Significance
Tropical ectotherms are considered especially vulnerable to climate change because they have narrow thermal tolerance ranges, such that even small increases in environmental temperature are likely to be detrimental. Although evolutionary adaptation may prevent extinction, it is unclear whether climate change generates selection on thermal physiology in nature or whether the strength of this selection is sufficient for rapid evolution to occur. When we transplanted lizards from their preferred habitat to a warmer and more thermally variable site, strong natural selection favored individuals that ran faster at warmer temperatures and across a broader range of temperatures. If thermal performance traits are heritable, some tropical species may be capable of rapid evolutionary adaptation to changing climates.
Keywords: Bahamas, thermoregulation
Abstract
Tropical ectotherms are thought to be especially vulnerable to climate change because they are adapted to relatively stable temperature regimes, such that even small increases in environmental temperature may lead to large decreases in physiological performance. One way in which tropical organisms may mitigate the detrimental effects of warming is through evolutionary change in thermal physiology. The speed and magnitude of this response depend, in part, on the strength of climate-driven selection. However, many ectotherms use behavioral adjustments to maintain preferred body temperatures in the face of environmental variation. These behaviors may shelter individuals from natural selection, preventing evolutionary adaptation to changing conditions. Here, we mimic the effects of climate change by experimentally transplanting a population of Anolis sagrei lizards to a novel thermal environment. Transplanted lizards experienced warmer and more thermally variable conditions, which resulted in strong directional selection on thermal performance traits. These same traits were not under selection in a reference population studied in a less thermally stressful environment. Our results indicate that climate change can exert strong natural selection on tropical ectotherms, despite their ability to thermoregulate behaviorally. To the extent that thermal performance traits are heritable, populations may be capable of rapid adaptation to anthropogenic warming.
Anthropogenic climate change may be the single most dramatic physical change our planet has experienced during human history (1). In the tropics, where species are adapted to relatively stable climates, the impacts of climate change are predicted to be especially severe (2–5) (but see refs. 6, 7). Because many species maintain a body temperature (Tb) that is already close to their thermal limits, even small increases in environmental temperature (Te) may produce large decreases in physiological performance that could push populations toward extinction (4).
In tropical environments, evolutionary adaptation may be one of the most important mechanisms by which populations can avoid extinction (8). As climates shift, fitness for many species will become increasingly linked to variation in traits important for performance in a warmer and more thermally variable world (4). Numerous theoretical, laboratory, and field studies demonstrate a capacity for rapid evolution on time scales similar to those over which global warming is predicted to occur (9–11). Indeed, recent work on lizards (12) and butterflies (13) has revealed rapid shifts in thermal physiology that appear to be directly associated with changing thermal environments. Despite mounting evidence that the capacity for evolution may fundamentally alter extinction probabilities in the face of anthropogenic climate change, the potential for rapid evolution is usually not considered in models that attempt to predict the impact of climate change on biological populations (8).
The rate at which thermal physiology can evolve in response to a changing climate depends on generation time (5), the additive genetic variances and covariances underlying phenotypic variation (14), and the strength of selection on traits that influence performance (15, 16). Strong selection is a prerequisite for rapid evolutionary adaptation to global warming, but many ectothermic species may shelter themselves from selection by using behavioral adjustments to maintain a preferred Tb in the face of environmental variation (17, 18). Thermoregulatory behavior may initially buffer individuals against thermal stress, but by reducing the opportunity for selection, these behaviors could also prevent populations from adapting to climate change over the long term (15). Despite the importance of natural selection in the evolutionary responses of organisms to global warming, direct measurements of climate-driven selection on the thermal physiology of natural populations have not previously been reported.
