Abstract
Cardiac valve surgery is life saving for many patients. The advent of minimally invasive surgical techniques has historically allowed for improvement in both post-operative convalescence and important clinical outcomes. The development of minimally invasive cardiac valve repair and replacement surgery over the past decade is poised to revolutionize the care of cardiac valve patients. Here, we present a review of the history and current trends in minimally invasive aortic and mitral valve repair and replacement, including the development of sutureless bioprosthetic valves.
Keywords: Aortic Valve, Mitral Valve, Minimally Invasive, Sutureless Cardiac Valves
Introduction
Cardiac valve repair and replacement surgery is highly effective in improving symptoms and mortality. Traditionally these operations were performed through full sternotomy incisions, which require significant convalescence during the recovery for several weeks. Recently there is an increasing adoption in minimally invasive surgical techniques that allow correction of the valvular disease with less pain and easier recovery.
While the advent of catheter-based treatments developed in the 1970s changed the way we approach ischemic heart disease, we currently stand on the brink of a revolutionary time when less-invasive surgical and catheter-based techniques will likely change the way we approach valvular heart disease. While much recent attention has been rightfully given to the development of catheter-based valve repair and replacement, important progress has also been made in less-invasive surgical valve repair and valve replacement.
Here, we will discuss the history and current trends in minimally invasive aortic and mitral valve repair and replacement including novel technologies to make these approaches more feasible.
Minimally Invasive Aortic Valve Surgery
The classic technique for aortic valve replacement (AVR) involves a median sternotomy, with cannulation of the right atrium (either a single two-stage cannula or bicaval cannulation) and distal ascending aorta for cardiopulmonary bypass. Aortic valve replacement is performed by placing multiple interrupted sutures in the aortic valve annulus after the diseased valve is excised. Sutures are then placed through a prosthetic valve and then each suture is tied to secure the valve in position. Isolated AVR has become a relatively safe procedure with an estimated mortality of less than 3%.[1–4] However, while a full sternotomy provides excellent exposure to the heart, patients seeking less post-operative pain and quicker recovery have driven surgeons to develop less-invasive approaches.
Seeking to avoid a full sternotomy, Cosgrove and Sabik began performing AVRs through a right paramedian incision in the mid 1990’s.[5, 2, 3, 6] This technique was not widely adopted for multiple reasons including significant challenges in wound healing, challenges in exposure when unexpected bleeding was encountered, and added the potential for vascular complications secondary to femoral arterial cannulation.
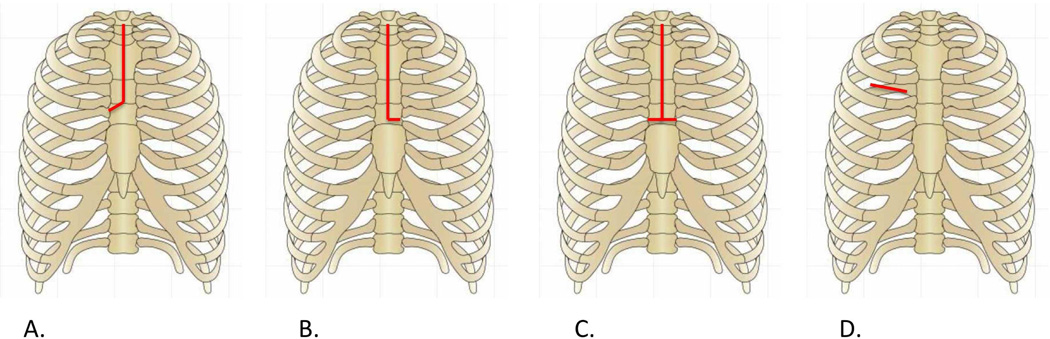
There are two common approaches to minimally invasive AVR today: partial sternotomy and right anterior thoracotomy. The traditional sternotomy extends from the sternal notch through the xiphoid process, while partial sternotomy starts at the sternal notch to the 3rd or 4th interspace and completed either to the patient’s right (J- sternal incision, Figure 1A), left (L-sternal incision, Figure 1B), or horizontally across the sternum (inverted T-sternal incision, Figure 1C) [3, 7–9]. For cardiopulmonary bypass, distal ascending aortic cannulation and femoral venous cannulation are utilized. Visualization is similar to a full sternotomy and this incision allows easy conversion to a complete incision should any challenges be encountered.
Figure 1.
Incisions for minimally invasive aortic valve replacement. J-hemisternotomy (A), L-hemisternotomy (B), inverted-T partial sternotomy (C), right anterior thoracotomy (D).
The right anterior thoracotomy approach preserves the entire sternum. A 3–6 cm right anterolateral thoracotomy is used for exposure (Figure 1D).[10] This technique has been gaining acceptance with potential disadvantages including challenging exposure particularly for suture placement, post-operative pain resulting from rib spreading, and difficulty in conversion to a full sternotomy if necessary.[11]
Any patient requiring an isolated AVR should be considered for a minimally invasive AVR. The most common reason a patient should not undergo a mini-AVR is the need for a concomitant procedure, including coronary artery bypass grafting (CABG) or Maze procedure for atrial fibrillation. In addition, patients with heavily calcified aortas, poor right ventricular function, or those with morbid obesity should be considered relative contraindications. Caution must be exercised when faced with a re-operative patient with previous bypass grafts to avoid injuring these during valve replacement.
