Most scientists are familiar with the basic tenet of evolutionary biology “mutation proposes, selection disposes,” where random variation generated by mutation is thought to provide the fuel for natural selection to direct evolutionary change. If this is truly a basic tenet, then why have prominent evolutionary biologists expressed concern about the relative importance given to variation or selection in the evolutionary process? For example, West-Eberhard states “There is prejudice against variation that is non-adaptive in studies of adaptive evolution, against variation that is non-genetic in studies of genetics, and against virtually all variation in studies of the genome in the worm, (Caenorhabditis elegans), the fly (Drosophila), and the frog (Xenopus).” (ref. 1, p. 205). In contrast, Bell (ref. 2, p. xix) states “A further weakness is that when selection is described, it is often treated as one of several possible mechanisms of evolution… This is surely unjustifiable.”
Their concern is about which of these two determines the rate and direction of evolutionary change (3–5); in other words, who’s in the evolutionary driver’s seat: variation or selection? By studying the developmental mechanisms underlying evolution of sex combs in the fruit fly Drosophila melanogaster, in PNAS, Malagón et al. provide new insight on this debate (6).
According to Gould (4), this debate has a long history that precedes Darwin’s Origin of Species, occurring between biologists who argue that natural selection is the creative force controlling both the rate and direction of evolution (functionalists) vs. those who argue that it is variation that controls them (structuralists). Variation in the eyes of the functionalist is small, continuous, copious, and random with respect to the environment, allowing natural selection to gradually mold phenotypes to a functional optimum as required by the environment. Functionalists acknowledge that limited genetic variation may slow the rate of natural selection, but this does not influence its ability to direct evolution. In contrast, variation in the eyes of the structuralist is discontinuous, structured and nonrandom and dictates the rate and direction of evolution. An example of extreme structuralist arguments denying selection any role for the origin of new species is Hugo de Vries’ “mutation theory” in 1903, which argues that new species arise in a single mutational step: a saltational jump to a fully functional and novel phenotype (7). Such extreme arguments, however, have largely been discredited.
Developmental constraint is a moderate version of structuralism that has been influential in modern evolutionary thought (8, 9). A developmental constraint is most often defined as “a bias on the production of variant phenotypes or a limitation on phenotypic variability caused by the structure, character, composition, or dynamics of the developmental system” (10). It can have positive (can bias variation) or negative (can limit variation) effects on evolutionary change. The relative contribution of developmental constraint and selection in determining the rate and direction of evolution has largely been a matter of philosophical debate (4, 9). However, with the emergence of Ecological Evolutionary Developmental Biology (Eco-Evo-Devo), we are now in a position to experimentally address this question (5, 11). Eco-Evo-Devo studies on butterfly eyespots (11), plate armor on three-spine stickleback fish (12), supersoldier ants (13), pigmentation in mice (14), male antennae in water striders (15), and now sex combs in flies (6), highlight the inextricable interplay between developmental constraints and selection during evolution.
In flies, multiple rows of bristles form on the legs. In some species, including D. melanogaster, some of these bristles have evolved in males to form sex combs, which are rows of thickened, pigmented bristles found on the distal parts of the forelegs (16). Sex combs mostly facilitate grasping of females by males during mating. Absence of sex combs results in reduction in the frequency of male mating success (17). Sex combs have evolved multiple times within Drosophilid flies, where extensive differences occur in the orientation of sex combs, the number of teeth, the degree of modification of the teeth, and their exact function among closely related species (16).
