Significance
A subpopulation of antibody-secreting cells, B-1 cells, provides early protection against several types of pathogens. Both the development and function differ between B-1 cells and the better known B-2 cells, and exclusively B-1 cells are lacking in mice deficient for the nuclear inhibitory κB protein, IκBNS. B-1 cells mature similar to B-2 cells via a transitional stage. We demonstrate here the existence of a phenotypically distinct B-1 transitional B (TrB)-cell population in the neonatal spleen of wild-type mice. This TrB-1a–cell subset was lost in the absence of IκBNS, thus revealing a requirement for intact NF-κB signaling via IκBNS during this stage of the development of B-1 cells. Learning more about the development of B-1 cells may reveal new targets for therapeutic intervention.
Keywords: B-1b, Nfkbid, NF-κB1, IκB-δ, NP-ficoll
Abstract
B-1 cells mediate early protection against infection by responding to T cell-independent (TI) antigens found on the surface of various pathogens. Mice with impaired expression of the atypical IκB protein IκBNS have markedly reduced frequencies of B-1 cells. We used a mouse strain with dysfunctional IκBNS derived from an N-ethyl-N-nitrosourea (ENU) screen, named bumble, to investigate the point in the development of B-1 cells where IκBNS is required. The presence of wild-type (wt) peritoneal cells in mixed wt/bumble chimeras did not rescue the development of bumble B-1 cells, but wt peritoneal cells transferred to bumble mice restored natural IgM levels and response to TI antigens. The bumble and wt mice displayed similar levels of fetal liver B-1 progenitors and splenic neonatal transitional B (TrB) cells, both of which were previously shown to give rise to B-1 cells. Interestingly, we found that a subset of wt neonatal TrB cells expressed common B-1a markers (TrB-1a) and that this cell population was absent in the bumble neonatal spleen. Sorted TrB-1a (CD93+IgM+CD5+) cells exclusively generated B-1a cells when adoptively transferred, whereas sorted CD93+IgM+CD5− cells gave rise to B-2 cells and, to a lesser extent, B-1b and B-1a cells. This study identifies a phenotypically distinct splenic population of TrB-1a cells and establishes that the development of B-1a cells is blocked before this stage in the absence of IκBNS.
T and B lymphocytes are central in the immune response to infections. After pathogen encounter, B cell responses to protein-based antigens are induced via help from T cells, whereas polysaccharide and/or particulate antigens can stimulate B cells to produce antibodies in a T cell-independent (TI) fashion, giving rise to a more immediate response. Antibodies to T cell-dependent (TD) antigens are mainly produced by follicular B cells, whereas marginal zone B (MZB) cells, B-1a cells, and B-1b cells, collectively referred to as innate-like B cells, facilitate rapid responses to TI antigens found on the surface of many classes of pathogens. These innate B cells play distinct, although sometimes overlapping, roles in pathogen confinement and presentation. In particular, MZB cells and B-1a cells both contribute to protection against Gram-negative bacteria by responding to LPSs (1, 2), whereas B-1b cells and MZB cells are required for optimal recall response against infection with encapsulated bacteria, such as Streptococcus pneumoniae (3, 4).
Conventional B (B-2) cells are replenished throughout life from a common precursor in the bone marrow. Differentiation into mature naive B cells takes place in the periphery upon exit of immature B cells from the bone marrow. The cells then migrate to the spleen, where they undergo transition and are subjected to selection (5). MZB and follicular B cells diverge at this B-cell transitional stage, dependent on the strength of signals mediated by the B-cell receptor (BCR), the B-cell–activating factor (BAFF) receptor, and Notch2, all of which involve the NF-κB pathways (6). Less is known about the development of B-1 cells, but it is well established that B-1 cells, in contrast to B-2 cells, are generated more abundantly from fetal liver than from the bone marrow and are maintained by self-renewal throughout the life span of the individual (7, 8). Studies on the early stages of the development of B-1 cells have identified B-1 progenitors (B-1p cells; Lin−CD93+CD19+B220lo/−) in fetal liver but also, at a lower frequency, in the bone marrow and spleen of neonatal as well as adult mice (9, 10). Recently, Montecino-Rodriguez and Dorshkind (11) proposed that B-1 cells develop through a transitional (CD93+IgM+CD23+/−) splenic intermediate population similar to that described for B-2 cells, with the exception that the transitional window of B-1 cells is limited to the neonatal stage. However, these studies did not provide information on how neonatal transitional B-1 (TrB-1) cells differ phenotypically or functionally from their TrB-2 counterparts.
TI antigens have traditionally been classified based on whether they induce antibodies in mice with a mutation in the gene coding for Bruton’s tyrosine kinase (xid/Btk) (TI-1) or not (TI-2) (12). Later, it was discovered that Btk interacting with phospholipase Cγ2 is needed for activation of the NF-κB transcription factor upon BCR ligation (13) and that NF-κB signaling also regulates Btk expression. This finding may partially explain why some of the immunological defects described for xid mice are also observed in mice where regulators of the NF-κB pathway are ablated. The NF-κB family includes NF-κB1 (p50), NF-κB2 (p52), RelA (p65), c-Rel, and RelB, which interact to form functional homo- or heterodimer complexes. The interplay between these components is regulated by IκB proteins, including IκBα, IκBβ, and IκBε, which are characterized by their ankyrin repeat structure (14). NF-κB activation is mediated by the p50, RelA, c-Rel, and NF-κB essential modulator (NEMO)-dependent classical pathway or by the alternative pathway, which involves p52/RelB complexes. Classical and alternative NF-κB pathways are implicated in multiple stages of B-cell life. Furthermore, function and survival signals mediated through the BAFF receptor and/or BCR require functional NF-κB signaling (15).
The development of B-cell subsets is differentially dependent on intact NF-κB pathways. Thus, B-1 cells and MZB cells were severely reduced in mice where classical NF-κB signaling components, including CARMA1, Bcl10, Malt1, and NF-κB1, were ablated, whereas follicular B-cell development and function were less affected under these conditions (15–17). In contrast, compound deficiencies in NF-κB1/NF-κB2 or c-Rel/RelA impair development of all mature B-cell subsets (18, 19). Also, the more recently identified atypical IκB proteins are involved in B-cell development (20–22). The atypical IκBs interact with NF-κB transcription factors in the nucleus rather than in the cytoplasm and, in contrast to traditional IκBs, are not only inhibitory but may either augment or repress transcriptional activity of target genes, depending on the cell type and conditions of activation that are studied (23). We previously identified a mouse strain with a nonsense mutation in the nfkbid gene encoding the atypical IκB protein, IκBNS, among a number of hits in a forward genetic N-ethyl-N-nitrosourea (ENU) mutagenesis screen for mice displaying B-cell response defects. The strain, which was named bumble, had reduced antibody responses to TI antigens and displayed reduced frequencies of peritoneal B-1 cells and spleen MZB cells (22).
In the present study, we used the bumble mice to investigate at which point in the development of B-1 cells NF-κB signaling via IκBNS is required. We demonstrate that bumble mice have largely normal frequencies of fetal liver B-1p and splenic neonatal transitional B cells, both of which have previously been described to give rise to B-1 cells. However, upon close examination of the splenic neonatal TrB cells, we found that they can be phenotypically divided into at least two sublineages, of which one predominantly gives rise to B-1a cells and the other to B-2 cells. We show here that bumble mice only harbor the latter population, and thus propose that the development of B-1a cells in the absence of IκBNS is blocked before this splenic transitional stage in the neonate. This study advances our understanding of the ontogeny of B-1 cells and identifies IκBNS as a central component required for the development of B-1 cells.
Results
Bumble Mice Lack B-1 Cells and Display Reduced Antibody Responses to TI Antigens.