Mini-Aortic Valve Replacement Procedure
Hemisternotomy
The skin is first incised from the sterno-manubrial junction 4–6 cm inferiorly. Following the creation of a J-sternotomy, a small Finochietto-type retractor is placed between the sternal edges. This provides excellent exposure for both the surgeon and assistant; a feature which lends itself nicely to the training of other surgeons (Figure 2A). Arterial cannulation of the aorta is achieved directly through the sternal incision, while a multi-stage venous cannula is placed from the femoral vein to the right atrium percutaneously using transesophageal echocardiographic (TEE) guidance. Suction-assisted drainage is employed to decrease the necessary size of the femoral cannula and ensure adequate venous drainage.
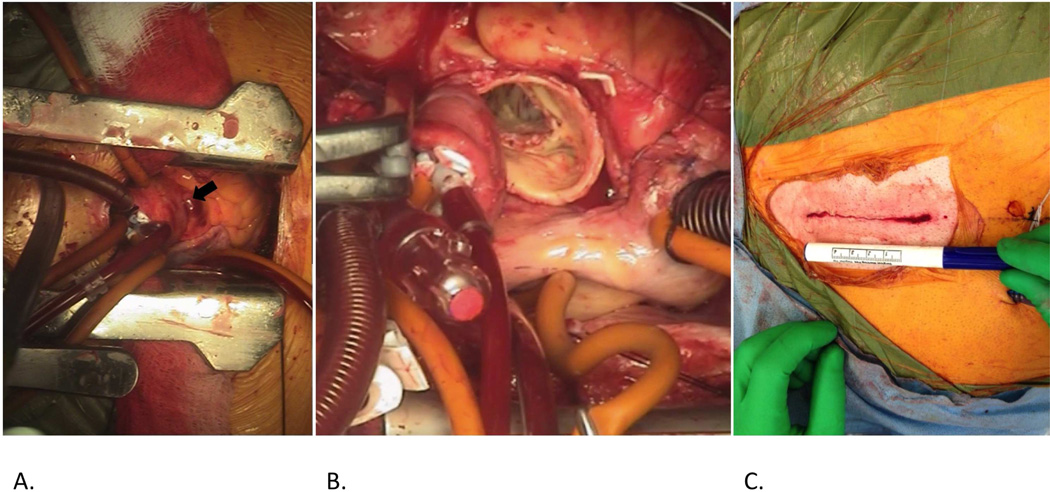
Figure 2.
Mini-AVR through a J-hemisternotomy revealing the exposed ascending aorta (A, arrow).Following cross-clamp placement, an aortotomy is made and the native valve is explanted (B). Skin closure following completion of the procedure (C). Ruler on the marking pen denotes 4 cm.
Once cardiopulmonary bypass (CPB) has been achieved, the aorta is directly cross-clamped and the heart can be arrested with the delivery of antegrade cardioplegia delivered directly and retrograde cardioplegia delivered through a catheter which is placed through the incision or percutaneously from the right internal jugular vein into the coronary sinus under TEE and fluoroscopic guidance.[12] The use of both antegrade and retrograde cardioplegia allows for optimal myocardial protection and a rapid arrest. Ice is placed on the heart if feasible and the field flooded with carbon dioxide gas to reduce the risk of air embolization.
The aorta is then opened usually transversely and the valve exposed. Using the aid of specialized long, low profile instruments, the valve can be excised (Figure 2B) and replaced. Sutures are placed as described above and extra care must be taken to assure proper seating of the new valve within the annulus. Improper seating of the valve leads to a paravalvular leak, which portends a worse long-term outcome.
The aorta is then closed, and the aortic cross clamp is removed restarting the heart. Ventricular fibrillation (VF) when transitioning off bypass can be of particular concern with minimally invasive approaches due to the inability to decompress the heart through the limited exposure. Should VF occur, the heart must be defibrillated with pediatric paddles or through transcutaneous patches. If these measures are unsuccessful, conversion to a full sternotomy may be necessary in order to protect the left ventricle from over-distention.
During weaning from cardiopulmonary bypass the heart is then de-aired through a small vent in the aortic root. The aorta is decannulated and the surgical field is inspected for hemostasis. Chest tubes are placed and the partial sternotomy is re-approximated with sternal wires and the incision closed (Figure 2C). Patients are typically able to be free from mechanical ventilation within 2–4 hours.
Right Anterior Thoracotomy
An alternative approach for minimally invasive AVR is through a right anterior thoracotomy. A 3–6 cm horizontal incision is made in the right second interspace just lateral to the costo-manubrial junction. Soft tissue is divided and a small Finochietto-type retractor is placed. The pericardium is then elevated and incised and pericardial retention sutures are placed. CPB is achieved by femoral venous and arterial cannulation through an inguinal incision. Once on full CBP support, the aorta is cross-clamped and the heart arrested with cardioplegia as described above. The aorta is transected and the native valve debrided. Annular sutures are then placed. Frequently, visualization of the annulus near the right coronary cusp is difficult with this exposure, and the annulus must be pulled into view in order to place these annular sutures. The annular sutures are then placed through the new aortic valve and the valve is seated into position. Ensuring proper seating of the valve is somewhat more difficult through this incision as the entire annulus cannot be visualized without placing traction on the annulus. The annular sutures are then tied and the aortotomy is closed. The aortic cross-clamp is removed and CPB is weaned. Chest tubes are placed and the incision is closed in several layers.
Due to the difficulty in placing the right coronary cusp annular sutures and in seating the valve, we prefer the hemisternotomy to the right anterior thoracotomy for AVR. In addition, the right anterior thoracotomy requires peripheral cannulation, which is not without vascular complications. In our experience, aortic cross clamp times are also longer with this technique as compared to the hemisternotomy approach discussed above.
