Summary
Exposure to sub-lethal levels of stress, or hormesis, is a means to induce longevity. By screening for mutations that enhance resistance to multiple stresses, we identified multiple alleles of alpha-1,2-mannosidase I (mas1) which, in addition to promoting stress resistance, also extend longevity. Longevity enhancement is also observed when mas1 expression is reduced via RNA interference in both Drosophila melanogaster and Caenorhabditis elegans. The screen also identified Edem1 (Edm1), a gene downstream of mas1, as a modulator of lifespan. Since double mutants for both mas1 and Edm1 showed no additional longevity enhancement, it appears that both mutations function within a common pathway to extend lifespan. Molecular analysis of these mutants reveals that the expression of BiP, a putative biomarker of dietary restriction (DR), is down-regulated in response to reductions in mas1 expression. These findings suggest that mutations in mas1 may extend longevity by modulating dietary restriction.
Keywords: alpha-1,2-mannosidase I; Edem1; longevity; dietary restriction; BiP; Drosophila; C. elegans
Introduction
Aging is a complicated process influenced by numerous genetic and environmental factors (Bishop & Guarente 2007). Several mechanisms have been proposed to regulate aging, including the accumulation of damage resulting from reactive oxygen species (ROS), the loss of genomic integrity, as well as the modulation of genetic pathways that control reproductive output, the ability to withstand environmental stress, and nutrient utilization (Zhang & Herman 2002; Lombard et al. 2005; Partridge et al. 2005; Sinclair 2005; Lim et al. 2006). During the natural aging process, the increased expression of stress-responsive genes is often observed and in many cases, long-lived individuals display increased resistance to environmental stressors (Vermeulen & Loeschcke 2007; Gems & Partridge 2008; Rattan 2008). In nematodes exposure to sub-lethal levels of stress results in enhanced longevity, a phenomenon referred to as hormesis or a hormetic state (Cypser & Johnson 2002), which also leads to lifespan extension (Gems & Partridge 2008).
In natural populations a common stressor is reduced nutrient availability. Inhibition of nutrient sensing pathways (Alcedo & Kenyon 2004; Libert & Pletcher 2007), as well as nutrient deficiency (due to dietary restriction (DR)), has been shown to extend longevity (Masoro 2005; Mattson 2008). It appears that Sir2-mediated life extension is modulated in part, via an endoplasmic reticulum (ER) stress response since changes in Sir2 activity result in the altered expression of ER stress responsive genes such as BiP/Grp78 and abu (activated in blocked unfolded protein response), and it has been shown that abu-1 and abu-11 can also modulate longevity (Urano et al. 2002; Viswanathan et al. 2005). BiP/Grp78 is also down-regulated in the liver of the DR-treated mouse (Heydari et al. 1995; Tillman et al. 1996; Dhahbi et al. 1997) as well as in resveratrol-treated N2 worms, suggesting that it could be a DR biomarker.
Mas1 is expressed in the ER, the Golgi apparatus, and the lysosome. It is a member of the class I glycosidases and is involved in N-linked glycosylation (Herscovics 2001). During the calnexin/calreticulin cycle, Mas1 removes mannose from permanently unfolded proteins, then the de-mannosed proteins are recognized by ER degradation-enhancing alpha-1,2-mannosidase-like protein (Edem), and degraded by ER-associated degradation (ERAD) (Hosokawa et al. 2001; Ellgaard & Helenius 2003; Olivari & Molinari 2007). Several lines of evidence indicate that Mas1 is important during the aging process. First, altered N-linked glycosylation affects the maturation rate of proteins that influence longevity, such as insulin and insulin-like growth factor-I receptors (Duronio et al. 1988). Furthermore, the expression of mas1 is decreased in aging and oxidatively-stressed Drosophila (Zou et al. 2000) as well as in the livers of aging mice and humans (Cingle et al. 1996; Zhu et al. 2006).
To identify genes involved in lifespan extension, we performed a genetic screen for resistance to multiple stressors which led to the isolation of mutants of mas1 and Edem1. Individuals with targeted mutations in these genes exhibit a longer lifespan. We also show that the mutant phenotype associated with mas1 mutants can be recapitulated via RNA interference not only in Drosophila but also in C. elegans. Molecular reduction in mas1 expression correlates with a reduction in BiP expression, suggesting that dietary regimen may be altered. In addition, these mutants show a decreased response to the beneficial effects of DR on lifespan extension. These results provide a novel link between ER-associated degradation and DR-related longevity.