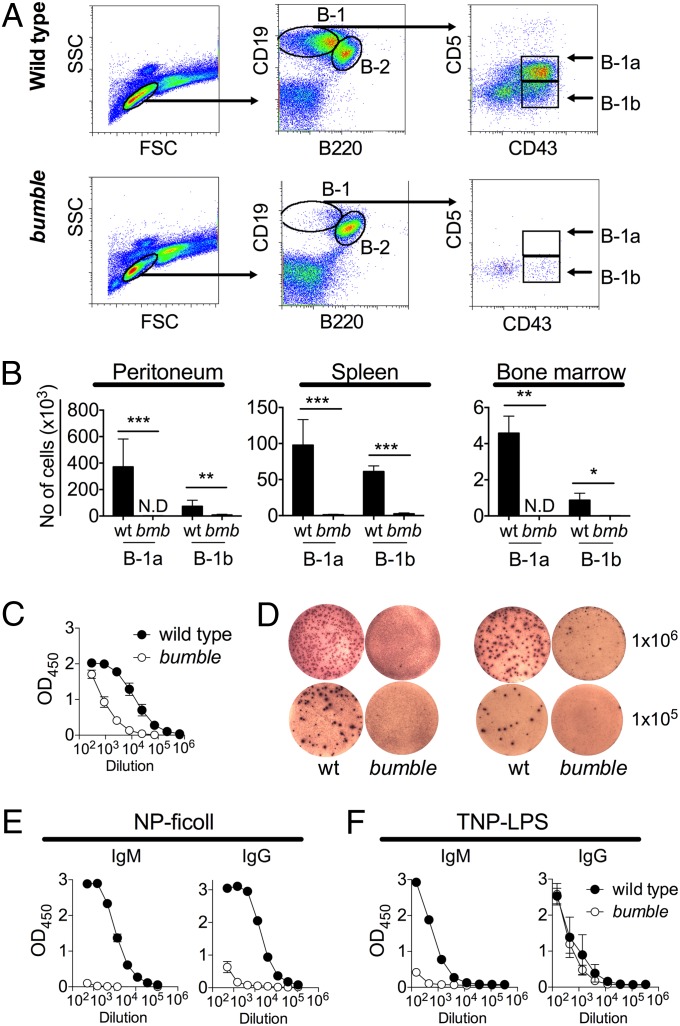
Mice with ablated IκBNS expression (bumble) were previously found to display normal development of pro/pre-, immature, and mature follicular B cells, whereas peritoneal B-1a cells were completely absent and the frequencies of B-1b cells were severely reduced (21, 22). There could be several possible reasons for the lack of peritoneal B-1 cells in bumble, including impaired B-1 development or maintenance, unsupportive microenvironment, and/or migrational defects. To investigate if the deficiency of B-1 cells in bumble was limited to the peritoneal cavity, we stained for B-1 cells in the major lymphoid organs. Similar to the case in the peritoneal cavity (Fig. 1A), no B-1a and very few B-1b cells were detected in the spleen and bone marrow of bumble mice (Fig. 1B), indicating that the lack of peritoneal B-1 cells in IκBNS-deficient mice is not simply due to a migration defect. B-1 cells produce most of the steady-state circulating natural IgM, and bumble mice displayed a severe reduction in total serum IgM (Fig. 1C), as well as IgM-secreting cells, in the spleen and bone marrow (Fig. 1D). B-1 cells are important for TI antibody responses, and bumble mice did not produce specific IgM when challenged with the TI type II antigen nitrophenylacetyl (NP)-Ficoll (22), and the IgG response was similarly reduced (Fig. 1E). The bumble mice also failed to produce a trinitrophenyl (TNP)-specific IgM antibody response but maintained the ability to generate TNP-specific IgG after administration of the TI type I antigen TNP-LPS (Fig. 1F).
Fig. 1.
Bumble mice have impaired frequencies of B-1 cells and responses to TI antigens. The wt and bumble (bmb) tissues were stained for B-1 cells identified as CD19hiB220loCD43+ (B-1b) and CD19hiB220loCD43+CD5+ (B-1a). (A) Representative plots showing the gating strategy for identification of peritoneal B-1a and B-1b cells. Peritoneal cells were isolated by flushing with 5 mL of PBS plus 1% fetal bovine serum (FBS). (B) Peritoneal, spleen, and bone marrow cells were isolated and stained for B-1 cells, and the absolute number of cells is shown as mean ± SD. (C) Total serum IgM was determined by ELISA. (D) IgM antibody secreting cells from spleen (Left) and bone marrow (Right) were examined by ELISpot. Plates were coated with anti-IgM, and the indicated numbers of wt and bumble cells were added in triplicate. IgM producing cells were determined 17 h later. Each picture shows one well from an ELISpot plate of standard size (96-wells, diameter: 6.4 mm). (E) Mice were evaluated for NP-specific antibodies by ELISA 10 d postadministration with 50 μg of NP-Ficoll. (F) Mice were evaluated for TNP-specific antibodies by ELISA 10 d after TNP-LPS administration (10 μg). Figures represent 8- to 14-wk-old mice with three to seven mice per group, and results are representative of at least two independent experiments. Graphs display mean ± SD. Statistically significant differences between bumble and wt mice are indicated by denoting *P < 0.05, **P < 0.01, or ***P < 0.001 as determined by an unpaired t test.
Transfer of Wild-Type Peritoneal Cavity Cells to Bumble Reconstitutes the B-1 Natural IgM Levels.
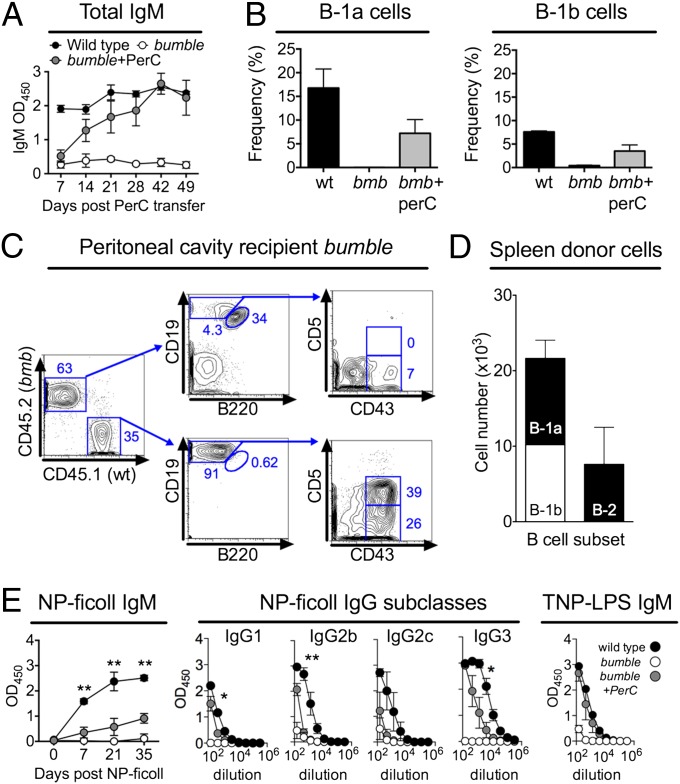
To investigate if bumble mice were capable of providing a supportive environment for the survival of mature B-1 cells, we transferred peritoneal cavity cells from wild-type (wt) mice into 3-wk-old nonirradiated bumble mice. We chose this age to minimize the risk of graft rejection. Because B-1 cells are major producers of natural IgM antibodies, we monitored serum IgM as an indication of successful transfer. Serum IgM levels of bumble peritoneal wt cell recipients reached wt levels at 6–7 wk posttransfer (Fig. 2A). Both B-1a and B-1b cells from the wt cell donors were recovered in the peritoneal cavity, and at 14 d posttransfer, the levels of B-1a and B-1b cells were 30% and 50%, respectively, of those levels in wt mice (Fig. 2B).