Comparison to Standard AVR
These minimally invasive approaches allow for aortic valve replacement with either tissue or mechanical valves, while providing a quicker return to activity and overall greater patient satisfaction as compared to standard AVR. [1, 13, 14] In a study from 2010, 90 patients undergoing minimally invasive AVR were matched to patients undergoing full sternotomy AVR and had shorter hospital stay and mechanical ventilation time despite longer operative times with mini-AVR.[15]
Potential pitfalls to mini-AVR include difficulty ensuring adequate left ventricular drainage and difficulty defibrillating. In addition, the risk of post-operative tamponade appears to be greater with mini-AVR (0.6 % standard, 4.6% mini-AVR), likely as a result of incomplete pericardiotomy and difficulty in assuring complete hemostasis through a smaller incision.[15] Nonetheless, mini-AVR appears to reduce the requirement for transfusion, and provides at least equivalent hospital stay and improved patient satisfaction.[15, 1, 14] The implementation of sutureless aortic valves (see below) may likely equilibrate the disparity seen in CBP and cross-clamp times between traditional and mini-AVR.
Minimally Invasive Mitral Valve Surgery
Minimally invasive mitral surgery is often more complex than minimally invasive aortic valve surgery for several reasons. First, due to its anatomic position, even the traditional surgical exposure of the mitral valve is much more challenging than for the aortic valve. In addition, while most patients undergoing aortic valve surgery undergo replacement, patients with mitral pathology often undergo mitral repair, which can be complicated. As such, depending on the particular mitral pathology, surgeons must be prepared to perform several techniques through small incisions. The mitral valve may be approached through a lower hemisternotomy; however, in our experience, exposure can be challenging with this approach.
Our preference is to utilize a small right lateral thoracotomy combined with the use of a thoracoscope to aid in visualization.[16, 17, 2, 18–20] This “port-access” approach allows for excellent visualization without significant alteration of the natural position of the mitral annulus, which can alter apparent coaptation and therefore the quality of repair. This approach has required several biomedical engineering innovations. One of the largest challenges in application of the port access technique is the ability to safely cross-clamp the aorta to arrest the heart through a right sided incision. Femoral arterial and venous cannulation can remove the need for central cannulation; however, development of a novel methods to cross-clamp the aorta proved somewhat challenging. Early in the development of minimally invasive mitral techniques, many surgeons turned to a large balloon-tipped catheter that could be guided by TEE into the distal ascending aorta (Endoaortic clamp, Heartport inc, Redwood City, CA). Early reports of balloon displacement and aortic rupture led many to seek alternative means to cross-clamp the aorta; however, newer versions of this device have addressed some of these concerns and are currently being used safely in experienced hands.[21–23]
The development of an intra-thoracic cross-clamp (Chitwood or Mohr clamp) allows for direct aortic clamping through a small stab incision.[17] Many other specialized long, low profile instruments have also been required to allow for facile exposure and manipulation of the mitral valve through the chest. In addition, as this requires peripheral cannulation, all patients undergo preoperative CT scans through the femoral vessels to allow for the development of an adequate cannulation strategy prior to the OR.
The challenge of tying down intracorporially placed sutures securely through minimally invasive incisions has also been recently aided by new device development. The Cor-Knot device (LSI Solutions, Victor, NY) allows for secure valve fixation without the difficulty of placing a finger deep in a small incision or the variability of using a laparoscopic knot-pusher. This device uses a titanium clip to secure sutures without the time or space required to seat a standard hand-tied knot.
Port-Access Mitral Valve Repair Technique
A double lumen endotracheal tube is placed (or bronchial blocker) along with transesophageal echocardiography. The procedure begins with the patient supine with a bump under the right side and both arms tucked. A 3–6 cm incision is made overlying the 4th intercostal space in the inframamary fold lateral to the right nipple and pectoralis major muscle. The right lung is deflated and the right chest is entered. Soft tissue retractors are placed in the thoracotomy to compress the soft tissue. A small chest wall retractor is then placed in the incision (Figure 3A). A standard VATS port is then placed in the mid-axillary line in the 5th intercostal space to provide a thoracoscopic view of the operative field. The right phrenic nerve is then identified and the pericardium is incised sharply well anterior to the nerve. Pericardial tacking sutures are then placed and brought out through the chest wall.
