Abstract
Several previous studies in mammalian systems have shown sexually dimorphic behaviors, neuroendocrine changes, and alterations in neurotransmitter release in response to stress. Additionally, men and women are differentially vulnerable to stress-related pathologies, which have led to the hypothesis that the stress response circuitry differs depending on sex. We used the genetic tractability of Drosophila to manipulate pre- or post-synaptic dopamine signaling in transgenic animals, which were assayed for several parameters of locomotion and heart rate following exposure to two environmental stressors, starvation and oxidative stress. Our results show significant differences in the stress response for males and females by analyzing heart rate, centering time, and high mobility in addition to other locomotor parameters with translational relevance. These data demonstrate that both pre- and post-synaptic neurons are differentially recruited into the dopaminergic stress response circuitry for males and females. Our results also show that the response circuits differ depending on the stressor and behavioral output. Furthermore, our studies provide a translatable Drosophila model for further elucidation of factors involved in the sexually dimorphic recruitment of neurons into the stress response circuitry.
Keywords: behavior, starvation, paraquat, locomotion, heart rate, oxidative stress
Introduction
Many studies have suggested that sex plays a role in individual vulnerability for development of affective disorders as a result of stressful experiences (reviewed in Oldehinkel and Bouma, 2011 and Figueira and Ouakinin, 2010). In addition, several of the health consequences resulting from environmental stressors display significant sexual dimorphism (Dedovic et al., 2009). Previous work in our lab has shown that mutant Drosophila with defects in specific areas of the brain known to be important for higher order functions responded differently to starvation and oxidative stress depending upon the sex of the population (Neckameyer and Matsuo, 2008). Furthermore, studies assessing the role of dopamine in the stress response showed that tyrosine hydroxylase (the rate limiting enzyme for dopamine synthesis) levels are altered in a sexually dimorphic manner, and that animals with decreased dopamine synthesis displayed altered locomotor and heart rate responses to starvation and oxidative stress compared to controls (Neckameyer and Weinstein, 2005).
To conclusively demonstrate that dopamine neurons are differentially recruited into the stress response circuitry for males and females, we utilized transgenic Drosophila with targeted decreases in dopamine synthesis either within subsets of dopamine neurons, or with decreased expression of individual dopamine receptors, to determine whether males and females differed in their behavioral output to starvation or oxidative stress. Starvation stress has been correlated with an increased incidence of depression and schizophrenia (Roseboom et al., 2001) in humans, and oxidative stress has been linked to schizophrenia and bipolar disorder (Marazziti et al., 2012). Additionally, both starvation and oxidative stress have been shown to alter tyrosine hydroxylase activity in Drosophila (Neckameyer and Weinstein, 2005) and rats (Philipp and Pirke, 1987; Kuter et al., 2007). Heart rate and locomotor parameters were assessed as the physiological outputs, since previous studies have shown that depression has a significant and negative impact on cardiac health (Plante, 2005), and basal locomotor activity and exploration are affected by stress in numerous animal models as well as in patients suffering from mental illness (Willner, 2005). Importantly, both heart rate and locomotion have been shown to be centrally regulated in Drosophila (Dulcis and Levin, 2003 and Strauss and Heisenberg, 1993, respectively) and altered in response to stress (Neckameyer and Weinstein, 2005, Neckameyer and Matsuo, 2008).
We hypothesized that if the stress response circuitry for a given stressor and output is composed of distinct subsets of dopamine neurons in males and females, then targeted manipulations of dopamine signaling in these neuronal populations should differentially alter locomotor and heart rate responses to starvation and oxidative stress. The resulting data show that the behavioral responses to starvation and oxidative stress in both sexually immature and sexually mature individuals are dependent on the population of dopamine neurons in the response circuit. Thus, Drosophila can serve as a model in which to elucidate factors involved in the modulation of a sexually dimorphic stress response circuit.
Materials and Methods
Fly Culture
Flies were maintained in glass pint bottles containing standard agar-cornmeal-yeast food at 25°C on a 12 hour light-dark cycle. Male and female progeny were collected immediately following eclosion and maintained separately in groups of <20 under identical conditions until needed for analysis.
Fly Strains
All stocks were obtained from the Bloomington Stock Center (Indiana University, Bloomington) unless otherwise noted. CSwu is a wild-type Canton S strain established in the laboratory of Martin Heisenberg (University of Wurzburg, Germany) in 1978 and was a gift from Dr. J. Steven de Belle (DART Neuroscience, San Diego, CA, USA). w* ;UAScytoβ-galactosidase (UAS β-galalactosidase) was a gift from Enrique Masa (Texas A&M University, Kingsville, TX, USA). w1118 is the parental strain for the Drosophila tyrosine hydroxylase (DTH) RNAi transgenic line. y[1] v[1] ; P{y[+t7.7]=CaryP}attP2 (pattP2) is the parental line for the dopamine receptor transgenics and was a gift from Exelexis (Harvard University, Boston, MA, USA). y1 v1 ; P{y+t7.7 v+t1.8 = TRiP.HM04077}attP2 carries a dsRNA transgene for the D1 dopamine receptor DopR under UAS control. y1 v1 ; P{y+t7.7 v+t1.8 = TRiP.JF02043}attP2 carries a dsRNA transgene for the D1 dopamine receptor DopR2 under UAS control. y1 v1 ; P{y+t7.7 v+t1.8 = TRiP.JF02025}attP2 carries a dsRNA transgene for the D2 dopamine receptor D2R under UAS control.
pP{w+mW.hs = GawB}elavC155 (elavC155) is a pan-neuronal Gal4 transgenic line which drives constitutive expression in post-mitotic neural tissue. Gal4 is one part of the Gal4/UAS system, which is used to target gene expression in Drosophila. Gal4 is a protein that binds to the upstream activating sequence (UAS) to activate transcription (Duffy, 2002). The DTH-Gal4 (TH-Gal4) line drives expression in the majority of dopaminergic neurons as well as in other tissues where tyrosine hydroxylase is expressed (generated by Serge Birman, Developmental Biology Institute of Marseille, Marseille, France). P{w+mW.hs = GawB}23y expresses Gal4 primarily in the mushroom body extrinsic neurons and was obtained from the University of Glasgow, UK (www.fly-trap.org). P{w+mW.hs =GawB}103y expresses Gal4 in the protocerebrum, mushroom body, antennal lobe, ellipsoid body and fan shaped body. P{w+mW.hs = GawB}201y expresses Gal4 primarily in mushroom bodies. P{w+mW.hs = GawB}drlPGAL8 (referred to by stock number 4669) primarily expresses Gal4 in the mushroom bodies and central complex. P{w+mW.hs = GawB}eyOK107 (referred to as stock number 854) expresses Gal4 primarily in the mushroom bodies. The DTH RNAi line (THK) was generated as previously described (Neckameyer and Bhatt, 2012) and used to decrease dopamine levels since tyrosine hydroxylase is the rate limiting step for dopamine synthesis.
