotropic glutamate receptor type 5. J Pharmacol Exp Ther. 1999;290:170–81. [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;193:283–94. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–9. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Hood LE, Olive MF. Attenuation of reinstatement of methamphetamine-, sucrose-, and food-seeking behavior in rats by fenobam, a metabotropic glutamate receptor 5 negative allosteric modulator. Psychopharmacology. 2013;225:151–9. doi: 10.1007/s00213-012-2804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006a;26:8531–6. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006b;31:1393–405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27:10546–55. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