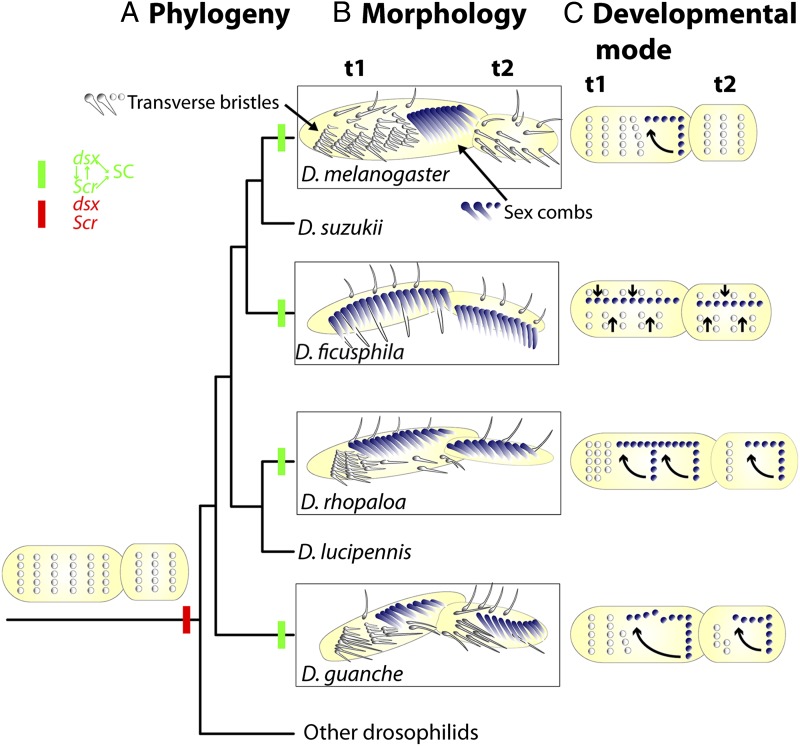
During development, transversely oriented rows of precursor cells give rise to both transverse rows of adult bristles and longitudinally oriented sex combs (Fig. 1). To accomplish this, the precursors that give rise to the sex combs undergo a 90° rotation. To allow room for this rotation the distal most transverse row is bent upward, implying the existence of a physical constraint of space between the sex combs and the transverse rows (18). Malagón et al. (6) use artificial selection, mutant analysis, and species comparisons to study the role of this space constraint in the evolution of sex combs. In D. melanogaster, they show that sex combs with small to moderate number of teeth are straight, whereas long sex combs are bent in the shape of a cane. They propose that this cane shape arises because rotation of the developing sex combs is obstructed by the developing transverse row. This suggests that there is a developmental constraint due to a conflict for space. Malagón et al. (6) then compare experimental observations in D. melanogaster to evolved differences in sex comb development in other species. Drosophila guanche, a species with long sex combs, exhibits bending or a break in sex combs, suggesting that this space conflict exists in nature (6). However, several other species have long sex combs that are straight implying that these species have apparently overcome the space conflict through the evolution of different developmental strategies: fewer bristles in the distal transverse row, fewer transverse rows, formation of longitudinal precursors, lesser space between transverse rows, rotating multiple transverse rows, or a longer tarsus (Fig. 1). Malagón et al. conclude that the interplay between developmental constraints and selection explains the evolution of different shapes and sizes of Drosophila sex combs (6).
Fig. 1.
Positive developmental constraint facilitates repeated evolution of long sex combs in Drosophilids. (A) Phylogenetic relationship between representative species. Green bar indicates functional role for doublesex (dsx) and Sex combs reduced (Scr) in long sex comb formation. Red bar represents absence of role in sex comb formation. (B) Morphology of tarsal segments 1 and 2 showing transverse rows of bristles (gray-white) and longitudinal sex combs (blue-white). (C) Mode of sex comb development in the respective species. Distal is on the right and anterior down.
While assessing the relative contributions of developmental constraint and selection, we often conflate its positive and negative effects and fail to consider multiple levels of biological organization (genes, cells, and phenotypes). For these reasons, Malagón et al. have yet to fully realize the grander implications of their study. There is an implicit assumption that the spatial conflict, embodied by the cane shape, confers lower fitness to males. If this was the case, the cane shape would limit the evolution of longer sex combs. Alternatively, if cane-shaped sex combs confers higher or no difference in fitness to males, then they may only represent an intermediate step toward evolving longer sex combs, and selection may well be on its way to resolving the space conflict. This alternative possibility is supported by the fact that there exist multiple solutions for resolving the space conflict, suggesting that selection had little difficulty in finding multiple solutions for evolving longer sex combs. Therefore, there may be no real negative effect of developmental constraint. However, when the phylogenetic history of the group, as well as the genes shown to be associated with the evolution of sex combs are incorporated into this story, Malagón et al.’s results can be interpreted as evidence for a positive developmental constraint (also called developmental bias).
Expression of the Hox gene Sex combs reduced and the sex-specific gene doublesex is strongly correlated to the presence and size of sex combs (16). This suggests a positive developmental constraint: the independent evolution of long sex combs is facilitated through the repeated use of these same genes. However, independent evolution of long sex combs has occurred through different cellular mechanisms in different species (18). This is strikingly similar to Khila et al. (19), where semiaquatic insects have independently evolved a novel leg length plan using the same gene ultrabithorax. In an analogous fashion, ultrabithorax has targeted different leg segments in different species. Therefore, in both cases, independent evolution of morphological traits occurs through the same gene(s), but uses different tissues and cells. This pattern may be driven by the functional specialization of both legs and sex combs in different species (19) and may reflect a general pattern in the interplay between developmental constraint and selection. The modular nature of tissues and cells may facilitate the ability of conserved developmental genes to use different combinations of cells and tissues (a process known as developmental recombination) (1) in different species to achieve functional specialization. Selection would be influenced by ancestral variation generated by conserved developmental genes, but at the same time, it could rapidly create multiple developmental solutions at the cell and tissue level to similar ecological challenges. Testing these ideas means there is much exciting work ahead in the field of Eco-Evo-Devo.