Immunohistochemistry
To establish the normal dopamine pattern, CSwu females were collected immediately following eclosion and aged for 3-5 days prior to dissection. Brains were dissected in phosphaste-buffered saline (PBS) and fixed overnight in 4% EM-grade formaldehyde in 1× PBS and washed thoroughly in PBT (1× PBS, 0.1% PBS, 0.1% protease-free bovine serum albumin, 0.1% Triton-X-100). Tissue was incubated with a primary antibody raised against DTH (Neckameyer et al., 2000), washed in PBT, and incubated with secondary antibody (Alexa Fluor 568 goat anti-rabbit 1:400 dilution; Invitrogen – Molecular Probes, Carlsbad, CA, USA). Tissues were then incubated in 4 mM sodium carbonate, mounted in 4% n-propyl gallate in 20 mM sodium carbonate and viewed under fluorescence. n = 42 individual brains total, analyzing different clusters from different individuals. The pattern of dopamine neurons was confirmed to be the same in males.
To establish subsets of dopamine neurons that overlapped with expression patterns for the Gal4 lines, individual lines (TH-Gal4, 23y, 103y, 201y, 4669, and 854) were used to drive expression of UAS β-galactosidase. Progeny were collected and dopamine neurons were detected using both the anti-DTH antibody and an anti-β-galactosidase monoclonal primary antibody (1:200 dilution; Promega Madison, WI); the former was visualized with Alex Fluor 568 (goat anti-rabbit 1:400 dilution; Invitrogen – Molecular Probes, Carlsbad, CA, USA), and the latter was visualized with an Alexa Fluor 488 (goat anti-mouse 1:400 dilution; Invitrogen – Molecular Probes, Carlsbad, CA, USA) to observe overlap between DTH and Gal4 driving β-galactosidase. Images were used to determine a consensus of where overlap most often occurred. n = 12-22 individual brains for each genotype utilizing different clusters from different individuals.
Stress Paradigms
Crosses were established between elavC155 and w1118 or THK to establish a negative control with normal dopamine expression (elavC155/w1118) and a positive control with globally decreased dopamine expression (elavC155/THK). Gal4 lines 23y, 103y, 201y, 4669, and 854 were crossed with THK to direct expression of the tyrosine hydroxylase RNAi transgene, and thus decrease dopamine synthesis, in the neurons that were previously found to express both dopamine and Gal4. elavC155/pattP2 served as a negative control for normal dopamine receptor expression. dsDopR, dsDopR2 and dsD2R were crossed with elavC155 to decrease dopamine receptor expression. DopR and DopR2 are D1-type receptors, and D2R is a D2-type receptor. On either the same day as collection (sexually immature, 1 day) or after 5 days (sexually mature), animals were placed in vials, with no more than 20 individuals per vial, containing either 2% yeast, 5% sucrose dissolved in 1mL deionized water (control for stress), water only (starvation stress), or 2% yeast, 5% sucrose, 30mM methyl viologen dichloride hydrate (paraquat) (Sigma-Aldrich St. Louis, MO, USA) dissolved in 1mL deionized water (oxidative stress) on a 2.1cm glass fiber filter circle (Fisher Scientific Waltham, MA, USA). Animals were maintained under these conditions for 24 hr at 25°C on a 12 hour light-dark cycle prior to behavioral analyses.
Heart Rate
Animals were anesthetized using FlyNap (Carolina Biological Supply), placed dorsal side up on a piece of sticky tape with the wings extended, and viewed under 200× magnification in a temperature-controlled room as previously described (Neckameyer and Matsuo, 2008). n = 40 individuals for each population (sexually immature and mature males and females), for each stress (starvation and oxidative stress) and control for stress condition.
Locomotion
The EthoVision XT tracking system (Noldus Information Technology Leesburg, VA, USA) was used to assess locomotor behaviors under normal room lighting. Flies were individually aspirated into a 60mm diameter petri dish in which the bottom portion of the dish was painted white to increase color contrast and reduce glare. Painting the arenas white also prevented the animals from being able to see out, which would reduce any effects of differences in eyesight amongst the genotypes. Although the transgenic lines displayed minor differences in eye pigmentation, which is critical for normal vision in Drosophila (Wu and Wong, 1977), all animals displayed red eyes and were thus assumed to have normal visual acuity. Arenas consisted of a 30mm diameter center zone and a 15mm diameter outer zone around the outer edge of the petri dish to allow for determination of time spent in the center.
Tracking videos were 20 minutes long. After an initial 5 minute set up period, the following 2 minutes was designated as exploratory locomotion, followed by an 8 minute period during which no tracking occurred. The last 5 minutes were designated as adapted, or basal, locomotion. Threshold velocities for the degree of mobility (stopping, walking, hopping/flying) were determined using velocities from the negative control animals (elavC155/w1118 for the targeted decreases in dopamine expression and elavC155/pattP2 for the dopamine receptor knockdowns). For the targeted decreases in dopamine expression, < 3.0mm/s was the threshold to stop, and > 3.1mm/s was the threshold to start walking. For the dopamine receptor knockdowns, < 3.2mm/s was the threshold to stop and > 3.3mm/s was the threshold to start walking. For all experiments, > 35mm/s was the threshold for high mobility, which would include very fast walking, hopping, and flying attempts. In our analysis 0 mm/s, the velocity which would correspond to absolutely no movement, was not used as the threshold for stopped because the flies are constantly making slight vibrations with their wings that are too small to be seen, yet are detected as having a velocity by the software we used. n = 45 individuals for each population (sexually immature and mature males and females) for each condition (control, starvation stress, and oxidative stress).
