Significance
The human form of forkhead box P2 (FOXP2) is the leading genetic candidate for human speech and language proficiency. We demonstrate that the introduction of the amino acid changes that occurred during human evolution into murine Foxp2 (Foxp2hum) profoundly affects learning and striatal neuroplasticity. Foxp2hum/hum mice learn stimulus–response associations more rapidly than WT mice when declarative (i.e., place-based) and procedural (i.e., response-based) forms of learning could interfere with one another. Dopamine levels, gene expression patterns, and synaptic physiology are oppositely affected in the striatal districts underpinning these learning forms, paralleling the behavioral change. We hypothesize that the human FOXP2 evolution led to differential tuning of corticostriatal systems involved in declarative and procedural learning and thus contributed to adapting the human brain for speech and language acquisition.
Keywords: dorsomedial striatum, dorsolateral striatum, T-maze, cross maze, learning strategy
Abstract
The acquisition of language and speech is uniquely human, but how genetic changes might have adapted the nervous system to this capacity is not well understood. Two human-specific amino acid substitutions in the transcription factor forkhead box P2 (FOXP2) are outstanding mechanistic candidates, as they could have been positively selected during human evolution and as FOXP2 is the sole gene to date firmly linked to speech and language development. When these two substitutions are introduced into the endogenous Foxp2 gene of mice (Foxp2hum), cortico-basal ganglia circuits are specifically affected. Here we demonstrate marked effects of this humanization of Foxp2 on learning and striatal neuroplasticity. Foxp2hum/hum mice learn stimulus–response associations faster than their WT littermates in situations in which declarative (i.e., place-based) and procedural (i.e., response-based) forms of learning could compete during transitions toward proceduralization of action sequences. Striatal districts known to be differently related to these two modes of learning are affected differently in the Foxp2hum/hum mice, as judged by measures of dopamine levels, gene expression patterns, and synaptic plasticity, including an NMDA receptor-dependent form of long-term depression. These findings raise the possibility that the humanized Foxp2 phenotype reflects a different tuning of corticostriatal systems involved in declarative and procedural learning, a capacity potentially contributing to adapting the human brain for speech and language acquisition.
The gene encoding the transcription factor forkhead box P2 (FOXP2) is a promising candidate for investigating the evolutionary basis of human speech and language capabilities. Humans carrying only one functional copy of this transcription factor experience difficulties in learning and performing complex orofacial movements and have receptive and expressive deficits in oral and written language, whereas other cognitive skills are less affected. These speech and language deficits are associated with functional impairments in cortico-basal ganglia and corticocerebellar circuits (1). Since the time that the human and chimpanzee lineages separated, approximately 6 Mya, two amino acid substitutions have occurred in FOXP2, a higher rate of change than expected given its conservation in mammals (2, 3). Mice in which the endogenous Foxp2 gene has been “humanized” for these two amino acid changes (Foxp2hum/hum mice) exhibit prominent neurochemical, neurophysiological, and neuroanatomical alterations in the striatum and related cortico-basal ganglia circuits (4, 5). These circuits are known to be essential for acquiring habits and other motor and cognitive behaviors (6), including vocal learning in songbirds (7) and speech and language capabilities in humans (8). However, whether learning behavior depending on these circuits is affected in Foxp2hum/hum mice has so far not been investigated.
A key functional distinction has been made between subregions of the striatum that underlie modes of learning also considered to be crucial for speech and language development and performance: declarative learning and procedural learning (9–12). These learning modes were first distinguished in human cognitive studies to differentiate between a conscious form of learning that can be “declared” and nonconscious forms of learning that require repetitive exposure (13). Equivalents for these two forms of learning have been suggested for animals in many pioneering studies, and terminology has been adapted depending on whether the motivational drive (action–outcome vs. stimulus–response; goal-directed vs. habit) or the task objective (place-based vs. response-based) is more central to the learning. In rodents, the two learning systems are often probed by tasks requiring motor learning, a type of learning thought to be mainly procedural, or by navigational maze tasks in which place-based learning is suggested to correspond to declarative learning and response-based learning is representative of procedural learning (13–17).
These systems are thought to interact dynamically to optimize behavior (17–22). Evidence suggests that these interacting systems have the capacity to compensate for each other if key components are pathologically affected (23, 24), but can also compete with each other under normal circumstances (14, 15, 17, 19, 25). In situations in which such competition occurs, learning is lessened but can be facilitated by attenuating one of the two competing learning strategies (19, 25). In a novel context, a fact-oriented, declarative type of learning predominates as the new environment is explored. With extended training, as beneficial behaviors are acquired, the procedural system becomes predominant.
