Significance
Mutation of isocitrate dehydrogenase 1 (IDH1) is believed to be the initiating event for the majority of secondary glioblastomas and lower-grade diffuse gliomas; however, the basis for tissue specificity of oncogenesis initiated by IDH1 mutation has not been apparent. We report evidence to suggest that specialization of human neocortex for glutaminergic neurotransmission provides a metabolic niche particularly suited for growth of IDH1R132H glioma. Our findings reveal that IDH1-mutant enzyme challenges growth of murine glioma progenitor cells but that these cells thrive if they are engineered to express the hominoid-specific brain enzyme GLUD2, a mitochondrial enzyme that converts glutamate to alpha-ketoglutarate in human cortex. The current findings raise the possibility that evolutionary changes contributing to human cognitive abilities may have conferred vulnerability to brain tumors driven by IDH1 mutation.
Keywords: tumor metabolism, astrocytoma, oligodendroglioma
Abstract
Somatic mutation of isocitrate dehydrogenase 1 (IDH1) is now recognized as the most common initiating event for secondary glioblastoma, a brain tumor type arising with high frequency in the frontal lobe. A puzzling feature of IDH1 mutation is the selective manifestation of glioma as the only neoplasm frequently associated with early postzygotic occurrence of this genomic alteration. We report here that IDH1R132H exhibits a growth-inhibitory effect that is abrogated in the presence of glutamate dehydrogenase 2 (GLUD2), a hominoid-specific enzyme purportedly optimized to facilitate glutamate turnover in human forebrain. Using murine glioma progenitor cells, we demonstrate that IDH1R132H exerts a growth-inhibitory effect that is paralleled by deficiency in metabolic flux from glucose and glutamine to lipids. Examining human gliomas, we find that glutamate dehydrogenase 1 (GLUD1) and GLUD2 are overexpressed in IDH1-mutant tumors and that orthotopic growth of an IDH1-mutant glioma line is inhibited by knockdown of GLUD1/2. Strikingly, introduction of GLUD2 into murine glioma progenitor cells reverses deleterious effects of IDH1 mutation on metabolic flux and tumor growth. Further, we report that glutamate, a substrate of GLUD2 and a neurotransmitter abundant in mammalian neocortex, can support growth of glioma progenitor cells irrespective of IDH1 mutation status. These findings suggest that specialization of human neocortex for high glutamate neurotransmitter flux creates a metabolic niche conducive to growth of IDH1 mutant tumors.
Malignant transformation is widely recognized to require metabolic reprogramming to enable rapid expansion of biomass (1). Reports that mutation or overexpression of metabolic enzymes can drive oncogenesis have spurred intense investigation into metabolic vulnerabilities that distinguish malignant and normal tissue (2); however, the extent to which specialized metabolism of normal differentiated tissues cooperates with particular oncogenes to facilitate tumor growth has largely escaped notice (3, 4).
Glioblastoma (GBM) is a highly aggressive brain malignancy and, until recently, all oncogenes identified in this tumor type constitute components of growth factor signaling pathways that activate anabolic processes. The discovery that mutation of isocitrate dehydrogenase 1 (IDH1), or the homologous gene IDH2, is the initiating event for the majority of secondary GBM and lower-grade diffuse glioma (5–7) suggests a novel mechanism for gliomagenesis. Oncogenic mutations altering IDH1 and IDH2 enzymes redirect metabolic flux to generate high concentrations of 2-hydroxyglutarate (2-HG) (8, 9), a metabolite that seems to initiate gliomagenesis via altered activity of α-ketoglutarate–dependent enzymes controlling hypoxia-inducible factor stability, epigenetic marks, and/or collagen maturation (10). Although IDH1 mutation promotes an undifferentiated phenotype (10, 11), the role of mutant enzyme in tumor growth is not clear (12, 13). Because IDH1-WT (IDH1WT) and IDH1-mutant GBMs are distinct diseases (14) and differ categorically in metabolic processes to support proliferation (15), effects of IDH1 mutation on IDH1WT glioma lines must be interpreted with caution. Herein, to gain greater understanding of the mechanisms by which IDH1-mutant gliomas support biomass expansion, we sought to identify determinants of growth in glioma progenitor cells bearing IDH1 mutation.