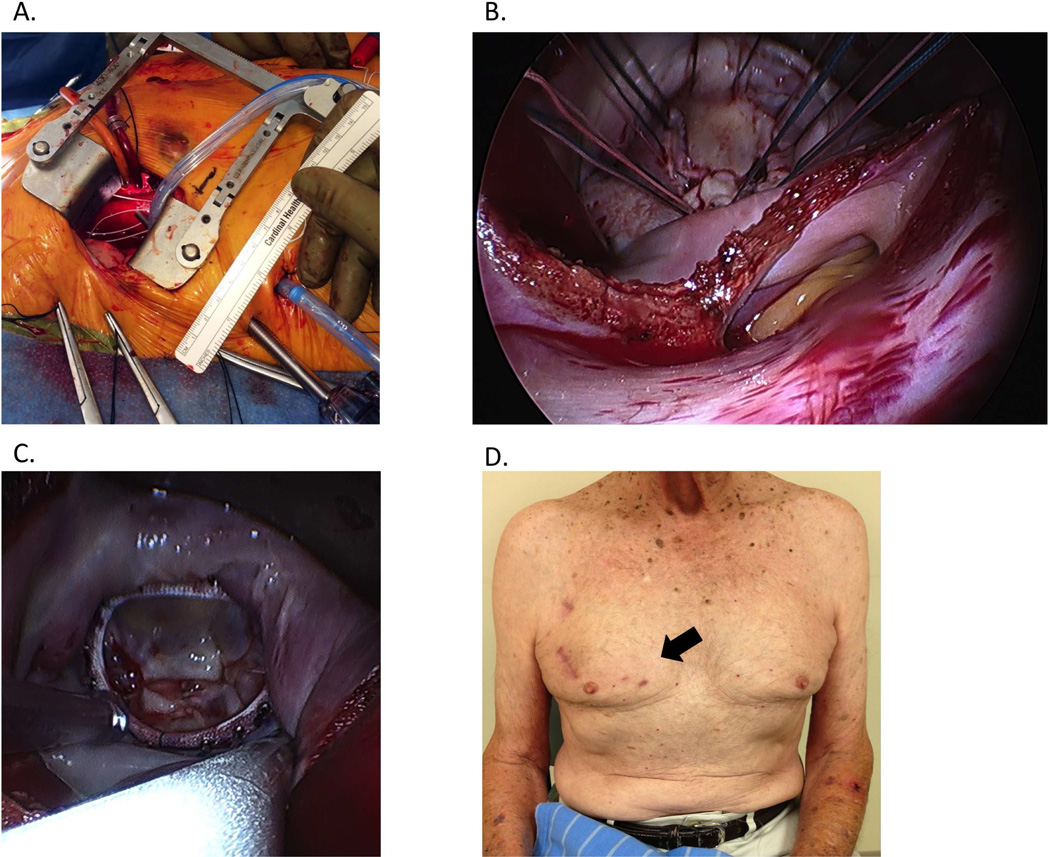
Figure 3.
Right 3rd intercostal anterior mini-thoracotomy for “port-access” mitral valve repair. View through the mini-thoracotomy (A) is greatly augmented by a laterally placed thoracoscope. Sutures are placed circumferentially (B) to secure a semi-rigid annuloplasty ring (C). Incision is seen 6 weeks postoperatively (arrow, D).
The femoral vessels are exposed through a small inguinal incision. The arterial cannula is then placed through a purse string suture over a guide-wire and into position in the iliac artery. A multi-stage venous cannula is then placed such that it traverses the right atrium in order to drain both the superior and inferior vena cava. Once adequate cardiopulmonary bypass has been established, a purse string suture is placed in the proximal ascending aorta and a specialized, long aortic vent is placed. The cross-clamp is inserted through a small stab incision in the 3rd intercostal space and guided into position on the ascending aorta. The clamp is carefully applied with special attention to the position of the tips of the clamp to ensure full cross clamping and the heart is arrested with antegrade cardioplegia. Additional myocardial protection can be achieved through retrograde cardioplegia through the coronary sinus as described above. A left atriotomy is then made and specialized atrial retractors are then placed through the thoracotomy incision.
The mitral valve leaflets and subvalvular apparatus can then be carefully examined with the aid of the thoracoscope (Figure 3B). With this technique, leaflet resections can be accomplished, as well as placement of neo-chordae if necessary. Ring annuloplasty can also easily be accomplished. Should replacement of the mitral valve be warranted, this can also be accomplished through the port access approach.
Following valve repair, the left ventricle is saline loaded to test the adequacy of repair (Figure 3C). The atrium is then closed with running suture. Prior to trying these sutures, the heart is filled and the lungs inflated to de-air the heart. The aortic cross clamp is removed. Once CBP has been weaned, the competency of the valve is confirmed by TEE. The heart is then further de-aired and the aortic root vent removed. Peripheral cannulae are then removed and the skin is closed in several layers. Chest tubes are placed and the thoracotomy and VATS ports are then closed in the standard fashion. The cosmetic result is typically excellent post-operatively (Figure 3D).
Port-Access vs. Traditional Mitral Repair/Replacement
Overall, surgeon and center experience with minimally invasive mitral techniques is quite variable. Overall, approximately 20% of isolated mitral valve operations in 2009 were performed minimally invasive; however, the median number by center was only 3, suggesting that the majority of these procedures were done at high-volume centers.[24, 25] A 2008 comparison of 2827 patients undergoing isolated mitral surgery with either full sternotomy or minimally invasive techniques demonstrated no difference in the rates of stroke or mortality, fewer re-operations for bleeding (OR 0.56, p=0.02) and a trend toward shorter length of stay (OR 0.73 days, p=0.7) in the minimally invasive group.[26] As with aortic valve surgery, minimally invasive mitral surgery requires longer times for CBP and aortic cross-clamp compared to sternotomy approaches.[26] Importantly, this increase has not been demonstrated to negatively affect either stroke or mortality in these patients.
The perception of reduced post-operative pain is a common reason for patients to seek out less invasive surgical options. Minimally invasive techniques are, however, not free from pain and theoretically may be more painful than traditional techniques given the need to spread the ribs. The reduction in post-operative pain does, however, appear to be significantly less in patients undergoing minimally invasive operations. [1, 27, 26, 28, 29] Additionally, several studies have demonstrated a more rapid return to baseline activity levels.[1, 27, 26]
Post-operative atrial fibrillation is known to increase both hospital length of stay as well as morbidity.[30–33, 26] As minimally invasive valve replacement became more popular, there was consideration that with less dissection and manipulation, these techniques may have a lower risk of post-operative atrial fibrillation. While some early studies showed no difference in rates of post-operative afiib, several more recent studies do indicate a lower than expected rate of afib after port access mitral valve surgery.[34] Additionally, the early results of currently enrolled studies appear to indicate a lower rate of blood product transfusion in those patients undergoing minimally invasive mitral valve surgery.