Fig. 2.
Peritoneal cells were isolated from 8- to 14-wk-old wt mice by flushing with 5 mL of PBS plus 1% FBS, and 4 × 106 cells were transferred i.p. to 3-wk-old bumble mice. (A) Total IgM levels in peritoneal cell recipient bumble mice were monitored by ELISA at various time points after cell transfer. (B) Frequency of peritoneal B-1a and B-1b cells at 14 d after peritoneal cell transfer. The same gating strategy as for Fig. 1A was used. The frequency of cells in the lymphocyte gate is shown. (C) Representative plot of bumble (CD45.2) mice that had received peritoneal cells from wt CD45.1 mice 50 d before analysis. (D) Absolute numbers of donor B-1a, B-1b, and B-2 cells in the spleen of recipient bumble mice 50 d after transfer of wt CD45.1 peritoneal cells. (E) Bumble mice that had received wt peritoneal cells 50 d earlier were immunized with NP-Ficoll or TNP-LPS, and NP-specific IgM and IgG subclass antibody responses were measured by ELISA at various time points after NP-Ficoll administration. NP-specific IgM responses were evaluated using a 1:1,350-fold serum dilution, and IgG subclasses were evaluated using threefold serial dilutions with a staring dilution of 1:150. IgG subclass responses are shown for day 21 after NP-Ficoll administration. TNP-specific IgM responses were determined 7 d after TNP-LPS immunization. Significant differences between wt mice and bumble mice that had received wt peritoneal cells are indicated by *P < 0.05 and **P < 0.01 as determined by an unpaired t test. Graphs display mean ± SD and are representative of two to three independent experiments with three mice per group. PerC, peritoneal cell.
To confirm the donor origin of the B-1 cells, we transferred wt peritoneal cells expressing the CD45.1 allotype. Recipient bumble mice (CD45.2) were analyzed 2 mo posttransfer. As expected, all B-1a cells and the majority of B-1b cells in the peritoneal cavity of reconstituted bumble mice were donor-derived (Fig. 2C). The donor B-1 cells were distributed to the spleen (Fig. 2D), indicating that mature B-1 cells migrate normally in bumble mice. In contrast to B-1 cells, few B-2 cells of donor origin were found in the peritoneum or spleen of bumble recipients (Fig. 2 C and D), as would be expected due to their inability to self-renew. These transfer experiments indicated that bumble mice support survival of mature wt B-1 cells.
Impaired Antibody Response to TI Antigens in Bumble Can Be Partially Attributed to the Lack of B-1 Cells.
B-1 and MZB cells respond in concert to challenge with TI antigens. We investigated if the lack of antibody response to the TI-2 antigen NP-Ficoll was restored after transfer of peritoneal wt cells to bumble mice. We chose to immunize bumble mice 50 d posttransfer, when the IgM levels had reached the levels observed in wt mice. Notably, transfer of wt peritoneal cells partially restored the NP-specific IgM and IgG response in bumble mice (Fig. 2E); however, the response did not reach the levels observed in NP-Ficoll–injected wt mice (P ≤ 0.05). Similar to the case in wt mice, NP-specific antibodies of the IgG3 subclass dominated the response in the bumble mice that had received wt peritoneal cells. Reconstitution of the B-1 compartment in bumble mice completely restored the IgM response to the TI-1 antigen TNP-LPS (Fig. 2E). These results suggest that the absence of TI antibody responses in bumble can be attributed, at least in part, to their lack of B-1 cells.
Lack of B-1 Cells in Bumble Mice Is Due to a Cell-Intrinsic Defect.
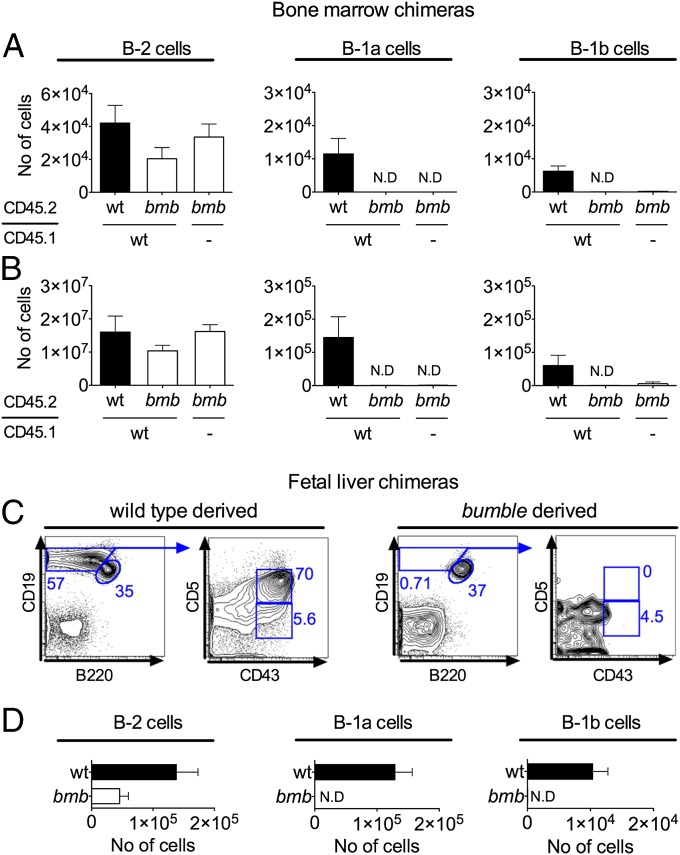
We next investigated whether the lack of B-1 cells in bumble was due to B cell-autonomous defects or alterations in the stromal microenvironment. As noted previously, initial studies showed that B-1 cells were generated from adult wt bone marrow when transferred into immunodeficient hosts (24–27). To distinguish between extrinsic and intrinsic contributions to the bumble phenotype, we mixed bone marrow from bumble (CD45.2) and wt (CD45.1) mice and transferred it into irradiated RAG1−/− mice. Control animals were RAG1−/− mice receiving bumble only or wt (CD45.1)/wt (CD45.2) cells. Fig. 3 shows the number of bumble or wt donor-derived CD45.2-expressing cells of the different B-cell subsets in the presence or absence of supplementing wt CD45.1 cells. The bumble bone marrow transferred alone gave rise to B-2 cells of similar numbers as those numbers observed in RAG1−/− mice that had received only wt cells. In the mixed bumble/wt chimeras, although bumble bone marrow generated nearly similar B-2 cell frequencies and numbers as the cotransferred wt counterpart, only the wt bone marrow gave rise to B-1 cells in the peritoneal cavity and spleen (Fig. 3 A and B).
Fig. 3.
Only B-1 cells of wt origin are generated in chimeras of wt and bumble. The wt (CD45.1) and bumble (CD45.2) bone marrow (50 × 106 cells of each) or fetal liver (3 × 106 cells of each) were mixed and transferred i.v. to irradiated (600 rad) RAG1−/− mice. Control chimeras were wt (CD45.1) mixed with wt (CD45.2) or bumble cells administered alone. The recipient mice were analyzed for B-1 and B-2 cells 8 wk later. The same gating strategy as for Fig. 1A was used after resolution of CD45.1/CD45.2 allotype. Absolute numbers of bone marrow transfer-derived CD45.2 (wt or bumble) B-2, B-1a, and B-1b cells derived from peritoneal cells (A) and splenocytes (B) are shown in the bar charts. Results are representative of three independent experiments. Fetal liver chimeras were generated and analyzed to confirm the cell-intrinsic defect. (C) Representative plot showing the gating strategy for identifying CD45.1 wt-derived (Left) or CD45.2 bumble-derived (Right) B-2, B-1a, and B-1b cells in the fetal liver chimeras. (D) Total number of fetal liver-derived peritoneal cells of the indicated B-cell subpopulations. The mean numbers of cells ± SEM are shown for three mice per group. N.D., not detected.
