Summary
Elacytarabine is a novel cytotoxic nucleoside analogue, independent of nucleoside transporters (e.g. human Equilibrative Nucleoside Transporter 1 [hENT1]) for cell uptake, and mechanisms of action similar to those of cytarabine. This Phase II study assessed the efficacy and safety of elacytarabine in patients with advanced stage acute myeloid leukaemia (AML). Patients received 2000 mg/m2/day continuously i.v. during days 1-5 every 3 weeks. Patients were matched by six risk factors with historical controls; remission rate (assessed after 1 or 2 cycles) and 6-month survival were compared. 61 patients, median age 58 years, were enrolled; 52% had five or six risk factors. The remission rate was 18% (95% confidence interval: 9%-30%) vs. 4% in controls (p< 0.0001), 6-month survival rate was 43%, median overall survival was 5.3 months (vs. 1.5 months); 10 patients (16%) were referred for stem cell transplantation after treatment. Side effects were predictable and manageable. The most common grade 3/4 non-haematological adverse events were febrile neutropenia, hypokalemia, fatigue, hyponatraemia, dyspnoea and pyrexia. 30-day all-cause mortality, after start of treatment, was 13% vs. 25% in controls. Elacytarabine has monotherapy activity in patients with advanced AML. This study provides proof-of-concept that lipid esterification of nucleoside analogues is clinically relevant.
Keywords: Elacytarabine, nucleoside analogue, lipid esterification
Introduction
Despite advances in supportive therapies, an increasing understanding of disease biology, and the use of prognostic factors to select appropriate treatment, the overall cure rate of patients with acute myeloid leukaemia (AML) is still disappointingly low (Giles et al, 2006). No standard of care exists for patients with advanced AML who continue to receive predominantly cytarabine-based relapse regimens. Cytarabine (ara-C, [1-ß-D-arabinofuranosylcytosine]), has been the cornerstone of care for patients with AML for several decades (Burnett et al, 2011), even though its activity varies markedly among the types and stages of leukaemia (Galmarini et al, 2002a). The hydrophilic molecule enters cells primarily as a false substrate through specialized nucleoside transporter proteins, mostly hENT1 (the human Equilibrative Nucleoside Transporter 1) (Damaraju et al, 2009, Galmarini et al, 2002b). Reduced hENT1 expression and activity is associated with adverse therapeutic outcomes and reduced cytotoxicity for patients treated with cytarabine (Galmarini et al, 2002a, Hubeek et al, 2005) or with other nucleoside analogues: e.g. decitabine (in AML or myelodysplastic syndromes [MDS]) (Qin et al, 2009); fludarabine (in chronic lymphocytic leukaemia and lymphoma) (Fernandez Calotti et al, 2008, Mackey et al, 2005); and gemcitabine (in pancreatic cancer; gastric cancer; lung cancer and mantle cell lymphoma) (Giovannetti et al, 2007, Marce et al, 2006, Oguri et al, 2007). Patients with AML and low hENT1 expression have reduced disease-free survival (Galmarini et al, 2002a, Galmarini et al, 2002b).
Elacytarabine (CP-4055), the lipophilic 5'-elaidic acid ester of cytarabine, enters cells independently of hENT1. Preclinical studies of elacytarabine demonstrate activity in cytarabine-resistant cell lines (Adams et al, 2008, Adema et al, 2011, Bergman et al, 2004, Jin et al, 2009) and in animal models with tumour xenografts (Adema et al, 2010a, Galmarini et al, 2009). It may therefore be an effective agent in patients for whom cytarabine is ineffective (Adema et al, 2010b, Breistol et al, 1999, Sandvold et al, 2010). In early clinical studies in patients with solid tumours (Dueland et al, 2009), myelosuppression was the dose-limiting toxicity (DLT, observed at 240 mg/m2/day) and antitumour activity was observed in patients with melanoma, ovarian and lung cancer. In a Phase I study conducted in patients with refractory haematological malignancies, elacytarabine had manageable and transient toxicities, similar to those of cytarabine, and a pharmacokinetic profile that allowed extended exposure of cytarabine and its active metabolites to leukaemic cells (Giles et al, 2012a, Giles et al, 2012b). Anti-leukaemia activity was reported in patients with refractory AML and recent prior failure to respond to cytarabine. The recommended dosing of single-agent elacytarabine for Phase II studies in patients with haematological malignancies was continuous infusion of 2000 mg/m2/day over five days (Giles et al, 2012b).
This Phase II study (CP4055-106/NCT00405743) evaluated elacytarabine as monotherapy for patients with refractory/relapsed AML following at least two prior induction regimens. The outcomes were compared to those observed in a cohort of 594 patients with relapsed/refractory AML (Giles et al, 2005). In that historical cohort, a multivariate analysis identified six adverse factors associated with achieving complete remission (CR) after second salvage therapy (described below).
