Significance
Inherited mutations in the tumor suppressor genes BRCA1 and BRCA2 predispose to very high risks of breast and ovarian cancer. For carriers of these mutations, risk-reducing surgery significantly reduces morbidity and mortality. General population screening for BRCA1 and BRCA2 mutations in young adult women could be feasible if accurate estimates of cancer risk for mutation carriers could be obtained. We determined that risks of breast and ovarian cancer for BRCA1 and BRCA2 mutation carriers ascertained from the general population are as high as for mutation carriers ascertained through personal or family history of cancer. General screening of BRCA1 and BRCA2 would identify many carriers who are currently not evaluated and could serve as a model for population screening for genetic predisposition to cancer.
Keywords: genomics
Abstract
In the Ashkenazi Jewish (AJ) population of Israel, 11% of breast cancer and 40% of ovarian cancer are due to three inherited founder mutations in the cancer predisposition genes BRCA1 and BRCA2. For carriers of these mutations, risk-reducing salpingo-oophorectomy significantly reduces morbidity and mortality. Population screening for these mutations among AJ women may be justifiable if accurate estimates of cancer risk for mutation carriers can be obtained. We therefore undertook to determine risks of breast and ovarian cancer for BRCA1 and BRCA2 mutation carriers ascertained irrespective of personal or family history of cancer. Families harboring mutations in BRCA1 or BRCA2 were ascertained by identifying mutation carriers among healthy AJ males recruited from health screening centers and outpatient clinics. Female relatives of the carriers were then enrolled and genotyped. Among the female relatives with BRCA1 or BRCA2 mutations, cumulative risk of developing either breast or ovarian cancer by age 60 and 80, respectively, were 0.60 (± 0.07) and 0.83 (± 0.07) for BRCA1 carriers and 0.33 (± 0.09) and 0.76 (± 0.13) for BRCA2 carriers. Risks were higher in recent vs. earlier birth cohorts (P = 0.006). High cancer risks in BRCA1 or BRCA2 mutation carriers identified through healthy males provide an evidence base for initiating a general screening program in the AJ population. General screening would identify many carriers who are not evaluated by genetic testing based on family history criteria. Such a program could serve as a model to investigate implementation and outcomes of population screening for genetic predisposition to cancer in other populations.
Inherited mutations in BRCA1 and BRCA2 predispose to high risks of breast and ovarian cancer. Among female mutation carriers, presymptomatic surgical measures significantly reduce morbidity and mortality (1, 2). In particular, risk-reducing salpingo-oophorectomy (i.e., the removal of ovaries and fallopian tubes from a woman without ovarian cancer) reduces risk both of breast cancer and of ovarian cancer, as well as overall mortality (1). However, for many mutation carriers identified following their first cancer diagnosis, genetic testing was not previously indicated because family history did not suggest inherited cancer predisposition (3–5, 6). From a prevention perspective, it is a missed opportunity to identify a woman as a BRCA1 or BRCA2 mutation carrier only after she develops cancer.
Among Ashkenazi (European) Jews (AJ), three mutations, BRCA1 185delAG, BRCA1 5382insC, and BRCA2 6174delT, account for the great majority of inherited cancer risk due to BRCA1 and BRCA2 (7). In the AJ population, 2.5% of persons carry one of these three mutations (8), and the mutations account for 11% of breast cancer (3) and 40% of ovarian cancer (9, 10). These observations suggest that genetic testing in the AJ population for these mutations fulfills WHO criteria for population screening (11, 12): The disease is an important public health burden to the target population; prevalence and attributable risk of disease due to the mutations are known; and effective interventions exist. However, one necessary piece of information remains unknown: What is the disease risk to mutation carriers ascertained from the general population, rather than carriers identified based on family history (13)?