Results
Mutations in alpha-1,2-mannosidase I (mas1) Extend Lifespan in Drosophila melanogaster
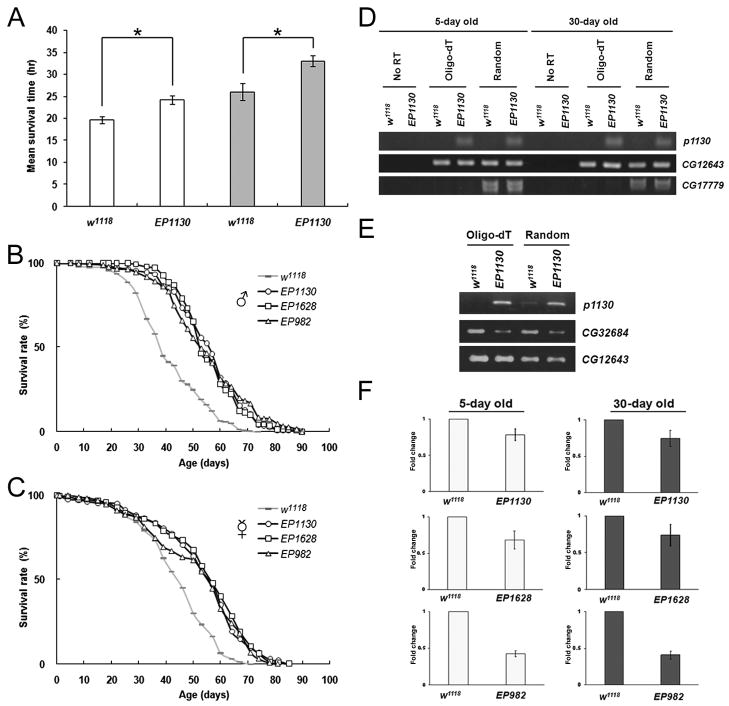
Long-lived organisms frequently display enhanced resistance to environmental stressors (Martin et al. 1996; Wang et al. 2004; Bubliy & Loeschcke 2005). To identify genes involved in lifespan extension, we screened a collection of transposon-mediated mutants (Rorth 1996) for enhanced resistance to paraquat and starvation, two common experimental stressors. Under these conditions, one of the mutant lines, EP1130, displayed an increase of about 60% in the mean survival time in both males and females relative to the control strain w1118 (Fig. 1A). The long-lived phenotype remained after out-crossing the EP1130 line to w1118 ten times confirming that the longevity was due to the insertion of the transposable element and not due to a background effect. The outcrossed mutant displayed a mean lifespan enhancement of 38% for males and 22% for females, and 15% maximum lifespan extension for both (Fig. 1B, 1C; Supplemental Table 1, 2).
Figure 1. Mutants with reduced mas1 (CG32684) expression exhibit extended lifespan.
(A) Both male and female EP1130 mutants survive longer than the control w1118 in the multiple-stress paradigm, 10mM paraquat and wet starvation. Open columns represent the mean survival time±SD of the male flies. Grey columns represent female. *P<0.05 by Student’s t test. (B) Male mas1 mutants display a similar fold of lifespan increase at 25°C. Grey bar represents w1118, open circle EP1130, open square EP1628, open triangle EP982. (C) Virgin female mutants also show enhanced lifespan. (D) A transcript, p1130, is up-regulated in EP1130 both in young (5-day old) and old (30-day old) flies as measured by RT-PCR either using oligo-dT or random primers in the RT reaction. The No RT reaction, which contains no primers in the RT reaction followed by PCR, is used to show the absence of genomic DNA in the PCR reaction. No difference in the expression of CG12643 and CG17779 genes near the EP1130 insertion site is detected. These were used as internal controls for the PCR reaction comparing w1118 and EP1130. (E) An increased expression of p1130 is correlated with the down-regulation of CG32684 in EP1130 by RT-PCR. The expression of CG12643 was used as an internal control. (F) A decreased expression of CG32684 is detected in both the young and old mutants by real-time PCR.
Since the insertion site of the transposon responsible for the mutation was known, the activity of genes in the vicinity of the insertion was monitored via RT-PCR by comparing gene expression in EP1130 and w1118. Only one transcript from the region was found to be differentially expressed in both young (5-day) and old (30-day) flies (Fig. 1D, row 1). This transcript, named p1130, was significantly up-regulated. Blast analysis of the RT-PCR fragment revealed no significant homology to any annotated genes. To obtain the full transcript, 5′ and 3′ RACE was performed resulting in the isolation of a 1.6-kb transcript, p1130 (Supplemental Fig. 1).
Since the 1.6-kb transcript does not appear to encode either a functional protein or a mature microRNA, the sequence was scanned for potential antisense homology to other genes. A 480-bp sequence (labeled in red in the Supplemental Fig. 1) at the 3′ end of p1130 is complementary to the 3′ UTR of CG32684 (mas1), suggesting that the expression of this transcript could down-regulate the expression of mas1. Indeed, in EP1130 decreased expression of mas1 is observed relative to w1118 (Fig. 1E, row 1 and 2), while the expression of CG12643 (another gene in the region used as an internal control), showed no changes between w1118 and EP1130 (Fig. 1E, row 3). PCR results were confirmed by Northern blot, which also revealed reduced CG32684 expression in EP1130 compared to w1118 (data not shown). Thus mas1 expression is altered in EP1130.
To determine whether the reduced expression of mas1 extends lifespan, we measured the lifespan of additional strains with insertions in this gene. Two lines with insertions in mas1, EP982 and EP1628, showed a similar level of life extension, 36% and 39% respectively (Fig. 1B-C, Supplemental Table 1 and 2). To confirm that the longevity changes were due to the down-regulation of mas1, the level of expression of this gene was examined in EP982, EP1628, and EP1130 in both young and old flies by real time PCR. It was found that the expression of mas1 is reduced in young and old flies in all three mutants (Fig. 1F). These results are consistent with the hypothesis that the down-regulation of mas1 extends lifespan. All the mutants displayed better resistance to individual stress, oxidative stress and starvation (Supplemental Table 3). EP1307, another insertion in mas1, isolated from an independent screen, was also found to have an increase in longevity, although the life extension was less striking in this line (Supplemental Fig. 2). The observation that three independent insertions into this gene extend lifespan strongly suggests that mas1 is important for modulating longevity.