Patients and methods
Study design and eligibility criteria
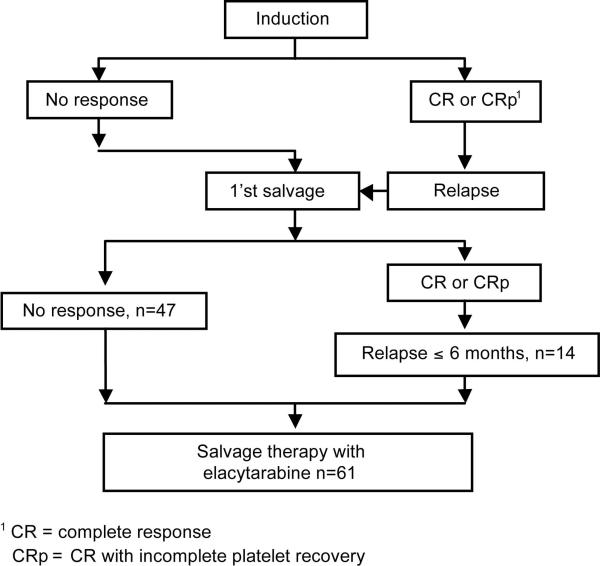
Adult patients (≥18 years of age) with AML (diagnosed according to the World Health Organization [WHO] classification [Vardiman et al 2009]), but not acute promyelocytic leukaemia, and who had failed at least two prior regimens of chemotherapy (Figure 1), were enrolled in this open-label, non-randomized, multicentre Phase II study (CP4055-106/NCT00405743) of single-agent elacytarabine, between April 2008 and March 2009.
Figure 1.
Patient responses to treatments before elacytarabine therapy
Patients were eligible for the study if they had more than 5% bone marrow myeloblasts, leukaemic blasts in peripheral blood or biopsy-proven extramedullary disease; Eastern Cooperative Oncology Group (ECOG)-WHO performance status ≤2; persistent chronic clinically significant symptoms from prior chemotherapy no more severe than Common Terminology Criteria for Adverse Events (CTCAE) grade 1, according to National Cancer Institute CTCAE version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf); were not receiving any other cytotoxic treatment, and had no stem cell transplantation (SCT) within 3 months of study inclusion. Patients who had received an allogeneic SCT had to be off immunosuppressive treatment for at least 4 weeks before study inclusion. In the absence of rapidly progressing disease, the interval from prior treatment to administration of study drug was to be at least 2 weeks for cytotoxic agents and at least 5 half-lives for other agents. Hydroxycarbamide had to be discontinued for at least 24 h before study drug was given. Patients had to have acceptable organ function as defined by serum creatinine ≤1.5 times the institutional upper limit of normal (ULN), total bilirubin ≤ 1.5 times the ULN unless considered due to Gilbert syndrome, and transaminases ≤ 2.5 times the ULN unless considered due to leukaemic involvement.
Patients were not eligible if they had leukaemia in the central nervous system (CNS), another active cancer within the last 5 years, were seropositive for human immunodeficiency virus or hepatitis B or C, had a history of allergic reactions to cytarabine (CTCAE grades 3 or 4) or eggs, uncontrolled intercurrent illness (e.g. active infection, cardiovascular disease or significant psychiatric disorders), active heart disease (e.g. myocardial infarction) within the previous 3 months, symptomatic coronary artery disease, any condition classified as New York Heart Association grade 3 or 4, or were pregnant or breast-feeding.
Study patients were compared to patients in an historic AML cohort (Giles, et al 2005). On multivariate analysis six adverse factors for complete remission (CR) attainment with second salvage therapy were identified in the historic cohort as follows: duration of first CR (CR1) <12 months, duration of second CR (CR2) <6 months, second salvage therapy not including SCT, non-inversion 16 AML, platelet count <50 × 109/l and leucocytosis (>50 × 109/l). The historic cohort of 594 patients with AML who had received at least two salvage chemotherapies who were included in this analysis formed the control population for this Phase II study of single agent elacytarabine. The six adverse prognostic factors formed the basis for defining four risk groups (Table 1).
Table 1.
Prognostic factors for complete remission
| Elacytarabine treatment | Control population | |
|---|---|---|
| N=61 (%) | N=594 (%) | |
| Six prognostic factors for CR | ||
| Duration of CR1 < 12 months | 71 | 73 |
| Duration of CR2 < 6 months | 100 | 85 |
| 2nd salvage therapy (no stem cell transplantation) | 100 | 85 |
| Non-inversion 16 AML | 93 | 96 |
| Platelet count < 50 × 109/l | 66 | 69 |
| Leucocytosis > 50 × 109/l | 16 | 11 |
| Risk groups (number of prognostic factors) | ||
| Low (1-2) | 2 | 8 |
| Intermediate 1 (3) | 7 | 20 |
| Intermediate 2 (4) | 39 | 38 |
| High (5-6) | 52 | 33 |
CR, complete remission; CR1, first CR; CR2, second CR; AML, acute myeloid leukaemia.
Treatment and procedures
The protocol was approved by the appropriate Institutional Review Board or Ethics Committees at each participating institution and published on ClinicalTrials.gov (NCT00405743). The study procedures adhered to Good Clinical Practice, applicable regulatory requirements and the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients before any study procedures were performed. Patients were enrolled on study from four US and seven European centres.
The ready-for-use vials (5 mg/ml) of elacytarabine ([5’-O-(trans-9”-octadecenoyl)-1-b-D-arabinofuranosyl cytosine; Clavis Pharma, Oslo, Norway), formulated as a liposomal dispersion for intravenous infusion, were usually diluted in 0.9% saline and injected into infusion bags, but could also be used undiluted. The product was administered to patients shortly after dilution and within 24 h. The amount of elacytarabine to be administered was determined from body surface area (BSA), calculated from actual heights and weights. An upper limit of BSA=2 m2 was defined for calculation of the patients’ dosage. Elacytarabine was administered at a dose of 2000 mg/m2/day intravenously over 24 h during days 1-5 of a three-week course – the regime deemed as optimal based on results of a prior study examining different elacytarabine schedules in patients with refractory leukaemia (Giles et al, 2012b). Patients with evidence of clinical benefit from elacytarabine could receive additional courses of the study drug.