Robotic Valve Surgery
Over the last 5 years, there has been an increase in the number of surgeons utilizing robotic (Da Vinci, Intuitive) methods to perform mitral valve surgery. While this is currently limited to high volume centers with great experience, this approach allows for excellent visualization of the mitral valve. The use of the robot however adds several layers of complexity and risk factors including the potential for exceedingly long operative times.
Sutureless Valves Replacement
The development of catheter-based aortic valve replacement (TAVR) has renewed a longstanding interest in the development of an artificial valve that does not require sutures to hold it in place. As early as 1962, Dr. George Magovern developed a novel sutureless heart valve both in the aortic and the mitral position. Manufactured from titanium and using a ball-in-cage design and small curved pins to hold the valve in place, the Magovern-Cromie valve sought to reduce the need for excessive CBP times which plagued early attempts at valve replacement.[35] While Magovern continued to implant his sutureless prosthesis through the early 1980’s, the use of this sutureless technology was held back, in part, by fear of paravalvular leak and valve embolization.[36, 37] Despite never gaining wide-spread popularity, reports of well-functioning Magovern valves up to 42 years following initial sutureless placement exist.[38]
As open valve replacement techniques improved, CPB times improved and the impetus to develop a sutureless prosthesis dissipated until the advent of catheter-based systems of aortic valve delivery. Manufactures used what had been learned about self-expanding and balloon expanding aortic stent grafts to apply radial force against the aortic wall and hold the valve in place. Unlike catheter-based valve replacement, a sutureless but minimally invasive surgical technique would allow for complete debridement of calcific debris, which may contribute to paravalvular leak and stroke risk in catheter-based valves.[39] As stated previously, both minimally invasive aortic and mitral valve replacement require longer CBP and cross-clamp times as compared to traditional valve replacement. The application of new sutureless prostheses in this setting may obviate that disparity and minimize the technical challenge of securely tying down the annular sutures that hold a traditional valve in place through a smaller incision. The development of this technology may also allow for new minimally invasive approaches and may allow for minimally invasive, multi-valve surgeries.
The first new-generation sutureless aortic valve was the 3F Enable aortic bioprosthesis (Medtronic, Minneapolis, MI). The valve is held in place by a balloon-expandable Nitinol frame. Studies examining the short-term function of the 3f aortic valve reported a mean systolic gradient of 8.62 mmHg and mean effective orifice area of 1.67 mm at one year.[40, 41] Of the 140 patients in early clinical application of the 3f valve, there were 3 major paravalvular leaks requiring valve explantation (2.1%) and a single embolic event (0.7%).[42] At the conclusion of this study, there were no reported cases of valve deterioration, significant hemolysis or thrombosis in 121.8 patient-years.[42]
The Perceval S sutureless aortic valve (Sorin, Saluggia, Italy) is a self-expanding, trileaflet bovine pericardial valve that features an inflow ring designed to sit at the level of the annulus and an outflow ring which sits at the sinotubular junction (Figure 4). Outward bowing struts connect these two rings and occupy the sinuses of Valsalva. The valve is the deployed, and the aortotomy closed. In a multi-center European trial, the mean CBP time of 59 +/− 21 minutes and mean aortic cross clamp time of 34 +/− 15 minutes: significantly less than expected for traditional aortic valve replacement.[43] At follow-up, the mean post-operative gradient was 10.4 mm Hg with mean effective orifice area of 1.4 cm2.[44] No patients in this study had more the mild paravavular leak.[45, 43]
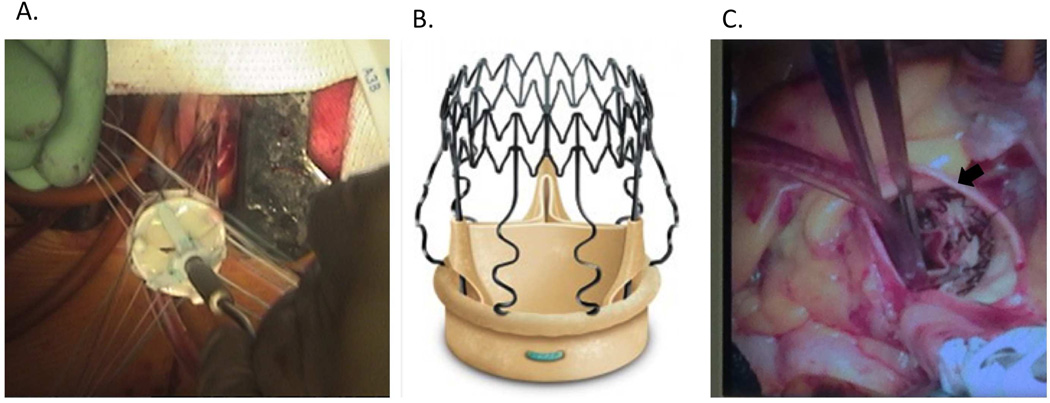
Figure 4.
Traditional sutured prosthetic aortic valve placement through a minimally invasive J-hemisternotomy (A). Percival S self-expanding aortic tissue valve with Sinus of Valsalva struts (B, from www.sorin.com/product/perceval-s). Perceval S valve placed in the aortic position (C, arrow).