Early suggestions that declarative learning solely depends on the temporal lobe and hippocampus, and procedural learning solely on the striatum and cerebellum, have been replaced by evidence that these functions are distributed. Within the striatum, moreover, strong evidence indicates that the declarative system operates early during learning in circuits engaging the dorsomedial striatum, when action–outcome associations are formed, whereas the eventual automatization or proceduralization of the behavior engages circuits interconnected with the dorsolateral striatum (17, 20–22, 26, 27). In brain imaging studies of humans lacking one functional copy of FOXP2, contrasting activation patterns have been reported for regions that are considered to be homologous to the dorsomedial and dorsolateral striatum in rodents (28, 29).
We took advantage of these findings by developing a panel of behavioral learning protocols adapted for mice to determine how humanized Foxp2 influences these two striatal learning systems.
Results
Motor Skill Learning Is Normal in Humanized Foxp2 Mice.
We first evaluated motor skill learning, given that mice lacking one functional allele of murine Foxp2 are reported to exhibit learning deficits on an accelerating rotarod and a tilted running wheel (30, 31). However, mice homozygous for humanized Foxp2 (Foxp2hum/hum) performed at levels equivalent to those of their WT (Foxp2wt/wt) controls when tested by these two tasks (n = 9–10 per genotype; Figs. S1 and S2), extending earlier findings based on different protocols (4). Hence, these types of motor skill learning are impaired in heterozygous murine Foxp2 KO mice (31), but they are not detectably affected by humanizing the Foxp2 protein in mice.
Learning Is Enhanced in Humanized Foxp2 Mice When Declarative and Procedural Systems Can Be Active.
We next performed a series of navigational maze experiments to probe declarative and procedural learning in the Foxp2hum/hum mice. We began by assessing learning in a context allowing place-based/declarative and response-based/procedural forms of learning. We trained Foxp2hum/hum and Foxp2wt/wt mice on a conditional T-maze task, in which distinctive learning-related activity patterns have been found in the dorsomedial and the dorsolateral striatum (22, 32). The mice were required to associate each of two sensory stimuli—a rough or smooth tactile flooring surface—with a food reward that could be found at either goal-arm of a T-maze. In addition, we surrounded the T-maze with salient spatial cues (Fig. 1A).
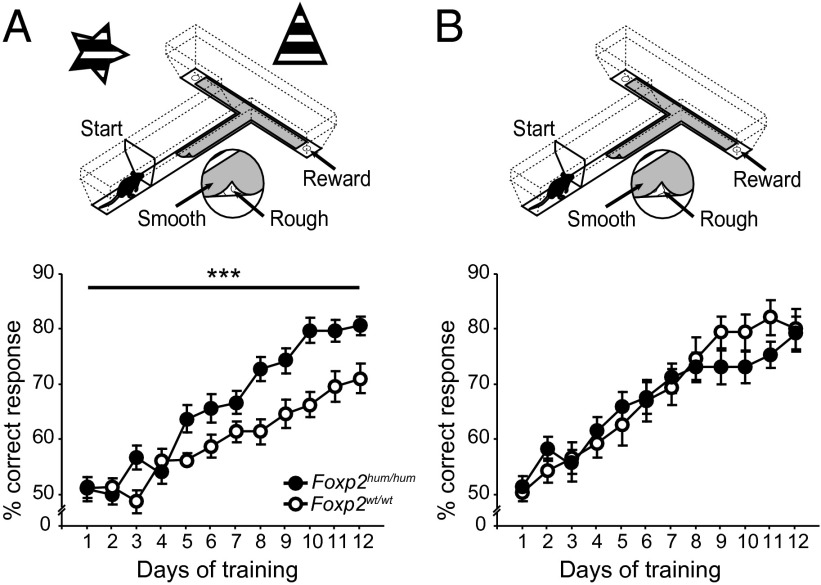
Fig. 1.
Foxp2hum/hum mice learn more rapidly than WT littermates in a conditional T-maze paradigm when spatial cues are present. (Upper) T-maze task with spatial cues (A), promoting place-based/declarative learning; or without spatial cues (B), promoting response-based/procedural learning. (Lower) Average percent correct responses for Foxp2hum/hum mice (black) and their WT littermates (white) in the two environments. Error bars indicate ±SEM (***P < 0.001).
The Foxp2hum/hum mice clearly learned faster than their WT littermates [n = 21–22 per genotype; repeated-measures ANOVA (RMA) days 1–12: F1,41 = 14.94, PGT < 0.001; F7.2,41 = 3.99, Pday*GT < 0.001; generalized linear mixed model days 1–12, z = −3.9, Pday*GT < 10−4; Fig. 1A, SI Materials and Methods, and Table S1]. Moreover, this faster learning in the Foxp2hum/hum mice was specific to the acquisition phase of training. Performance during overtraining, as correct performance was reached and then maintained at greater than 72.5%, did not differ between genotypes (n = 14–15 per genotype; RMA overtraining days 1–10: F1,27 = 0.11, PGT = 0.74; F9,27 = 1.14, Pday*GT = 0.34; Fig. S3).