Previous studies assessing cancer risks due to mutations in BRCA1 and BRCA2 ascertained carriers through high-incidence families (14), through a single index case with breast or ovarian cancer (3, 15) or through both affected and unaffected carriers (16). In a 1997 study of AJ volunteers, most index cases had no previous cancer diagnosis, but the percentage of index cases with a family history of breast cancer was approximately double that of unselected AJs (17). In principle, these strategies could have yielded risk estimates different from those of carriers ascertained from the local host population, if cancer risk in BRCA1 or BRCA2 carriers were influenced by familial factors other than the BRCA1 or BRCA2 mutation, such as modifier genes or shared environment (18). In addition, in almost all of these studies, risk estimates were based on imputing carrier status, rather than on direct genetic testing of BRCA1 and BRCA2. This year, the Recommendation Statement on BRCA Testing from the US Preventive Services Task Force recommended against population screening for BRCA1 and BRCA2 mutations, because cancer risk to mutation carriers in the general population was not yet known (19). To address this gap, in this study we assessed breast and ovarian cancer risks in confirmed carriers of BRCA1 and BRCA2 mutations ascertained from the general population. The study was undertaken in the AJ population, because screening for only three founder mutations is sufficient to capture nearly all inherited cancer risk in this population due to BRCA1 and BRCA2 (7).
Results
Index Subjects as Surrogates for the General AJ Population.
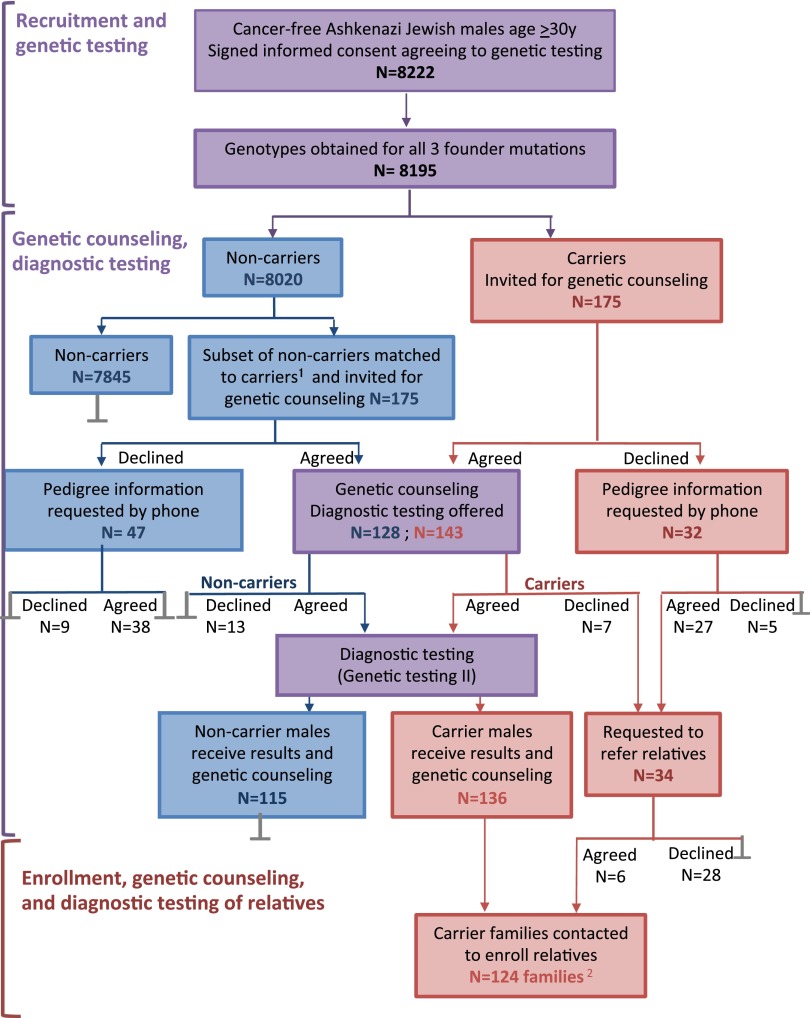
Of 8,222 male index subjects enrolled, DNA samples from 8,195 subjects (99.7%) were successfully genotyped for the three AJ mutations in BRCA1 and BRCA2 (Fig. 1). Of the 8,195 subjects, 175 carried a mutant allele: 91 in BRCA1, 81 in BRCA2, and 3 in both BRCA1 and BRCA2. Carrier frequencies were 1.14% (94/8195) for BRCA1, 1.03% (84/8195) for BRCA2, and 2.17% (178/8195) for the three alleles combined. Approximately equal numbers of male mutation carriers were members of families with high incidence or low incidence of breast or ovarian cancer (SI Appendix, Table S3), a profile also observed in other studies of AJ families harboring BRCA1 and BRCA2 mutations (3, 5, 6).