Footnotes
The authors declare no conflict of interest.
See companion article on page E4103.
References
- 1.West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford, UK: Oxford Univ Press; 2003. p xx. [Google Scholar]
- 2.Bell G. Selection: The Mechanism of Evolution. New York: Chapman & Hall; 1997. p xxiii. [Google Scholar]
- 3.Brakefield PM, et al. Development, plasticity and evolution of butterfly eyespot patterns. Nature. 1996;384(6606):236–242. doi: 10.1038/384236a0. [DOI] [PubMed] [Google Scholar]
- 4.Gould SJ. The Structure of Evolutionary Theory. Cambridge, MA: The Belknap Press of Harvard Univ Press; 2002. p xxii. [Google Scholar]
- 5.Losos JB. Convergence, adaptation, and constraint. Evolution. 2011;65(7):1827–1840. doi: 10.1111/j.1558-5646.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 6.Malagón JN, et al. Evolution of Drosophila sex comb length illustrates the inextricable interplay between selection and variation. Proc Natl Acad Sci USA. 2014;111:E4103–E4109. doi: 10.1073/pnas.1322342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provine WB. The Origins of Theoretical Population Genetics. Chicago: Univ of Chicago Press; 1971. [Google Scholar]
- 8.Alberch P, Gould SJ, Oster GF, Wake DB. Size and Shape in ontogeny and phylogeny. Paleobiology. 1979;5(3):296–317. [Google Scholar]
- 9.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proc R Soc Lond B Biol Sci. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 10.Maynard-Smith J, et al. Developmental constraints and evolution: A perspective from the Mountain Lake Conference on Development and Evolution. Q Rev Biol. 1985;60(3):265–287. [Google Scholar]
- 11.Beldade P, Koops K, Brakefield PM. Developmental constraints versus flexibility in morphological evolution. Nature. 2002;416(6883):844–847. doi: 10.1038/416844a. [DOI] [PubMed] [Google Scholar]
- 12.Barrett RD, Rogers SM, Schluter D. Natural selection on a major armor gene in threespine stickleback. Science. 2008;322(5899):255–257. doi: 10.1126/science.1159978. [DOI] [PubMed] [Google Scholar]
- 13.Rajakumar R, et al. Ancestral developmental potential facilitates parallel evolution in ants. Science. 2012;335(6064):79–82. doi: 10.1126/science.1211451. [DOI] [PubMed] [Google Scholar]
- 14.Steiner CC, Römpler H, Boettger LM, Schöneberg T, Hoekstra HE. The genetic basis of phenotypic convergence in beach mice: Similar pigment patterns but different genes. Mol Biol Evol. 2009;26(1):35–45. doi: 10.1093/molbev/msn218. [DOI] [PubMed] [Google Scholar]
- 15.Khila A, Abouheif E, Rowe L. Function, developmental genetics, and fitness consequences of a sexually antagonistic trait. Science. 2012;336(6081):585–589. doi: 10.1126/science.1217258. [DOI] [PubMed] [Google Scholar]
- 16.Kopp A. Drosophila sex combs as a model of evolutionary innovations. Evol Dev. 2011;13(6):504–522. doi: 10.1111/j.1525-142X.2011.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng CS, Kopp A. Sex combs are important for male mating success in Drosophila melanogaster. Behav Genet. 2008;38(2):195–201. doi: 10.1007/s10519-008-9190-7. [DOI] [PubMed] [Google Scholar]
- 18.Atallah J, et al. Cell dynamics and developmental bias in the ontogeny of a complex sexually dimorphic trait in Drosophila melanogaster. Evol Dev. 2009;11(2):191–204. doi: 10.1111/j.1525-142X.2009.00319.x. [DOI] [PubMed] [Google Scholar]
- 19.Khila A, Abouheif E, Rowe L. Comparative functional analyses of ultrabithorax reveal multiple steps and paths to diversification of legs in the adaptive radiation of semi-aquatic insects. Evolution. 2014;68(8):2159–2170. doi: 10.1111/evo.12444. [DOI] [PubMed] [Google Scholar]