RNA-interference-mediated Reduction in mas1 Expression Increases Lifespan in Drosophila and C. elegans
To determine whether mas1 (CG32684) down-regulation is sufficient for the life extension, we generated an RNAi line of transgenic flies, UAS-CG32684RNAi, to knock-down mas1 expression. We observed the effect of downregulation of mas1, by crossing UAS-CG32684RNAi transgenic flies with the ubiquitous driver line, da-Gal4. The mean lifespan of the progeny was 39% greater than that of control flies (Fig. 2A, Supplemental Table 4), further supporting the hypothesis that reduction of the activity of mas1 extends lifespan.
Figure 2. The RNA interference knockdown of mas1 extends lifespan in Drosophila melanogaster and C. elegans.
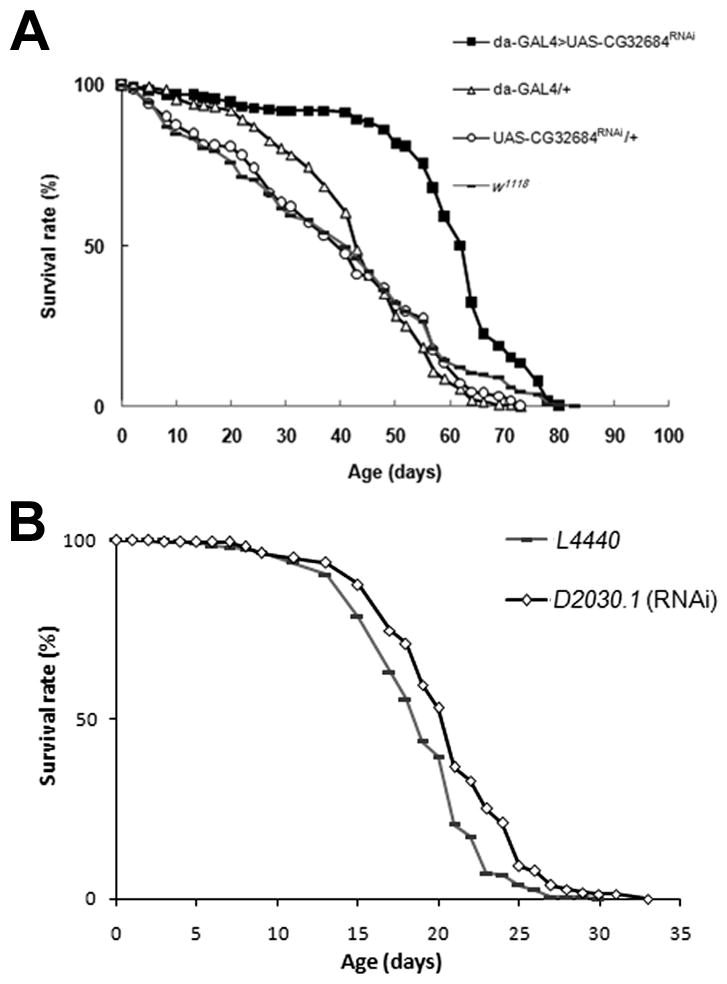
(A) Flies with knock-downed CG32684 expression prolong lifespan. Solid square represents the da-GAL4>UAS-CG32684RNAi knock-downed flies, open triangle da-GAL4/+, open circle UAS-CG32684RNAi/+, grey bar w1118. (B) N2 worms fed with E. coli expressing double-stranded RNA against D2030.1 exhibit enhanced lifespan at 20°C. Grey bar represents N2 worms fed with E. coli containing L4440 (vector only) as a control, open diamond represents N2 worms fed with E. coli expressing double-stranded RNA against D2030.1. P<0.01 calculated by log rank test.
To examine whether reduction of mas1 can extend lifespan in an organism other than Drosophila, we tested the effect of down-regulating the expression of D2030.1, the gene most homologous to mas1 in C. elegans. We measured the lifespan of the wild-type, N2 strain, fed either E. coli producing double-stranded RNAi against D2030.1, or E. coli containing only expression vector (L4440) as a control. As in the fly, the knockdown of D2030.1 extended lifespan, although the result was less striking in the worm with 9% extension in mean lifespan (P<0.0001; Figure 2B, Supplemental Table 4). However, the effect is specific to this homologue since the knockdown of the other paralogues of mas1 in the nematode by RNAi did not significantly extend lifespan (Supplemental Table 4). These results suggest that a conserved role of lifespan enhancement exists for this gene in both flies and worms.
Down-regulation of Edem1 Extends Lifespan
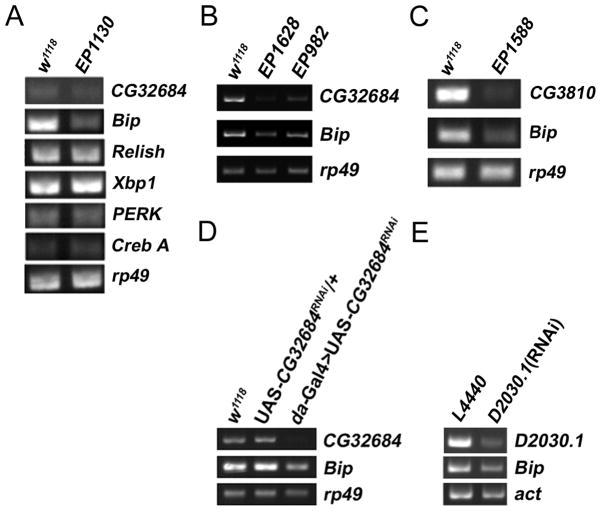
To determine whether the mechanism of life extension of the mas1 mutants is modulated via its known function in the ERAD pathway, we tested whether other mutants obtained in the screen affected components of this pathway. One mutant identified (EP1588) contained a transposable element inserted into CG3810 (Edem1), a gene downstream of mas1 in the ERAD pathway (Ellgaard & Helenius 2003; Olivari & Molinari 2007). The expression level of Edem1 was significantly lower in the mutant line relative to the control (Fig. 3A) and the mean lifespan of both male and female mutant flies was increased by more than 30% (Figs. 3B and 3C, Supplemental Tables 1 and 2). Since both genes are known to act in a common pathway, the hypothesis that they mediate their effects via this pathway can be tested by looking for genetic interactions. Typically, when two genes function in different pathways double mutants show synergistic effects, while genes that function in the same pathway produce no additional enhancement.