Fig. 1.
Study design: the testing protocol and the number of participants at each step. Purple indicates stages common to all males index subjects; blue indicates stages for noncarriers only; red indicates stages for carriers only; gray bars indicate end of participation (1). A subset of noncarriers was matched to carriers for age, area of residence, and recruitment locale (2). Of the 175 male index subjects with mutations, 3 were related to another carrier, so 172 families were represented.
An important consideration in generalizing from this study population to the Israeli AJ population as a whole was whether these 8,195 male index subjects were representative of the general AJ population with respect to family history of breast and ovarian cancer. The concern was that males with family histories of breast or ovarian cancer might be more likely to consent to participation, leading to more severely affected families in the study cohort than in the population as a whole. Three lines of evidence addressed this question. First, carrier rates for the founder mutations among index males were similar to carrier rates reported by other studies of the Israeli AJ population (8). Second, among the male index subjects born between 1940 and 1975, 9.6% reported a diagnosis of breast cancer in their mothers. In comparison, for the Israeli AJ female population born between 1920 and 1950 (i.e., the generation of the mothers of the index subjects), the Israeli Cancer Registry reported a cumulative incidence of breast cancer to the year 2009 of 9.4% (SI Appendix, SI Methods). Third, parental origin of mutation could be determined for 78 index males carrying mutations. Inheritance was maternal for 41 index males (53%) and paternal for 37 index males (47%), close to the 50:50 ratio expected if index male carriers participated without regard to family history. Combined, these observations suggest that the 8,195 male index subjects were representative of the general Israeli AJ population with respect to BRCA1 and BRCA2 genotypes and with respect to family history of breast cancer.
Analysis BRCA1 and BRCA2 Mutations in Female Relatives.
The principal goal of the study was to determine the risks of breast and ovarian cancer among women with BRCA1 or BRCA2 mutations ascertained through an unaffected male index subject. The 175 male index subjects with mutations were asked to provide new DNA samples to confirm mutation status, to undergo genetic counseling, and to refer their female relatives to the study (Fig. 1). These 175 male index subjects represented 172 different families. From these families, 629 informative relatives (431 women and 198 men) were ultimately enrolled, among whom 211 female mutation carriers were identified.
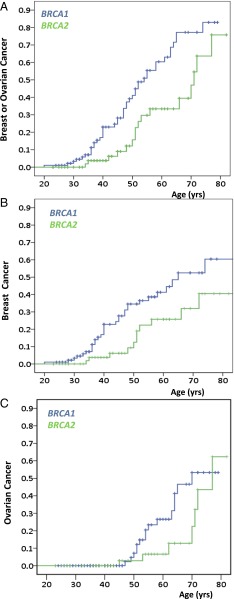
Risks of breast cancer, ovarian cancer, and either breast or ovarian cancer to these female mutation carriers are shown in Table 1 and Fig. 2. Cumulative incidences of either breast or ovarian cancer, by ages 60 and 80 respectively, were 0.60 (± 0.07) and 0.83 (± 0.07) for carriers of BRCA1 mutations and 0.33 (± 0.09) and 0.76 (± 0.13) for carriers of BRCA2 mutations (Fig. 2A). Cumulative incidences specifically of breast cancer, by ages 60 and 80, respectively, were 0.41 (± 0.06) and 0.60 (± 0.10) for carriers of BRCA1 mutations and 0.26 (± 0.08) and 0.40 (± 0.11) or carriers of BRCA2 mutations (Fig. 2B). Cumulative incidences specifically of ovarian cancer, by ages 60 and 80, respectively, were 0.27 (± 0.07) and 0.53 (± 0.11) for carriers of BRCA1 mutations and 0.07 (± 0.05) and 0.62 (± 0.18) for carriers of BRCA2 mutations (Fig. 2C).
Table 1.