Statistical Analysis
All statistical analyses were performed using SPSS for Windows from IBM Corporation (Armonk, NY, USA). All analyses are ANOVAs, 3-way ANOVA (Genotype × Sex × Stress), 2-way ANOVA (Sex × Stress), ANOVA (Stress). For all ANOVAs, p < 0.05 was considered to be significant. ANOVAs with p > 0.05 for the corrected model were considered not significant. A 95% confidence interval was used for all analyses. All error bars are standard error of the mean.
Results
Identification of Gal4 lines overlapping with dopamine neurons
Prior to determination of overlap between the Gal4 lines and tyrosine hydroxylase neurons, the normal dopamine pattern was established in wild-type (CSwu) animals using an antibody raised against tyrosine hydroxylase (the rate limiting enzyme for dopamine synthesis). The pattern was consistent between males and females ranging from 1-6 days of age, and is shown as the pattern of dopamine neurons in our negative control line, elavC155/w1118 (Figure 1A-C). To determine overlapping expression patterns with tyrosine hydroxylase-expressing neurons, several Gal4 lines were used to target expression of a UAS line for β-galactosidase. Double label immunohistochemistry was used to visualize overlap between neurons expressing both tyrosine hydroxylase and β-galactosidase. The TH-Gal4, 23y, 103y, 201y, 4669 and 854 GAL4 lines were all found to have overlapping expression with tyrosine hydroxylase-expressing neurons. For these Gal4 lines, additional brains were analyzed to determine a consensus of the specific neurons expressing both Gal4 and tyrosine hydroxylase. Resulting patterns of overlap are shown as the solid black neurons (Figure 1G-X). With the exception of TH-Gal4, these are all neuronal drivers; differences in overlap with tyrosine hydroxylase-expressing cells would be the only cells affected by a tyrosine hydroxylase RNAi. While TH-Gal4 drives expression in peripheral tissues, such as the gut and cuticle, these tissues would not mediate centrally modified behavior.
Figure 1.
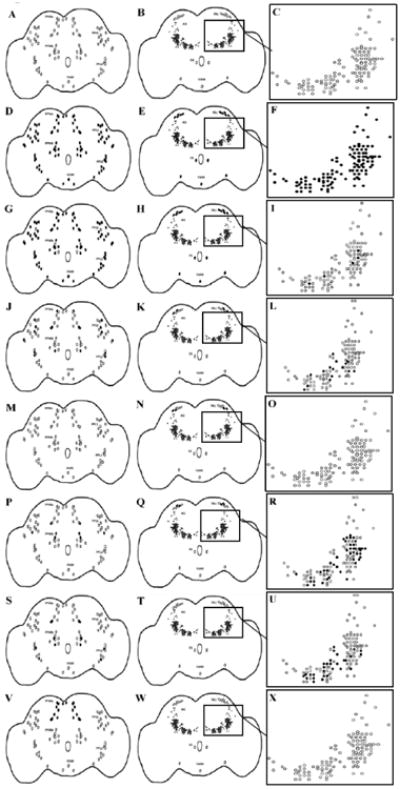
Cartoon depictions of the Gal4 expressing neurons found to overlap with tyrosine hydroxylase-expressing neurons in a series of Gal4 lines. Column 1, focus posterior, column 2, focus anterior, column 3, focus AG magnified to show individual neurons. Black filled neurons represent those expressing both Gal4 and tyrosine hydroxylase, whereas open neurons represent those expressing only tyrosine hydroxylase. (A-C) a parental control line (elavC155/w1118); (D-F) a panneuronal GAL4 (elavC155); (G-I) THGal4; (J-L) 23y Gal4; (M-O) 103y Gal4; (P-R) 201y Gal4; (S-U) 4669 Gal4; (V-X) 854 Gal4. The pattern of overlap is bilaterally symmetrical. PPM (protocerebral posterior medial), PPL (protocerebral posterior lateral), EG (esophageal), VUM (ventral unpaired medial), PAL (protocerebral anterior lateral), AG (antennal glomeruli). n = 42 to establish the normal pattern of dopamine neurons in wild-type (CSwu) animals. n = 12-22 to determine overlap between tyrosine hydroxylase and Gal4 expression.
TH-Gal4 was found to have overlap in the majority of posterior neurons; however, there were a few tyrosine hydroxylase-expressing neurons that did not express Gal4 in the protocerebral posterior medial (PPM) b and protocerebral posterior lateral (PPL) 1 clusters. There was also overlap with the protocerebral anterior lateral (PAL), esophageal (EG), and ventral unpaired medial (VUM) neurons in the anterior, but there was very little overlap within the antennal glomeruli (AG) (Figure 1G-I). 23y showed scattered neurons with overlapping expression in all posterior clusters except for the VUM, as well as some overlap in the posterior PAL and AG (Figure 1J-L). 103y showed no overlapping expression in the posterior, and only two neurons overlapping in the PAL in the anterior (Figure 1M-O). 201y showed sparse neurons with overlapping expression in the posterior PPL1 and PPMa and PPMb and anterior PAL, with abundant overlap in the AG (Figure 1P-R). 4669 showed sparse overlap in the PPL2 and PPMa and PPMb as well as some overlap in the AG (Figure 1S-U). 854 showed a limited overlap pattern consisting of all neurons in the PPMa, but no others (Figure 1V-X). Although each of these Gal4 lines had a unique overlap pattern, some of the lines directed expression in some of the same neuronal clusters. For the PPMa cluster, there are neurons with coinciding overlap with TH-Gal4, 23y, 201y, 4669, and 854; for the PPMb cluster, there was overlap with TH-Gal4, 23y, 201y, and 4669; for the PPL1 cluster, TH-Gal4, 23y, and 201y; PPL2 cluster TH-Gal4, 23y, and 4669; and the PAL cluster had neurons with coinciding overlap with TH-Gal4, 23y, 103y, and 201y. No observable differences were found in Gal4 expression levels or expression pattern for any of the lines used. In addition to the above mentioned GAL4 lines, elavC155, a pan-neuronal GAL4, was also used as a positive control (Figure 1D-F).