Figure 3. Edem1 mutant (EP1588) also displays lifespan extension.
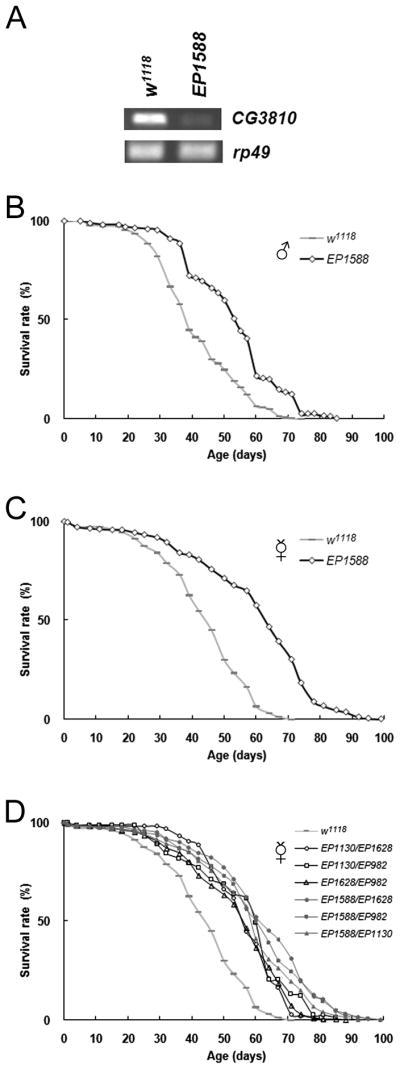
(A) Decreased expression of CG3810 (Edem1) is detected by RT-PCR in the mutant EP1588 which is also isolated from the multiple-stress screen. Edem1 is a downstream gene of mas1 in the ERAD pathway. The internal control is rp49. (B) Male Edem1 mutant exhibits increased lifespan. Grey bar represents w1118, open diamond EP1588. (C) Virgin female Edem1 mutants also exhibit extended lifespan. (D) Trans-heterozygous mutants of Edem1 and mas1 display similar fold levels of lifespan extension. Grey bar represents w1118, open circle EP1130/EP1628, open square EP1130/EP982, open triangle EP1628/EP982, solid circle EP1588/EP1628, solid square EP1588/EP982, solid triangle EP1588/EP1130.
Since the longevity phenotypes of EP1130, EP1628, EP982, and EP1588 are dominant (Supplemental Table 5), the effects of combining the various mutations can be readily observed by crossing the mutant strains. If the mutations act through a common pathway, the phenotype of a fly mutant for two different genes is expected to be similar to that generated by crossing two independent mutations in one of the genes. To test this prediction, mutant flies affecting mas1 were crossed to Edem1 mutants. The transheterozygotes for mas1 and Edem1 showed similar lifespan profiles to those resulting from crossing for different alleles of mas1 (Fig. 3D, Supplemental Table 5). These results support the hypothesis that the two genes act to extend longevity through a common genetic pathway.
Mutations in mas1 and Edem1 Exhibit Reduced BiP Expression
Mas1 is involved in the process of N-linked glycosylation (Herscovics 2001). Abolishing N-glycosylation causes unfolded protein accumulation and leads to an ER stress response (Elbein 1991; Lawson et al. 1998). In C. elegans the modulation of two ER stress response genes, abu-1 and abu-11, affects lifespan (Urano et al. 2002; Viswanathan et al. 2005). To examine whether ER stress plays a role in lifespan extension, we measured the mRNA levels of several ER stress response genes, including relish, perk, BiP/Grp78, xbp1, and crebA (Greene et al. 2005) in both EP1130 and w1118 and found that only BiP expression levels were altered (Fig. 4A). BiP expression was also reduced in EP1628, EP982, and EP1588 relative to w1118 (Fig 4A–C). To demonstrate that BiP down-regulation occurs as a result of the change in mas1 expression, the effect of knockdown by RNAi was measured. In both Drosophila and C. elegans, the treatment resulted in a reduction in BiP expression (Fig. 4D–E).
Figure 4. Reduced BiP expression, an indication of dietary restriction, is detected in the longevity mas1 and Edem1 mutant flies.
(A) The expression levels of ER stress response genes, BiP, Relish, Xbp1, PERK, Creb A, were measured in the w1118 and EP1130 by RT-PCR. Decreased BiP expression is found in EP1130. No changes appear in the expression of the other ER stress response genes. (B–C) Lowered BiP expression is also observed in the other longevity mutants EP1628, EP982, and EP1588 via RT-PCR. (D–E) Reduced BiP expression is detected in the RNAi knockdown of mas1 and D2030.1 in the fly and worm by RT-PCR.