Because B-1 cells are predominantly generated from the fetal liver, we next tested if B-1 cells would develop from bumble fetal liver progenitors in the presence of wt fetal liver cells. Analogous to the bone marrow transfers, fetal liver chimeras were made by mixing equal numbers of wt (CD45.1) and bumble (CD45.2) cells and transferring these into irradiated RAG1−/− recipient mice. Confirming the results in bone marrow chimeras, all B-1a and B-1b cells in the fetal liver chimeras were wt-derived (Fig. 3 C and D). The bumble B-2 cell numbers were also reduced in the fetal liver chimeras, although the difference was not statistically significant (P = 0.07). In summary, supplementation of wt cells did not restore the deficiency in bumble bone marrow or fetal liver precursor cells to differentiate into B-1 cells, illustrating that the reduction in bumble B-1 cells is due to a cell-intrinsic defect.
Neonatal Bumble Mice Lack B-1 Cells.
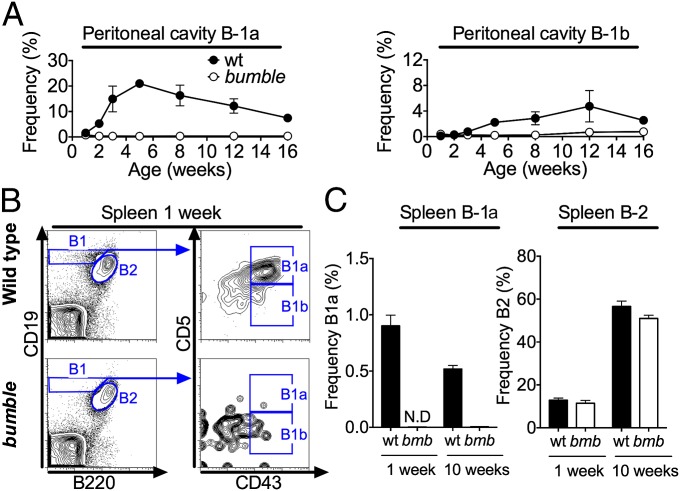
Most B-1 cells are generated at the neonatal stage and are then maintained by self-renewal. It was therefore possible that the lack of B-1 cells in adult bumble mice was due to a limited capacity for mature B-1 cells to self-renew. We therefore tested in a kinetic study whether bumble B-1 cells were present at the neonatal stage and wane with increasing age (Fig. 4A). Only very low frequencies of B-1 cells were detected in the peritoneal cavity of wt mice at 1 wk of age (<2% of cells in the lymphocyte gate). In contrast, 2-wk-old wt mice had a distinct CD19hiB220lo peritoneal population of B-1 cells, of which most were CD43+CD5+ B-1a cells. The frequency of B-1a cells peaked at 5 wk of age and then declined, whereas B-1b cells were maintained at similar frequencies from 8 to 16 wk of age. No peritoneal B-1a cells and only a few B-1b cells were detected in bumble mice at any time point. In wt mice, B-1a cells appeared earlier in the spleen than in the peritoneal cavity, because B-1 cells, mostly of the CD43+CD5+ B-1a phenotype, were already readily detected 1 wk after birth (Fig. 4B). Neonatal wt splenocytes displayed a higher frequency of B-1a cells than adult mice (Fig. 4C). Conversely, the frequency of B-2 cells was greatly increased in adult mice. The bumble mice did not have any detectable splenic B-1 cells at 1 wk of age (Fig. 4 B and C), similar to adult bumble mice. These data suggest that rather than being lost due to an incapability of self-renewal, mature B-1a cells were not generated in bumble mice and B-1b cells were severely reduced.
Fig. 4.
B-1 cells are impaired already in neonatal bumble mice. The wt and bumble mice of different ages were stained for B-1 and B-2 cells using the same gating strategy as for Fig. 1A. (A) Peritoneal cells were isolated by flushing with 1–10 mL of PBS plus 1% FBS, and the frequencies of B-1a (Left) and B-1b cells (Right) are shown. (B) Representative plot of splenocytes from 1-wk-old mice stained for B-1 cells. (C) Frequencies of splenic B-1a and B-2 cells in 1-wk-old and 10-wk-old wt and bumble mice. The mean frequencies of cells in the lymphocyte gate ± SEM are shown for three mice per group. Results are representative of two independent experiments.
B-1 Progenitors and Neonatal Splenic Transitional B Cells Are Present in Bumble Mice.
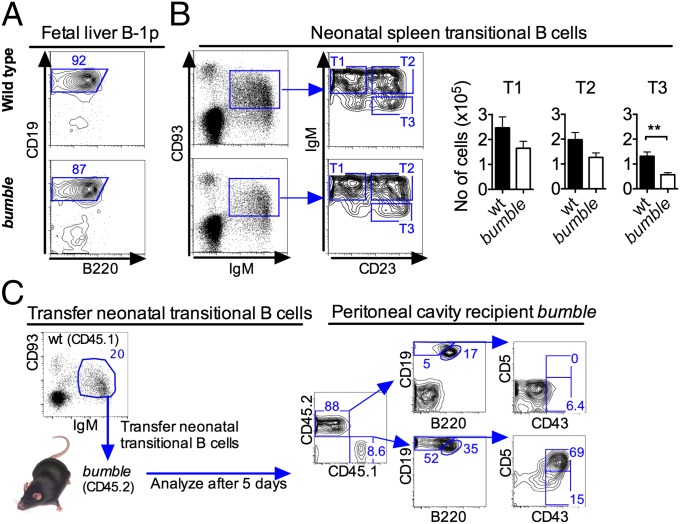
These results prompted us to investigate at which stage in B-cell development lymphopoiesis of B-1 cells fails in bumble mice. A B-1p population defined as Lin−CD93+CD19+B220lo/− was described as present in the mouse fetal liver peaking at the age of embryonic day (E) 15 (9). When analyzing bumble fetal livers of E15 embryos for putative B-1p cells, we readily detected Lin−CD93+CD19+B220lo/− cells of similar frequencies as in wt mice (Fig. 5A). It was also recently demonstrated that transitional (IgM+CD93+CD23+/−) B cells in the spleen of neonatal mice predominantly give rise to B-1 cells when transferred into SCID mice (11). Staining for neonatal TrB cells in 1-wk-old bumble splenocytes revealed similar overall frequencies of IgM+CD93+ cells as in wt mice, although bumble mice had a trend toward lower IgMhiCD93+CD23− (T1) and IgMhiCD93+CD23+ (T2) cell numbers and significantly lower IgM+CD93+CD23+ (T3) cell numbers (Fig. 5B), suggesting a partial block in B-cell development at the T2 stage. Notably, the skewed distribution of TrB cells may be at least partially explained by the increased surface IgM expression on bumble B cells, as previously described (21, 22). We then investigated if peritoneal B-1 cells develop from neonatal splenic TrB cells by transferring sorted IgM+CD93+ cells into nonirradiated 3-wk-old bumble recipients and analyzing for donor-derived B-1 cells 5–7 d later. The transferred wt CD45.1 neonatal TrB cells predominantly generated B-1a cells, but B-2 cells and a few B-1b cells were also observed in the peritoneum of reconstituted bumble mice (Fig. 5C). The similar frequencies of neonatal transitional IgM+CD93+ B cells in bumble and wt mice, coupled with the finding that most neonatal TrB cells give rise to B-1 cells, suggested that IκBNS is dispensable for the development of B-1 cells until after the B transitional stage.