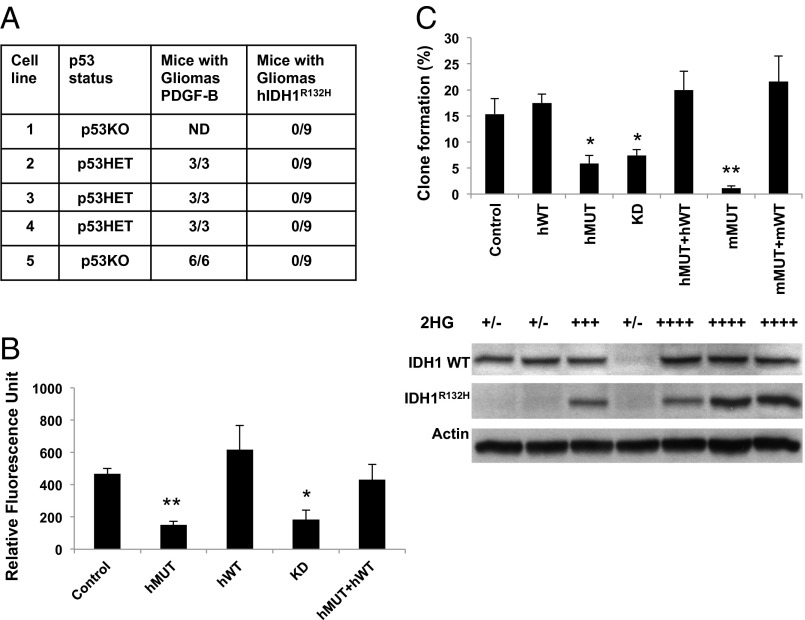
Because the majority of IDH1-mutant GBMs harbor mutations in p53 and display proneural gene expression signature (16), we chose for study a model system of p53-deficient cells competent to generate proneural gliomas at high efficiency (17). Cultured neural stem cells from brains of newborn Nestin-tva p53 −/− or Nestin-tva p53 +/− mice formed gliomas with 100% penetrance following infection with replication-competent avian sarcoma leukosis virus long terminal repeat with splice acceptor (RCAS) vector encoding PDGF-B (PDGF/RCAS) and subsequent implantation into forebrain of immunocompromised mice (Fig. 1A) and hence are referred to as glioma progenitor cells. Whereas cultures infected with PDGF/RCAS generated gliomas that caused all host mice to become symptomatic within 2–4 wk postimplantation, cultures infected with RCAS vector encoding IDH1R132H (IDH1R132H/RCAS) failed to generate expanding grafts for a period of 6 mo following implantation (Fig. 1A).
Fig. 1.
IDH1R132H inhibits growth of murine glioma progenitor cells. (A) Incidence of glioma formation with p53 +/− or p53 −/− neural stem cultures expressing PDGF-B or human IDH1R132H (hIDH1R132H). ND, not determined. (B) CyQuant assay of glioma progenitors (line 5 in A) expressing either no exogenous protein (control), human IDH1R132H (hMUT), human IDH1WT (hWT), shRNA to murine IDH1 (KD), or hMUT and hWT (hMUT + hWT). For B and C, bars represent mean ± SEM for three or more determinations; *P < 0.005, **P < 0.0005 vs. control, t test. (C) (Top) Clone formation assay. mMUT, a clonal line expressing murine IDH1R132H; mMUT + mWT, mMUT clonal line rescued with IDH1WT/RCAS. (Middle) 2-HG concentrations. +/−, basal levels; ++, three- to fivefold; +++, 5- to 10-fold; ++++, 10- to 50-fold, relative to control (see Fig. S1). (Bottom) Western blots for IDH1WT and IDH1R132H.
Examining growth of p53 −/− glioma progenitors in vitro, we observed that both knockdown of IDH1 or expression of IDH1R132H strongly decreased bulk culture growth and clone formation (Fig. 1 B and C). As expected, supernatants of cultures expressing IDH1R132H showed marked elevation of 2-HG (Fig. 1C). Surprisingly, growth and clonogenicity of the cultures expressing human or mouse IDH1R132H were rescued to levels indistinguishable from parental cultures by introduction of vector encoding IDH1WT, despite enhanced accumulation of 2-HG (Fig. 1 B and C). These findings are consistent with the suggestion that the growth deficit induced by mutant enzyme may be mediated by diversion of cytosolic α-ketoglutarate (α-KG) from IDH1WT (18), rather than by toxicity of 2-HG.