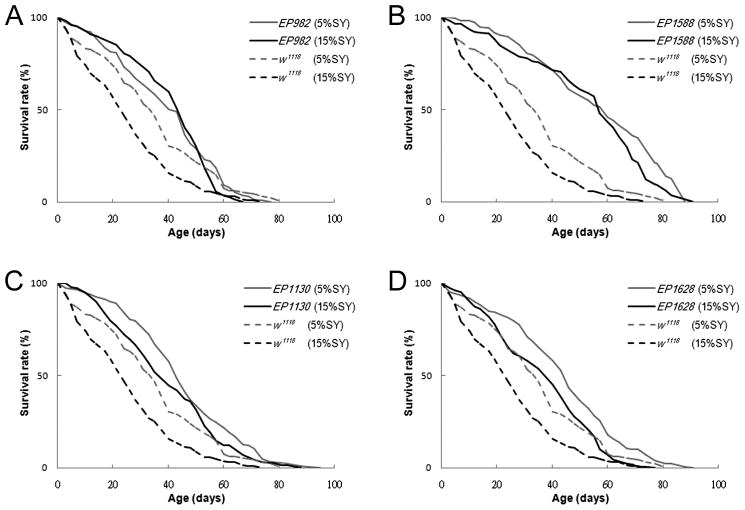
Since reduction in BiP/Grp78 expression is observed in long-lived mice under DR (Heydari et al. 1995; Tillman et al. 1996; Dhahbi et al. 1997), we hypothesized that the effect of down-regulating mas1 might be involved in modulation of the DR pathway in Drosophila. Drosophila raised under various nutrient regimens display dramatic changes in longevity and mutations that affect DR show an altered response to nutrient alteration. Therefore, mutations that affect DR-related pathways will display a non-proportional change in longevity on restricted (DR) media relative to control flies (Tatar 2007). To test whether mas1 may be involved in the modulation of the DR pathway in flies, the lifespan of the mutant and control flies was measured under two dietary conditions previously shown to lie in the linear range of lifespan increase for wild-type flies: abundant (15%-sugar/yeast (15%SY)) and restricted (5%-sugar/yeast (5%SY)) food (Wood et al. 2004; Libert et al. 2007). As expected the control flies, w1118, lived substantially longer under DR conditions (36% increase, Fig. 5A, Supplemental Table 6). Interestingly, the mutants were longer-lived than the controls under both conditions, and the mutants’ longevity enhancement relative to controls was dramatically increased on the abundant diet. As shown in Figure 5, these increases range from a 1.6-fold increase for EP1628 (26% extension on restricted versus 42% extension on abundant) to a 3.1-fold increase for EP982 (17% extension on restricted versus 54% on abundant) (Fig. 5A–D, Supplemental Table 6), consistent with mas1 and Edem1 interacting with the dietary restriction pathway. These results are also summarized on supplemental figure 3 where the non-proportionality of the reaction norm is illustrated by plotting mean lifespan of mutant and control flies on the two media. To determine whether the mutations abrogated DR plasticity or shifted the response to DR, the longevity of the various lines was compared under both sets of conditions (Supplemental Fig. 3; Supplemental table 6). For three of the four lines, a smaller but statistically significant enhancement was observed under DR conditions, while one of the lines, EP982, showed no significant difference. Thus it would appear that these mutations while interacting with the DR pathway do maintain some degree of responsiveness to DR.
Figure 5. Mutants in mas1 and Edem1 extend lifespan via dietary restriction pathway.
(A–D) The lifespan of the longevity mutants EP1130, EP1628, EP982, EP1588 and the control w1118 female flies on the abundant (15%SY) and restricted (5%SY) food at 25°C. No further lifespan enhancement was observed in EP982 and EP1588 under the restricted food; EP1130 and EP1628 only display half of the fold increase than that in control w1118. A dashed grey line represents the lifespan curve of w1118 on the restricted food, dashed black line is w1118 on the abundant food, solid grey line indicates mutants on the restricted food, solid black line is mutants on the abundant food.
To further examine the relationship of mas1 mutation and DR, the lifespan of the mas1-RNAi-knockdowned and control flies were measured under the abundant and restricted food conditions. Similar to the P-element mutants, the mas1-RNAi-knockdowned flies by RNAi also show the best life-extension on abundant media (roughly a 2-fold enhancement 19% under restricted versus 37% on abundant, Supplemental Fig. 4). Finally, to ensure that the restricted food represents DR conditions, we measured the fecundity of the mutant and control flies on both the restricted and abundant food. A similar pattern of reduced fecundity was observed for each fly strain on the restricted food compared to the abundant food (Supplemental Fig. 5), demonstrating that the restricted food environment results in DR conditions for both the mutant and control flies.