Cumulative incidence of breast or ovarian cancer among women with mutations in BRCA1 or BRCA2, ascertained via unaffected males
| To age, y | BRCA1 (SE) | BRCA2 (SE) | |
| Risk of breast cancer | |||
| 30 | 0.02 (0.02) | 0 | |
| 40 | 0.17 (0.04) | 0.04 (0.03) | |
| 50 | 0.35 (0.06) | 0.09 (0.05) | |
| 60 | 0.41 (0.06) | 0.26 (0.08) | |
| 70 | 0.52 (0.08) | 0.32 (0.09) | |
| 80 | 0.60 (0.10) | 0.40 (0.11) | |
| Risk of ovarian cancer | |||
| 40 | 0 | 0 | |
| 50 | 0.05 (0.03) | 0.03 (0.03) | |
| 60 | 0.27 (0.07) | 0.07 (0.05) | |
| 70 | 0.47 (0.10) | 0.13 (0.07) | |
| 80 | 0.53 (0.11) | 0.62 (0.18) | |
| Risk of either breast or ovarian cancer | |||
| 30 | 0.03 (0.02) | 0 | |
| 40 | 0.23 (0.05) | 0.04 (0.03) | |
| 50 | 0.41 (0.06) | 0.16 (0.06) | |
| 60 | 0.60 (0.07) | 0.33 (0.09) | |
| 70 | 0.77 (0.07) | 0.47 (0.11) | |
| 80 | 0.83 (0.07) | 0.76 (0.13) |
Fig. 2.
Cumulative incidence of breast and ovarian cancer among women with mutations in BRCA1 and BRCA2. Cumulative incidence rates were estimated for confirmed female mutation carriers from fully genotyped sibships. Blue indicates BRCA1 carriers; green indicates BRCA2 carriers. (A) Cumulative risk of developing either breast or ovarian cancer. Risk differed significantly for carriers of mutations in BRCA1 vs. BRCA2 (P = 0.004). (B) Cumulative risk of breast cancer. Risk differed significantly for carriers of mutations in BRCA1 vs. BRCA2 (P = 0.02). (C) Cumulative risk of developing ovarian cancer. Difference in risk for carriers of mutations in BRCA1 vs. BRCA2 was not significant (P = 0.16).
These cumulative incidence estimates were based on fully genotyped sibships. To address potential biases resulting from exclusion of incompletely genotyped sibships, cumulative incidences were also estimated for all sibships, after imputing probabilities of carrier status for women who were not genotyped. Imputation was carried out independently based on risks estimated from two previous studies (3, 20) as well as from this one. Analyses based on fully genotyped sibships and analyses based on all sibships yielded very similar risk estimates for breast cancer and ovarian cancer in BRCA1 carriers and for breast cancer in BRCA2 carriers (Table 1 and SI Appendix, Table S5). Estimates of ovarian cancer risk by age 80 in BRCA2 carriers were higher based on fully genotyped sibships (0.62 ± 0.18; Table 1) than estimates based on all sibships (0.37–0.45 ± 0.08–0.12; SI Appendix, Table S5).
Effect of Birth Cohort on Risk Among Mutation Carriers.