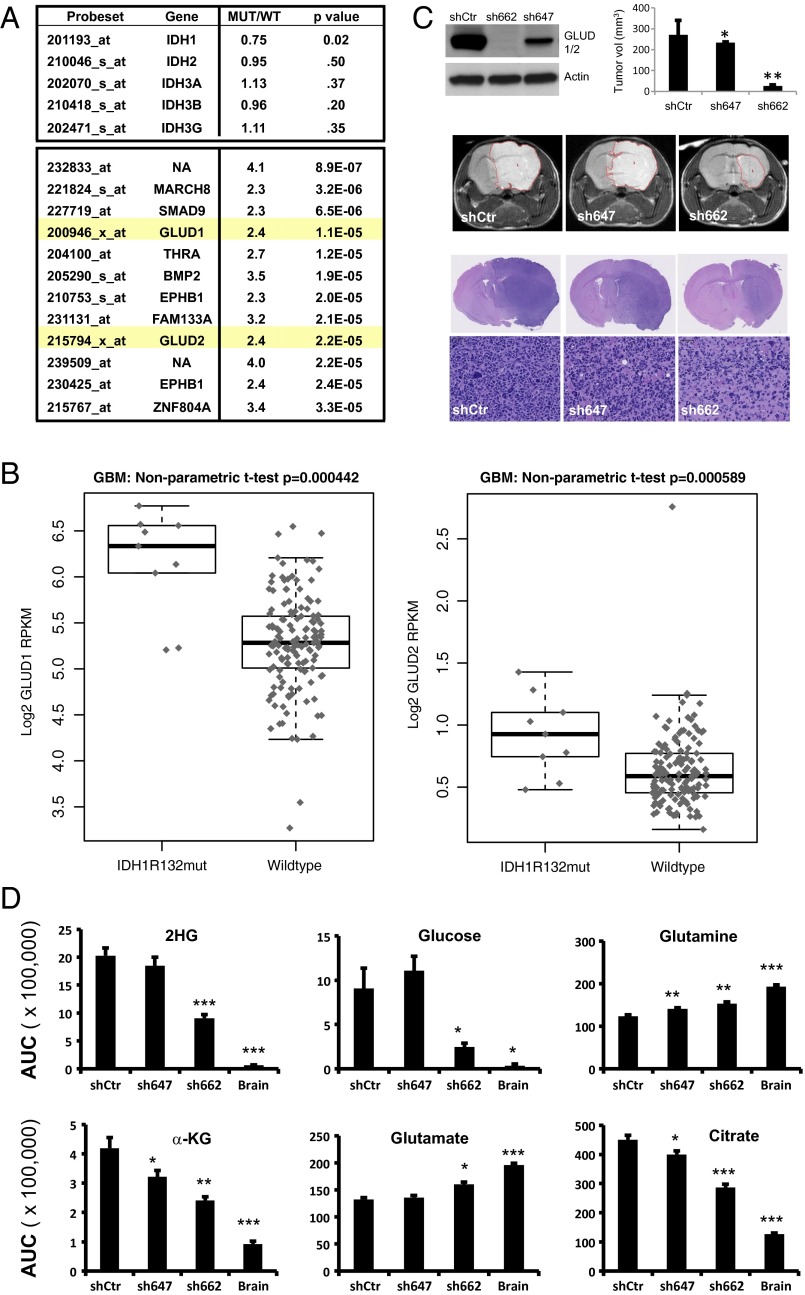
Previous studies have demonstrated that IDH1R132H human gliomas maintain normal concentrations of α-KG but have not identified a mechanism that compensates for diverted flux of α-KG into 2-HG (8, 9, 18, 19). In human tumors, mutations in IDH1 and IDH2 are mutually exclusive and invariably heterozygous (7). To examine whether IDH1R132H human gliomas up-regulate expression of WT IDH enzymes to negate growth-inhibitory effect of mutant enzyme, we compared expression profiling data from three series of histologically matched IDH1R132H and IDH1WT high-grade gliomas. Our analysis revealed no increase in mRNA signals for IDH1, IDH2, or IDH3 subunits in IDH1R132H tumors (Fig. 2A). Among probesets for which signal was most consistently up-regulated in IDH1R132H gliomas, the only probesets corresponding to enzymes were ones annotated as glutamate dehydrogenase 1 (GLUD1) and glutamate dehydrogenase 2 (GLUD2) (Fig. 2A). Analysis of RNA sequencing (RNAseq) data from The Cancer Genome Atlas (TCGA) GBM specimens confirmed that mRNA for both GLUD1 and GLUD2 are increased in IDH1R132H GBM relative to IDH1WT GBM (Fig. 2B). To determine whether GLUD1 or GLUD2 contributes to growth of IDH1R132H glioma, we examined effects of shRNA to GLUD1/2 on orthotopic grafts of an IDH1R132H human glioma line. Two shRNA targeting constructs to GLUD1/2 were used: sh647, which elicited partial reduction of GLUD1/2 protein, and sh662, which resulted in nearly complete elimination of detectable GLUD1/2 protein (Fig. 2C). Using T2-MRI to compare tumor volumes we found that grafts expressing sh647 or sh662 demonstrated a statistically significant reduction in tumor volume relative to grafts of cells transduced with control vector (Fig. 2C and Fig. S2). In addition, sh662-expressing grafts displayed reduction of apparent tumor cell density in H&E-stained sections (Fig. 2C). Consistent with growth effects of the targeting vectors, sh662, and to a lesser extent sh647, attenuated alterations in metabolite levels that distinguished tumors from host brain (Fig. 2D and Table S1). These results reveal a dependence of tumor growth on GLUD1 and/or GLUD2 but do not reveal the relative contributions of each of these highly homologous proteins to tumor growth.