To determine whether and how thermal physiology is subject to natural selection, we measured survival as a function of the thermal sensitivity of sprint speed in two populations of Anolis sagrei lizards from The Bahamas. We first quantified the relationship between thermal performance and survival of 85 males from an unmanipulated population on Kidd Cay, Great Exuma (hereafter, the “reference” population). Next, to test whether a simulated change in thermal environment would increase or otherwise alter selection on thermal performance, we repeated this study on a population of 80 males from the island of Eleuthera that we transplanted from an interior forested habitat (hereafter, the “transplant-source” site) to a warmer, more thermally variable site ∼1 km away (hereafter, the “transplant” population) (Fig. S1). We measured natural selection on three composite traits that describe the shape of thermal performance curves (TPCs) (Fig. 1). These traits—maximal performance (Pmax), the thermal optimum (Topt), and the thermal performance breadth (Tbr)—represent three primary axes of variation along which TPCs can evolve (19). Specifically, we tested the predictions that (i) an increase in mean operative Te is associated with selection for higher Topt, (ii) an increase in temperature variability is associated with selection for wider Tbr, and (iii) Pmax is subject to positive directional selection in all thermal environments.
Fig. 1.
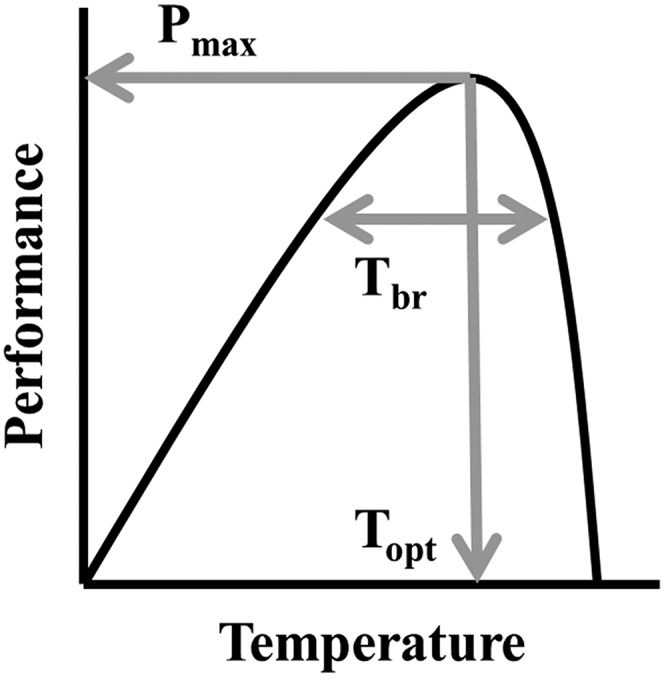
Hypothetical TPC with the characteristic values (thermal performance traits) labeled. The Topt is the temperature at which Pmax is achieved. Tbr is the range of temperatures across which at least 80% of Pmax is achieved.
Results
Thermal Variation Among Sites.
The operative thermal environments (the Te distribution available to each population, as measured with copper models) differed among sites (ANOVA: F2,76 = 46.69, P < 0.001). Mean Te at the transplant site was 2.7 °C higher than at the reference site (Tukey post hoc comparison: P < 0.001) and 2.3 °C higher than at the transplant-source site (Tukey post hoc comparison: P < 0.001), differences that are similar to the projected change in mean temperature in the Caribbean for the next 90 y (20). Daily and seasonal variance in Te was also higher at the transplant site in a manner congruent with predictions for changes in temperature variability resulting from climate change (20). By contrast, mean Te did not differ between the reference and transplant-source sites (Tukey post hoc comparison: P = 0.58), and seasonal variation in Te was also broadly similar between these two sites (Figs. S2 and S3). Thus, before the transplantation, populations on both islands should have experienced similar selective environments with respect to thermal traits (Table S1).
Lizard Tb.
Differences in operative thermal environments among sites were associated with differences in lizard Tb (ANOVA: F2,175 = 8.13, P < 0.01; Fig. S4). Mean and maximum Tb of lizards at the transplant site (mean Tb = 34.4 ± 0.21 °C, maximum Tb = 38.6 °C) were significantly higher than at the reference site (mean Tb = 32.9 ± 0.24 °C, maximum Tb = 36.4 °C; Tukey post hoc comparison for difference in mean: P = 0.002) and the transplant-source site (mean Tb = 33.3 ± 0.18 °C, maximum Tb = 38.1 °C; Tukey post hoc comparison for difference in mean: P = 0.002). By contrast, mean Tb did not differ between the reference and transplant-source sites (Tukey post hoc comparison: P = 0.57).