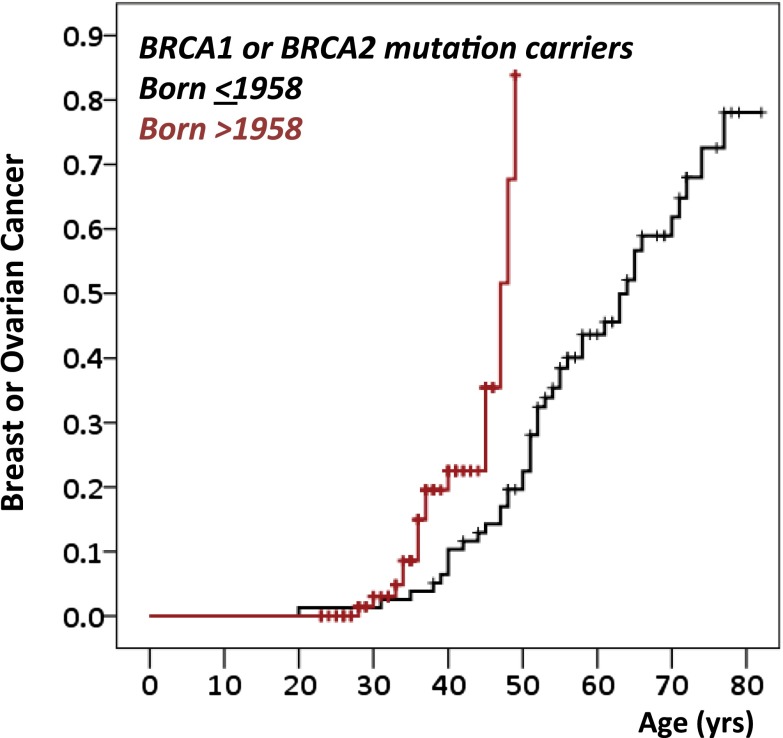
Previous studies reported higher breast cancer risks among BRCA1 and BRCA2 mutation carriers from more recent, compared with earlier, birth cohorts (3, 15, 21). Similarly, in the present study, combined breast and ovarian cancer incidence was higher for carriers in more recent birth cohorts (Fig. 3). Among women born in or before 1958 (the median year of birth for mutation carriers), cumulative incidence of breast or ovarian cancer was 0.22 by age 50 and 0.78 by age 80. For women born after 1958, cumulative incidence of breast or ovarian cancer was 0.84 by age 50. Age-specific cancer risks, controlled for gene, were 3.8-fold higher for carriers in the younger vs. the older birth cohort (P = 0.006; Fig. 3). Per year of later birth, hazard ratios, controlled for gene, were 1.041 (P = 0.04) for ovarian cancer and 1.033 (P = 0.01) for breast cancer.
Fig. 3.
Time trends in cumulative incidence for either breast or ovarian cancer among women with mutations in BRCA1 or BRCA2. Risk to carriers has increased with time, illustrated by cumulative risk of either breast cancer or ovarian cancer for carriers born after 1958 (the median birth year of carriers in the study) vs. carriers born in 1958 or earlier (P = 0.006).
Discussion
To evaluate whether evidence supports population screening for BRCA1 and BRCA2 mutations, we assessed breast and ovarian cancer risks among female mutation carriers ascertained through healthy males. The males who comprised the screening series were representative of the general Israeli AJ population with respect to mutation prevalence and family history of cancer. Risks of breast and ovarian cancer to females with BRCA1 mutations ascertained via these healthy males were comparable to risk estimates in previous studies with ascertainment through affected index cases (SI Appendix, Table S6) (3, 20, 22). In contrast, a notable difference between this and other studies is that BRCA2 mutation carriers in this study have a higher risk of ovarian cancer and a lower risk of breast cancer than do BRCA2 mutation carriers in other studies (SI Appendix, Tables S6 and S7) (2, 16). Differences in site of first cancer may reflect a lower prevalence in the Israeli population of nongenetic risk factors for breast cancer, such as late age at first pregnancy, and therefore reduced competing risk. In comparison with studies of BRCA2 in non-AJ populations, a second contributing factor may be the location of BRCA2 6174delT in the BRCA2 “ovarian cancer cluster region,” where mutations may be associated with higher ovarian cancer risks (23). To some extent, risks of breast and ovarian cancer likely compensate for one another, yielding combined risks for developing either breast or ovarian cancer that are similar across studies. Breast cancer risks among mutation carriers in Israel are rapidly increasing (Fig. 3), with the time trend particularly marked for BRCA2 carriers. This increase is likely due both to improvements in screening for breast cancer and to trends toward escalating nongenetic risk factors for breast cancer, such as younger age at menarche and older age at first childbirth. These strong birth cohort effects suggest that for both BRCA1 and BRCA2, most published risk estimates, which reflect multiple generations of carriers, underestimate risk to carriers among present-day young women.