Fig. 5.
Bumble mice have B-1p and neonatal TrB cells. (A) B-1p cells were identified in the fetal livers of 15-d-old bumble and wt fetuses as CD19+B220lo after gating on non–B-cell lineage-depleted CD93+IgM− cells. Representative staining of fetuses pooled from one litter is shown. (B, Left) Representative plots of IgM+CD93+ TrB cells resolved into T1, T2, and T3 populations from wt and bumble mice. (B, Right) Numbers of the different TrB cell populations from bumble and wt mice. Data represent seven mice per group, and error bars show SEM. Statistically significant differences between bumble and wt mice are indicated by **P < 0.01, as determined by an unpaired t test. (C) Sorted wt neonatal TrB cells (3 × 105 cells) were transferred i.p. into 3-wk-old bumble (CD45.2) mice, and the recipient mice were analyzed 5 d later for donor-derived CD45.1 B-1 cells in the peritoneal cavity. Data show two recipient mice per group and are representative of two independent experiments.
Transitional B Cells from Neonatal Mice Can Be Divided into Subsets Displaying B-1a or B-2 Markers, and Only the Latter Are Found in Bumble Mice.
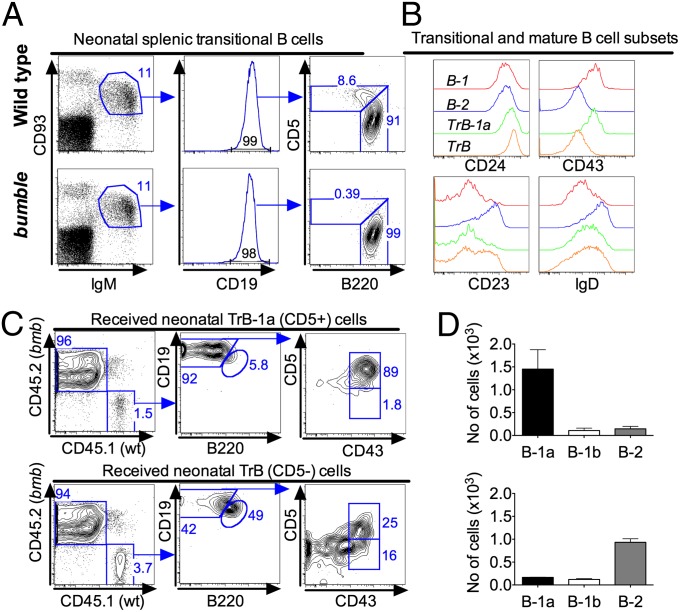
B-1p cells, as well as mature B-1 cells, express low levels of B220. Curiously, we observed that similar to mature CD93− B cells, CD93+IgM+CD19+ TrB cells could be divided into B220+ and B220lo populations and that the B220lo cells expressed CD5. In 1-wk-old wt mice the fraction of B220loCD5+ cells was 7–12% of TrB cells (Fig. 6A). Strikingly, a CD93+IgM+CD19+B220loCD5+ cell population could not be detected in bumble mice (Fig. 6A). This finding led us to suggest that transitional cells giving rise to B-1a cells may be phenotypically distinct from transitional cells giving rise to B-2 cells and that these two B-cell populations may be distinguished based on B220 and CD5 expression similar to other cells of B-1a/B-2 lineage. Furthermore, the lack of B220lo/CD5+ TrB cells in bumble mice indicates that the development of B-1a cells in IκBNS-deficient mice is blocked before this transitional stage of B-1a cells. We defined the neonatal splenic B220loCD5+ TrB cells as TrB-1a cells and characterized these cells further by comparing their surface marker expression with that of CD93+IgM+CD19+B220+CD5− TrB cells and mature B-1 and B-2 cells (Fig. 6B). Both TrB-1a and TrB cells were CD24hi. In contrast to TrB cells, the TrB-1a population also expressed the common B-1 marker CD43 (Fig. 6B). B-2 cells undergo transitional stages expressing CD93 and various levels of IgM and CD23. Notably, only the TrB cells could be separated into CD23−/+ populations corresponding to T1 and T2 B cells, respectively, whereas TrB-1a cells were CD23−. In contrast, TrB-1a and TrB cells expressed similar levels of IgD, which were higher than in mature B-1 cells and lower than in mature B-2 cells.
Fig. 6.
Neonatal TrB cells with B-1a potential constitute a phenotypically distinct population and are lacking in bumble mice. TrB cells from bumble and wt neonatal mice were evaluated for common markers of B-1 cells. (A) Distinct CD93+IgM+CD19+B220loCD5+ TrB-cell population (abbreviated TrB-1a) was observed in neonatal wt mice and not in bumble mice. (B) To characterize this TrB-cell population further, CD93+IgM+CD19+B220lo B cells from 1-wk-old wt mice were stained for the indicated surface markers. The histograms show the surface markers on transitional CD93+IgM+CD19+B220lo (TrB-1a), CD93+IgM+CD19+B220+ (TrB), and mature CD93−IgM+CD19+B220lo (B-1) and CD93−IgM+CD19+B220+ (B-2) cells. The histograms were created using the offset mode in FlowJo. Stainings represent three to four mice per group, and studies were repeated at least two times. (C) Different subsets of IgM+CD93+ TrB cells from 1-wk-old wt (CD45.1) mice were evaluated for in vivo B-1 differentiation capacity. IgM+CD93+ cells were sorted into CD5+ (TrB-1a) and CD5− fractions (TrB), and 2–3 × 104 cells were transferred into bumble (CD45.2) mice. Five to 7 d posttransfer, recipient mice were analyzed for donor-derived B-1a, B-1b, and B-2 cells in the peritoneal cavity. (D) Absolute number of donor-derived cells of the different B-cell subsets in the peritoneal cavity of bumble mice receiving wt TrB-1a (Upper, CD5+) and TrB (Lower, CD5−) cells, respectively. Cell transfer studies were performed two times with two to three mice per group. Error bars indicate SEM.
Transfer of Neonatal Transitional Cells Reveals at Least Two Subsets That Give Rise to B-1 Cells.