The INTUITY Valve System (Edwards Lifesciences, Irvine, CA) is a trileaflet, bovine pericardial valve that is based on the Magna Ease aortic valve prosthesis. It is held in place by a single, cloth-covered stainless steel frame, which is balloon expandable and sits at the inflow aspect of the valve. Following native valve removal, the INTUITY valve is guided into its proper supra-annular position by 3–4 guiding sutures that are tied down. The stent portion of the valve is expanded with a balloon. The TRITON trial examined 152 patients undergoing placement of the INTUITY valve and demonstrated a 96.1% technical success rate.[46] Average CPB and cross-clamp times were 76 and 41 minutes, respectively for isolated AVR and mean gradient was 8.4 +/− 3.4 mm Hg and mean effective orifice area was 1.7 +/− 0.2 cm2 at one year.[46] Four early (2.7%) and two late (1.9%) thromboembolic complications were reported. Three paravalvular leaks were observed and remained stable at 1-year follow up.
While sutureless aortic valve technology has led the way, several sutureless mitral valve options are currently under development. The CardiaAQ Valve (CardiaAQ Valve Technologies, Irvine, CA) is a sutureless, self-expandable tissue valve that can be placed either with or without full CPB support.[47] Currently, very high-risk patients requiring mitral valve repair may be treated with catheter-based valve repair with a MitraClip (Abbott Vascular, Menlo Park, CA) device. In the future, transcatheter mitral valve replacement may also be feasible in these patients. While transcatheter mitral valve replacement is still in its infancy, the application of what has been learned in the development of transcatheter aortic valves will certainly speed the development and application of such technology.
Future Developments in Minimally Invasive Valve Technology
Minimally invasive valve surgery is a burgeoning field. As our experience with minimally invasive techniques grows, more technically complex and challenging patients will likely be offered a minimally invasive approach. The adoption of sutureless valves may play a key role in shortening minimally invasive CPB times to allow for multi-valve procedures. Additionally, several centers have begun using robotic surgical systems to add 3-dimensional visual enhancement and improved dexterity during minimally invasive valve replacement. Translational research conducted in the laboratory has and will continue to prove invaluable to these efforts toward continued improvement and development of new minimally invasive technologies.
Conclusions
Minimally invasive surgical approaches to aortic and mitral valve repair and replacement offer reduced post-operative pain, a faster return to baseline activity and at least equivalent morbidity and mortality as traditional valve operations. In addition, surgical techniques allow for the removal of existing valve tissue and debris, which may improve paravalvular leak rates. While minimally invasive aortic and mitral valve replacement currently require longer CBP and aortic cross-clamp times, the development of sutureless aortic and mitral valves may reduce these times and further improve outcomes. Minimally invasive techniques should be considered in many patients requiring isolated valve repair or replacement.
References
- 1.Cohn LH, Adams DH, Couper GS, Bichell DP, Rosborough DM, Sears SP, Aranki SF. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Annals of surgery. 1997;226(4):421–426. doi: 10.1097/00000658-199710000-00003. discussion 427–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosgrove DM, 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. The Annals of thoracic surgery. 1998;65(6):1535–1538. doi: 10.1016/s0003-4975(98)00300-2. discussion 1538–1539. [DOI] [PubMed] [Google Scholar]
- 3.Johnston WF, Ailawadi G. Surgical management of minimally invasive aortic valve operations. Seminars in cardiothoracic and vascular anesthesia. 2012;16(1):41–51. doi: 10.1177/1089253211431647. [DOI] [PubMed] [Google Scholar]
- 4.LaPar DJ, Ailawadi G, Bhamidipati CM, Stukenborg G, Crosby IK, Kern JA, Kron IL. Small prosthesis size in aortic valve replacement does not affect mortality. The Annals of thoracic surgery. 2011;92(3):880–888. doi: 10.1016/j.athoracsur.2011.04.105. discussion 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosgrove DM, 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. The Annals of thoracic surgery. 1996;62(2):596–597. [PubMed] [Google Scholar]
- 6.Navia JL, Cosgrove DM., 3rd Minimally invasive mitral valve operations. The Annals of thoracic surgery. 1996;62(5):1542–1544. doi: 10.1016/0003-4975(96)00779-5. [DOI] [PubMed] [Google Scholar]
- 7.Svensson LG. Minimal-access "J" or "j" sternotomy for valvular, aortic, and coronary operations or reoperations. The Annals of thoracic surgery. 1997;64(5):1501–1503. doi: 10.1016/S0003-4975(97)00927-2. [DOI] [PubMed] [Google Scholar]
- 8.Tam RK, Almeida AA. Minimally invasive aortic valve replacement via partial sternotomy. The Annals of thoracic surgery. 1998;65(1):275–276. doi: 10.1016/s0003-4975(97)01204-6. [DOI] [PubMed] [Google Scholar]