Integration of genomic advances into public health requires well-characterized genetic tests for clearly actionable mutations (24). In the AJ population, evidence supporting testing for the unambiguously damaging founder mutations in BRCA1 and BRCA2 is provided by the results of this study, by previous studies of the AJ alleles (2, 3, 22), and by the existence of effective intervention for carriers (1, 2). A great advantage of population screening is identification of all carriers, not only those from families with a significant cancer history. In this study, 51% (85 of 167) families harboring BRCA1 or BRCA2 mutations had little or no history of relevant cancer (SI Appendix, Table S3). These families were small and included few females with mutations who had reached the ages of highest cancer risk. Young women in these families would not have been tested in the absence of a general screening program. Population screening also enables carriers to be identified regardless of their relatives' willingness to divulge information on cancer diagnosis or genetic test results. It also enables carriers to be identified independent of physician referral, a potentially important consideration. A recent survey in the United States revealed that only 19% of primary care physicians accurately assessed family history for BRCA1/BRCA2 testing (25). In BRCA1/BRCA2 families in France, most eligible relatives are not referred for testing (26). In the present study in Israel, only 35% (29 of 82) of high-cancer-incidence families had been referred previously for genetic counseling, despite its availability within the Israeli universal single-payer health care system.
Implementation of a population-screening program for cancer genetics presents new challenges. Traditional cancer genetic counseling including both pretest and posttest counseling is impractical on a large scale. In this study, this two-stage process was retained only for a small subset of index males. This study design provided mutation carriers with another opportunity to reconsider their decision to be tested, reflecting early concerns about possible negative effects of knowledge of carrier status. These concerns have lessened as testing for cancer predisposition has gained acceptance and its medical benefits have become apparent. There is already some evidence that receiving limited pretest information does not affect satisfaction with BRCA1 and BRCA2 testing (27), suggesting that extensive posttest counseling can be limited to carriers. Genetic screening will need to be integrated into adult primary care, entailing special outreach to family practitioners and to community-based physicians, as well as to the public. The AJ community has considerable experience with genetic carrier screening for autosomal recessive pediatric disorders. Such screening is widely accepted and has led to significant prevention of diseases such as Tay-Sachs (28). However, genetic screening for an autosomal dominant, common adult-onset disease has not yet been attempted, and raises unique issues: Carriers are themselves at risk, and their offspring are at 50% risk of inheriting any mutation identified, regardless of partner choice. Further research would be necessary to optimize cost-effective implementation, which may vary between cultures and medical systems (29).
This study was performed in an AJ population, but its results are widely applicable. Among persons of any ethnicity undergoing clinical exome or genome sequencing for conditions other than cancer, damaging mutations in BRCA1 and BRCA2 identified incidentally are already considered to be sufficiently actionable to require reporting (30). Furthermore, it is now possible to identify, in one test and at reasonable cost, all actionable mutations in BRCA1 and BRCA2, as well as in all other known breast and ovarian cancer genes (31, 32). Such analyses reveal all variation: both unambiguously damaging mutations and variants of unknown significance. The AJ population is unusual in that the mutational burden of BRCA1 and BRCA2 is essentially limited to three unambiguously damaging alleles. We suggest that population screening efforts focus on clearly damaging mutations, such as those evaluated in this project. BRCA1 and BRCA2 were identified in the mid-1990s, and patent issues that in the United States previously limited complete genomic analysis of BRCA1 and BRCA2 have been largely resolved (33). We suggest that the time has come to apply our knowledge of these genes to consideration of a general screening program, with the aim of reducing the burden of breast and ovarian cancer.
Methods
Participants.