Natural Selection on Thermal Performance.
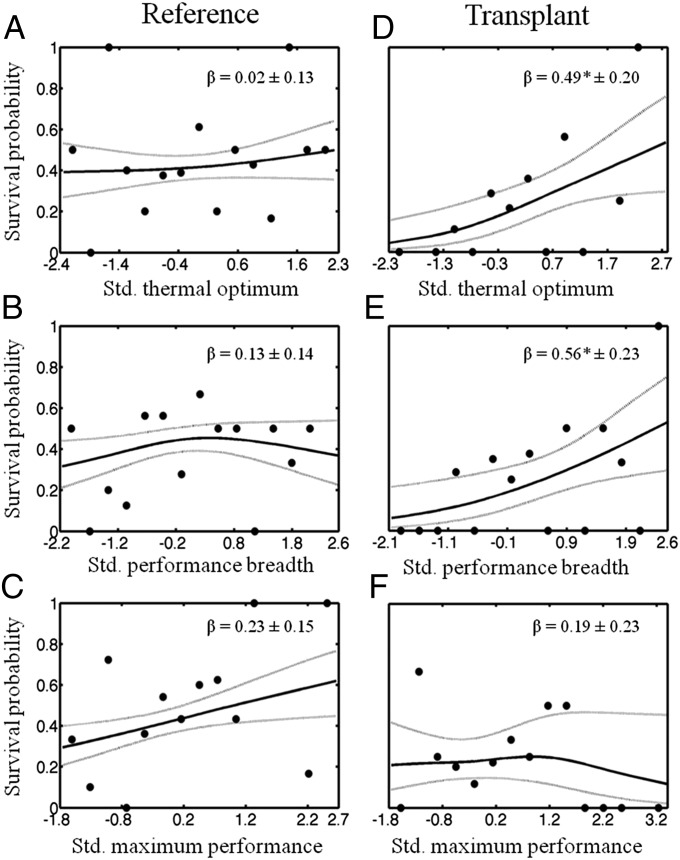
At the reference and transplant sites, 45% and 22% of lizards survived the breeding season, respectively. Selection did not occur on any performance trait at the reference site (Fig. 2 A–C and Table 1), but lizards transplanted to the warmer and more thermally variable site underwent strong directional selection for higher Topt (β = 0.49 ± 0.20, χ2 = 5.47, P = 0.02; Fig. 2D) and wider Tbr (β = 0.56 ± 0.23, χ2 = 5.33, P = 0.02; Fig. 2E). We did not detect nonlinear (stabilizing or disruptive) selection on any trait at either site. Selection gradients generated by resampling the trait distributions (1,000 bootstrap iterations sampling with replacement) were very similar to the observed selection gradients for each population (Table S2). Moreover, a comparison of regression slopes at the two sites revealed significant differences in directional selection acting on Topt (Topt × Site: χ2 = 7.80, P = 0.005) and Tbr (Tbr × Site: χ2 = 5.11, P = 0.02). As predicted, Pmax was under weak (but nonsignificant) positive selection at both sites (reference: β = 0.23 ± 0.15, χ2 = 2.51, P = 0.11; transplant: β = 0.19 ± 0.23, χ2 = 0.81, P = 0.36), and selection on Pmax did not differ between sites (Pmax × Site: χ2 = 0.002, P = 0.96; Table 1). Two alternative methods of fitting TPCs (Materials and Methods) produced similar results (Table S3).
Fig. 2.
Univariate fitness surfaces (cubic splines from 500 bootstrap iterations) illustrating survival as a function of standardized values for the Topt, Tbr, and Pmax, traits that describe the shape of the TPC. Significant selection did not occur on any trait in the reference population (A–C), whereas positive directional selection (β) occurred on both the Topt (D) and Tbr (E) in the transplant population. Asterisks denote statistically significant gradients based on generalized linear regressions (Table 1). Points represent average survival probabilities for phenotypic values grouped into 15 equal-sized bins. Dotted lines represent ±1 SE. Std., standard.