Behavior
The GAL4 lines that were previously analyzed for overlap between Gal4 and tyrosine hydroxylase were used to target expression of a tyrosine hydroxylase RNAi line (THK). The filled neurons in Figure 1 represent the overlap between GAL4 and tyrosine hydroxylase and thus those in which dopamine synthesis was decreased with THK. Animals from these genotypes were exposed to stress either immediately following eclosion (sexually immature) or 5 days post-eclosion (sexually mature) for 24 hours prior to locomotor and heart rate analysis. Several behaviors were found that showed significant differences between the level of sexual maturity, sex and stress; however, the behaviors with significant interactions between genotype, sex, and stress serve as evidence that depending on whether an individual is male or female, different populations of dopamine neurons are recruited into the stress response. The results of the 3-way interactions (Genotype × Sex × Stress) are shown in Table 1. A significant p value for Genotype × Sex × Stress demonstrates that the stress response varies between the sexes when dopamine synthesis is manipulated in different subpopulations of neurons. The locomotor parameters include total distance moved, mean velocity, walking duration, walking frequency, and mean duration of each bout of walking, all which describe the animals' exploration of the arena. Time spent in the center zone was also assessed since it has been used in other studies as a measure of anxiety. Stopped duration, stopped frequency, and mean duration of each stopped bout are measures of diminished mobility. Highly mobile duration, highly mobile frequency, and mean duration of bouts of high mobility include hops or flying attempts, which are limited due to the size of the arena.
Table 1.
Genotype × Sex × Stress Interactions Following Presynaptic Manipulation of Dopamine Neurons
| Age (days) | Stress | Heart Rate | |||
| 1 | Starve | *** | |||
| Paraquat | *** | ||||
| 5 | Starve | *** | |||
| Paraquat | *** | ||||
| Age (days) | Stress | Locomotor Phase | Distance Moved | In Zone Duration | Mean Velocity |
| 1 | Starve | Exploratory | ns | ** | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | ** | * | |
| Basal | *** | ns | * | ||
| 5 | Starve | Exploratory | ** | ns | ** |
| Basal | * | ns | ns | ||
| Paraquat | Exploratory | *** | ns | *** | |
| Basal | *** | ns | *** | ||
| Age (days) | Stress | Locomotor Phase | Walking Duration | Walking Frequency | Walking Mean |
| 1 | Starve | Exploratory | ns | ns | ns |
| Basal | * | ns | ns | ||
| Paraquat | Exploratory | ns | ns | ns | |
| Basal | *** | ns | ns | ||
| 5 | Starve | Exploratory | * | * | ns |
| Basal | * | * | ns | ||
| Paraquat | Exploratory | *** | *** | *** | |
| Basal | *** | *** | ns | ||
| Age (days) | Stress | Locomotor Phase | Stopped Duration | Stopped Frequency | Stopped Mean |
| 1 | Starve | Exploratory | ns | ns | ** |
| Basal | * | ns | ns | ||
| Paraquat | Exploratory | ns | ns | * | |
| Basal | *** | ns | ns | ||
| 5 | Starve | Exploratory | * | * | * |
| Basal | * | * | ns | ||
| Paraquat | Exploratory | *** | *** | ns | |
| Basal | *** | *** | ns | ||
| Age (days) | Stress | Locomotor Phase | Highly Mobile Duration | Highly Mobile Frequency | Highly Mobile Mean |
| 1 | Starve | Exploratory | ** | *** | ns |
| Basal | ns | *** | ns | ||
| Paraquat | Exploratory | *** | *** | ns | |
| Basal | ns | *** | ns | ||
| 5 | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | *** | ns | ns | |
| Basal | * | * | ns | ||
Sexually immature and mature males and females were assayed for heart rate and locomotion following a 24 hour exposure to starvation or oxidative stress. Exploratory and basal locomotion refer to the first 2 and last 5 minutes of a 15 minute period, respectively. Mean for walking, stopped, and highly mobile is a calculation of the mean duration of each bout. n = 40 individuals for each population (for heart rate) and n = 45 individuals for each population (for locomotion). 3-way ANOVA (Genotype × Sex × Stress),
p < 0.05,
p < 0.01,
p < 0.001.
Given the high number of behavioral parameters that showed a significant Genotype × Sex × Stress interaction, only a small but representative subset are shown. These analyses depict which of the genotypes displayed sexually dimorphic behaviors, as well as the specific differences between the males and females for those genotypes. In several cases, the control animals (elavC155/w1118) showed sexually dimorphic responses to stress; however, differences were seen in the direction and extent of responses when comparing these flies with the male and female animals from the other genotypes. A selected subset of the behaviors is described below. Each stressor and behavioral response defines a unique circuit; therefore, the same behaviors would not be affected by the two stressors and within a given behavior, we expected the affected genotypes should differ, based on our past studies (Neckameyer and Weinstein, 2005; Neckameyer and Matsuo, 2008. For sexually mature animals assayed for heart rate in response to oxidative stress, the genotypes with significant interactions between sex and stress were elavC155/w1118 (samples 1 and 2, p < 0.013), elavC155/THK (samples 3 and 4, p < 0.000), 23y/THK (samples 7 and 8, p < 0.000), 103y/THK (samples 9 and 10, p < 0.000), and 4669/THK (samples 13 and 14, p < 0.000). Within these genotypes, elavC155/w1118 males decreased heart rate (sample 2, p < 0.000), while females did not show significant effects (sample 1, p = 0.917) in response to oxidative stress. elavC155/THK females showed a significant increase in heart rate in response to oxidative stress (sample 3, p < 0.000), whereas males did not display a significant effect (sample 4, p = 0.249). 23y/THK females showed a significant decrease in heart rate in response to oxidative stress (sample 7, p < 0.000), while males showed a significant increase in heart rate in response to oxidative stress (sample 8, p < 0.000). 103y/THK females and males both increase their heart rate in response to oxidative stress (samples 9 and 10, p < 0.000 for both). 4669/THK males increased heart rate in response to oxidative stress (sample 14, p < 0.000) and females did not display a significant effect (sample 13, p = 0.222). (Figure 2).
Figure 2.