Supportive therapies, such as erythropoietin, analgesics, blood transfusions, antimicrobials, and haematopoietic colony-stimulating factors for treatment of cytopenias, were permitted, as was adequate systemic antiemetic treatment with 5-HT3 antagonists and corticosteroids (or alternatively prochlorperazine) administered according to study centre routines. Prophylactic use of haematopoietic colony-stimulating factors or administration of other anticancer therapies, investigational cytotoxic agents, radiation, or biological therapy was not allowed while the patient was in the study. During the first 21 days of the study, patients could receive leucopheresis, to control elevated blast and/or platelet counts (not more than three procedures per week, and not more than five procedures in total). Hydroxycarbamide was allowed (up to a maximum of 5 g/day for a maximum of 5 days), as was intrathecal chemotherapy to treat isolated CNS involvement of leukaemia, diagnosed during the study.
Assessments
CR was defined as an absolute neutrophil count ≥ 1 × 109/l, platelet count ≥ 100 × 109/l and normal marrow differential (≤ 5% blasts) per International Working Group criteria (Cheson et al 2003). CRp was as per CR, but with incomplete platelet recovery i.e. platelet count <100 × 109/l. Partial Response (PR) was defined as all haematological values of a CR with 50% decrease in bone marrow blasts to 6–25% blast cells in the marrow aspirate (Cheson, et al 2003).
Patients were followed for relapse and survival for at least 6 months. An Independent Data Monitoring Committee (IDMC) reviewed every 20-patient cohort for toxicity and futility. Safety assessments were based on data that were available from all cycles, and toxicity was graded according to CTCAE v.3.0. Physical examinations, haematology, chemistry and urinalysis were recorded at regular intervals.
Criteria for evaluation and statistical analyses
Giles et al (2005) analysed 594 historical AML patients who received at least two salvage chemotherapies. These patients were used as the control for this Phase II study in an analysis defined a priori powered to detect an increase in PR or better (CR or CRp) from 5% in the historical control group to 10% or higher in the study cohort. Primary endpoints were 6-month survival rate and incidence of CR and CRp after one or two courses of therapy. Giles et al (2005) used a logistic regression model to perform a multivariate analysis of prognostic factors for CR/CRp (CR1 duration < 12 months, CR2 <6 months, platelet count <50 × 109/l, leucocyte count >50 × 109/l, non-inversion 16 AML, no SCT). When defining the prognostic factors for patients in the current study, CR duration was set at 0 months for patients who had not achieved a prior first CR or second CR. The methods reported by Berry (1989, 2006) were used to monitor this Phase II study. The S in the Giles et al (2005) model was used as a score, namely, S = exp(Z)/[1+exp(Z)]. Here, S was defined as the probability of achieving CR/CRp, and is used as a prognosis index. This was applied to the patients in this Phase II study, using the following model, Logit(P) = α + Z, where P is the probability of achieving CR/CRp in this study, and α is an unknown parameter to measure the difference in patients CR/CRp rates between this study and the historical data.
The prior distribution of α was set to be normal, with mean 0 and standard deviation 3, and was updated as the Phase II data in this study accumulated. The goal of increasing CR/CRp rate from 5% to 10% corresponded to α=0.75. The statistical focus of the trial was Pr(α > 0.75|data), the probability that the CR/CRp rate was greater than 10%.
Stopping rules for toxicity and futility were applied in a review by the IDMC after every 20-patient cohort:
Futility: If Pr(α > 0.75|data) < 0.02, stop for futility.
Toxicity. If Pr(Tox > 0.33|data) > 0.95, then terminate the study.
The actual stopping boundaries in terms of number of patients experiencing CR/CRp depended on the covariates of patients in the actual study. Toxicity was defined as unexpected clinically significant elacytarabine-attributable grade 3 or 4 nonhaematological toxicities. Excessive toxicity was defined as toxicity in 11 out of 20 patients, 19 out of 40 patients and 27 out of 60 patients.
Results
Patient characteristics
A total of 61 patients with median age, 58 years (range 25-82 years) were enrolled – baseline patient characteristics are presented in Table 2. Secondary AML was identified in seven patients (11%), five secondary to MDS and two secondary to other antecedent haematological diagnoses. Forty-seven patients had primary refractory AML (Figure 1). The average number of treatment courses per patient was 1.4. Ninety-five percent of the patients treated in course 1 and 77% of patients treated with a second course received ≥80% of the assigned dose.
Table 2.
Patient characteristics (N=61)
| Number of patients | 61 |
| Age (years) | |
| Median | 58 |
| Range | 25-82 |
| Sex | |
| Male | 40 (66%) |
| Female | 21 (34%) |
| Eastern Cooperative Oncology Group Performance Status | |
| 0 | 14 (23%) |
| 1 | 36 (59%) |
| 2 | 11 (18%) |
| Number of previous chemotherapy regimens | |
| 2 | 49 (80%) |
| 3 | 9 (15%) |
| 4 | 2 (3%) |
| Concomitant use of medication | |
| Antineoplastic agents (hydroxycarbamide) | 9 (15%) |
| Immunostimulants (granulocyte colony-stimulating factor) | 14 (23%) |
Response to treatment
CR or CRp was achieved in 11 patients (18%); 5 had a CR and 6 a CRp. PR was seen in 2 patients (Table 3). Eight of the 11 patients achieved CR or CRp after the first treatment course and 3 after the second course. Of these 3 patients, 2 had documented PR at the end of course 1. The test for futility was performed on the final study data and Pr(α > 0.75|data) was estimated to 0.99830. The median time from start of treatment to remission was 48 days (range 28-114 days). The median duration of remission was 95 days (range 4 – 230 days). Of the 11 patients with CR or CRp, 2 were still in remission after 6 months. Median duration of neutrophil count recovery in peripheral blood was available for 9 of the patients in remission and was 34 days (range 22-40 days).