Table 1.
Selection gradients, associated SEs, and significance values for individual and pooled populations
| Reference | Transplant | Tests for site differences | |||||||
| Trait | β/γ | SE | P | β/γ | SE | P | Interaction | χ2 | P |
| Topt | 0.02 | 0.13 | 0.87 | 0.49 | 0.20 | 0.02 | Topt × Site | 7.80 | 0.0005 |
| Pmax | 0.23 | 0.15 | 0.11 | 0.19 | 0.23 | 0.36 | Pmax × Site | 0.002 | 0.96 |
| Tbr | 0.13 | 0.14 | 0.35 | 0.56 | 0.23 | 0.02 | Tbr × Site | 5.11 | 0.02 |
| Topt2 | 0.18 | 0.20 | 0.37 | −0.30 | 0.30 | 0.13 | Topt2 × Site | 8.95 | 0.003 |
| Pmax2 | 0.08 | 0.22 | 0.71 | −0.14 | 0.32 | 0.62 | Pmax2 × Site | 0.96 | 0.32 |
| Tbr2 | −0.40 | 0.22 | 0.06 | −0.34 | 0.36 | 0.41 | Tbr2 × Site | 0.11 | 0.74 |
| Site | 0.70 | 0.38 | |||||||
Significant selection gradients are shown in boldface. Linear gradients are partial regression coefficients from a multiple regression including only linear terms for all three traits. Quadratic gradients were calculated by doubling quadratic regression coefficients (and their associated SEs) from a separate model that included both linear and quadratic (but not cross-product) terms.
Discussion
In the face of rapid anthropogenic change, evolutionary adaptation may be an important means by which species can avoid extinction (8). The strength and form of selection acting on phenotypic variation are major determinants of whether a population can evolve rapidly (21), yet behavioral thermoregulation is often assumed to reduce the strength of selection on thermal performance traits. Nevertheless, when we simulated a rapid change in the thermal environment by transplanting a population of lizards to a warmer and more thermally variable habitat, we observed strong natural selection on thermal physiology. Our results imply that rapid climate change may result in directional selection on thermal physiology, even in species whose thermoregulatory behaviors are thought to shelter them from natural selection.
It is likely that lizards in the transplanted population experienced a stressful thermal environment; we observed a 1.5 °C increase in mean Tb relative to a 2.7 °C increase in mean operative Te. In this novel thermal environment, natural selection favored those individuals that maximized performance at warmer temperatures (Fig. 2D). The thermal environment of the transplant site was also more temporally variable, and selection favored individuals that maximized performance across a broader range of temperatures (Fig. 2E). Although we did not replicate our experiment at the population level, differences in both Tb and Te among sites (Table 1, Figs. S2–S4, and Table S1) were consistent with the interpretation that climatic variation was responsible for the observed differences in selection. Moreover, we detected weak positive directional selection on Pmax at both sites, suggesting that faster lizards were more likely to survive. This pattern is consistent with previous studies (22, 23) and supports the common assertion that sprint speed is an ecologically relevant measure of performance in lizards.
Changes in operative temperature may have interacted with several aspects of lizard biology to drive natural selection on thermal performance. First, operative temperatures at the transplant site were often well above Topt at midday (Table S1), and these harsh conditions may have forced some individuals into retreat sites to avoid thermal stress. The transplanted lizards that maximized performance at warmer (and more variable) temperatures may have been able to maintain activity during longer periods of the day, permitting more time for energy intake and increasing their chances of surviving the breeding season (24). Second, lizards with higher thermal optima, and therefore above-average sprint capacities at the warmer transplant site, may have been more successful in outcompeting rivals for access to resources (through either interference or exploitative competition) (23). Third, individuals with higher thermal optima may have been better at evading predators (22, 23). It is also possible that the thermal sensitivity of sprint speed is correlated with other measures of performance (e.g., digestive efficiency, immune function) that influenced fitness but that we did not measure directly. The precise mechanism by which variation in the thermal sensitivity of locomotor performance influences the adaptive landscape as thermal environments change remains a promising topic for future study.