Fig. 2.
GLUD1 and GLUD2 are overexpressed in IDH1R132H human GBM, and knockdown of GLUD1/2 inhibits orthotopic growth of an IDH1R132H glioma line. (A) List of all probesets showing twofold change and P value less than 1 × 10−4 (t test) in each of three separate comparisons of IDH1R132H vs. IDH1WT high-grade glioma. Values reported represent mean fold change and P value for three comparisons. (B) RNAseq data for GLUD1 and GLUD2 comparing IDH1R132H vs. IDH1WT GBM. (C) Effects of shRNA to GLUD1/2 on orthotopic growth of IDH1R132H human glioma line BT142. (Top Left) Western blot showing effects of shRNAs on immunoreactivity for GLUD1/2. (See Fig. S3 for demonstration that GLUD1 and GLUD2 are both detected by Western blot). (Top Right) Volume (mean + SEM) of orthotopic BT142 grafts at 3 mo postimplantation. T2-weighted MRI. *P < 0.05, **P < 0.0001, with comparison with shCtr. (Middle) Representative T2 MRI images. Dotted line denotes tumor–brain boundary (Fig. S2). (Bottom) Images of H&E-stained sections of representative brains of grafted mice. (D) Concentrations of selected metabolite concentrations in IDH1R132H tumor grafts following GLUD1/2 knockdown. *P < 0.05, **P < 0.005, ***P < 0.0005, for comparison with shCtr, t test. AUC, area under the curve. (See Table S1 for all metabolites profiled.)
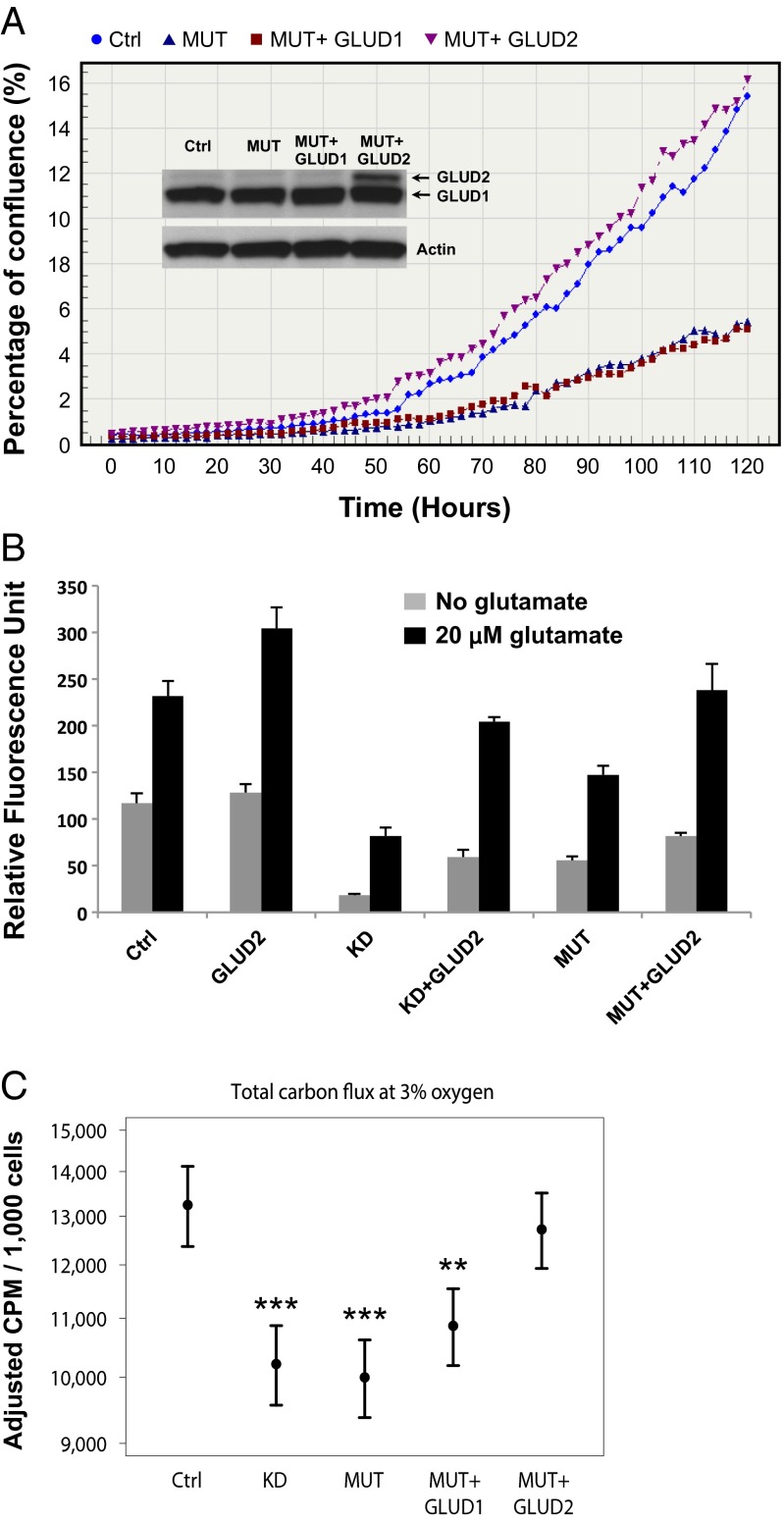
GLUD1 and GLUD2 are mitochondrial enzymes that catalyze the conversion of glutamate to α-KG and lie immediately upstream from IDH1 and/or IDH2 in a reductive glutaminolysis pathway critical for lipogenesis and growth under conditions of hypoxia or mitochondrial dysfunction (20–22). To determine whether either GLUD1 or GLUD2 rescues growth-inhibitory effects of mutant IDH1 enzyme, we expressed each of these enzymes in glioma progenitor cells. Cultures of control murine glioma progenitor cells demonstrated robust immunoreactivity in Western blots using an antibody recognizing GLUD1 and GLUD2 (Fig. 3A and Fig. S3). Because GLUD2 is a hominoid-specific gene, immunoreactivity in control murine glioma progenitor cells is presumed to reflect GLUD1 protein. Although expression of human GLUD1 did not promote growth of IDH1R132H murine glioma progenitors, GLUD2 rescued growth of these cultures (Fig. 3A). In contrast to the robust effects of GLUD2 on growth of cultures expressing IDH1R132H, GLUD2 had no consistent effect on growth of parental cultures (Fig. 3B).
Fig. 3.