Table 3.
Best response by risk groups (N=61)
| Response1 | Total | Low / Intermediate 1 risk group | Intermediate 2 risk group | High risk group |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| CR | 5 (8) | 2 (40) | 3 (13) | |
| CRp | 6 (10) | 1 (4) | 5 (16) | |
| PR | 2 (3) | 1 (4) | 1 (3) | |
| NR | 40 (66) | 3 (60) | 16 (67) | 21 (66) |
| NE | 8 (13) | 3 (13) | 5 (16) | |
| Total | 61 (100) | 5 (8) | 24 (39) | 32 (52) |
CR = complete response
CRp = CR with incomplete platelet recovery
PR = Partial response
NR = No response
NE = Not evaluable
Prognostic factors for CR and survival compared to historical control
Patients were grouped into risk groups low, intermediate 1, intermediate 2, and high. The distribution of prognostic factors and risk groups were compared to the described historical control group (see Patients and Methods) (Table 1). The majority of patients were in the intermediate 2 or high risk group, both in the study (91%), and in the control group (71%). In the study, 52% were in the high-risk group alone compared to 33% in the control. The remission rate of 18%, when matched on the distribution of prognostic factors, was significantly better than that seen in the control population (4%): p<0.0001.
Survival and referral to stem cell treatment
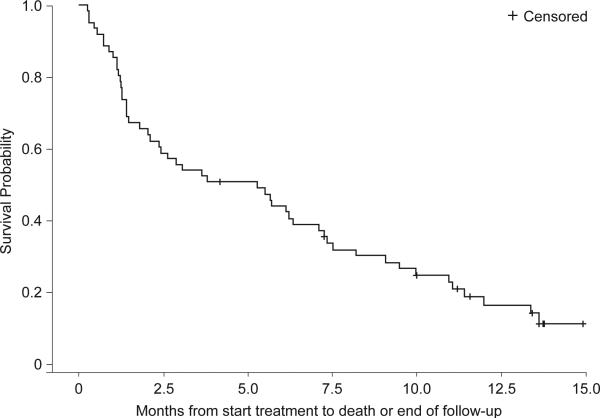
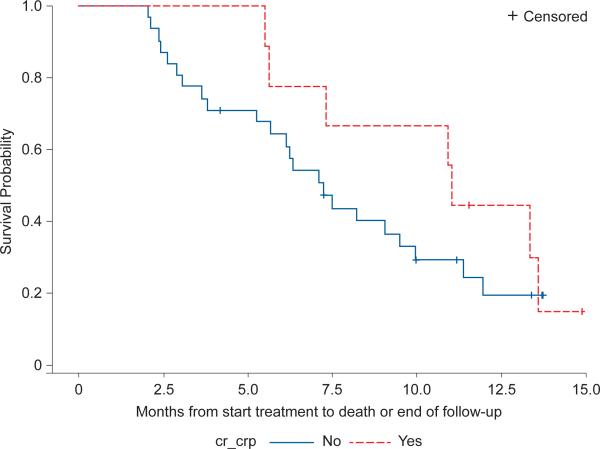
The 6-month survival rate was 43% and the median overall survival was 5.3 months (range 0.3 -14.9+ months) (Figure 2). For those with a CR or CRp, the 6-month survival rate was 64% and the median survival was 10.9 months (13.1 months for the CR population alone) (Figure 2). Median overall survival in the control population was 1.5 months.
Figure 2.
a Survival (n=61)
b Survival per response status, CR/CRp vs. others, (n=40) in patients still alive after 2 months
Ten patients (16%) patients were referred for SCT after achieving remission or experiencing clinical benefit compared to 12% in the control population. Four patients had achieved remission prior to SCT.
Safety and patient tolerance
Of the 61 patients on study, 60 had at least one AE; 51 had an AE that was attributed to elacytarabine by the Investigator and 40 had at least one AE with CTCAE grade 3 or 4 of possible relation to elacytarabine. The most commonly reported AEs were gastrointestinal; nausea (43 patients; 71%), diarrhoea (38 patients; 62%), vomiting (27 patients; 44%) and myelosuppression; thrombocytopenia (26 patients; 43%), leucopenia (25 patients; 41%) and anaemia (22 patients; 36%), hypokalaemia (25 patients; 41%), pyrexia (24 patients; 39%), fatigue (23 patients; 38%), and febrile neutropenia (22 patients; 36%).
The most commonly reported AEs with CTCAE grades 3 or 4 are presented in Table 4, and were mainly haematological: thrombocytopenia, leucopenia, anaemia, and neutropenia. Of non-haematological AEs of CTCAE grades 3 or 4, the most frequent (>5% of the patients) were: febrile neutropenia, hypokalaemia, fatigue, hyponatraemia, dyspnoea and pyrexia. Taken together, the side effects of elacytarabine were predictable and manageable.
Table 4.