Although we have demonstrated that rapid changes in thermal environments can generate strong natural selection on TPCs, evolutionary change will not occur unless thermal performance traits are heritable (25). Typical heritability estimates for thermal performance traits in other species range from 0.2–0.6 (26–29), suggesting that selection acting on Topt and Tbr could drive a rapid evolutionary response to climate change. Assuming these values for heritability, the change in mean Topt and Tbr after a sudden 2.7 °C increase in operative temperature would range from 0.25–0.75 °C and 0.5–1.5 °C, respectively. Thus, even if the amount of warming expected through the end of the century (20) occurred during a single breeding season, this species could hypothetically compensate for as much as 30% of that environmental change through evolutionary adaptation alone. Nevertheless, it is not currently known whether thermal performance traits are heritable in A. sagrei. Indeed, there is a paucity of data describing the genetic architecture underlying TPCs for most species (30), and these data will be critical for predicting the evolutionary responses of ectotherms to climate change.
Our results show that despite their ability to thermoregulate behaviorally, some terrestrial ectotherms may experience strong natural selection in response to climate change. Assuming that the standing crop of additive genetic variance in thermal performance traits is sufficient, many populations may rapidly adapt to changing climates (12, 31). We suggest that future studies consider the importance of climate change as a selective force when assessing the adaptive capacity of ectotherms around the globe.
Materials and Methods
Study Sites and Experimental Design.
We measured natural selection using males from two wild populations of A. sagrei on two islands in The Bahamas. We focused on males because A. sagrei is extremely sexually dimorphic, such that selection often acts in a sex-specific manner (32, 33). One population (the reference population), measured in 2011, occurred naturally on Kidd Cay (21), near Georgetown, Great Exuma. Kidd Cay is a small (∼1,600 m2) island connected to Great Exuma by a concrete causeway. The second population (the transplant population), measured in 2012 on the island of Eleuthera (33), was experimentally transplanted from an interior forest habitat to a small (∼1,300 m2) peninsular site 1 km away (Fig. S1). The majority of resident males were removed from the peninsula before introducing the study population.
At the onset of the breeding season (May), we measured the thermal sensitivity of locomotor performance (sprint speed) for 108 and 98 adult males at the reference and transplant sites, respectively. Only those individuals that were reliably measured at four of five temperatures (including the lowest and highest temperatures) were included in selection analyses, resulting in final sample sizes of 85 and 80 males, respectively. Sprint speed is an ecologically relevant trait that is known to correlate with survival (22, 23) and may also provide a representative index of whole-organism physiological performance (34). Near the end of the breeding season (late August), we returned to both sites and attempted to recapture all surviving individuals to estimate viability selection. Dispersal was not a concern for the reference site, which was an island. For the peninsular transplant population, we sampled 100 m beyond the dirt road separating the study site from the remainder of the peninsula (Fig. S1). All recaptured individuals were found on the study site except for two, which were found less than 5 m north of it. Thus, we assumed the majority of individuals did not disperse out of the study site. Typical recapture rates for adult males in these populations are 90–95% (21, 33).
Estimating Thermal Performance Traits.
We used a curve-fitting procedure (see below) to estimate three traits for each individual: (i) Pmax, the predicted maximum sprint speed; (ii) Topt, the temperature at which performance is predicted to be maximal; and (iii) Tbr, the range of temperatures over which an individual is predicted to achieve at least 80% of Pmax. These traits are the characteristic values that describe the shape of the TPC (30).
After capturing each lizard by noose or hand and allowing it to acclimate to a stable indoor environment for 24 h, we measured its sprint speed five times at each of five temperatures (in a random order for each individual): 15 °C, 22 °C, 28 °C, 36 °C, and 42 °C, except in the case of 10 individuals for which we were unable to obtain sprint speeds at 22 °C. These temperatures bound the performance curve at its cool and warm extremes, and include estimates of running speed in the region of the curve where the instantaneous rate of change is fastest (30 °C and 36°C, which are near Topt). We brought individuals to these target temperatures by heating or cooling them in a thermostat-controlled temperature chamber, and we verified that they had achieved the target temperature using a cloacal thermometer. After a lizard achieved its target temperature, we motivated it to run along a 3-cm diameter wooden dowel (demarcated every 5 cm), inclined at an angle of 20° to discourage hopping (22). We filmed trials with high-speed (60 frames per second) digital video. We always provided lizards a minimum of 2 h of rest between trials at different temperatures, and we never held them in captivity for more than 60 h. We did not feed lizards during this time, and we provided water ad libitum.