GLUD2 promotes growth and metabolite flux to lipid in IDH1R132H glioma progenitors. In vitro behavior of uninfected glioma progenitor culture (Ctrl) was compared with the same line infected with RCAS vector(s) engineered to express murine IDH1R132H (MUT), human GLUD1 (GLUD1), GLUD2 (GLUD2), shRNA to murine IDH1 (KD), or sequential infections with MUT followed by GLUD1 or GLUD2. (A) IncuCyte growth assay and Western blot (Inset) using electrophoresis conditions optimized for separation of GLUD1 and GLUD2 (see SI Methods and Fig. S3). (B) CyQuant assay after 4 d of growth. For all conditions, P < 0.05 for 0 vs. 20 μM glutamate, t test. (C) Total carbon flux to lipids from glutamine and glucose with [14C]glutamine or [14C]glucose tracer for cultures maintained in standard conditions (3% oxygen). Means ± 95% confidence intervals, n ≥ 3. **P < 0.01, ***P < 0.001 vs. control, mixed linear effects.
Because glutamate is a substrate for GLUD1 and GLUD2 and is a neurotransmitter abundant in brain extracellular space (23), we next sought to determine whether extracellular glutamate could influence growth. The addition of 20 μM glutamate to culture media enhanced growth of glioma progenitor cultures regardless of whether or not cultures had been engineered to express IDH1R132H, shRNA to IDH1, GLUD1, or GLUD2 (Fig. 3B). These findings suggest that extracellular glutamate acts independently of IDH1 and GLUD2 enzymes to support growth.
Given previous reports that IDH1-mutant protein is deficient in reductive carboxylation (19) and that IDH1-mutant glioma is particularly sensitive to inhibition of glutaminase (24), we hypothesized that growth of IDH1R132H glioma progenitors may be limited by the ability to use glutamine as a carbon source for lipogenesis. Using [14C]glucose or [14C]glutamine to monitor carbon labeling from glucose or glutamine, we find that total fractional labeling from these metabolites into lipids was consistently reduced by either knockdown of endogenous IDH1 or introduction of IDH1R132H (Fig. 3C and Fig. S4). Labeling of lipids in cultures of glioma progenitors expressing IDH1R132H was minimally influenced by introduction of human GLUD1. However, GLUD2 rescued flux in IDH1R132H cultures to levels similar to that of parental cultures (Fig. 3C and Fig. S4). Thus, growth effects exerted by IDH1R132H and GLUD2 in glioma progenitor cells are paralleled by alterations in fractional labeling of carbon derived from glucose or glutamine to lipids.
Given our finding that extracellular glutamate can support growth of glioma progenitors, we next sought to determine whether these cells use glutamate as a carbon source to support lipogenesis. Although incorporation of 14C from extracellular glutamate into lipids was detected in all cultures, only low levels of carbon flux to lipids were observed (Fig. S4), suggesting that additional mechanisms, such as extracellular signaling, may contribute to the effect of glutamate on cell growth.
