Significance
The evolution of the biota and of atmospheric composition have been tightly coupled throughout Earth history. Of key importance has been the establishment of an oxygen-rich atmosphere, and this has apparently occurred in two major steps. The second step occurred during the Neoproterozoic, at the time when various lines of evidence suggest a significant expansion of terrestrial life and soil development. The present paper links these two events in a unique way, not through increased oxygen production on land, but through increased consumption in biotic soils that until compensated by higher atmospheric oxygen levels, restricted the supply of oxygen to the weathering environment.
Keywords: Precambrian, terrestrial biota, carbon isotopes, paleosol
Abstract
Considerable geological, geochemical, paleontological, and isotopic evidence exists to support the hypothesis that the atmospheric oxygen level rose from an Archean baseline of essentially zero to modern values in two steps roughly 2.3 billion and 0.8–0.6 billion years ago (Ga). The first step in oxygen content, the Great Oxidation Event, was likely a threshold response to diminishing reductant input from Earth’s interior. Here I provide an alternative to previous suggestions that the second step was the result of the establishment of the first terrestrial fungal–lichen ecosystems. The consumption of oxygen by aerobes respiring this new source of organic matter in soils would have necessitated an increase in the atmospheric oxygen content to compensate for the reduced delivery of oxygen to the weathering environment below the organic-rich upper soil layer. Support for this hypothesis comes from the observed spread toward more negative carbon isotope compositions in Neoproterozoic (1.0–0.542 Ga) and younger limestones altered under the influence of ground waters, and the positive correlation between the carbon isotope composition and oxygen content of modern ground waters in contact with limestones. Thus, the greening of the planet’s land surfaces forced the atmospheric oxygen level to a new, higher equilibrium state.
Colonization of the Land Surface
Although vascular plants evolved much later, microfossils in 1.0 billion years ago (Ga) cherts have been interpreted as terrestrial bacteria that affected soil chemistry and weathering rates on land (1). Molecular clock determinations argue for the origin of fungi by 0.85 Ga (2), and controversial body fossil evidence for terrestrial lichens dates back to 0.6 Ga (3). An apparent increase in the proportion of pedogenic clay minerals in Neoproterozoic mudstones has been attributed to a growing stabilization of soils by organic matter derived from an early terrestrial soil biota that generated a moist, cation-rich environment for precipitating clay minerals (4). These observations indicate that life on land may have become well established during the Neoproterozoic.
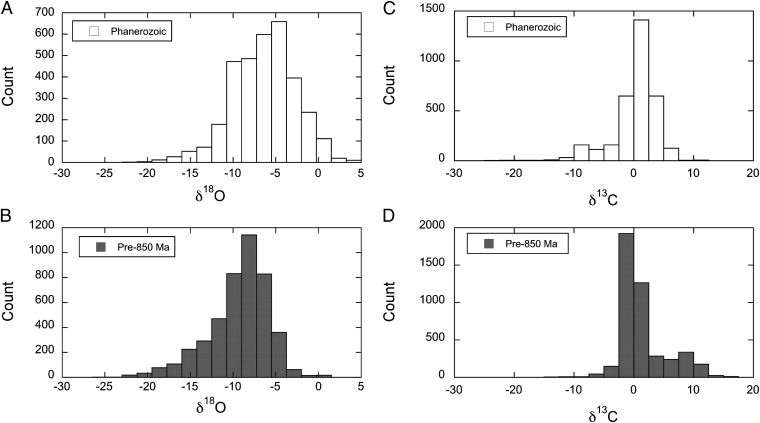
Perhaps the most compelling evidence for a Neoproterozoic expansion of terrestrial ecosystems and their influence on the soil weathering environment comes from the carbon and oxygen isotopic composition of marine limestones and dolostones (5). Carbonate rocks deposited before 0.85 Ga show a wide range of δ18O values, indicating significant alteration by meteoric waters and burial diagenesis, but lack the low δ13C values characteristic of Phanerozoic rocks which have been affected by the respiration of isotopically light organic matter in the soil environment (Fig. 1). The inference is that only after 0.85 Ga were terrestrial ecosystems sufficiently well established that they were able to alter the soil gas and water chemistry through aerobic respiration (5), increasing the pCO2, and, importantly, decreasing the pO2 compared with the atmosphere.
Fig. 1.