- 9.Woo YJ. Minimally invasive valve surgery. The Surgical clinics of North America. 2009;89(4):923–949. x. doi: 10.1016/j.suc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Benetti FJ, Mariani MA, Rizzardi JL, Benetti I. Minimally invasive aortic valve replacement. The Journal of thoracic and cardiovascular surgery. 1997;113(4):806–807. doi: 10.1016/S0022-5223(97)70246-0. [DOI] [PubMed] [Google Scholar]
- 11.Sharony R, Grossi EA, Saunders PC, Schwartz CF, Ribakove GH, Culliford AT, Ursomanno P, Baumann FG, Galloway AC, Colvin SB. Minimally invasive aortic valve surgery in the elderly: a case-control study. Circulation. 2003;108(Suppl 1):II43–II47. doi: 10.1161/01.cir.0000087446.53440.a3. [DOI] [PubMed] [Google Scholar]
- 12.Menasche P, Kural S, Fauchet M, Lavergne A, Commin P, Bercot M, Touchot B, Georgiopoulos G, Piwnica A. Retrograde coronary sinus perfusion: a safe alternative for ensuring cardioplegic delivery in aortic valve surgery. The Annals of thoracic surgery. 1982;34(6):647–658. doi: 10.1016/s0003-4975(10)60904-6. [DOI] [PubMed] [Google Scholar]
- 13.Estrera AL, Reardon MJ. Current approaches to minimally invasive aortic valve surgery. Current opinion in cardiology. 2000;15(2):91–95. doi: 10.1097/00001573-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mihaljevic T, Cohn LH, Unic D, Aranki SF, Couper GS, Byrne JG. One thousand minimally invasive valve operations: early and late results. Annals of surgery. 2004;240(3):529–534. doi: 10.1097/01.sla.0000137141.55267.47. discussion 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinkman WT, Hoffman W, Dewey TM, Culica D, Prince SL, Herbert MA, Mack MJ, Ryan WH. Aortic valve replacement surgery: comparison of outcomes in matched sternotomy and PORT ACCESS groups. The Annals of thoracic surgery. 2010;90(1):131–135. doi: 10.1016/j.athoracsur.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 16.Chitwood WR, Jr, Elbeery JR, Moran JF. Minimally invasive mitral valve repair using transthoracic aortic occlusion. The Annals of thoracic surgery. 1997;63(5):1477–1479. doi: 10.1016/s0003-4975(97)00242-7. [DOI] [PubMed] [Google Scholar]
- 17.Chitwood WR, Jr, Wixon CL, Elbeery JR, Moran JF, Chapman WH, Lust RM. Video-assisted minimally invasive mitral valve surgery. The Journal of thoracic and cardiovascular surgery. 1997;114(5):773–780. doi: 10.1016/S0022-5223(97)70081-3. discussion 780–772. [DOI] [PubMed] [Google Scholar]
- 18.Falk V, Walther T, Diegeler A, Wendler R, Autschbach R, van Son JA, Siegel LC, Pompilli MF, Mohr FW. Echocardiographic monitoring of minimally invasive mitral valve surgery using an endoaortic clamp. The Journal of heart valve disease. 1996;5(6):630–637. [PubMed] [Google Scholar]
- 19.Stevens JH, Burdon TA, Peters WS, Siegel LC, Pompili MF, Vierra MA, St Goar FG, Ribakove GH, Mitchell RS, Reitz BA. Port-access coronary artery bypass grafting: a proposed surgical method. The Journal of thoracic and cardiovascular surgery. 1996;111(3):567–573. doi: 10.1016/s0022-5223(96)70308-2. [DOI] [PubMed] [Google Scholar]
- 20.Stevens JH, Burdon TA, Siegel LC, Peters WS, Pompili MF, St Goar FG, Berry GJ, Ribakove GH, Vierra MA, Mitchell RS, Toomasian JM, Reitz BA. Port-access coronary artery bypass with cardioplegic arrest: acute and chronic canine studies. The Annals of thoracic surgery. 1996;62(2):435–440. discussion 441. [PubMed] [Google Scholar]
- 21.Hesselvik JF, Ortega RA, Treanor P, Shemin RJ. Intraoperative rupture of the endoaortic clamp balloon in a patient undergoing port-access mitral valve repair. Journal of cardiothoracic and vascular anesthesia. 1999;13(4):462–465. doi: 10.1016/s1053-0770(99)90222-7. [DOI] [PubMed] [Google Scholar]
- 22.Ius F, Mazzaro E, Tursi V, Guzzi G, Spagna E, Vetrugno L, Bassi F, Livi U. Clinical results of minimally invasive mitral valve surgery: endoaortic clamp versus external aortic clamp techniques. Innovations (Phila) 2009;4(6):311–318. doi: 10.1097/IMI.0b013e3181c490e5. [DOI] [PubMed] [Google Scholar]
- 23.Schneider F, Falk V, Walther T, Mohr FW. Control of endoaortic clamp position during Port-Access mitral valve operations using transcranial Doppler echography. The Annals of thoracic surgery. 1998;65(5):1481–1482. doi: 10.1016/s0003-4975(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 24.Bolling SF, Li S, O'Brien SM, Brennan JM, Prager RL, Gammie JS. Predictors of mitral valve repair: clinical and surgeon factors. The Annals of thoracic surgery. 2010;90(6):1904–1911. doi: 10.1016/j.athoracsur.2010.07.062. discussion 1912. [DOI] [PubMed] [Google Scholar]
- 25.Gammie JS, Zhao Y, Peterson ED, O'Brien SM, Rankin JS, Griffith BP. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. The Annals of thoracic surgery. 2010;90(5):1401–1408. 1410, e1401. doi: 10.1016/j.athoracsur.2010.05.055. discussion 1408–1410. [DOI] [PubMed] [Google Scholar]
- 26.Modi P, Hassan A, Chitwood WR., Jr Minimally invasive mitral valve surgery: a systematic review and meta-analysis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2008;34(5):943–952. doi: 10.1016/j.ejcts.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 27.Glower DD, Landolfo KP, Clements F, Debruijn NP, Stafford-Smith M, Smith PK, Duhaylongsod F. Mitral valve operation via Port Access versus median sternotomy. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1998;14(Suppl 1):S143–S147. doi: 10.1016/s1010-7940(98)00123-7. [DOI] [PubMed] [Google Scholar]