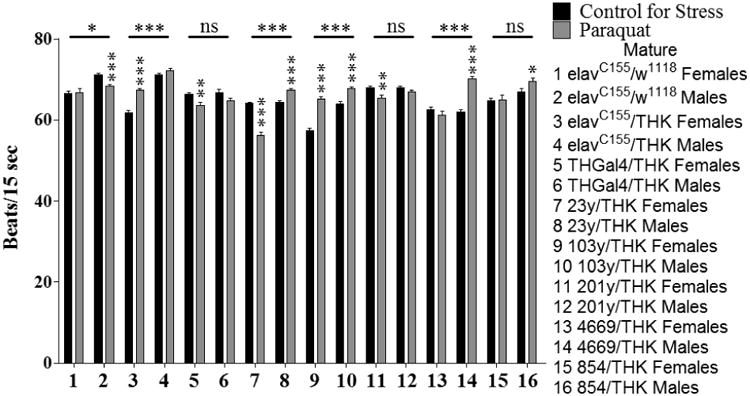
Sexually mature males and females were assayed for heart rate following a 24 hour exposure to oxidative stress. n = 40 individuals for each population (males and females with either control for stress or oxidative (paraquat) stress conditions for each genotype). Individual heart rates were the average of 5 intervals of 15 seconds each with 15 seconds between each interval. 2-Way ANOVA (Sex × Stress) displayed on lines above the corresponding bars, ANOVA (Stress) displayed directly above the bars, * p < 0.05, ** p < 0.01, *** p < 0.001.
For sexually immature animals assayed for time spent in the center zone during exploratory locomotion in response to starvation stress, elavC155/w1118 (samples 1 and 2, p = 0.047), elavC155/THK (samples 3 and 4, p = 0.001), THGal4/THK (samples 5 and 6, p = 0.029), and 103y/THK (samples 9 and 10, p = 0.015) had significant interactions between sex and stress. Within these genotypes, elavC155/w1118 and 103y/THK females showed an increase in time spent in the center zone in response to starvation stress (sample 1, p = 0.003 and sample 9, p = 0.022, respectively), whereas males did not show a significant effect (sample 2, p = 0.495 and sample 10, p = 0.084, respectively). elavC155/THK females showed an increase in time spent in the center zone with starvation stress (sample 3, p < 0.000), whereas males decreased their time spent in the center zone (sample 4, p = 0.029). THGal4/THK males displayed a decrease in time spent in the center zone with starvation stress (sample 6, p = 0.046), whereas females did not show a significant effect (sample 5, p = 0.34) (Figure 3).
Figure 3.
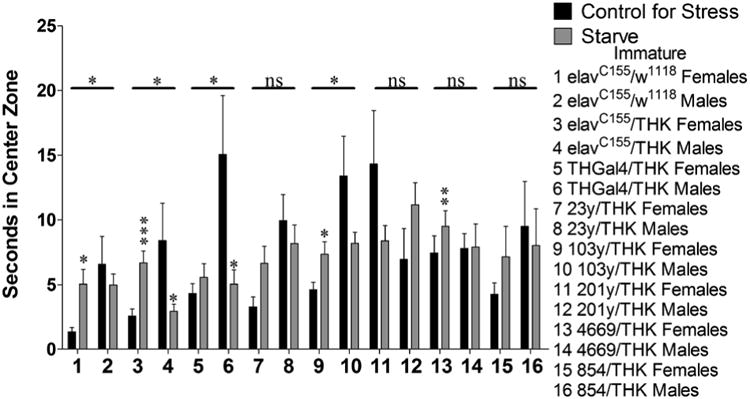
Sexually immature animals were assayed for time (seconds) spent in the center zone during exploratory locomotion following a 24 hour exposure to starvation stress. n = 45 (for each population). 2-way ANOVA (Sex × Stress) displayed on lines above the corresponding bars, ANOVA (Stress) displayed directly above the bars * p < 0.05, ** p < 0.01, *** p < 0.001.
Manipulation of dopamine receptors
The above analyses were limited to manipulations of presynaptic dopamine neurons. To assess whether there was a sexual dimorphism in the postsynaptic dopamine signaling within the response circuits, expression of the three Drosophila dopamine receptors DopR (type 1), DopR2 (type 1), and D2R (type 2) were reduced in the central nervous system by targeting the corresponding RNA is with elavC155 Gal4. The progeny of elav Gal4 crossed with the parental line for the generation of these RNAi lines, denoted here as pattP2, served as a control. Heart rate and the locomotor behaviors described above were assayed following a 24 hour exposure to starvation or oxidative stress. Many of these parameters displayed significance in a 3-way interaction between genotype, sex, and stress, and provide evidence for sexually dimorphic recruitment of postsynaptic dopamine neurons in the stress response circuitry (Table 2).
Table 2.
Genotype × Sex × Stress Interactions Following Postsynaptic Manipulation of Dopamine Signaling
| Age (days) | Stress | Heart Rate | |||
| 1 | Starve | ns | |||
| Paraquat | ns | ||||
| 5 | Starve | *** | |||
| Paraquat | *** | ||||
| Age (days) | Stress | Locomotor Phase | Distance Moved | In Zone Duration | Mean Velocity |
| 1 | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | * | ns | * | |
| Basal | ns | ns | ns | ||
| 5 | Starve | Exploratory | ns | ns | * |
| Basal | ** | ns | *** | ||
| Paraquat | Exploratory | ** | ns | ** | |
| Basal | *** | ns | *** | ||
| Age (days) | Stress | Locomotor Phase | Walking Duration | Walking Frequency | Walking Mean |
| 1 | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | ns | * | |
| Basal | ns | ns | ns | ||
| 5 | Starve | Exploratory | ns | ** | * |
| Basal | * | *** | ns | ||
| Paraquat | Exploratory | * | *** | * | |
| Basal | * | * | ns | ||
| Age (days) | Stress | Locomotor Phase | Stopped Duration | Stopped Frequency | Stopped Mean |
| 1 | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | * | ns | ns | |
| Basal | ns | ns | ns | ||
| 5 | Starve | Exploratory | ns | ** | ns |
| Basal | * | *** | * | ||
| Paraquat | Exploratory | * | *** | ns | |
| Basal | * | * | ns | ||
| Age (days) | Stress | Locomotor Phase | Highly Mobile Duration | Highly Mobile Frequency | Highly Mobile Mean |
| 1 | Starve | Exploratory | *** | *** | ns |
| Basal | ns | * | ns | ||
| Paraquat | Exploratory | ns | ns | ns | |
| Basal | * | ns | ns | ||
| 5 | Starve | Exploratory | ns | * | ns |
| Basal | *** | * | ns | ||
| Paraquat | Exploratory | *** | *** | ns | |
| Basal | * | *** | ns | ||
Sexually immature and mature males and females were assayed for heart rate and locomotion following a 24 hour exposure to starvation or oxidative stress. Exploratory and basal locomotion refer to the first 2 and last 5 minutes of a 15 minute period, respectively. Mean for walking, stopped, and highly mobile is a calculation of the mean duration of each bout. n = 40 individuals for each population (for heart rate) n = 45 individuals for each population (for locomotion). 3-way ANOVA (Genotype × Sex × Stress),
p < 0.05,
p < 0.01,
p < 0.001.