Histograms showing the distribution of δ18O and δ13C values for altered marine carbonate rocks deposited before 0.85 Ga (B and D, respectively) and during the Phanerozoic (A and C). The oxygen isotope distributions (A and B) are similarly shaped although the pre-850-Ma distribution has a stronger negative skewness (−0.86 vs. −0.32 for the Phanerozoic) and is shifted toward the left (lighter values), reflecting the higher degree of alteration of these rocks. The carbon isotope distributions (C and D) contrast markedly, with the pre-850-Ma skewness positive (0.79) but shifted slightly to the left (lighter values) and the Phanerozoic skewness negative (−1.6), a reflection of the larger negative tail of 13C-depleted isotope values in post-Neoproterozoic rocks. Data from ref. 5. Note that the analyses of Neoproterozoic rocks younger than 850 Ma are excluded from these figures to highlight the contrast between more ancient and “postgreening” diagenetic effects and avoid the complications of the anomalous values associated with Neoproterozoic glaciation.
Effect of Modern Soil Biota on Oxygen and Carbon Isotopes
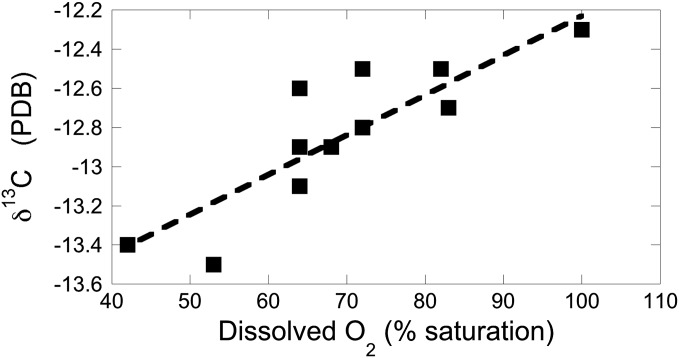
Support for this inference comes from a study of carbonate ground waters and springs in central Pennsylvania (6, 7; Fig. 2). Excluding those samples that were argued to be impacted by human wastewater, there is a strong correlation between δ13C of dissolved inorganic carbon and dissolved oxygen content of ground waters, with waters saturated with atmospheric O2 the heaviest (δ13C ∼ −12.2 ‰) and, through extrapolation, those with complete O2 depletion ∼2 ‰ lighter as a result of addition of low δ13C CO2 from organic matter decay by the aerobic soil biota. All of these waters have experienced considerable limestone dissolution, and presumably have evolved from equilibration with an initial soil gas source that was isotopically depleted (δ13C < −21 ‰) to heavier values with the addition of carbonate ions derived from limestone dissolution (6).
Fig. 2.
Positive correlation (r2 = 0.73) between the δ13C of Central Pennsylvania carbonate ground waters and their oxygen concentration, indicating that as organic matter is respired to CO2 in the subsurface, the aqueous carbon species are increasingly influenced by the low δ13C of this respired carbon. Data from refs. 6–7.
The implication of these observations from modern ground waters is that the appearance of strongly 13C-depleted isotope values in carbonate rocks after 0.85 Ga was the result of increased photosynthetic organic carbon production on land and its respiration in the soil environment. One consequence of this “greening” of the land surface was limited oxygen penetration into the soil. Assuming that the rate of oxidation of crustal materials during Proterozoic weathering was just sufficient to oxidize all of the reduced minerals but limited by the supply of oxygen by gaseous diffusion or rainwater percolation through the soil (8), the consumption of oxygen by weathering would have been temporarily reduced with the intensification of aerobic soil respiration and reduction in soil oxygen content. The natural response would have been an increase in atmospheric oxygen content until the rate of oxygen supply to the subsurface and attendant oxidative weathering rate was restored to its preperturbation level.
Estimating Atmospheric Oxygen Levels
One can use the formulation of Holland and Zbinden (8) to estimate atmospheric pO2 in the Proterozoic before the establishment of a significant terrestrial biota (>0.85 Ga). The calculation is based on the following: (i) an assumed steady state for atmospheric pO2 where the rate of O2 production by organic carbon burial (1013 mol/y; 9) was balanced by the consumption of oxygen during weathering; (ii) that diffusion through the soil atmosphere controlled the supply of O2 to the weathering environment, according to the following equation:
| [1] |
where Dsoil is the soil O2 diffusivity (0.016 cm2/s), pO2,air is the atmospheric oxygen partial pressure and pO2,L is the O2 partial pressure at the water table, L is the typical depth to water table (1,000 cm), and 1.4 × 103 is a constant that converts mol to liter gas and seconds to years (8); (iii) the assumption that just enough oxygen was provided by diffusion through the soil to consume all of the oxygen at the base of the oxidative weathering zone (at the water table; i.e., pO2,L = 0) and thus oxidize essentially all of the reduced soil minerals; and (iv) the simplification that the Proterozoic land area was roughly equivalent to today’s (148 × 106 km2). Then, setting the rate of oxygen production equal to the rate of oxygen consumption by oxidative weathering according to Eq. 1, and accepting the uncertainties in Eq. 1 as presented by ref. 8, which largely arise because of variations in soil diffusivity in modern soils and in soil thicknesses, one can solve for the atmospheric pO2:
| [2] |
where 1010 is the number of cm2/km2, the uncertainty is a factor of 36, and PAL is the present atmospheric level (0.21 atm).