For each trial at each temperature, we computed the fastest running speed over 10 cm using frame-by-frame analysis in the motion analysis software program Eagle Eye Pro Viewer. If lizards did not perform a “good” run by the time 30 s had elapsed, we did not include that trial in further analyses. A good run was one in which the individual remained on the vertical surface of the dowel without falling off. If the lizard could not run at least 10 cm without stopping, we considered that to be a speed of 0 m/s.
To estimate the TPC of each individual lizard, we fit a set of left-skewed parabolic equations built into the program TableCurve 2D (Systat Software, Inc.) to the raw sprint data (Fig. S5). We chose these equations based on the established general shape of TPCs, which are presumably structured by the thermodynamics of enzyme function (19, 35). We chose the best fit for each individual using Akaike’s Information Criterion (AIC) (36). If two equations did not differ significantly in their AIC score, we chose the one with fewer parameters. If curves did not differ in AIC score or the number of parameters, we chose the curve with the highest r2 value. As a complementary approach (Table S3), we combined data from all individuals (mean performance at each temperature for each animal) and used the AIC to select the single model (in this case, a four-parameter log-normal function) that best fit the entire dataset. We then estimated TPCs for each individual using this model, which reduced final sample sizes to 56 (reference) and 54 (transplant) lizards, because the model could not be optimized for ∼35% of the individuals in each population. This conservative approach yielded patterns of selection that were similar to those generated by fitting a separate best-fit model to each individual (Table S3).
Measuring the Thermal Environment.
We deployed operative temperature models (OTMs) at each of our study sites to generate distributions of instantaneous Te (37). To achieve rapid thermal equilibration, OTMs were built with type-M (thin-walled) copper piping (30). Models were cut to the mean length and mass of A. sagrei and painted to approximate the appropriate photo-spectrum absorbance (Fig. S6). Ibutton data loggers (Embedded Data Systems) were suspended in nonconductive acrylic mesh within each OTM and set to record temperatures every 1.5 h. We only included temperatures recorded during the diel activity period of A. sagrei (between 0600 and 1800 hours) in our analyses. At the reference site, the transplant site, and the source site for the transplanted population, we deployed 27, 28, and 25 OTMs, respectively. Each OTM was distributed at a random height in the vegetation (0.5–2 m in 0.5-m intervals) and in a random direction (North, East, South, or West) and distance (0–5 m in 1-m intervals) from haphazardly chosen points covering the greatest area possible. At the reference site, OTMs were deployed from May 16 to August 19, 2011. At the transplant and transplant-source sites, OTMs were deployed from May 5 to August 23, 2012. At each site, field-active Tb were measured with a cloacal thermometer (Omega Engineering, Inc.) between 0800 and 1800 hours. For comparisons of Tb among sites, we only included measurements taken during days with “normal” weather (i.e., low cloud cover, no rain) and during time periods when sample sizes were equivalent (0900–1700 hours).
Selection on Thermal Performance Traits.
We estimated viability selection by standardizing trait distributions to a mean of zero and unit variance and by standardizing survival values to population means (25). Before performing analyses, we first tested for problems associated with multicollinearity among traits and found none. Variance inflation factors were all <2, and pairwise correlation coefficients between traits were small (Table S4). We estimated linear and nonlinear selection gradients from separate multivariate regressions, incorporating the three traits that describe the shape of the TPC (Pmax, Topt, and Tbr). We doubled nonlinear regression coefficients and their respective SEs to estimate quadratic selection. We did not include cross-product terms (i.e., correlational selection) in any models because sample sizes were insufficient to produce robust estimates of these coefficients (25). Significance values for selection gradients were estimated from generalized linear models with a logit link function to account for the binomial-dependent variable (live/dead). We used log-link functions to account for Poisson distributions of relative fitness in between-population comparisons.