Grade 3-4 Adverse events occurring in > 5% of all patients1, N=61
| Event | % All Patients (n = 61) | % Patients ≥ 65 years (n=15) |
|---|---|---|
| Anaemia | 31 | 13 |
| Febrile neutropenia | 36 | 27 |
| Leucopenia | 41 | 27 |
| Lymphopenia | 10 | 20 |
| Neutropenia | 30 | 20 |
| Thrombocytopenia | 43 | 53 |
| Diarrhoea | 7 | 7 |
| Nausea | 7 | 0 |
| Fatigue | 13 | 0 |
| Pyrexia | 11 | 13 |
| Alanine aminotransferase increased | 7 | 7 |
| Aspartate aminotransferase increased | 10 | 7 |
| Hyperbilirubinaemia | 10 | 13 |
| Cellulitis | 5 | 13 |
| Pneumonia | 10 | 0 |
| Sepsis | 10 | 13 |
| Staphylococcal sepsis* | 3 | 13 |
| Hypoalbuminaemia | 7 | 0 |
| Hypokalaemia | 26 | 7 |
| Hyponatraemia | 13 | 7 |
| Hypophosphataemia | 8 | 0 |
| Dyspnea | 15 | 7 |
| Hypoxia | 10 | 0 |
| Hypotension | 7 | 7 |
In addition Grade 3-4 adverse events occurring more than once in patients ≥ 65 years
Fifteen patients aged 65 years or older were enrolled into the study. The most commonly reported AEs with CTCAE grades 3 or 4 for this group of patients are presented in Table 4. The oldest patient, 82 years of age, tolerated 5 courses of elacytarabine therapy administered over six months.
Fifty-one (84% of patients) deaths were recorded, of which 32 (53%) were attributed to progressive disease, two (3%) were due to adverse events (not including symptoms of disease progression) and 17 (28%) were categorized as “other”. Seventeen patients experienced AEs of CTCAE grade 5, with death occurring at a mean of 33.0 days. One patient developed pneumonia that was assigned a possible causal relationship. All other AEs of CTCAE grade 5 were either unrelated or unlikely to be related to the study treatment.
30-day mortality after start of treatment was 13% (8/61), compared to 25% in the historical control group.
Discussion
In this Phase II study in patients with relapsed/refractory AML, elacytarabine monotherapy resulted in a higher remission rate of 18% and improved survival of 5.3 months when compared to a large historical control group. The majority of patients (91%) on study were in the intermediate 2 or high risk groups, based on adverse prognostic factors established previously (Giles et al, 2005). The 6-month survival rate for the total population was 43%. For those with a CR or CRp, the 6-month survival rate was 64% and the median survival was 10.9 months (13.1 months for the CR population alone). The study enrolled patients with very refractory AML and a short life expectancy. Ten (16%) patients achieved remission or clinical benefit enabling them to receive SCT post-elacytarabine therapy, compared to 12% in the historical control cohort. SCT represents a significant change in goal of therapy from response to potential cure.
The AE profile of elacytarabine was consistent with the profile reported in Phase I studies (Giles et al, 2012a, Giles et al, 2012b), similar to that seen with cytarabine therapy and expected for patients with advanced AML. Patients up to 82 years were treated with elacytarabine 2000 mg/m2/day, a dose that is equivalent to 1 g of cytarabine (elaidic acid/cytarabine, 50% w/w). The absence of neurological (and specifically cerebellar) toxicity was consistent with findings in Phase I studies and is particularly encouraging in light of recent recommendations that most patients up to 80 years of age with AML should be considered for intensive therapy (Juliusson et al, 2009). Given that many of the AEs reported in this study are very common in patients with advanced AML, it is difficult to distinguish between those associated with the drug and those associated with the underlying leukaemia. Elevation of liver function tests, including bilirubin, was mostly transient and consistent with findings in the Phase I studies. Many events, such as infections, anaemia and fatigue, often occur as a consequence of the disease. Although elacytarabine was administered continuously at high doses, 2000 mg/m2/day, over 5 days, the treatment-related mortality, measured as 30-day mortality, was only 13%. This is significantly better than that seen in the historical control group (25%) where high dose cytarabine was one of the treatment options (Giles et al, 2005).
The assessment per 20 patient cohorts did not stop the study for futility or toxicity. Clinical activity was seen consistently throughout the study cohorts. The goal was to achieve an increase in the remission rate from 5% to 10%, and after the third cohort review this was reached. The study was designed to enroll up to 200 patients but was stopped early due to highly promising activity through improved remission rates and survival. It was decided to proceed to a pivotal Phase III randomized study.
It remains an important future question to investigate the relationship between the independence of elacytarabine from the need for nucleoside transporters and its clinical activity in AML. Reduced hENT1 expression and activity is associated with adverse therapeutic outcomes and reduced cytotoxicity for patients treated with cytarabine (Galmarini et al, 2002a, Hubeek et al, 2005) as for those treated for other haematological malignancies with other nucleoside analogues (Fernandez Calotti et al, 2008, Giovannetti et al, 2007, Macke et al, 2005, Marce et al, 2006, Oguri et al, 2007, Qin et al, 2009).