This estimate, a minimum to provide sufficient oxygen by diffusion for oxidative weathering to balance the oxygen production from organic carbon burial, is lower than the upper limits based on observations from the marine record of Fe deposition and sulfur-isotope fractionation (5–18% PAL; 10). Moreover, it is likely an underestimate of the minimum because it presumes that the entire land surface was involved in oxidative weathering.
After the establishment of biotic soils, this value would represent the minimum value at the base of the soil; atmospheric oxygen levels (at and above the soil–air interface) had to have been higher than this because of consumption of oxygen by the soil biota. The extent to which modern soil respiration reduces the oxygen content of the soil atmosphere (ΔpO2 = pO2,atm − pO2,soil; PAL) ranges from very small depletions in upland ridges even in tropical locations (ΔpO2 ∼ 10% PAL; 11), to larger depletions in moister tropical valleys (ΔpO2 ∼ 50% PAL; 11), and poorly drained peat soils (ΔpO2 ∼ 75% PAL; 12). Water-saturated soils are often anoxic. Nevertheless, and consistent with the calculation above, the supply of oxygen to the weathering zone today greatly exceeds the demand, such that oxidative weathering is essentially complete (13). Modeling suggests that pO2,atm must be below 10% PAL for O2-dependent feedbacks on weathering to be important in the regulation of atmospheric O2 (13).
Clearly the extent of oxygen depletion depends on a host of environmental and soil parameters beyond terrestrial biotic productivity, especially the water content of the soil, so it is unclear what the ΔpO2 of the Neoproterozoic soil biota was. The observed shift in carbonate δ13C (Fig. 1 and ref. 5) from pre-Neoproterozoic to Phanerozoic of ∼2 ‰ is consistent with an O2 depletion capacity comparable to today’s. The Neoproterozoic depletion likely was considerably smaller, but if even a fraction of today’s, would require a substantial increase in atmospheric pO2.
This is not the first suggestion that the greening of the land surface affected the oxygen content of the atmosphere. Lenton and Watson (14) proposed that biotic enhancement of weathering in the Neoproterozoic led to the enhanced delivery of phosphorus to the ocean, promoting increased marine productivity and organic carbon burial, a resulting stepwise increase in atmospheric oxygen, and drawdown of atmospheric CO2, perhaps triggering Neoproterozoic glaciation. Knauth and Kennedy (5) also focused on the greening’s effect on oxygen production rather than oxygen consumption as done here, speculating that biotically enhanced weathering and clay mineral production led to an increase in nutrient and clay mineral supply to marine environments, promoting marine biological productivity and providing enhanced burial preservation of organic matter protected from degradation by adsorption onto the clays. Neither of these hypotheses, which involve changes in the mechanism of oxygen production, precludes the effect described here that acts on the oxygen consumption side of the balance.
Hypothesis for the Stepwise Oxygenation of the Atmosphere
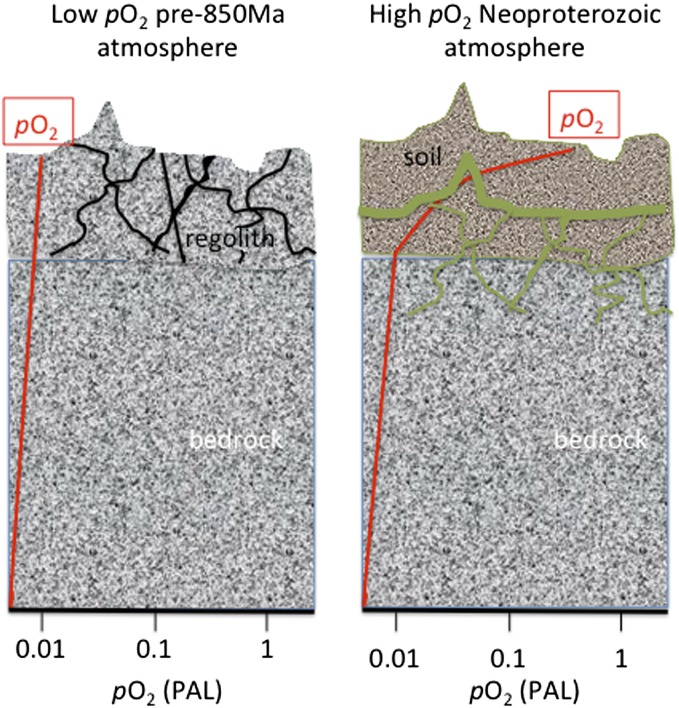
The emerging picture of atmospheric oxygen regulation is a system that has evolved with the evolution of the solid Earth and biosphere. Archean oxygen levels were vanishingly low because of an excess of sinks for oxygen arising from more reducing volcanic (15–18) and/or metamorphic (19) fluids. A diminution of this volcanic–metamorphic sink, to below the net source of oxygen associated with the sequestration of organic matter produced by oxygenic photosynthesis, occurred at or near the Archean–Proterozoic transition as the continents stabilized and became largely subaerially exposed. This Great Oxidation Event may have been somewhat protracted (20) as oxidative weathering of a