DR related life-extension can be achieved via a number of mechanisms, including reduced food consumption, reduced metabolic output, and reduced fertility (Wood et al. 2004; Sinclair 2005). Each of these parameters was examined and the results are shown in Table 1. No difference in food consumption was observed between EP1130 and w1118 in both young and old flies. As younger flies usually eat more than older flies, to ensure that the lack of change is not due to a limiting amount of radioisotope in the food, we used similar aged females as a control since they eat more than their male peers. Female flies show a much higher dose of radiation, indicating the radioisotope in the food is in excess. Likewise, no change in metabolic rate, as measured by carbon dioxide output, was found between EP1130 and w1118 (Table 1). Interestingly, a slight increase in fecundity was observed in the mutants relative to the wild-type (Table 1), a finding that has also been reported for Resveratrol (Wood et al., 2004). Since Resveratrol also improves the activity of high calorie diet-fed mice on a treadmill or rotarod test (Baur et al. 2006), we measured the climbing activity of EP1130 mutants and found that the mutants display enhanced climbing activity relative to w1118 (Table 1). These results show that like Resveratrol treatment, reduced mas1 activity provides DR-like benefits without some of the associated, undesirable tradeoffs.
Table 1.
Food uptake, fecundity, CO2 output, and climbing activity in EP1130
| Strain | Food uptake (CPM) | CO2 output (ppm/g) | Fecundity (egg number) | Climbing activity (PI) | |
|---|---|---|---|---|---|
| young | old | ||||
| w1118 ♂ | 4470±103 | 2734±108 | 286±7.1 | 215 | 25% |
| EP1130 ♂ | 4629±137 | 2843±63 | 291±8.9 | 243 | 50% |
| w1118 ♀ | 8022 | 5592 | |||
The young flies are 6 days old and the old flies are 20 days old.
Discussion
A number of previous studies have suggested that modulation of glycosylation via alpha-mannosidase can influence longevity. The expression of mas1 decreases with age in Drosophila (Zou et al. 2000) while in mammals, the activity of mannosidases is lower in the livers of both aging mice and humans (Cingle et al. 1996; Zhu et al. 2006). We have also detected lower expression levels of mas1A, the mouse CG32684 orthologue, in the livers of aged (28-month) mice relative to young (3-month) mice (data not shown). Direct studies aimed at examining the role of these genes have revealed that alpha-mannosidase-II over-expression results in extended lifespan in Drosophila (Landis et al. 2001). However, none of these studies provide a mechanism for the action of alpha-mannosidase nor do they address the robustness of the effects.
We provide compelling evidence that the down-regulation of a specific alpha mannosidase, mas1, can extend longevity in both Drosophila and C. elegans, suggesting that this is likely a conserved role for modulating longevity. Furthermore, we show that this effect is robust, observed in animals either heterozygous or homozygous for the mutation, as well as when down-regulation is achieved via RNA interference. These results suggest that beneficial effects of mas1 can be obtained over a wide range of gene activity. We have initiated studies aimed at replicating our results using pharmacological intervention. Using Mas1 inhibitors such as 1-deoxymannojirimycin and kifunensine on wild-type flies we have obtained promising results. In preliminary studies we have observed increases in lifespan of approximately 18% (data not shown); however due to high experimental variability, these results have failed to meet statistical significance. There are several possible explanations for these observations. First, the inhibitors may not be effective in the fly, or the conditions under which they were delivered were suboptimal. Alternatively, adverse side effects of the drugs may exist that mask the otherwise beneficial effects or the drugs target a wider range of molecules than we are genetically testing, since genetic or biochemical redundancy may exist for In mas1, since a null mutant can overcome its defect and synthesize the full range of N-linked oligosaccharides in Drosophila melanogaster (Roberts et al. 1998). Further studies using targeted RNAi may allow for the identification of specific tissues or life stages in which interventions are most effective. This work in combination with continued pharmacological studies may prove highly useful in identifying optimal intervention strategies.
Several lines of evidence suggest that the mechanism by which mas1 modulates longevity overlaps with those that regulate dietary restriction. First, the mutants obtained show greatly enhanced longevity under the abundant diet conditions relative to control flies and much less dramatic longevity phenotypes under DR, consistent with what would be expected if the mutants functioned within that pathway. In addition, it is known that the ERAD pathway interacts with many components of the DR pathway, and we have shown that a second gene in the ERAD pathway, Edem1, produces phenotypes indistinguishable from those of mas1 and fails to genetically interact with mas1 mutants. Likewise, modulation of Sir2 via Resveratrol administration can activate DR-induced pathways in a number of organisms (Howitz et al. 2003; Wood et al. 2004), and in the nematode resveratrol reduces the expression of worm mas1 (D2030.1) (Viswanathan et al. 2005), which we show increases longevity when down-regulated.
In addition, we find that the down-regulation of BiP/Grp78 expression, which occurs both in response to DR and to the resveratrol treatment (Heydari et al. 1995; Tillman et al. 1996; Dhahbi et al. 1997; Viswanathan et al. 2005) also occurs in response to reduced activity of mas1. BiP is a chaperone which maintains PERK and IRE1-alpha in an inactive state. BiP overexpression attenuates PERK and IRE1 activity and represses the unfolded protein response (UPR), whereas BiP downregulation activates the UPR (Bertolotti et al. 2000; Okamura et al. 2000). Therefore, a reduced BiP expression may trigger the ER stress response.
All of the mutants described in this work were shown to down-regulate the level of expression of BiP specifically without altering many other genes in the ER stress response pathway. These results suggest that BiP may be a particularly valuable biomarker for DR-related activity as mediated via the ERAD pathway. Dietary restriction protects against carcinogenesis in mammals. Recent study suggests that combination therapy with reduced BiP/Grp78 expression may be a novel approach to eradicate residual tumors (Lee 2007; Pyrko et al. 2007). Our discovery that reducing mas1 or Edem1 reduces BiP expression not only provides a new aspect of anti-aging study but also may thus offer a possible new target for cancer therapy.