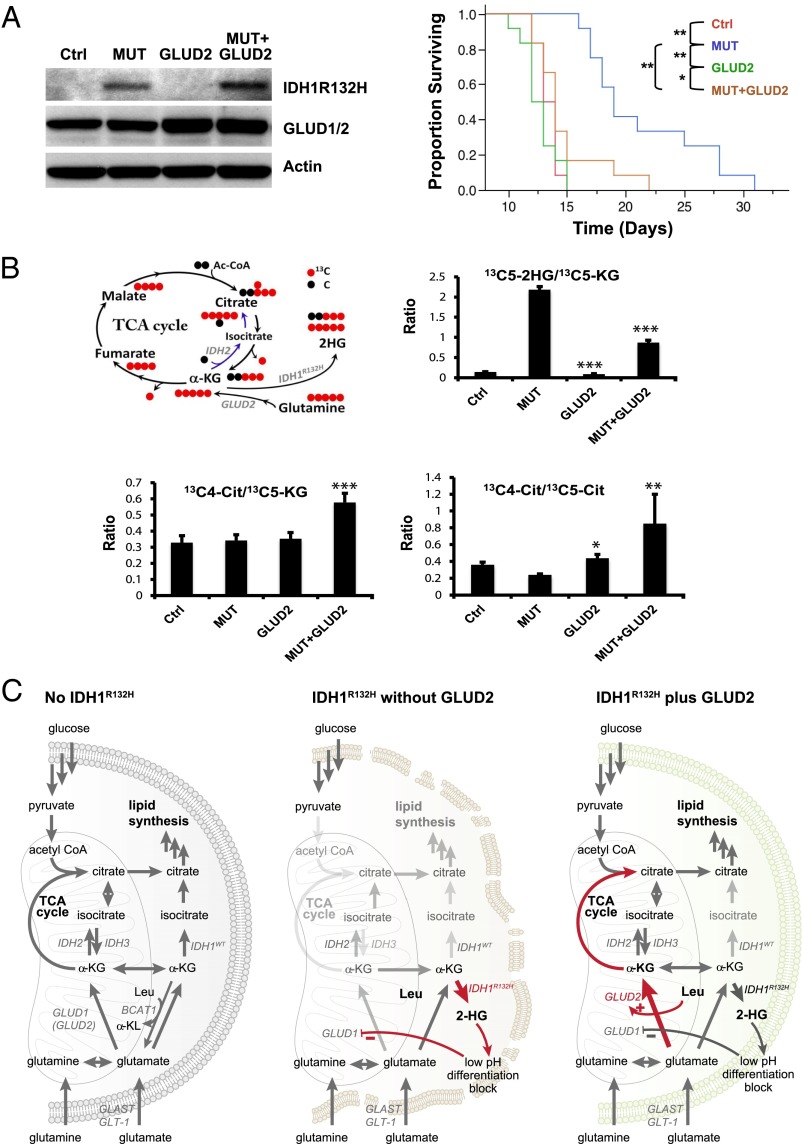
We next sought to determine whether GLUD2 antagonizes growth-inhibitory effects of IDH1R132H in vivo. Using PDGF/RCAS to drive gliomas from p53−/− Nestin-tva glioma progenitor cultures, we observed that infection of cultures with IDH1R132H/RCAS before implantation led to a marked prolongation in survival of engrafted mice (Fig. 4A). GLUD2/RCAS had no effect on outcome of mice engrafted with parental PDGF-driven gliomas; however, infection with GLUD2/RCAS completely abrogated the negative effect of IDH1R132H on tumor aggressiveness (Fig. 4A). Thus, in this model of in vivo glioma growth, GLUD2 exerts growth-promoting effects that are specific to tumors expressing IDH1-mutant protein. Mass isotopomer distribution analysis using 13C stable isotopes in the cultures used for grafting revealed evidence that introduction of GLUD2 into cells bearing IDH1R132H diverts glutamine-derived α-KG toward oxidative generation of citrate in the tricarboxylic acid (TCA) cycle of mitochondria at the expense of flux to 2-HG in the cytosol (Fig. 4B and Fig. S5).
Fig. 4.