large, accumulated reservoir of reduced crustal materials put a damper on oxygen accumulation (21). Eventually this damper was overwhelmed, and atmospheric oxygen levels may have significantly overshot (22–24) their intermediate Proterozoic steady state of ca. 1–10% PAL (10), until weathering feedbacks were able to stabilize atmospheric oxygen levels within this range. Then, the Neoproterozoic greening of the land surface caused a temporary reduction in the sink associated with terrestrial weathering by choking off the supply of O2 to the subsurface. Atmospheric oxygen levels rose such that at the base of the soil, oxygen concentrations were roughly equivalent to the pregreening atmosphere, restoring the original oxygen supply to ground waters (Fig. 3).
Fig. 3.
Cartoon depicting the distribution of oxygen in the regolith and bedrock of pre-850-Ma weathering environments contrasted with those of the postgreening Neoproterozoic. Neoproterozoic weathering environments incorporate biotic soils that deplete soil oxygen levels (red curve) through respiration, reducing the effective oxygen concentration in the weathering environment to that of the pre-850-Ma world.
Unfortunately, a systematic study of the supply of oxygen to the weathering environment, as it is affected by varying degrees of respiratory consumption in the organic-rich upper soil, and its consumption during oxidative weathering has not been made. As a result, the hypothesis presented here awaits more definitive field tests.
Any viable explanation for stepwise oxygen evolution must involve a fundamental change in the way the Earth system operates; perturbations, even massive organic carbon burial events, bolide impacts, or flood basalt eruptions, in themselves cannot effect permanent change in Earth’s surface environment [unless the system is bistable (25, 26); the persistently anoxic Archean suggests that bistability, if it existed, developed as a result of secular evolution]. Temporal trends in solar luminosity, heat flow, and the supply of reductant from Earth’s interior can drive the Earth system through thresholds, leading to stepwise change. Biological innovation can also exert lasting effects. In this context, the advent, expansion, and diversification of terrestrial ecosystems are likely to have had a global, lasting effect on surface conditions, leading to a stepwise and permanent increase in atmospheric oxygen levels.
Acknowledgments
This paper was greatly improved through discussions with Stephanie Olson, Jeff Havig, Kyle Rybacki, Aviv Bachan, Peter Swart, and Amanda Oehlert, and in response to two thorough anonymous reviews. The author acknowledges support from the National Aeronautics and Space Administration Astrobiology Institute, the US National Science Foundation Geobiology and Low-Temperature Geochemistry Program, and the Canadian Institute for Advanced Research.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Horodyski RJ, Knauth LP. Life on land in the Precambrian. Science. 1994;263(5146):494–498. doi: 10.1126/science.263.5146.494. [DOI] [PubMed] [Google Scholar]
- 2.Heckman DS, et al. Molecular evidence for the early colonization of land by fungi and plants. Science. 2001;293(5532):1129–1133. doi: 10.1126/science.1061457. [DOI] [PubMed] [Google Scholar]
- 3.Retallack GJ. Ediacaran life on land. Nature. 2013;493(7430):89–92. doi: 10.1038/nature11777. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy M, Droser M, Mayer LM, Pevear D, Mrofka D. Late Precambrian oxygenation; inception of the clay mineral factory. Science. 2006;311(5766):1446–1449. doi: 10.1126/science.1118929. [DOI] [PubMed] [Google Scholar]
- 5.Knauth LP, Kennedy MJ. The late Precambrian greening of the Earth. Nature. 2009;460(7256):728–732. doi: 10.1038/nature08213. [DOI] [PubMed] [Google Scholar]