We performed further analyses for a representative subset of parameters to demonstrate which genotypes showed significant interactions between sex and stress, as well as the specific nature of the sexual dimorphisms. In these analyses there were several cases in which sexual dimorphisms could be observed in the control animals (elavC155/pattP2); however, the visual representations below, in conjunction with the results of the 3-way ANOVAS in Table 2, illustrate the differences in the sexual dimorphisms amongst the genotypes. Sexually mature animals assayed for heart rate following a 24 hour exposure to starvation displayed significant 2-way interactions (Sex × Stress) for elavC155/pattP2 (samples 1 and 2, p = 0.007), elavC155/dsDopR (samples 3 and 4, p < 0.000), elavC155/dsDopR2 (samples 5 and 6, p = 0.026), and elavC155/dsD2R (sample 7 and 8, p = 0.017). elavC155/dsDopR2 showed a non-significant corrected model (samples 5 and 6, p = 0.076) and was removed from further analysis. elavC155/pattP2 females and males both decreased their heart rates in response to starvation stress, but to varying degrees (sample 1, p = 0.003 and sample 2, p < 0.000, respectively). elavC155/dsDopR females decreased their heart rate in response to starvation stress (sample 3, p = 0.007), whereas males increased theirs (sample 4, p < 0.000). elavC155/dsD2R males decreased their heart rates in response to starvation stress (sample 8, p < 0.000), whereas females did not display a significant effect (sample 7, p = 0.206) (Figure 4).
Figure 4.
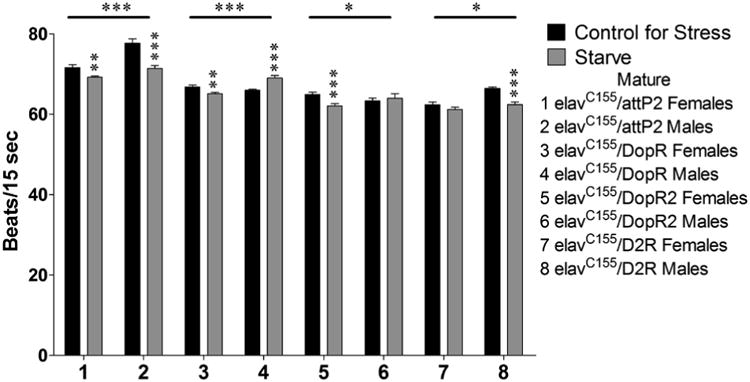
Sexually mature animals were assayed for heart rate as above following a 24 hour exposure to starvation stress. n = 40 (for each population). 2-way ANOVA (Sex × Stress) displayed on lines above the corresponding bars, ANOVA (Stress) displayed directly above the bars * p < 0.05, ** p < 0.01, *** p < 0.001.
Sexually mature animals assayed for time spent highly mobile during exploratory locomotion following a 24 hour exposure to oxidative stress displayed significant 2-way interactions (Sex × Stress) were seen for elavC155/dsDopR (sample 3 and 4, p < 0.000) and elavC155/dsDopR2 (samples 5 and 6, p = 0.04). elavC155/dsDopR females decreased their time spent highly mobile in response to oxidative stress (sample 3, p < 0.000), while males showed no significant effect (sample 4, p = 0.464). elavC155/dsDopR2 females increased their time spent highly mobile in response to oxidative stress (sample 5, p < 0.000) and males displayed no significant effect (sample 6, p = 0.277) (Figure 5).
Figure 5.
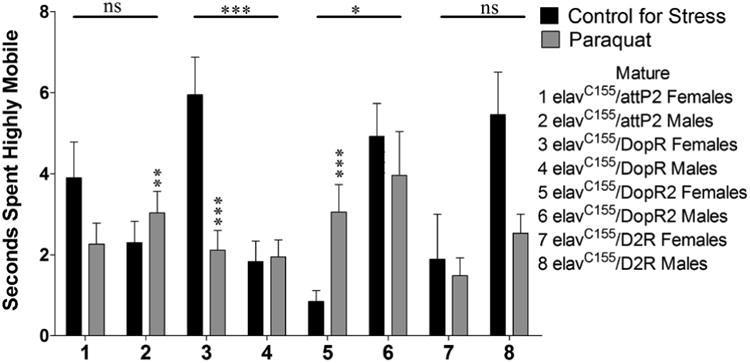
Sexually mature animals were assayed for time spent highly mobile during exploratory locomotion following a 24 hour exposure to oxidative stress. n = 45 (for each population). 2-way ANOVA (Sex × Stress) displayed on lines above corresponding bars, ANOVA (Stress) displayed directly above bars * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
It has been assumed that, due to observed sexually dimorphic differences in susceptibility to stress and stress-related disorders, the stress response circuitry likely differs in males and females (Rhodes and Rubin, 1999). Consistent with this expectation, previous work from our lab has shown that tyrosine hydroxylase levels were altered in response to stress in a manner that was dependent on sex, age, and reproductive status of the population being studied (Neckameyer and Weinstein, 2005), and that mutant lines carrying specific anatomical defects in the brain responded differently depending upon these same parameters (Neckameyer and Matsuo, 2008). In this study, we continue our analysis of the sexually dimorphic nature of the dopamine circuitry. These kinds of genetic manipulations, and this type of high throughput, in depth analysis of the behavioral output to stress, are only possible in a genetically tractable organism such as Drosophila. Here, our analyses consisted of three-way ANOVAs to assess the interactions between genotype, sex, and stress. By reducing dopamine levels in select populations of dopamine neurons, we were able to demonstrate a significant interaction amongst genotypes, sex, and stress, which supports the conclusion that there are sex differences in the recruitment of dopamine neurons into the stress response circuitry. Similar results were observed by decreasing levels of dopamine receptors, showing that the dopamine signaling in total is sexually dimorphic. The inclusion of two distinct stressors and diverse behavioral outputs permits an extension of our conclusions to show that a sexually dimorphic stress response circuitry is a general phenomenon, not limited to one specific stress, one age group, or a single behavioral output to stress.