- 28.Walther T, Falk V, Metz S, Diegeler A, Battellini R, Autschbach R, Mohr FW. Pain and quality of life after minimally invasive versus conventional cardiac surgery. The Annals of thoracic surgery. 1999;67(6):1643–1647. doi: 10.1016/s0003-4975(99)00284-2. [DOI] [PubMed] [Google Scholar]
- 29.Yamada T, Ochiai R, Takeda J, Shin H, Yozu R. Comparison of early postoperative quality of life in minimally invasive versus conventional valve surgery. Journal of anesthesia. 2003;17(3):171–176. doi: 10.1007/s00540-003-0176-6. [DOI] [PubMed] [Google Scholar]
- 30.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, Tarazi R, Shroyer AL, Sethi GK, Grover FL, Hammermeister KE. Atrial fibrillation after cardiac surgery: a major morbid event? Annals of surgery. 1997;226(4):501–511. doi: 10.1097/00000658-199710000-00011. discussion 511–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Postoperative atrial fibrillation independently predicts prolongation of hospital stay after cardiac surgery. The Journal of cardiovascular surgery. 2005;46(6):583–588. [PubMed] [Google Scholar]
- 32.Jung W, Meyerfeldt U, Birkemeyer R. Atrial arrhythmias after cardiac surgery in patients with diabetes mellitus. Clinical research in cardiology : official journal of the German Cardiac Society. 2006;95(Suppl 1):i88–i97. doi: 10.1007/s00392-006-1120-1. [DOI] [PubMed] [Google Scholar]
- 33.Kim MH, Eagle KA. Postoperative atrial fibrillation after heart surgery: What are the goals of prevention? American heart journal. 2001;141(5):691–693. doi: 10.1067/mhj.2001.114969. [DOI] [PubMed] [Google Scholar]
- 34.Glower DD, Siegel LC, Frischmeyer KJ, Galloway AC, Ribakove GH, Grossi EA, Robinson NB, Ryan WH, Colvin SB. Predictors of outcome in a multicenter port-access valve registry. The Annals of thoracic surgery. 2000;70(3):1054–1059. doi: 10.1016/s0003-4975(00)01748-3. [DOI] [PubMed] [Google Scholar]
- 35.Magovern GJ, Cromie HW. Sutureless Prosthetic Heart Valves. The Journal of thoracic and cardiovascular surgery. 1963;46:726–736. [PubMed] [Google Scholar]
- 36.Gott VL, Alejo DE, Cameron DE. Mechanical heart valves: 50 years of evolution. The Annals of thoracic surgery. 2003;76(6):S2230–S2239. doi: 10.1016/j.athoracsur.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Magovern GJ, Liebler GA, Park SB, Burkholder JA, Sakert T, Simpson KA. Twenty-five-year review of the Magovern-Cromie sutureless aortic valve. The Annals of thoracic surgery. 1989;48(3 Suppl):S33–S34. doi: 10.1016/0003-4975(89)90629-2. [DOI] [PubMed] [Google Scholar]
- 38.Zlotnick AY, Shiran A, Lewis BS, Aravot D. Images in cardiovascular medicine. A perfectly functioning Magovern-Cromie sutureless prosthetic aortic valve 42 years after implantation. Circulation. 2008;117(1):e1–e2. doi: 10.1161/CIRCULATIONAHA.107.699991. [DOI] [PubMed] [Google Scholar]
- 39.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. The New England journal of medicine. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 40.Cox JL, Ad N, Myers K, Gharib M, Quijano RC. Tubular heart valves: a new tissue prosthesis design--preclinical evaluation of the 3F aortic bioprosthesis. The Journal of thoracic and cardiovascular surgery. 2005;130(2):520–527. doi: 10.1016/j.jtcvs.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 41.Leyh R, Yildirim C, Buck T, Sommer S, Herold U, Jakob H. Early single-center experience with the 3F-enable aortic valve bioprosthesis. Herz. 2006;31(5):423–428. doi: 10.1007/s00059-006-2837-3. [DOI] [PubMed] [Google Scholar]
- 42.Martens S, Sadowski J, Eckstein FS, Bartus K, Kapelak B, Sievers HH, Schlensak C, Carrel T. Clinical experience with the ATS 3f Enable(R) Sutureless Bioprosthesis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011;40(3):749–755. doi: 10.1016/j.ejcts.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 43.Shrestha M, Folliguet T, Meuris B, Dibie A, Bara C, Herregods MC, Khaladj N, Hagl C, Flameng W, Laborde F, Haverich A. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. The Journal of heart valve disease. 2009;18(6):698–702. [PubMed] [Google Scholar]
- 44.Folliguet TA, Laborde F, Zannis K, Ghorayeb G, Haverich A, Shrestha M. Sutureless perceval aortic valve replacement: results of two European centers. The Annals of thoracic surgery. 2012;93(5):1483–1488. doi: 10.1016/j.athoracsur.2012.01.071. [DOI] [PubMed] [Google Scholar]
- 45.Flameng W, Herregods MC, Hermans H, Van der Mieren G, Vercalsteren M, Poortmans G, Van Hemelrijck J, Meuris B. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. The Journal of thoracic and cardiovascular surgery. 2011;142(6):1453–1457. doi: 10.1016/j.jtcvs.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Kocher AA, Laufer G, Haverich A, Shrestha M, Walther T, Misfeld M, Kempfert J, Gillam L, Schmitz C, Wahlers TC, Wippermann J, Mohr FW, Roth M, Skwara A, Rahmanian P, Wiedemann D, Borger MA. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. The Journal of thoracic and cardiovascular surgery. 2013;145(1):110–115. doi: 10.1016/j.jtcvs.2012.07.108. discussion 115–116. [DOI] [PubMed] [Google Scholar]
- 47.Quadri AIA, Ratz BJ, Liao Y, Bavaria J. Self-anchoring, suture-less mitral valve replacement. Paper presented at the American Association for Thoracic Surgery Mitral Conclave; May 2, 2013; New York, NY. 2013. [Google Scholar]