- 6.Deines P, Langmuir DL, Harmon RS. Stable carbon isotope ratios and the existence of a gas phase in the evolution of carbonate ground waters. Geochim Cosmochim Acta. 1974;38(7):1147–1164. [Google Scholar]
- 7.Langmuir D. The geochemistry of some carbonate ground waters in central Pennsylvania. Geochim Cosmochim Acta. 1971;35(10):1023–1045. [Google Scholar]
- 8.Holland HD, Zbinden EA. Paleosols and the evolution of the atmosphere: Part I. In: Lerman A, Meybeck M, editors. Physical and Chemical Weathering in Geochemical Cycles. New York: Kluwer Academic; 1988. pp. 61–82. [Google Scholar]
- 9.Holser WT, Schidlowski M, Mackenzie FT, Maynard JB. Biogeochemical cycles of carbon and sulfur. In: Gregor CB, Garrels RM, Mackenzie FT, Maynard JB, editors. Chemical Cycles in the Evolution of the Earth. New York: Wiley; 1988. pp. 105–173. [Google Scholar]
- 10.Canfield DE. The early history of atmospheric oxygen: Homage to Robert M. Garrels. Annu Rev Earth Planet Sci. 2005;33:1–36. [Google Scholar]
- 11.Silver WL, Lugo AE, Keller M. Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry. 1999;44(3):301–328. [Google Scholar]
- 12.Magnusson T. Studies of the soil atmosphere and related physical characteristics in peat forest soils. For Ecol Manage. 1994;67(1-3):203–224. [Google Scholar]
- 13.Bolton WE, Berner RA, Petsch ST. The weathering of sedimentary organic matter as a control on atmospheric O2: II. Theoretical modeling. Am J Sci. 2006;306(8):575–615. [Google Scholar]
- 14.Lenton TM, Watson AJ. Biotic enhancement of weathering, atmospheric oxygen and carbon dioxide in the Neoproterozoic. Geophys Res Lett. 2004 doi: 10.1029/2003GL018802. [DOI] [Google Scholar]
- 15.Holland HD. Volcanic gases, black smokers, and the great oxidation event. Geochim Cosmochim Acta. 2002;66(21):3811–3826. [Google Scholar]
- 16.Kasting JF, Eggler DH, Raeburn SP. Mantle redox evolution and the oxidation state of the Archean atmosphere. J Geol. 1993;101(2):245–257. doi: 10.1086/648219. [DOI] [PubMed] [Google Scholar]
- 17.Kump LR, Kasting JF, Barley ME. Rise of atmospheric oxygen and the “upside-down” archean mantle. Geochem Geophys Geosyst. 2001 doi: 10.1029/2000gc000114. [DOI] [Google Scholar]
- 18.Kump LR, Barley ME. Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature. 2007;448(7157):1033–1036. doi: 10.1038/nature06058. [DOI] [PubMed] [Google Scholar]
- 19.Catling DC, Zahnle KJ, McKay C. Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science. 2001;293(5531):839–843. doi: 10.1126/science.1061976. [DOI] [PubMed] [Google Scholar]
- 20.Murakami T, Sreenivas B, Das Sharma S, Sugimori H. Quantification of atmospheric oxygen levels during the Paleoproterozoic using paleosol compositions and iron oxidation kinetics. Geochim Cosmochim Acta. 2011;75(14):3982–4004. [Google Scholar]
- 21.Konhauser KO, et al. Aerobic bacterial pyrite oxidation and acid rock drainage during the Great Oxidation Event. Nature. 2011;478(7369):369–373. doi: 10.1038/nature10511. [DOI] [PubMed] [Google Scholar]
- 22.Kump LR, et al. Isotopic evidence for massive oxidation of organic matter following the great oxidation event. Science. 2011;334(6063):1694–1696. doi: 10.1126/science.1213999. [DOI] [PubMed] [Google Scholar]
- 23.Bekker A, Holland HD. Oxygen overshoot and recovery during the early Paleoproterozoic. Earth Planet Sci Lett. 2012;317–318:295–304. [Google Scholar]
- 24.Canfield DE, et al. Oxygen dynamics in the aftermath of the Great Oxidation of Earth’s atmosphere. Proc Natl Acad Sci USA. 2013;110(42):16736–16741. doi: 10.1073/pnas.1315570110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldblatt C, Lenton TM, Watson AJ. Bistability of atmospheric oxygen and the Great Oxidation. Nature. 2006;443(7112):683–686. doi: 10.1038/nature05169. [DOI] [PubMed] [Google Scholar]
- 26.Zerkle AL, Claire MW, Domagal-Goldman SD, Farquhar J, Poulton SW. A bi-stable organic-rich atmosphere on the Neoarchaean Earth. Nat Geosci. 2012;5:359–363. [Google Scholar]