Patients with refractory AML have a poor prognosis. Most of the reports and the literature regarding salvage therapy are limited to patients with either primary refractory AML or those in first relapse, thus focusing on the results of first salvage therapy. Very few studies limit eligibility to AML in second relapse. Relevant recent literature has investigated the use of cytarabine with a novel agent and eligibility was quite restricted (Chevallier et al, 2008, Cortes et al, 2011, Eom et al, 2010, Kohrt et al, 2010, Montillo et al, 2009, Perl et al, 2009, Schimmer et al, 2009, Wierzbowska et al, 2008). Four recent reports investigating the use of cytarabine with novel agents that did include patients in second relapse or greater included 3 of 82 patients in an ECOG Trial (Litzow et al, 2010), and 3 of 60 patients in a Cancer and Leukemia Group B Study (Stone et al, 2011). In one trial investigating the use of azacitidine with cytarabine, multiply relapsed patients were included. The median number of prior therapies was 2 (range 0-6). The response rate was 33% in minimally pretreated patients but was 0% in multiply relapsed patients (Borthakur et al, 2010). A recent trial, describing the regimen of granulocyte colony-stimulating factor, clofarabine and high-dose cytarabine conducted in 46 patients, concluded that this was a highly active regimen. Nevertheless, of 5 patients in 3rd or greater salvage (analogous to the current group), no remissions occurred (Becker et al, 2011). Most patients with multiply relapsed disease are excluded from clinical trials because of their known poor prognosis. The current population had a CR rate that was over 4 times that predicted based on the results in comparative patients from the historical control cohort. This suggests the significant increase in potency expected with elacytarabine by virtue of its ability to enter AML blasts independent of nucleoside transporters.
Further investigations with elacytarabine single agent and in combination with an anthracycline are ongoing in refractory or relapsed AML. Based on this study's data, elacytarabine single agent is being compared with standard of care in patients with advanced AML in a randomized Phase III trial (NCT01147939, CLAVELA study). Elacytarabine is also being combined with idarubicin (NCT01035502) in patients refractory to one course of standard induction therapy, 7+3 (cytarabine + anthracycline).
Acknowledgements
S O'Brien, DA Rizzieri, Norbert Vey, F Ravandi, UO Krug, MA Sekeres, M. Dennis, A Venditti, DA Berry, TF Jacobsen, K Staudacher, T Bergeland and FJ Giles performed the research, collected and analysed data, wrote the paper and approved its final version. S O'Brien, DA Berry and FJ Giles designed the research.
We are thankful to the patients and study teams for their dedication and essential contributions; Bo I Nilsson, MD, PhD, deceased, for his continuous medical expertise and personal engagement in the study; Athos Gianella-Borradori, MD, Clavis Pharma for review of the manuscript.
Footnotes
Conflict of interest:
S O'Brien, DA Rizzieri, N Vey, U Krug, MA Sekeres, M Dennis, A Venditti, FJ Giles have received research funding from Clavis Pharma, TF Jacobsen, K Staudacher and T Bergeland are/were Clavis Pharma employees.
References
- Adams DJ, Sandvold ML, Myhren F, Jacobsen TF, Giles F, Rizzieri DA. Anti proliferative activity of ELACYT (CP-4055) in combination with cloretazine (VNP40101M), idarubicin, gemcitabine, irinotecan and topotecan in human leukemia and lymphoma cells. Leukemia Lymphoma. 2008;49:786–797. doi: 10.1080/10428190801935752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adema AD, Laan AC, Myhren F, Fichtner I, Verheul HM, Sandvold ML, Peters GJ. Cell cycle effects of fatty acid derivatives of cytarabine, CP-4055, and of gemcitabine, CP-4126, as basis for the interaction with oxaliplatin and docetaxel. International Journal of Oncology. 2010a;36:285–294. [PubMed] [Google Scholar]
- Adema AD, Losekoot N, Smid K, Kathmann I, Myhren F, Sandvold ML, Peters GJ. Induction of resistance to the lipophilic cytarabine prodrug elacytarabine (CP-4055) in CEM leukemic cells. Nucleosides Nucleotides Nucleic Acids. 2010b;29:394–399. doi: 10.1080/15257771003741166. [DOI] [PubMed] [Google Scholar]
- Adema AD, Smid K, Losekoot N, Honeywell RJ, Verheul HM, Myhren F, Sandvold ML, Peters GJ. Metabolism and accumulation of the lipophilic deoxynucleoside analogs elacytarabine and CP-4126. Investigational New Drugs. 2011 doi: 10.1007/s10637-011-9756-8. 10.1007/s10637-011-9756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker PS, Kantarjian HM, Appelbaum FR, Petersdorf SH, Storer B, Pierce S, Shan J, Hendrie PC, Pagel JM, Shustov AR, Stirewalt DL, Faderl S, Harrington E, Estey EH. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. British Journal of Haematology. 2011;155:182–189. doi: 10.1111/j.1365-2141.2011.08831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman AM, Kuiper CM, Voorn DA, Comijn EM, Myhren F, Sandvold ML, Hendriks HR, Peters GJ. Antiproliferative activity and mechanism of action of fatty acid derivatives of arabinofuranosylcytosine in leukemia and solid tumor cell lines. Biochemical Pharmacology. 2004;67:503–511. doi: 10.1016/j.bcp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Berry DA. Monitoring accumulating data in a clinical trial. Biometrics. 1989;45:1197–1211. [PubMed] [Google Scholar]