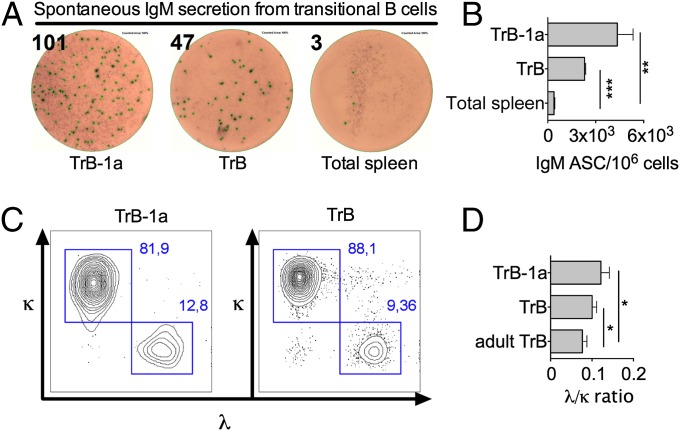
To gain more insight into which neonatal TrB-cell population gives rise to B-1a, B-1b, and B-2 cells, we repeated the transfer of TrB cells into bumble mice, this time sorting cells based on TrB-cell markers in addition to CD5, which, in mice, is exclusively expressed by B-1a cells and not by other cells of the B lineage (Fig. 6C). The IgM+CD93+ cells from neonatal wt mice were purified into CD5− and CD5+ populations, transferred into bumble mice, and analyzed 4–6 d later. The purity of the sorted populations was 95–97%. As expected, transfer of TrB-1a (IgM+CD93+CD5+) cells almost exclusively gave rise to CD19+B220loCD43+CD5+ B-1a cells, which were recovered in the peritoneal cavity of recipient bumble mice (Fig. 6C). Transfer of IgM+CD93+CD5− neonatal transitional cells yielded mostly B-2 cells. However, donor-derived peritoneal B-1a and B-1b cells could also be observed in the bumble recipients (Fig. 6D). These data suggest the existence of at least two different subsets of TrB-1 cells in the neonatal spleen and suggest that expression of CD5, the hallmark surface marker for B-1a cells, is initiated at the TrB-cell stage. Spontaneous secretion of natural IgM antibodies is a hallmark function of B-1a cells. In contrast, adult TrB cells do not spontaneously secrete IgM antibodies. Interestingly, both wt neonatal TrB-1a and TrB cells spontaneously produced IgM antibodies after 17 h of incubation, although the number of IgM+ antibody-secreting cells in the TrB-1a population was twice as high compared with TrB cells (Fig. 7A). Another phenotypic characteristic of B-1a cells is increased λ-light chain use compared with B-2 cells (28). Neonatal TrB-1a cells and, to a lesser extent, TrB cells displayed a significantly higher λ- to κ-light chain ratio than TrB cells from adult mice (Fig. 7B). Overall, these data indicate that the IgM+CD93+CD5+ TrB-1a cells represent a direct precursor of mature B-1a cells, whereas the neonatal wave of IgM+CD93+CD5− TrB cells contain immature B cells of both B-1 and B-2 lineage.
Fig. 7.
Neonatal TrB cells spontaneously secrete IgM and display increased λ light chain use compared with adult TrB cells. (A and B) Transitional IgM+CD93+ cells were sorted by FACS into CD5+ (TrB-1a) or CD5− (TrB) cells and examined for antibody secreting cells (ASCs) spontaneously producing IgM by ELISpot by incubating 2 × 104 cells for 17 h. Samples were pooled from one litter of wt mice. (C) Representative plot of TrB-1a and TrB cells expressing λ or κ light chains from neonatal wt mice. (D) Ratio of λ/κ using neonatal or adult TrB cells. The λ/κ ratio was determined in three independent experiments with three to four wt mice. Statistically significant differences are indicated *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by an unpaired t test.
Discussion
A principal component of B cell activation and antibody production is specific recognition of antigen by the BCR, combined with signals received through interaction with T cells. With the discovery of Toll-like receptors and other pathogen recognition receptors on the surface of B cells, it became increasingly clear how activation signals and antibody production may also be stimulated by innate immune system activation in the absence of T-cell help. However, the different B cell subsets differ in terms of responsiveness to TI signals, with the innate-like B cells, MZB cells, and B-1 cells, being most responsive (29). Pathogenic microorganisms are complex, expressing both TD and TI antigens, requiring both the innate and adaptive arms of the immune systems to act in concert to facilitate a protective immune response. B-1 cells constitute an important first line of defense by recognizing TI antigens found on the surface of pathogens and rapidly responding by secreting antibodies to confine the infectious agent and activate other arms of the immune system. These events are illustrated upon infection with influenza viruses (30).
Although the development of B-2 cells and MZB cells has been investigated extensively, less is known about the development of B-1 cells. Recent years have witnessed an increased interest in this area, particularly with the identification of B-1p cells in murine fetal liver, bone marrow, and spleen (9, 10). The present study advances our knowledge of the later steps in the development of B-1 cells and establishes that IκBNS is required for lymphopoiesis of B-1a cells rather than for maintenance of mature B-1 cells. We would like to emphasize the following findings. First, the development of B-1a cells occurs via a splenic transitional intermediate, which is phenotypically distinct but shares some characteristics with the well-established splenic TrB cells that predominantly give rise to B-2 cells. Second, mice deficient in the atypical nuclear IκB protein IκBNS lack B-1 cells due to a cell-intrinsic mechanism, and the lack of B-1 cells explains, at least in part, the impaired antibody responses to TI antigens. Finally, IκBNS is required for the development of B-1a cells after the B-1p stage but before the herein identified B-1a transitional stage. The possibility that B-1 cells develop through a transitional stage was only recently demonstrated by Montecino-Rodriguez and Dorshkind (11), who showed that splenic TrB cells (IgM+CD93+CD23+/−) from neonatal but not adult mice differentiated into B-1a and B-1b cells. We have confirmed and extended this finding by identifying phenotypic characteristics, which can be used to distinguish transitional cells with B-1a potential from their B-2 counterparts. In line with studies that demonstrated B-1a cells to be generated as an early wave (8), TrB cells of B-1a lineage were found in the spleen of neonatal rather than adult mice. Similar to adult B-2 transitional cells, the B-1a–specified TrB-1a cells expressed CD93 and IgM on the surface; however, they were unique in expressing CD43 and only low levels of B220. In addition, the TrB-1a cells expressed CD5, and the majority of mature peritoneal B-1 cells generated from these precursors were B-1a cells.
Neonatal wt CD93+IgM+CD5− TrB cells predominantly matured into B-2 cells when transferred to bumble mice. Interestingly, this population also gave rise to some B-1b cells and even B-1a cells, illustrating an unappreciated heterogeneity of the neonatal TrB-cell population. Interestingly, neonatal TrB cells spontaneously secreted IgM and displayed increased λ-light chain use compared with their adult counterparts. These features are characteristics of B-1a cells, further suggesting the potential of TrB cells to develop into B-1a cells. The finding that both CD5− and CD5+ TrB cells give rise to B-1a cells suggests that CD5 expression is initiated at the immature/TrB-cell stage. This observation is also in line with the finding that B-1p cells are CD5−. Following from these data, it seems likely that the identified CD5+ TrB-1a cells constitute a direct descendant of CD5− TrB cells, and thus represent a late transitional stage in the development of B-1a cells. However, it remains possible that different developmental pathways may lead to the generation of cells with a B-1a phenotype. In this regard, it was recently shown that both Lin−CD19+B220lo and Lin−CD19−B220− fetal liver B-1p cells generate B-1 cells but that B-1a progeny differed functionally in terms of N-nucleotide addition patterns (31).
The reduced frequency of B-1 cells in several NF-κB–deficient mouse strains has long been a conundrum. The finding that B-1 cells develop from neonatal TrB cells, coupled with the fact that the latter are found in similar frequencies in wt and NF-κB1–deficient mice, led to the hypothesis that signaling via the classical NF-κB pathway is not needed for the development of B-1 cells but rather for the maintained self-renewal capability of mature B-1 cells (11). Interestingly, we observed that the transitional cells in neonatal wt mice could be phenotypically separated into at least one additional subset (IgM+CD93+B220loCD43+CD5+) in addition to the previously established T1, T2, and T3 cells and that bumble mice were devoid of this newly identified subset of TrB-1a cells. This finding suggests that IκBNS is required during the development of B-1a cells before the identified TrB-1a stage. The lack of IgM+CD93+B220loCD43+CD5+ TrB-1a cells that we demonstrate for IκBNS-deficient mice might also hold true for other strains deficient in classical or alternative NF-κB signaling, which is of interest to examine. It should also be noted that although our findings demonstrate that IκBNS is required at the B-1a developmental stage, IκBNS might also be required for the maintenance of mature B-1 cells.