The study was approved by the institutional review boards of all participating institutions and by Israel's National Human Subjects Committee. All participants provided written informed consent. Male index subjects and their relatives of those index subjects with BRCA1 or BRCA2 mutations were recruited between June 2004 and December 2010, as outlined in Fig. 1 and SI Appendix, SI Methods. Males visiting health-related settings throughout Israel were offered participation if they were age 30 or older, identified all four grandparents as AJ, and had no personal history of cancer (SI Appendix, Table S1). Family history of cancer was not a criterion for or against participation. Males who chose to participate provided a blood or buccal sample from which DNA was extracted and genotyped for BRCA1 185delAG, BRCA1 5382insC and BRCA2 6174delT (SI Appendix, Table S2). All males carrying at least one of these mutations were recontacted and invited for genetic counseling. Institutional review board requirements did not allow results to be disclosed to participants until the participant received genetic counseling and the test was repeated. Therefore, to maintain blindness of the participants to the test results, an equal number of males with none of these BRCA1 or BRCA2 mutations, matched to mutation carriers for age, town of residence, and recruitment locale, were similarly recontacted and invited for genetic counseling. All recontacted subjects were told that they had a 50% chance of being a carrier. At the follow-up visit, full counseling was provided by a genetic counselor who was unaware of the results of the first test. Counseled participants interested in receiving test results gave consent for a second (repeat) test. Each counseled participant provided a detailed family history and a blood sample, which was tested for all three AJ mutations. All results were identical for the original and repeat tests. Results were reported to participants in a posttest counseling session. Participants identified as carriers were asked to refer all adult female relatives for genetic counseling and testing. Matched subjects without mutations were not asked to participate further. For each family carrying a BRCA1 or BRCA2 mutation, family history of cancer was obtained through personal report and verified whenever possible through the Israel Cancer Registry (ICR) and hospital records. Family history was assessed both from information provided by the male index subject at study entry and subsequently from information provided by all counseled relatives. Living relatives were counseled and tested for the three AJ mutations. For deceased relatives, genotypes were obtained by testing archived pathology specimens and by testing surviving relatives.
Statistical Methods.
Characteristics of index subjects with vs. without mutations were compared by t test for continuous variables, χ2 tests for categorical variables, and McNemar tests for categorical variables in paired subjects. All P values were two-tailed and confidence intervals calculated at the 95% level. Cumulative incidences of breast cancer and of ovarian, fallopian tube, or primary peritonieal cancer among female mutation carriers were determined using Kaplan–Meier analysis and Cox proportional hazard models. Ages of affected women were censored at age of first diagnosis. Ages of unaffected women were censored at age at death, age at most recent follow-up, or age at risk-reduction surgery, whichever came first. Women were excluded from analysis if born before 1910 or if cause of death was unknown. Results are shown for fully genotyped sibships, i.e., those in which genotypes were confirmed for all sisters, affected and unaffected. Fully genotyped sibships are likely to be smaller and younger than other sibships, but are less prone to survival bias, if deceased relatives could not be genotyped, or to refusal bias, if presence or absence of cancer diagnosis influenced consent to be tested. An alternate analysis including all female carriers, with genotypes identified either directly by genetic testing or imputed statistically, is given in the SI Appendix, SI Results. A sensitivity analysis to assess the possible effect on risk estimates of consent bias based on family history of breast cancer is also given in SI Appendix, SI Results and Table S4.
Supplementary Material
Acknowledgments
We thank Dr. Lital Keynan-Boker and Dr. Micha Barchana for providing data from the Israel Cancer Registry; Dr. Nava Epstein, Dorina Katzenellenbogen, Pesya Ashki, and Haya Hackett for help with study coordination and recruitment; and Hafez Jabara, Anat Yadin, Ariella Tomer, Irit Kisslov, and Hila Gadelki for laboratory and database assistance. We thank the participants and their families for their willingness and cooperation. This work was supported by the Breast Cancer Research Foundation, the Israel National Institute for Health Policy Research, the Israel Cancer Association, and National Institutes of Health Grant R01CA157744.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415979111/-/DCSupplemental.