- Berry DA. Bayesian clinical trials. Nature Reviews in Drug Discovery. 2006;5:27–36. doi: 10.1038/nrd1927. [DOI] [PubMed] [Google Scholar]
- Borthakur G, Huang X, Kantarjian H, Faderl S, Ravandi F, Ferrajoli A, Torma R, Morris G, Berry D, Issa JP. Report of a phase 1/2 study of a combination of azacitidine and cytarabine in acute myelogenous leukemia and high-risk myelodysplastic syndromes. Leukemia & Lymphoma. 2010;51:73–78. doi: 10.3109/10428190903318329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breistol K, Balzarini J, Sandvold ML, Myhren F, Martinsen M, De Clercq E, Fodstad O. Antitumor activity of P-4055 (elaidic acid-cytarabine) compared to cytarabine in metastatic and s.c. human tumor xenograft models. Cancer Research. 1999;59:2944–2949. [PubMed] [Google Scholar]
- Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. Journal of Clinical Oncology. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of Clinical Oncology. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Chevallier P, Delaunay J, Turlure P, Pigneux A, Hunault M, Garand R, Guillaume T, Avet-Loiseau H, Dmytruk N, Girault S, Milpied N, Ifrah N, Mohty M, Harousseau JL. Long-term disease-free survival after gemtuzumab, intermediate-dose cytarabine, and mitoxantrone in patients with CD33(+) primary resistant or relapsed acute myeloid leukemia. Journal of Clinical Oncology. 2008;26:5192–5197. doi: 10.1200/JCO.2007.15.9764. [DOI] [PubMed] [Google Scholar]
- Cortes J, Kantarjian H, Ball ED, Dipersio J, Kolitz JE, Fernandez HF, Goodman M, Borthakur G, Baer MR, Wetzler M. Phase 2 randomized study of p53 antisense oligonucleotide (cenersen) plus idarubicin with or without cytarabine in refractory and relapsed acute myeloid leukemia. Cancer. 2011;118:418–427. doi: 10.1002/cncr.26292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju VL, Sawyer MB, Mackey JR, Young JD, Cass CE. Human nucleoside transporters: biomarkers for response to nucleoside drugs. Nucleosides Nucleotides Nucleic Acids. 2009;28:450–463. doi: 10.1080/15257770903044499. [DOI] [PubMed] [Google Scholar]
- Dueland S, Aamdal S, Lind MJ, Thomas H, Sandvold ML, Gaullier JM, Rasch W. Intravenous administration of CP-4055 (ELACYT) in patients with solid tumours. A Phase I study. Acta Oncologica. 2009;48:137–145. doi: 10.1080/02841860802183620. [DOI] [PubMed] [Google Scholar]
- Eom KS, Min WS, Kim HJ, Cho BS, Choi SM, Lee DG, Lee S, Min CK, Kim YJ, Cho SG, Lee JW, Kim CC. FLANG salvage chemotherapy is an effective regimen that offers a safe bridge to transplantation for patients with relapsed or refractory acute myeloid leukemia. Medical Oncology. Suppl. 2010;281:462–470. doi: 10.1007/s12032-010-9653-6. [DOI] [PubMed] [Google Scholar]
- Fernandez Calotti P, Galmarini CM, Canones C, Gamberale R, Saenz D, Avalos JS, Chianelli M, Rosenstein R, Giordano M. Modulation of the human equilibrative nucleoside transporter1 (hENT1) activity by IL-4 and PMA in B cells from chronic lymphocytic leukemia. Biochemical Pharmacology. 2008;75:857–865. doi: 10.1016/j.bcp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Galmarini CM, Thomas X, Calvo F, Rousselot P, El Jafaari A, Cros E, Dumontet C. Potential mechanisms of resistance to cytarabine in AML patients. Leukemia Research. 2002a;26:621–629. doi: 10.1016/s0145-2126(01)00184-9. [DOI] [PubMed] [Google Scholar]
- Galmarini CM, Thomas X, Calvo F, Rousselot P, Rabilloud M, El Jaffari A, Cros E, Dumontet C. In vivo mechanisms of resistance to cytarabine in acute myeloid leukaemia. British Journal of Haematology. 2002b;117:860–868. doi: 10.1046/j.1365-2141.2002.03538.x. [DOI] [PubMed] [Google Scholar]
- Galmarini CM, Myhren F, Sandvold ML. CP-4055 and CP-4126 are active in ara-C and gemcitabine-resistant lymphoma cell lines. British Journal of Haematology. 2009;144:273–275. doi: 10.1111/j.1365-2141.2008.07467.x. [DOI] [PubMed] [Google Scholar]
- Giles F, O'Brien S, Cortes J, Verstovsek S, Bueso-Ramos C, Shan J, Pierce S, Garcia-Manero G, Keating M, Kantarjian H. Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer. 2005;104:547–554. doi: 10.1002/cncr.21187. [DOI] [PubMed] [Google Scholar]
- Giles F, Verstovsek S, Garcia-Manero G, Thomas D, Ravandi F, Wierda W, Ferrajoli A, Kornblau S, Jabbour E, Shan J, O'Brien S, Albitar M, Kantarjian H. Validation of the European Prognostic Index for younger adult patients with acute myeloid leukaemia in first relapse. British Journal of Haematology. 2006;134:58–60. doi: 10.1111/j.1365-2141.2006.06106.x. [DOI] [PubMed] [Google Scholar]