To validate selection estimates further, ensuring that they were robust to the inclusion or exclusion of individuals, we performed a bootstrap analysis of the distribution of trait values in each population (1,000 iterations, resampling with replacement). We calculated mean values of the bootstrapped selection gradients (and SEs) for each trait.
We visualized univariate fitness surfaces for each trait using cubic splines (38). In most instances, these univariate splines accurately illustrate the form of selection estimated from multivariate gradients (Table 1), except in the case of weak directional selection on Pmax in the transplant population, which is not evident from the spline.
Supplementary Material
Acknowledgments
We thank B. Calsbeek for help with bootstrap analyses. We acknowledge N. Bottomley, A. Schultz, K. Knight, R. Knight, S. Aland, the Cape Eleuthera Institute, and the Island School for helping with logistics and facilitating the successful completion of this project. Comments and contributions from M. Augat, E. D. Brodie III, B. Calsbeek, C. Cox, L. Culler, M. Duryea, S. Fey, A. Hanninen, M. Hague, R. Huey, A. Kahrl, K. Keegan, N. Lany, O. Molnar, A. Reedy, B. Reinke, B. Sanderson, and C. Wood helped us improve the manuscript. This project was carried out under research permits issued by the Bahamas Environment, Science, and Technology Commission and the Bahamas Ministry of Agriculture in 2011 and 2012.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404885111/-/DCSupplemental.
References
- 1.Schrag DP. Geobiology of the Anthropocene. In: Knoll AH, Konhauser KO, editors. Fundamentals of Geobiology. Chichester, UK: John Wiley & Sons; 2012. [Google Scholar]
- 2.Tewksbury JJ, Huey RB, Deutsch CA. Ecology. Putting the heat on tropical animals. Science. 2008;320(5881):1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- 3.Huey RB, et al. Why tropical forest lizards are vulnerable to climate warming. Proc Biol Sci. 2009;276(1664):1939–1948. doi: 10.1098/rspb.2008.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105(18):6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huey RB, et al. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci. 2012;367(1596):1665–1679. doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingsolver JG, Diamond SE, Buckley LB. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct Ecol. 2013;27(6):1415–1423. [Google Scholar]
- 7.Vasseur DA, et al. Increased temperature variation poses a greater risk to species than climate warming. Proc Biol Sci. 2014;281(1779):1–8. doi: 10.1098/rspb.2013.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. 2011;470(7335):479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- 9.Hoekstra HE, et al. Strength and tempo of directional selection in the wild. Proc Natl Acad Sci USA. 2001;98(16):9157–9160. doi: 10.1073/pnas.161281098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsolver JG, Diamond SE. Phenotypic selection in natural populations: What limits directional selection? Am Nat. 2011;177(3):346–357. doi: 10.1086/658341. [DOI] [PubMed] [Google Scholar]
- 11.Partridge L, Barrie B, Barton NH, Fowler K, French V. Rapid laboratory evolution of adult life-history traits in Drosophila melanogaster in response to temperature. Evolution. 1995;49(3):538–544. doi: 10.1111/j.1558-5646.1995.tb02285.x. [DOI] [PubMed] [Google Scholar]
- 12.Leal M, Gunderson AR. Rapid change in the thermal tolerance of a tropical lizard. Am Nat. 2012;180(6):815–822. doi: 10.1086/668077. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JK, MacLean HJ, Buckley LB, Kingsolver JG. Geographic differences and microevolutionary changes in thermal sensitivity of butterfly larvae in response to climate. Functional Ecology. 2013;28(4):982–989. [Google Scholar]
- 14.Hoffmann AA. Physiological climatic limits in Drosophila: Patterns and implications. J Exp Biol. 2010;213(6):870–880. doi: 10.1242/jeb.037630. [DOI] [PubMed] [Google Scholar]