B-1a and B-1b cells share many phenotypic features but have largely different functions. The bumble mice were deficient in generating B-1a cells but were not completely devoid of peritoneal B-1b cells, with 5–15% of wt levels consistently observed. Whether B-1a and B-1b cells have a different requirement for IκBNS or develop through different pathways (32) remains unknown. Our mixed bone marrow and fetal liver chimera experiments clearly demonstrated that the development of B-1 cells from bumble bone marrow is intrinsically impaired. It is interesting to note that whereas bumble bone marrow transferred alone to RAG1−/− mice gave rise to some B-1b cells, only wt B-1b cells were observed in the mixed wt/bumble chimeras, illustrating a competitive disadvantage for bumble B-1b cells.
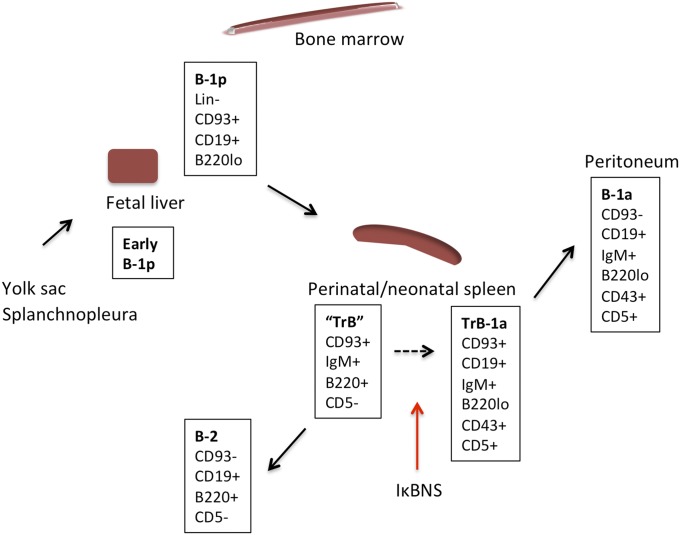
The identification of splenic TrB-1a cells indicates that development of B-1a cells, similar to that of B-2 cells, requires an intact spleen. This assumption is supported by the finding of drastically reduced frequencies of peritoneal B-1a cells in Hox11-null mice lacking a spleen and in splenectomized wt mice (33, 34). The finding that final maturation of B-1 cells occurs in the spleen is also consistent with our observation of a clear population of B-1a cells in the spleen of 1-wk-old neonatal mice, a time point where we failed to detect a clear population of B-1a cells in the peritoneal cavity (Fig. 4). Based on the current study and on recent findings of B-1p cells in various lymphoid organs, we propose the following developmental pathway for B-1 cells, as indicated in Fig. 8.
Fig. 8.
Schematic of steps of the development of B-1a cells. Early B-1p cells exist in 9-d-old fetal yolk sac and splanchnopleura (39, 40). Markers for these cells have not been identified to date. From day 11 of gestation, B-1p cells can be identified in the fetal liver as Lin−CD93+CD19+B220lo/− (9). Cells with a similar phenotype can be found at low frequencies in the neonatal and adult bone marrow and spleen (9, 10). B-1p cells undergo transitional development into mature B-1a cells, initiating CD5 expression in the neonatal spleen and possibly in other secondary lymphoid organs. The B220+CD5− neonatal TrB-cell population gave rise to mostly B-2 cells but also some B-1 cells. Herein, we refer to this neonatal TrB-cell population as “TrB” to reflect their apparent capacity to give rise to both B-1a and B-2 cells, suggesting that they are a heterogeneous population. As indicated by the stippled arrow, it is likely that the TrB cells develop into B-1a cells via the TrB-1a intermediate. The red arrow indicates that IκBNS is required for B-1p cells to progress to the stage of TrB-1a cells.
B-1 cells are important responders to TI antigens, but the contribution of these cells vs. MZB cells for the response to polysaccharides from encapsulated bacteria, as well as other TI antigens, is not completely clear. Preponderant evidence supports MZB cells as the major responder to the TI antigen NP-Ficoll (35, 36). The lack of both of MZB and B-1 cells may explain the impaired TI antibody responses in bumble mice, but because transfer of wt peritoneal cells to bumble partially restored antibody responses to NP-Ficoll, our data illustrate that B-1 cells mediate at least some of the response to this antigen, as has previously been demonstrated (37).
In humans, the existence or phenotype of B-1 cells is still a matter of debate, although recent reports suggest the existence of a cell type that at least shares many of the functions attributed to B-1b cells in mice (38). Our study may therefore assist future studies aimed at identifying human B-1 cells by prompting evaluation of neonatal TrB cells for the proposed human B-1 markers. In sum, we show that B-1a cells develop via a transitional intermediate, which can be phenotypically distinguished from that giving rise mainly to B-2 cells. Furthermore, we demonstrate that IκBNS-deficient mice are devoid of these B-1a transitional cells, thus revealing a requirement for this factor for normal development of B-1a cells before the B-1a transitional stage.
Materials and Methods
Mice.
Mice were housed and bred at the animal research facility of the Department of Microbiology, Tumor, and Cell Biology, Karolinska Institutet. IκBNS-deficient bumble mice, generated by ENU mutagenesis of C57BL/6J mice, and their wt C57BL/6J counterparts were described previously (22). CD45.1 and RAG1−/− mice on a C57BL/6J background were bred locally. Mice were studied at 8–14 wk of age or at the indicated age. All animal studies were conducted with the approval of the Committee for Animal Ethics (Stockholms Norra djurförsöksetiska nämnd).
Tissue Preparation.
Single-cell suspensions of splenocytes and fetal livers were prepared by a 70-μm cell strainer using a syringe plunger. Peritoneal cells were isolated by flushing with cold PBS plus 1% FBS (1–10 mL, depending on mouse age). Peritoneal cells were discarded if contaminated with blood. Femurs and tibias were flushed with a 26-gauge needle. Cell suspensions were diluted in RPMI 1640 supplemented with 2 mM l-glutamine, penicillin (100 IU)-streptomycin (100 μg/mL), and 10% FBS (complete RPMI). Cell suspensions were washed once in Ca2+-free, Mg2+-free PBS and treated with RBC lysis buffer before further processing. RBC lysis buffer was omitted for peritoneal cells and when preparing cells for bone marrow or fetal liver chimeras.
Immunization.
Mice were injected i.p. with 10–20 μg of 2,4,6, Trinitrophenyl hapten conjugated to LPS or TNP (10)-LPS (0111:B4) or with 50 μg of NP (40)-Ficoll (Biosearch Technologies) in 100 μL of PBS.
ELISA.
Antigen-specific ELISA was performed by coating ELISA plates (Nunc) with 500 ng per well of NP (25) or TNP (20) conjugated with BSA (Biosearch Technologies) and incubated overnight (4 °C). Following washing (PBS plus 2% Tween 20) and blocking for 1 h with PBS containing 2% (wt/vol) dry milk, serum was added in threefold serial dilutions in blocking buffer and incubated for 1.5 h at room temperature (RT) before addition of secondary antibody HRP-coupled IgM, IgG1, IgG2b, IgG2c, or IgG3 (all from Southern Biotech). The assay was developed with 3,3′,5,5′-tetramethylbenzidine substrate (KPL) followed by 1 M H2SO4, and the OD at 450 nm was read using an Asys Expert 96 ELISA reader (Biochrom).
Enzyme-Linked Immunosorbent Spot Assay for Detection of Antibody-Secreting Cells.