- Giles F, Rizzieri D, Ravandi F, Swords R, Jacobsen TF, O'Brien S. Elacytarabine, a novel 5′-elaidic acid derivative of cytarabine, and idarubicin combination is active in refractory acute myeloid leukemia. Leukemia Research. 2012a;36:71–73. doi: 10.1016/j.leukres.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles FJ, Vey N, Rizzieri D, Ravandi F, Prebet T, Borthakur G, Jacobsen TF, Hagen S, Nilsson B, O'Brien S. Phase I and pharmacokinetic study of elacytarabine, a novel 5′-elaidic acid derivative of cytarabine, in adults with refractory hematologic malignancies. Leukemia. 2012b Jan 6; doi: 10.1038/leu.2012.1. doi: 10.1038/leu.2012.1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Giovannetti E, Mey V, Loni L, Nannizzi S, Barsanti G, Savarino G, Ricciardi S, Del Tacca M, Danesi R. Cytotoxic activity of gemcitabine and correlation with expression profile of drug-related genes in human lymphoid cells. Pharmacology Research. 2007;55:343–349. doi: 10.1016/j.phrs.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Hubeek I, Stam RW, Peters GJ, Broekhuizen R, Meijerink JP, van Wering ER, Gibson BE, Creutzig U, Zwaan CM, Cloos J, Kuik DJ, Pieters R, Kaspers GJ. The human equilibrative nucleoside transporter 1 mediates in vitro cytarabine sensitivity in childhood acute myeloid leukaemia. British Journal of Cancer. 2005;93:1388–1394. doi: 10.1038/sj.bjc.6602881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Matsushita H, Asai S, Tsukamoto H, Ono R, Nosaka T, Yahata T, Takahashi S, Miyachi H. FLT3-ITD induces ara-C resistance in myeloid leukemic cells through the repression of the ENT1 expression. Biochemical Biophysical Research Communications. 2009;390:1001–1006. doi: 10.1016/j.bbrc.2009.10.094. [DOI] [PubMed] [Google Scholar]
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, Tidefelt U, Wahlin A, Hoglund M. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- Kohrt HE, Patel S, Ho M, Owen T, Pollyea DA, Majeti R, Gotlib J, Coutre S, Liedtke M, Berube C, Alizadeh AA, Medeiros BC. Second-line mitoxantrone, etoposide, and cytarabine for acute myeloid leukemia: a single-center experience. American Journal of Hematology. 2010;85:877–881. doi: 10.1002/ajh.21857. [DOI] [PubMed] [Google Scholar]
- Litzow MR, Othus M, Cripe LD, Gore SD, Lazarus HM, Lee SJ, Bennett JM, Paietta EM, Dewald GW, Rowe JM, Tallman MS. Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: a report from the Eastern Cooperative Oncology Group. British Journal Haematology. 2010;148:217–225. doi: 10.1111/j.1365-2141.2009.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey JR, Galmarini CM, Graham KA, Joy AA, Delmer A, Dabbagh L, Glubrecht D, Jewell LD, Lai R, Lang T, Hanson J, Young JD, Merle-Beral H, Binet JL, Cass CE, Dumontet C. Quantitative analysis of nucleoside transporter and metabolism gene expression in chronic lymphocytic leukemia (CLL): identification of fludarabine-sensitive and -insensitive populations. Blood. 2005;105:767–774. doi: 10.1182/blood-2004-03-1046. [DOI] [PubMed] [Google Scholar]
- Marce S, Molina-Arcas M, Villamor N, Casado FJ, Campo E, Pastor-Anglada M, Colomer D. Expression of human equilibrative nucleoside transporter 1 (hENT1) and its correlation with gemcitabine uptake and cytotoxicity in mantle cell lymphoma. Haematologica. 2006;91:895–902. [PubMed] [Google Scholar]
- Montillo M, Ricci F, Tedeschi A, Cafro AM, Nosari AM, Nichelatti M, Marbello L, Morra E. Twice daily fludarabine/Ara-C associated to idarubicin, G-CSF and ATRA is an effective salvage regimen in non-promyelocytic acute myeloid leukemia. Leukemia Research. 2009;33:1072–1078. doi: 10.1016/j.leukres.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Oguri T, Achiwa H, Muramatsu H, Ozasa H, Sato S, Shimizu S, Yamazaki H, Eimoto T, Ueda R. The absence of human equilibrative nucleoside transporter 1 expression predicts nonresponse to gemcitabine-containing chemotherapy in non-small cell lung cancer. Cancer Letters. 2007;256:112–119. doi: 10.1016/j.canlet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Perl AE, Kasner MT, Tsai DE, Vogl DT, Loren AW, Schuster SJ, Porter DL, Stadtmauer EA, Goldstein SC, Frey NV, Nasta SD, Hexner EO, Dierov JK, Swider CR, Bagg A, Gewirtz AM, Carroll M, Luger SM. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clinical Cancer Research. 2009;15:6732–6739. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2'-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvold ML, Galmarini C, Myhren F, Peters G. The activity of the lipophilic nucleoside derivatives elacytarabine and CP-4126 in a panel of tumor cell lines resistant to nucleoside analogues. Nucleosides Nucleotides Nucleic Acids. 2010;29:386–393. doi: 10.1080/15257771003729625. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS, Altman JK, Karp JE, Kassis J, Hedley DW, Brandwein J, Xu W, Mak DH, LaCasse E, Jacob C, Morris SJ, Jolivet J, Andreeff M. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. Journal of Clinical Oncology. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RM, Moser B, Sanford B, Schulman P, Kolitz JE, Allen S, Stock W, Galinsky I, Vij R, Marcucci G, Hurd D, Larson RA. High dose cytarabine plus gemtuzumab ozogamicin for patients with relapsed or refractory acute myeloid leukemia: Cancer and Leukemia Group B study 19902. Leukemia Research. 2011;35:329–333. doi: 10.1016/j.leukres.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of. myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Holowiecki J, Kyrcz-Krzemien S, Grosicki S, Giebel S, Skotnicki AB, Piatkowska-Jakubas B, Kuliczkowski K, Kielbinski M, Zawilska K, Kloczko J, Wrzesien-Kus A. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. European Journal of Haematology. 2008;80:115–126. doi: 10.1111/j.1600-0609.2007.00988.x. [DOI] [PubMed] [Google Scholar]