Specific Gal4 driver lines were chosen for these experiments to provide distinct patterns of overlap with tyrosine hydroxylase expressing neurons. We observed some individual variability in the pattern of expression with some of these lines. The pattern of dopamine neurons was largely consistent from one individual to the next, yet where the Gal4 expression overlapped was occasionally variable. The depictions shown in Figure 1 were a consensus of the most often observed expression patterns. The 103y Gal4 driver was the most variable. In the image shown in Figure 1M, there are no posterior neurons with overlap between Gal4 and tyrosine hydroxylase; however, there was overlap in the PPL1 and PPM2 clusters in approximately 15-20% of individuals. However, individual differences have no impact on our findings, since these lines were used only to generate different manipulations of the dopamine circuitry, and determination of the specific neurons important for the stress response circuitry in males and females was not relevant to our hypothesis. Inconsistencies between the Gal4 lines may also include variability in relative strength both between the Gal4 lines and between different neurons within a single Gal4 line. While comparative levels of expression could not be quantified, all of the Gal4 lines have been shown to direct robust expression, suggesting that dopamine synthesis was definitely reduced in these neurons.
Contrary to the original report that THGal4 recapitulates the pattern of dopamine neurons in the adult brain (Friggi-Grelin et al., 2003), certain individual dopamine neurons detected in wild type (CSwu) flies, as well as in the other Gal4 lines, were not observed when the THGal4 line was used to direct expression. Our immunohistochemical analyses also showed ectopic expression of neurons using the THGal4 driver. While the ectopic expression is important to note, it would have no effect on our conclusions since THGal4 was used in combination with the tyrosine hydroxylase RNAi line, which would only affect neurons synthesizing dopamine. The neurons not identified by THGal4 are likely critical in the stress response circuitry, since THGal4/THK behavior differed from that of elavC155/THK in most circumstances. This is consistent with previous data showing that TH-Gal4 does not fully recapitulate the pattern of dopamine neurons (Mao and Davis, 2009).
Starvation and oxidative stress were chosen because, while they are unrelated stressors, both have been linked to the development of specific psychiatric disorders and to have sexually dimorphic effects (see Du et al., 2009 and Viña et al., 2011 for examples). Similarly, the behaviors highlighted in this manuscript were chosen because they have been shown to change as a consequence of stress, depression and anxiety in mammals, and have also been shown to be sexually dimorphic. High stress loads can increase risk for, and progression of, cardiovascular disease (reviewed in Menezes et al., 2011), and can alter parameters of locomotor behavior. In rodents, high emotionality inhibits exploration (Lester, 1968). In Drosophila and mammals, there is decreased locomotion after adaptation to a novel environment, which is sexually dimorphic (Liu et al., 2007; Neckameyer and Matsuo, 2008). Centrophobism is the tendency to spend more time in closer proximity to the perimeter of the environment rather than in the central open area. In rodents, animals that spend more time in the central zone are considered less fearful and less anxious (Treit and Fundytus, 1989). In Drosophila this behavior is sexually dimorphic, with females typically displaying more avoidance of the center than males (Besson and Martin, 2005).
A recent study in Drosophila suggested that time spent close to the boundaries of the enclosure may be due to a desire to explore the walls rather than an avoidance of the center of the arena (Soibam et al., 2012); however, in our experiments we observed only limited vertical movement along the walls. One possible explanation for this could be that in our experiments the petri dishes used for the open field were painted white, so that the animals could not see outside of their enclosure. Soibam et al. also concluded that the preference for the boundaries of the enclosure was not due to centrophobism because the animals also preferred to be in close proximity to the walls when the arena was divided into concentric circles regardless of whether the wall was the outer- or inner-most. However, it must be considered that flies may not have the ability to view their arena on such a global scale and that what is important is the tactile sensation of being close to the wall rather than knowing where they are in terms of the whole arena. Although the specific motivation for this behavior in Drosophila has not yet been determined, we did find that this behavior is altered in response to stress in a sexually dimorphic manner. Additionally, as with the other behaviors assayed, there is no qualitative assessment of whether increased time spent in the center zone is indicative of an adapative or maladaptive stress response, anxiety, or fear, only that this behavior is altered in response to stress.
Other parameters of locomotion that we observed included duration of time spent stopped, or freezing, which is considered a startle response, and is adaptive to prevent detection by a potential predator (Denenberg, 1969). Previous reports in mice have suggested that females may have an increased startle response compared to males when threatened by predation (Adamec et al., 2006). By highlighting specific behaviors that are often used in mammalian models for stress, depression, and anxiety, this analysis establishes Drosophila as a useful model for elucidating the sexually dimorphic effects of stress and affective disorders.
The aim of this study was not to determine what constitutes a “normal” stress response, but rather to focus on demonstrating that the response circuits for stress are different for males and females. Although direct comparisons between the different stressors and behaviors were not shown, differences in the responses clearly indicate that not only are the response circuits sexually dimorphic, but they are also unique for a given stressor and specific behavioral response. The same is also true for the various behavioral parameters. This can be clearly observed by noting differences in the genotypes with significant sexual dimorphisms for each stress and for each behavioral output, and by observing differences in whether the stress resulted in an increase or decrease of a specific response. While directionality of change was given for each of the specific behaviors, the quality of the responses and any effects of the animals' health and survival were not determined.
Although identification of the specific neurons important for the behavioral response for females and males was beyond the scope of our study, this data set provided clusters that would be of particular interest in beginning to specify neurons important for the sexually dimorphic stress response. From the heart rate data, the dopamine neurons targeted by 23y and 201y would be of interest for female heart rate since these lines displayed decreased heart rate in response to oxidative stress, whereas most other lines displayed increases. Additionally, the neurons targeted by 103y, although limited, copied the increase in heart rate in response to oxidative stress that was observed for elavC155/THK females. For male heart rate, the neurons targeted by 23y, 103y, 4669, and 854 stood out since these all resulted in increased heart rate following oxidative stress. For time spent in the center zone in response to starvation stress in females, the neurons targeted by 103y and 4669 stood out as having possible importance. Interestingly, these are the same lines that were identified for heart rate in response to oxidative stress, suggesting that these subsets could be involved in the female stress response in general rather than being important for a specific behavior or stressor. It should also be noted that elavC155/THK females displayed an increase in time spent in the center zone that was absent in TH-Gal4/THK females, indicating that the dopamine neurons not targeted by TH-Gal4 could be key for this behavioral response. For the receptor heart rate data, D2R was observed to be important for females, whereas DopR and DopR2 seemed to have greater importance for males. For time spent highly mobile, both DopR and DopR2 appeared to be important for females, whereas knockdown of all three receptors altered male behavior.