Detection of total IgM-producing cells was performed using an enzyme-linked immunosorbent spot (ELISpot) assay. MultiScreen-IP filter plates (Millipore) were pretreated with 70% ethanol and washed in sterile PBS. Plates were coated with 5 μg/mL anti-mouse IgM (Southern Biotech) diluted in PBS and incubated overnight at 4 °C. The following day, plates were washed in sterile PBS and blocked in complete RPMI medium with 50 μM 2-mercaptoethanol and 10 mM Hepes for 1 h at 37 °C, and the indicated cell numbers were added. Plates were incubated for 17 h at 37 °C in 5% CO2. Cells were then removed by washing in PBS, and 0.1 μg per well of biotinylated anti-mouse IgM (Mabtech) diluted in PBS was added to the wells. After 2 h of incubation at RT, plates were washed and developed with 100 μL of 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium-plus substrate (Mabtech). The reaction was stopped when distinct spots could be observed, by rinsing the plates extensively in tap water. Spots were counted in an ELISpot reader (Cellular Technology Limited).
Flow Cytometry and Cell Sorting.
Bone marrow and splenic RBCs were lysed before Fc blockade (anti-CD16/32; Becton Dickinson), washing in PBS plus 1% FBS, and staining with fluorochrome or biotin-conjugated monoclonal antibodies in washing buffer. The antibodies used were as follows: CD19 phycoerythrin (PE), B220 peridinin-chlorophyll protein (PERCP), CD23 biotin, CD24 PE, CD43 allophycocyanin (APC), CD45.1 APC-Cy7, CD45.2 V450, λ1- to λ3-light chain biotin, and κ-light chain PerCP-Cy5.5 (all from Becton Dickinson); B220 APC-eFluor 780, IgM eFluor 450, CD93 APC, and IgD FITC (all from eBioscience), IgM FITC (Southern Biotech); and CD5 biotin (Biolegend). In panels containing biotin-coupled antibodies, cells were washed and stained again with streptavidin conjugated to AF488 (Invitrogen) or PerCP-Cy5.5 (eBioscience). Before staining for fetal liver B-1p cells, fetal liver cells were prepared using Lymphoprep (Axis–Shield), and non–B-lineage cells were depleted by means of the B-cell isolation kit (STEMCELL Technologies) according to the manufacturer’s instructions. Cells were analyzed on a Becton Dickinson FACSCalibur or LSRII instrument. Cell sorting was performed on a Moflo XDP instrument (Beckman Coulter). To obtain highly pure populations, the sorted cells were resuspended in PBS plus 1% FBS and resorted. The purity of the sorted populations constituted 95–97% as determined by a presorted sample run in parallel and reanalysis of the sorted populations. Data were analyzed in FlowJo version 9.6.4 or version X.0.7 (TreeStar). Flow cytometry plots depict log10 fluorescence.
Adoptive Cell Transfer.
Peritoneal cells (4 × 106 cells) from adult C57BL/6J CD45.1 or CD45.2 mice were resuspended in 100 μL of PBS and transferred i.p. into 3-wk-old nonirradiated bumble recipient mice. Mice were killed at different time points posttransfer and analyzed for successful grafting. To evaluate the TI-2 antibody response in grafted bumble mice, these mice were immunized with NP-Ficoll 50 d after cell transfer. For transfer of neonatal TrB cells, 1–3 × 105 C57BL/6J CD45.1 cells from 1-wk-old mice were sorted as CD93+IgM+ and transferred i.p. into 3-wk-old bumble mice. In some studies, the TrB cells were further sorted into CD5−/+ subsets and 2–3 × 104 cells were transferred. The bumble mice that had received sorted TrB cells were analyzed 4–6 d posttransfer. No signs of graft-versus-host disease were observed in recipient mice.
Bone Marrow and Fetal Liver Chimeras.
Bone marrow and fetal liver chimeras were generated by transferring cells from CD45.1 wt mice mixed 1:1 with CD45.2 wt or bumble mice i.v. into nonlethally irradiated RAG1−/− mice (600 rad, 137Cs source). For bone marrow and fetal liver chimeras, 50 × 106 and 3 × 106 cells, respectively, were transferred from each mouse strain. The recipient mice were given antibiotics in the drinking water for 21 d. Chimeras were analyzed 8–12 wk after reconstitution.
Statistics.
Differences between groups were analyzed by a two-tailed unpaired t test (Prism version 6.0d; GraphPad).
Acknowledgments
We thank the personnel at the animal facility and the FACS facility at the Department of Microbiology, Tumor, and Cell Biology (Karolinska Institutet). This work was supported by grants from the Bill and Melinda Gates Foundation (to G.B.K.H. and B.B.) and the Swedish Research Council (to G.B.K.H.) and by a fellowship from the Wenner–Gren Foundations (to G.K.P.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The bumble strain (C57BL/6J-Nfkbid<m1Btlr>; 036725-MU) is available from the Mutant Mouse Regional Resource Centers.
References
- 1.Rauch PJ, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335(6068):597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2(5):323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 3.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 4.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23(1):7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Allman D, et al. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167(12):6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 6.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9(11):767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa K, Hardy RR, Stall AM, Herzenberg LA, Herzenberg LA. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7(3):293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 10.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci USA. 2011;108(7):2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montecino-Rodriguez E, Dorshkind K. Formation of B-1 B cells from neonatal B-1 transitional cells exhibits NF-kappaB redundancy. J Immunol. 2011;187(11):5712–5719. doi: 10.4049/jimmunol.1102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosier DE, Mond JJ, Goldings EA. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X-linked B cell defect. J Immunol. 1977;119(6):1874–1878. [PubMed] [Google Scholar]
- 13.Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton’s tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191(10):1745–1754. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden MS, Ghosh S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4(5):348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 16.Pohl T, et al. The combined absence of NF-kappa B1 and c-Rel reveals that overlapping roles for these transcription factors in the B cell lineage are restricted to the activation and function of mature cells. Proc Natl Acad Sci USA. 2002;99(7):4514–4519. doi: 10.1073/pnas.072071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25(5):701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Grossmann M, et al. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 2000;19(23):6351–6360. doi: 10.1093/emboj/19.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzoso G, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Paun A, Claudio E, Wang H, Siebenlist U. The tumor promoter and NF-κB modulator Bcl-3 regulates splenic B cell development. J Immunol. 2013;191(12):5984–5992. doi: 10.4049/jimmunol.1300611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Touma M, et al. Impaired B cell development and function in the absence of IkappaBNS. J Immunol. 2011;187(8):3942–3952. doi: 10.4049/jimmunol.1002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold CN, et al. A forward genetic screen reveals roles for Nfkbid, Zeb1, and Ruvbl2 in humoral immunity. Proc Natl Acad Sci USA. 2012;109(31):12286–12293. doi: 10.1073/pnas.1209134109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster M, Annemann M, Plaza-Sirvent C, Schmitz I. Atypical IκB proteins—Nuclear modulators of NF-κB signaling. Cell Commun Signal. 2013;11(1):23. doi: 10.1186/1478-811X-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39(9):2383–2394. doi: 10.1002/eji.200838920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann A, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8(7):695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 26.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106(14):5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Düber S, et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114(24):4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: Genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986;16(4):450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 29.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- 30.Baumgarth N. The double life of a B-1 cell: Self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 31.Holodick NE, Vizconde T, Rothstein TL. B-1a cell diversity: Nontemplated addition in B-1a cell Ig is determined by progenitor population and developmental location. J Immunol. 2014;192(5):2432–2441. doi: 10.4049/jimmunol.1300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosn EE, et al. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci USA. 2012;109(14):5394–5398. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195(6):771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretschmer K, Stopkowicz J, Scheffer S, Greten TF, Weiss S. Maintenance of peritoneal B-1a lymphocytes in the absence of the spleen. J Immunol. 2004;173(1):197–204. doi: 10.4049/jimmunol.173.1.197. [DOI] [PubMed] [Google Scholar]
- 35.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1(1):31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 36.Samardzic T, et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur J Immunol. 2002;32(2):561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 37.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci USA. 2006;103(15):5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70- J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lièvre F, Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364(6432):67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2011;108(4):1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]