GLUD2 rescues in vivo growth of murine gliomas expressing IDH1R132H. (A) Murine glioma progenitors driven to form tumors by human PDGFB/RCAS were treated with no additional vector (Ctrl), IDH1R132H/RCAS (MUT), GLUD2/RCAS (GLUD2), or IDH1R132H/RCAS followed by GLUD2/RCAS (MUT + GLUD2). (Left) Western blot for IDH1R132H and GLUD1/2. (Right) Survival of mice bearing grafts of the engineered cells. *P < 0.05, **P < 0.001 vs. Ctrl, log rank. (B) Effects of GLUD2 on ratios of metabolites derived from 13C5 glutamine. (Upper Left) Schematic diagram of carbon flux in TCA cycle starting from 13C5–α-KG. (Upper Right) Decreased 2-HG production from glutamine-derived α-KG. (Lower Left) Increased citrate production from TCA cycle. (Lower Right) Increased citrate production through oxidative phosphorylation over through reductive glutamine metabolism. *P < 0.05, **P < 0.01, ***P < 0.001 for GLUD2 effect compared with Ctrl (for GLUD2) or MUT (for MUT + GLUD2). (C) Model of proposed actions of GLUD2 to rescue growth of glioma progenitors expressing IDH1R132H. The arrows in red highlight the effects of IDH1R132H (Center) and of GLUD2 in the presence of IDH1R132H (Right).
Our findings suggest a model in which GLUD2 expression in human brain renders cells resistant to growth-inhibitory effects of IDH1R132H by supplying α-KG to fuel the citric acid cycle and support lipid synthesis (Fig. 4C). The relative importance of lipid synthesis vs. citric acid cycle in limiting growth of IDH1-mutant tumors cannot be determined from the current findings, nor can we eliminate other α-KG–dependent processes as underlying the growth-promoting effects of GLUD2 observed in the presence of mutant IDH1 enzyme. Restricted expression of GLUD2 (25) may provide a potential explanation for the tissue specificity of oncogenesis in patients with occurrence of IDH1R132H in multiple cell lineages; the majority patients with Ollier disease and Maffuci syndrome display evidence of early postzygotic occurrence of IDH1R132H mutation, yet glioma is the only malignancy occurring at increased frequency in these individuals (26, 27). Further, the absence of a GLUD2 gene in nonhominoid species may account for the reported difficulties in identification or generation of animal models of IDH1 mutant glioma (28, 29).
Although it is tempting to speculate that the growth-inhibitory effects of IDH1-mutant protein contribute to the better prognosis of IDH1-mutant human GBM, it is important to recognize that IDH1-WT and IDH1-mutant human GBMs reflect distinct disease entities that likely originate from separate cells of origin (5, 14) and, as such, numerous differences in biology likely contribute to differences in aggressiveness of the two tumor types. Our results do, however, underscore differences in metabolic vulnerability of human IDH1-mutant and IDH1-WT gliomas and suggest that therapeutic approaches targeting glutamate metabolism or availability of α-KG might be applicable to IDH1-mutant gliomas.
Although previous work implicates glutamate dehydrogenase as a regulator of GBM growth (30), our findings reveal an unexpected lack of redundancy between the capabilities of GLUD1 and GLUD2. Although our results do not allow us to determine whether GLUD1 contributes to growth of IDH1-mutant cells, they do clearly reveal that GLUD2 promotes growth of IDH1-mutant cells in a manner that is not duplicated by overexpression of GLUD1. Whether the unique actions of GLUD2 observed are due to subcellular localization or biochemical properties of the enzyme cannot be determined from the current findings. Intriguingly, the GLUD2 gene is unique to hominoids and has undergone rapid evolutionary selection concomitant with expansion of prefrontal cortex (25, 31), the site at which IDH1R132H glioma most frequently occurs (14). The prevailing belief is that selection pressure during hominoid evolution for higher flux of glutamate transmitter in prefrontal cortex led to optimization of GLUD2 for degradation of glutamate in the nervous system environment. We speculate that the amino acid substitutions in GLUD2 that confer lower pH optimum and lack of negative regulation by GTP not only optimize the enzyme’s ability to support glutamate transmitter flux in the normal human brain (25, 31) but also account for the ability of this enzyme to support growth of IDH1R132H glioma progenitors. In particular, we point to the likelihood that the pH optimum of GLUD2 is better suited than that of GLUD1 for glioma progenitors acidified by high intracellular 2-HG. Of note, the recent finding that IDH1R132H GBMs lack expression of Branched Chain Amino acid Transaminase-1 (BCAT1) (15) offers a mechanism to sustain high intracellular concentrations of both α-KG, a product of GLUD2, and leucine, an activator of GLUD2 (25).
In addition to revealing a role for GLUD2 in supporting growth of IDH1R132H glioma progenitor cells, our results point to the likelihood that extracellular glutamate resulting from neurotransmitter release also contributes to growth of IDH1R132H glioma. Thus, metabolic specialization in both tumor cell-of-origin and the stromal niche seems to contribute to vulnerability of human forebrain to formation and growth of IDH1R132H glioma, raising the possibility that adaptations that facilitate human cognition may have come at the cost of increased susceptibility to this tumor type.