Additionally, we observed greater statistical significance for the presynaptic compared to the postsynaptic studies. This interpretation is somewhat complicated by data suggesting that similar to some mammalian dopamine receptors, the D2R receptor may be located presynaptically and act as an autoreceptor which regulates dopamine release (Vickrey and Venton, 2011). In spite of this complication, the difference in statistical significance between the two data sets could suggest that postsynaptic dopamine targets are not as sexually dimorphic as presynaptic dopaminergic neurons in the stress response circuitry, or effects with the receptors cancelled each other out since type 1-like and type 2-like receptors have opposing actions.
Conclusions
These data illustrate the importance of considering sex differences in the analysis of stress and related illnesses, since the behavioral response circuits differ between males and females. The paradigms that we have established illustrate sex differences in the behavioral response circuits for stress in Drosophila, and will provide an excellent model to further elucidate factors that are important for the establishment of sexually dimorphic circuitry.
References
- Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiology & Behavior. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Besson M, Martin JR. Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. Journal of Neurobiology. 2005;62:386–396. doi: 10.1002/neu.20111. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Wadiwalla M, Engert V, Pruessner JC. The role of sex and gender socialization in stress reactivity. Developmental Psychology. 2009;45:45–55. doi: 10.1037/a0014433. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Open-field behavior in the rate: what does it mean? Annals of the New York Academy of Sciences. 1969;159:852–859. doi: 10.1111/j.1749-6632.1969.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, Kochanek PM, Jenkins LW, Ren J, Gibson G, Chu CT, Kagen VE, Clark RSB. Starving neurons show sex difference in autophagy. Journal of Biological Chemistry. 2009;284:2383–2396. doi: 10.1074/jbc.M804396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticists's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Levine RB. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. Journal of Comparative Neurology. 2003;465:560–578. doi: 10.1002/cne.10869. [DOI] [PubMed] [Google Scholar]
- Figueira ML, Ouakinin S. Gender-related endocrinological dysfunction and mental disorders. Current Opinion in Psychiatry. 2010;23:369–372. doi: 10.1097/YCO.0b013e3283399b86. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. Journal of Neurobiology. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Kuter K, Smialowska M, Wierońska J, Zieba B, Warda J, Pietraszek M, Nowak P, Biedka I, Roczniak W, Konieczny J, Wolfarth S, Ossowska K. Toxic influence of subchronic paraquat administration on dopaminergic neurons in rats. Brain Research. 2007;1155:196–207. doi: 10.1016/j.brainres.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Lester D. The effect of fear and anxiety on exploration and curiosity: toward a theory of exploration. Journal of General Psychology. 1968;79:105–120. doi: 10.1080/00221309.1968.9710458. [DOI] [PubMed] [Google Scholar]
- Liu L, David RL, Roman G. Exploratory activity in Drosophila requires the kurtz nonvisual arrestin. Genetics. 2007;175:1197–1212. doi: 10.1534/genetics.106.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Frontiers in Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, Catena Dell'Osso M. Psychiatric disorders and mitochondrial dysfunctions. European Review for Medical and Pharmacological Sciences. 2012;16:270–275. [PubMed] [Google Scholar]
- Menezes A, Lavie C, Milani R, O'Keefe J, Lavie T. Psychological risk factors and cardiovascular disease: Is it all in your head? Postgraduate Medicine. 2011;123:165–176. doi: 10.3810/pgm.2011.09.2472. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Bhatt P. Neurotrophic actions of dopamine on the development of a serotonergic feeding circuit in Drosophila melanogaster. BMC Neuroscience. 2012;13:26. doi: 10.1186/1471-2202-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, Matsuo H. Distinct neural circuits reflect sex, sexual maturity, and reproductive status in response to stress in Drosophila melanogaster. Neuroscience. 2008;156:841–856. doi: 10.1016/j.neuroscience.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Neckameyer W, Weinstein J. Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress. 2005;8:117–132. doi: 10.1080/10253890500147381. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiology of Aging. 2000;21:145–152. doi: 10.1016/s0197-4580(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Bouma EMC. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: A review of gender differences. Neuroscience and Biobehavioral Reviews. 2011;35:1757–1770. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Philipp E, Pirke KM. Effects of starvation on hypothalamic tyrosine hydroxylase activity in adult male rats. Brain Research. 1987;413:53–59. doi: 10.1016/0006-8993(87)90153-3. [DOI] [PubMed] [Google Scholar]
- Plante GE. Depression and cardiovascular disease: a reciprocal relationship. Metabolism. 2005;54:45–48. doi: 10.1016/j.metabol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Research Reviews. 1999;30:135–152. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Rooseboom TJ, Painter RC, van Abeelen AFM, Veenendaal MVE, Rooij SR. Hungry in the womb: What are the consequences? Lessons from the Dutch famine. Muturitas. 2011;70:141–145. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Soibam B, Mann M, Liu L, Tran J, Lobaina M, Kang YY, Gunaratne GH, Pletcher S, Roman G. Open-field arena boundary is a primary object of exploration for Drosophila. Brain and Behavior. 2012;2:97–108. doi: 10.1002/brb3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. Journal of Neuroscience. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test of anxiolytic activity in rats. Pharmacology, Biochemistry, and Behavior. 1989;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Vickrey TL, Venton BJ. Drosophila Dopamine2-like receptors function as autoreceptors. ACS Chemical Neuroscience. 2011;2:723–729. doi: 10.1021/cn200057k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viña J, Gambini J, Lopen-Grueso R, Abdelaziz KM, Jove M, Borras C. Females live longer than males: role of oxidative stress. Current Pharmaceutical Design. 2011;17:3959–3965. doi: 10.2174/138161211798764942. [DOI] [PubMed] [Google Scholar]
- Willner P, Hale A, Argyropoulos S. Dopaminergic mechanism of antidepressant actions in depressed patients. Journal of Affective Disorders. 2005;86:37–45. doi: 10.1016/j.jad.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Wu CF, Wong F. Frequency characteristics in the visual system of Drosophila. Journal of General Physiology. 1977;69:705–724. doi: 10.1085/jgp.69.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]