Experimental procedures
Fly and worm strains
Independent EP-element insertion lines EP1130, EP1628, EP982, EP1588 (Rorth 1996), were all out-crossed with the control fly w1118 ten times, then re-established as homozygous lines. The location of the transposon in each line was confirmed by inverse PCR. To generate UAS-CG32684RNAi, the pWIZ vector was used to express the double-stranded RNA of a 220 base-pair sequence from CG32684 cDNA amplified by PCR primers MA220 FOR and MA220 REV in an inverted orientation cloned into pWIZ. The construct was verified by DNA sequencing before the micro-injection to generate the RNAi transgenic flies UAS-CG32684RNAi. A ubiquitous Gal4 driver, da-Gal4, was used to cross with UAS-CG32684RNAi to express double-stranded RNA. All flies were raised and maintained in standard fly food, unless special mention elsewhere, incubated at 25°C, 65% humidity, 12-hr day/night cycle incubator.
N2 worms were fed E. coli HT115 containing either the vector L4440 alone or the construct expressing double-stranded RNAi against D2030.1 (mas1 orthologue in worm), C52E4.5 or T03G11.4 (mas1 paralogues). The lifespan of RNAi-treated worms was measured as described in (Viswanathan et al. 2005).
Lifespan measurement of flies on regular and dietary restriction (DR) conditions and stress assays
Newly eclosed flies (4-days old) raised in standard food were collected by sex and maintained at a density of thirty per vial on the appropriate food, standard fly food (Caltech recipe by Professor Edward Lewis) for standard lifespan, and abundant food (agar 2%, yeast 15%, sucrose 15%) or the restricted food (agar 2%, yeast 5%, sucrose 5%) for DR experiments as described (Wood et al. 2004). The flies were maintained at 25°C/65% in a humidity controlled incubator, transferred to new food every three or four days until all were dead. At least three independent repeats were carried out for all experiments.
Young flies about 4-days old were collected at a density of 25 flies per vial for stress assays. For oxidative stress test, the flies were fed with 5mM paraquat in 5% sucrose water and counted dead fly number every four hours till all dead. For starvation, the young flies were kept in 1% agar vials and counted dead fly number every six hours till all flies were dead.
Fly character measurements
To measure food intake, 6-day- and 20-day-old control and EP1130 flies were separated by sex with 20 flies per vial and fed with the standard fly food containing 32P isotopes (7μl of 10μCi 32P-dCTP mixed in 50ml fly food) for 24 hours, and then collected in a vial to measure the radioactive emission values as described in (Brummel et al. 2004). Carbon dioxide output, an indicator of metabolism, is measured by using groups of ten flies in 2.2-ml glass vials in the TR-2 CO2 gas respirometer (Sable Systems International) and analyzed by DATACAN software (Walker & Benzer 2004). The measurement of fecundity was performed as described in (Wang et al. 2004). Ten virgin female flies were mated with ten males of the same genotype for 1 day in a vial, the mated females were collected to place in a cage to lay eggs on a grape-juice agar plate for 24 hours. A new grape-juice agar plate was replaced every 24 hours for four consecutive days. The cumulative egg number within the four days was calculated to check the fecundity. The fly climbing activity, negative geotaxis, was carried out by the countercurrent apparatus with a 15-second interval (Gargano et al. 2005). Approximately 50 6-day-old male flies were placed into a plastic test tube (17×100 mm, Falcon Cat#2057) fit into the countercurrent apparatus. After two-minute rest, the flies were mechanically agitated and tested for climbing activity. The performance index was calculated based on the ratio of flies climbing over the 100-mm-length tube within 15 seconds to the total number of flies.
RT-PCR and Real time PCR
Total RNA isolation and reverse transcription followed by polymerase chain reaction were described in (Wang et al. 2004). Complementary DNA for each sample were obtained by RT and then used in real time PCR by SYBR GREEN PCR Master Mix and quantified using an ABI PRISM 7500 sequence detector system (Applied Biosystem). The PCR was performed in triplicate reactions with 4 μl of 1/50 diluted cDNA and 2.5 μM of both primers in a final volume of 15 μl containing 7.5 μl of 2X SYBR GREEN PCR Master Mix. The average Ct value of each gene was calculated and normalized by the average Ct of the rp49 gene as an internal standard among different samples. The information of all primers is available upon request.
Supplementary Material
Acknowledgments
We thank Drs. Micheline Laurent and William Ja for the critical reading and editing of the manuscript. We thank Dr. Yi-Chun Wu for providing the materials for the worm work, and Dr. Ting-Fen Tsai for the liver tissues from the different aged mice. We are grateful for the suggestions from Drs. Jui-Chou Hsu and Tzu-Kang Sang on the manuscript. We thank Miss Yi-Yun Wang for the graphic assistance. The work is supported by the grants from National Science Counsel (NSC 94-2311-B-007-008, 94-2311-B-007-015, 95-2311-B-007-006, 96-2311-B-007-015) and in part by the APEX funding 97N2504E1 from BRC at National Tsing Hua University to H.-D. Wang and a grant from the National Institute of Aging (R15 AG027749) to T.J. Brummel.