Methods
Glioma progenitor cultures were established from brains of newborn mice resulting from breedings of Nestin-tva-TG.p53+/−.B6 × Nestin-tva-TG.p53−/−.B6 adults. Cultures were created by dissociation of whole forebrain and maintained in neurosphere media in 5% CO2 and 3% O2, unless otherwise indicated. With the exception of the experiment reported in Fig. 1A, a single Nestin-tva/p53−/− culture (line 5) was the parental culture used for all experiments using glioma progenitors. Glioma progenitor cultures were engineered to express proteins or shRNA constructs by in vitro infection with appropriate RCAS vectors. Bulk culture growth and clone formation assays were conducted in 3% oxygen and monitored by CyQuant and microscopic inspection, respectively. In addition, culture growth in 20% oxygen was monitored by CyQuant or Incucyte. Concentrations of 2-HG in culture supernatants and cell pellets were determined by liquid chromatography coupled with tandem mass spectrometry. For in vivo glioma growth studies, 5 × 105 mouse glioma progenitor cells infected in vitro with RCAS vectors or 1 × 105 cells of human heterozygous IDH1R132H line BT142 (SRC-4002; American Type Culture Collection) infected in vitro with lentiviral vectors were implanted into right striatum of female CD1 nude mice or NOD/SCID mice. Protocols for all in vivo studies were reviewed and approved by the Genentech Institutional Animal Care and Use Committee.
Two replicate in vivo experiments were conducted with IDH1R132H and GLUD2 expression in murine glioma progenitor grafts and a single in vivo experiment was conducted with GLUD1/2 shRNA knockdown in the human BT142 line. Tumor growth was monitored by host survival (murine grafts) or T2-weighted MRI (BT142) and, in all experiments, presence or absence of tumor was verified by histological examination. Expression of mRNA differences between IDH1R132H and IDH1WT human gliomas was determined by analysis of microarray data generated in previous studies (14, 32) from two sets of GBM and one set of grade III astrocytoma as well as from TCGA RNAseq data from GBMs.
See SI Methods for detailed methods.
Supplementary Material
Acknowledgments
We thank M. Gao for technical assistance on mass spectrometry, A. Bruce for graphics, E. McNamara for animal care, N. van Bruggen for MRI consultation, and L. Gilmour and the cell sorting laboratory for assistance with cell cloning.
Footnotes
Conflict of interest statement: All authors are paid employees or consultants of Genentech, Inc. The subject matter of this manuscript does not relate to any products under development by the company.
*This Direct Submission article had a Prearranged Editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409653111/-/DCSupplemental.
References
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491(7424):364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 3.DeBerardinis RJ. Good neighbours in the tumour stroma reduce oxidative stress. Nat Cell Biol. 2012;14(3):235–236. doi: 10.1038/ncb2449. [DOI] [PubMed] [Google Scholar]
- 4.Yuneva MO, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15(2):157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 6.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan H, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward PS, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losman JA, Kaelin WG., Jr What a difference a hydroxyl makes: Mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev. 2013;27(8):836–852. doi: 10.1101/gad.217406.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bralten LB, et al. IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol. 2011;69(3):455–463. doi: 10.1002/ana.22390. [DOI] [PubMed] [Google Scholar]
- 13.Rohle D, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai A, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tönjes M, et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med. 2013;19(7):901–908. doi: 10.1038/nm.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhaak RG, et al. Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai C, et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navis AC, et al. Increased mitochondrial activity in a novel IDH1-R132H mutant human oligodendroglioma xenograft model: In situ detection of 2-HG and α-KG. Acta Neuropathol Commun. 2013;1(1):18–29. doi: 10.1186/2051-5960-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonardi R, Subramanian C, Jackowski S, Rock CO. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem. 2012;287(18):14615–14620. doi: 10.1074/jbc.C112.353946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise DR, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA. 2011;108(49):19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen AR, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481(7381):385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481(7381):380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erecińska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35(4):245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 24.Seltzer MJ, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shashidharan P, Plaitakis A. The discovery of human of GLUD2 glutamate dehydrogenase and its implications for cell function in health and disease. Neurochem Res. 2014;39(3):460–470. doi: 10.1007/s11064-013-1227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amary MF, et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet. 2011;43(12):1262–1265. doi: 10.1038/ng.994. [DOI] [PubMed] [Google Scholar]
- 27.Pansuriya TC, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011;43(12):1256–1261. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitman ZJ, et al. IDH1 and IDH2 hotspot mutations are not found in canine glioma. Int J Cancer. 2010;127(1):245–246. doi: 10.1002/ijc.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki M, et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev. 2012;26(18):2038–2049. doi: 10.1101/gad.198200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, et al. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69(20):7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burki F, Kaessmann H. Birth and adaptive evolution of a hominoid gene that supports high neurotransmitter flux. Nat Genet. 2004;36(10):1061–1063. doi: 10.1038/ng1431. [DOI] [PubMed] [Google Scholar]
- 32.Phillips HS, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.