- 15.Huey RB, Hertz PE, Sinervo B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am Nat. 2003;161(3):357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw WE, Holzapfel CM. Genetic response to rapid climate change: It’s seasonal timing that matters. Mol Ecol. 2008;17(1):157–166. doi: 10.1111/j.1365-294X.2007.03509.x. [DOI] [PubMed] [Google Scholar]
- 17.Huey RB, Slatkin M. Cost and benefits of lizard thermoregulation. Q Rev Biol. 1976;51(3):363–384. doi: 10.1086/409470. [DOI] [PubMed] [Google Scholar]
- 18.Kearney M, Shine R, Porter WP. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc Natl Acad Sci USA. 2009;106(10):3835–3840. doi: 10.1073/pnas.0808913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angilletta MJ, Wilson RS, Navas CA, James RS. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol. 2003;18(5):234–240. [Google Scholar]
- 20.IPCC Climate Change. 2013 2013: the Physical Science Basis (Cambridge University Press, New York), p 1535. [Google Scholar]
- 21.Calsbeek R, Cox RM. Experimentally assessing the relative importance of predation and competition as agents of selection. Nature. 2010;465(7298):613–616. doi: 10.1038/nature09020. [DOI] [PubMed] [Google Scholar]
- 22.Calsbeek R, Irschick DJ. The quick and the dead: Correlational selection on morphology, performance, and habitat use in island lizards. Evolution. 2007;61(11):2493–2503. doi: 10.1111/j.1558-5646.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 23.Miles DB. The race goes to the swift: Fitness consequences of variation in sprint performance in juvenile lizards. Evol Ecol Res. 2004;6(1):63–75. [Google Scholar]
- 24.Sinervo B, et al. Erosion of lizard diversity by climate change and altered thermal niches. Science. 2010;328(5980):894–899. doi: 10.1126/science.1184695. [DOI] [PubMed] [Google Scholar]
- 25.Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37(6):1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 26.Ketola T, Kellermann V, Kristensen TN, Loeschcke V. Constant, cycling, hot and cold thermal environments: Strong effects on mean viability but not on genetic estimates. J Evol Biol. 2012;25(6):1209–1215. doi: 10.1111/j.1420-9101.2012.02513.x. [DOI] [PubMed] [Google Scholar]
- 27.Mousseau TA, Roff DA. Natural selection and the heritability of fitness components. Heredity (Edinb) 1987;59(Pt 2):181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- 28.Ottaviano E, Sari Gorla M, Pè E, Frova C. Molecular markers (RFLPs and HSPs) for the genetic dissection of thermotolerance in maize. Theor Appl Genet. 1991;81(6):713–719. doi: 10.1007/BF00224979. [DOI] [PubMed] [Google Scholar]
- 29.Meffe GK, Weeks SC, Mulvey M, Kandl KL. Genetic differences in thermal tolerance of eastern mosquitofish (Gambusia holbrooki Poeciliidae) from ambient and thermal ponds. Can J Fish Aquat Sci. 1995;52(12):2704–2711. [Google Scholar]
- 30.Angilletta MJ. Thermal Adaptation. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 31.Gunderson AR, Leal M. Geographic variation in vulnerability to climate warming in a tropical Caribbean lizard. Funct Ecol. 2012;26(4):783–793. [Google Scholar]
- 32.Connallon T, Cox RM, Calsbeek R. Fitness consequences of sex-specific selection. Evolution. 2010;64(6):1671–1682. doi: 10.1111/j.1558-5646.2009.00934.x. [DOI] [PubMed] [Google Scholar]
- 33.Cox RM, Calsbeek R. Sex-specific selection and intraspecific variation in sexual size dimorphism. Evolution. 2010;64(3):798–809. doi: 10.1111/j.1558-5646.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- 34.Arnold SJ. Morphology, performance, and fitness. Am Zool. 1983;23(2):347–361. [Google Scholar]
- 35.Angilletta MJ. Estimating and comparing thermal performance curves. J Therm Biol. 2006;31(7):541–545. [Google Scholar]
- 36.Akaike H. Factor-analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 37.Bakken GS. Measurement and application of operative and standard operative temperatures in ecology. Am Zool. 1992;32(2):194–216. [Google Scholar]
- 38.Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42(5):849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.