References
- Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nature reviews. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Brummel T, Ching A, Seroude L, Simon AF, Benzer S. Drosophila lifespan enhancement by exogenous bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12974–12979. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubliy OA, Loeschcke V. Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. Journal of evolutionary biology. 2005;18:789–803. doi: 10.1111/j.1420-9101.2005.00928.x. [DOI] [PubMed] [Google Scholar]
- Cingle KA, Kalski RS, Bruner WE, O’Brien CM, Erhard P, Wyszynski RE. Age-related changes of glycosidases in human retinal pigment epithelium. Current eye research. 1996;15:433–438. doi: 10.3109/02713689608995834. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. The journals of gerontology. 2002;57:B109–114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Tillman JB, Walford RL, Spindler SR. Dietary energy tissue-specifically regulates endoplasmic reticulum chaperone gene expression in the liver of mice. J Nutr. 1997;127:1758–1764. doi: 10.1093/jn/127.9.1758. [DOI] [PubMed] [Google Scholar]
- Duronio V, Jacobs S, Romero PA, Herscovics A. Effects of inhibitors of N-linked oligosaccharide processing on the biosynthesis and function of insulin and insulin-like growth factor-I receptors. The Journal of biological chemistry. 1988;263:5436–5445. [PubMed] [Google Scholar]
- Elbein AD. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. Faseb J. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Experimental gerontology. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell metabolism. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Andrews LA, Parker TJ, Pallanck LJ. Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Human molecular genetics. 2005;14:799–811. doi: 10.1093/hmg/ddi074. [DOI] [PubMed] [Google Scholar]
- Herscovics A. Structure and function of Class I alpha 1,2-mannosidases involved in glycoprotein synthesis and endoplasmic reticulum quality control. Biochimie. 2001;83:757–762. doi: 10.1016/s0300-9084(01)01319-0. [DOI] [PubMed] [Google Scholar]
- Heydari AR, Conrad CC, Richardson A. Expression of heat shock genes in hepatocytes is affected by age and food restriction in rats. J Nutr. 1995;125:410–418. doi: 10.1093/jn/125.3.410. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Wada I, Hasegawa K, Yorihuzi T, Tremblay LO, Herscovics A, Nagata K. A novel ER alpha-mannosidase-like protein accelerates ER-associated degradation. EMBO Rep. 2001;2:415–422. doi: 10.1093/embo-reports/kve084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Landis G, Bhole D, Lu L, Tower J. High-frequency generation of conditional mutations affecting Drosophila melanogaster development and life span. Genetics. 2001;158:1167–1176. doi: 10.1093/genetics/158.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson B, Brewer JW, Hendershot LM. Geldanamycin, an hsp90/GRP94-binding drug, induces increased transcription of endoplasmic reticulum (ER) chaperones via the ER stress pathway. Journal of cellular physiology. 1998;174:170–178. doi: 10.1002/(SICI)1097-4652(199802)174:2<170::AID-JCP4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- Libert S, Pletcher SD. Modulation of longevity by environmental sensing. Cell. 2007;131:1231–1234. doi: 10.1016/j.cell.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science (New York, NY. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Lim HY, Bodmer R, Perrin L. Drosophila aging 2005/06. Experimental gerontology. 2006;41:1213–1216. doi: 10.1016/j.exger.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mechanisms of ageing and development. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing research reviews. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Kimata Y, Higashio H, Tsuru A, Kohno K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochemical and biophysical research communications. 2000;279:445–450. doi: 10.1006/bbrc.2000.3987. [DOI] [PubMed] [Google Scholar]
- Olivari S, Molinari M. Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett. 2007;581:3658–3664. doi: 10.1016/j.febslet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mechanisms of ageing and development. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing research reviews. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Roberts DB, Mulvany WJ, Dwek RA, Rudd PM. Mutant analysis reveals an alternative pathway for N-linked glycosylation in Drosophila melanogaster. European journal of biochemistry/FEBS. 1998;253:494–498. doi: 10.1046/j.1432-1327.1998.2530494.x. [DOI] [PubMed] [Google Scholar]
- Rorth P. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12418–12422. doi: 10.1073/pnas.93.22.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mechanisms of ageing and development. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Tatar M. Diet restriction in Drosophila melanogaster. Design and analysis. Interdiscip Top Gerontol. 2007;35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- Tillman JB, Mote PL, Dhahbi JM, Walford RL, Spindler SR. Dietary energy restriction in mice negatively regulates hepatic glucose-regulated protein 78 (GRP78) expression at the posttranscriptional level. J Nutr. 1996;126:416–423. doi: 10.1093/jn/126.2.416. [DOI] [PubMed] [Google Scholar]
- Urano F, Calfon M, Yoneda T, Yun C, Kiraly M, Clark SG, Ron D. A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol. 2002;158:639–646. doi: 10.1083/jcb.200203086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen CJ, Loeschcke V. Longevity and the stress response in Drosophila. Experimental gerontology. 2007;42:153–159. doi: 10.1016/j.exger.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Developmental cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Walker DW, Benzer S. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10290–10295. doi: 10.1073/pnas.0403767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Herman B. Ageing and apoptosis. Mechanisms of ageing and development. 2002;123:245–260. doi: 10.1016/s0047-6374(01)00349-9. [DOI] [PubMed] [Google Scholar]
- Zhu M, Lovell KL, Patterson JS, Saunders TL, Hughes ED, Friderici KH. Beta-mannosidosis mice: a model for the human lysosomal storage disease. Human molecular genetics. 2006;15:493–500. doi: 10.1093/hmg/ddi465. [DOI] [PubMed] [Google Scholar]
- Zou S, Meadows S, Sharp L, Jan LY, Jan YN. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13726–13731. doi: 10.1073/pnas.260496697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.