References
- 1.Domchek SM, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelman BS, et al. Breast and ovarian cancer risk and risk reduction in Jewish BRCA1/2 mutation carriers. J Clin Oncol. 2012;30(12):1321–1328. doi: 10.1200/JCO.2011.37.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King MC, Marks JH, Mandell JB. New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 4.Møller P, et al. Genetic epidemiology of BRCA mutations—family history detects less than 50% of the mutation carriers. Eur J Cancer. 2007;43(11):1713–1717. doi: 10.1016/j.ejca.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Weitzel JN, et al. Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA. 2007;297(23):2587–2595. doi: 10.1001/jama.297.23.2587. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe KA, et al. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28(3):387–391. doi: 10.1200/JCO.2009.25.0712. [DOI] [PubMed] [Google Scholar]
- 7.Frank TS, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: Analysis of 10,000 individuals. J Clin Oncol. 2002;20(6):1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 8.Roa BB, Boyd AA, Volcik K, Richards CS. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996;14(2):185–187. doi: 10.1038/ng1096-185. [DOI] [PubMed] [Google Scholar]
- 9.Hirsh-Yechezkel G, et al. Population attributes affecting the prevalence of BRCA mutation carriers in epithelial ovarian cancer cases in israel. Gynecol Oncol. 2003;89(3):494–498. doi: 10.1016/s0090-8258(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 10.Risch HA, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson J, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. [Google Scholar]
- 12.Khoury MJ, McCabe LL, McCabe ER. Population screening in the age of genomic medicine. N Engl J Med. 2003;348(1):50–58. doi: 10.1056/NEJMra013182. [DOI] [PubMed] [Google Scholar]
- 13.Levy-Lahad E. Population-based BRCA1/BRCA2 screening in Ashkenazi Jews: A call for evidence. Genet Med. 2009;11(9):620–621. doi: 10.1097/GIM.0b013e3181b765d3. [DOI] [PubMed] [Google Scholar]
- 14.Ford D, et al. The Breast Cancer Linkage Consortium Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. 1998;62(3):676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniou A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mavaddat N, et al. EMBRACE Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 17.Struewing JP, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB. On the use of familial aggregation in population-based case probands for calculating penetrance. J Natl Cancer Inst. 2002;94(16):1221–1226. doi: 10.1093/jnci/94.16.1221. [DOI] [PubMed] [Google Scholar]
- 19.Moyer VA. U.S. Preventive Services Task Force Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281. doi: 10.7326/M13-2747. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tryggvadottir L, et al. Population-based study of changing breast cancer risk in Icelandic BRCA2 mutation carriers, 1920-2000. J Natl Cancer Inst. 2006;98(2):116–122. doi: 10.1093/jnci/djj012. [DOI] [PubMed] [Google Scholar]
- 22.Antoniou AC, et al. Breast and ovarian cancer risks to carriers of the BRCA1 5382insC and 185delAG and BRCA2 6174delT mutations: A combined analysis of 22 population based studies. J Med Genet. 2005;42(7):602–603. doi: 10.1136/jmg.2004.024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gayther SA, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15(1):103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- 24.Khoury MJ, et al. The continuum of translation research in genomic medicine: How can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9(10):665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 25.Bellcross CA, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61–66. doi: 10.1016/j.amepre.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Pujol P, et al. Lack of referral for genetic counseling and testing in BRCA1/2 and Lynch syndromes: A nationwide study based on 240,134 consultations and 134,652 genetic tests. Breast Cancer Res Treat. 2013;141(1):135–144. doi: 10.1007/s10549-013-2669-9. [DOI] [PubMed] [Google Scholar]
- 27.Metcalfe KA, et al. Patient satisfaction and cancer-related distress among unselected Jewish women undergoing genetic testing for BRCA1 and BRCA2. Clin Genet. 2010;78(5):411–417. doi: 10.1111/j.1399-0004.2010.01499.x. [DOI] [PubMed] [Google Scholar]
- 28.Klugman S, Gross SJ. Ashkenazi Jewish screening in the twenty-first century. Obstet Gynecol Clin North Am. 2010;37(1):37–46. doi: 10.1016/j.ogc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Khoury MJ, Gwinn ML, Glasgow RE, Kramer BS. A population approach to precision medicine. Am J Prev Med. 2012;42(6):639–645. doi: 10.1016/j.amepre.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green RC, et al. American College of Medical Genetics and Genomics ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh T, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci USA. 2010;107(28):12629–12633. doi: 10.1073/pnas.1007983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong HK, et al. The validation and clinical implementation of BRCAplus: A comprehensive high-risk breast cancer diagnostic assay. PLoS ONE. 2014;9(5):e97408. doi: 10.1371/journal.pone.0097408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kesselheim AS, Cook-Deegan RM, Winickoff DE, Mello MM. Gene patenting—the Supreme Court finally speaks. N Engl J Med. 2013;369(9):869–875. doi: 10.1056/NEJMhle1308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.