We designed experiments to determine whether this enhancement of learning speed in the Foxp2hum/hum mice reflected enhanced place-based/declarative learning, enhanced response-based/procedural learning, or an altered interaction of these learning systems. An altered interaction, for example, caused by an attenuated declarative system, could enhance performance by accelerating the transition toward the procedural system, an interaction that has been proposed to occur during striatum-dependent learning tasks (17, 18, 21, 22, 27). In the original T-maze surrounded by spatial cues, the mice were provided with at least three learning possibilities. They could associate a sensory stimulus (rough or smooth) with a reward at a constant place (place-based/declarative learning), associate the stimulus with a body turn (procedural/response-based strategy), or shift from a declarative to a procedural strategy during the course of the training. We tested these three alternatives individually.
First, we changed the T-maze task to favor procedural learning by removing extramaze spatial cues (Fig. 1B), and we tested acquisition in new, naïve cohorts of mutant and WT mice. In this context, the Foxp2hum/hum and WT mice learned equally well (n = 13–14 per genotype; RMA days 1–12: F1,25 = 0.07, PGT = 0.795; F11,25 = 1.439, Pday*GT = 0.156; Fig. 1B and Table S2). Analyses of the combined data for the two task paradigms showed that the presence of spatial cues had clearly a different effect on learning in Foxp2hum/hum mice and their WT controls (RMA days 1–12: F7.85,68 = 4.04, Pday*GT*setup < 0.001). This difference appears to reflect less efficient learning by WT mice in the presence of spatial cues (Fig. 1). This possibility is in accord with reports of less efficient learning in an environment in which the two learning strategies of declarative/place-based and procedural/response-based learning can interact competitively (25) and that WT C57BL/6 mice are “essentially place learners” (33–35). By this view, the abundance of spatial cues in the original maze task did not impair the performance of the Foxp2hum/hum mice, which might have dealt more effectively with competition between the two available learning strategies.
Given this result, we turned to a cross-maze task often used to discriminate place-based from response-based learning (15, 17, 25). We chose a Tolman variation of the task (16, 36), tailored for our purposes, because the cross-maze variation by Packard and McGaugh (15) has been reported to be difficult for mice (33–35). In this cross-maze paradigm, we were able to test declarative/place-based learning and procedural/response-based learning separately as well as to challenge the interaction between them by testing the ability to change between place-based and response-based learning. The mice started from either of two opposing arms of the maze (north or south), with reward available after a specific response (e.g., right turn; Fig. 2A, Left) or at a fixed place (e.g., east arm; Fig. 2A, Right).
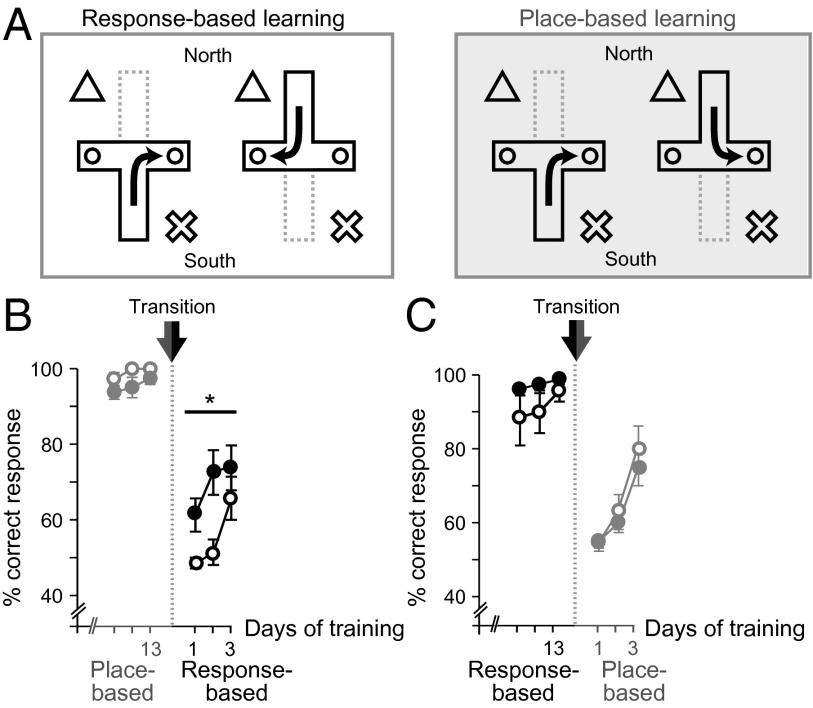
Fig. 2.
Foxp2hum/hum mice exhibit enhanced ability to make transitions from a declarative to a procedural mode of learning. (A) Response-based/procedural (Left) and place-based/declarative (Right) versions of the cross-maze task. (B and C) Average percent correct responses (±SEM) for Foxp2hum/hum (filled dots) and Foxp2wt/wt (open dots) mice successively trained on the two cross-maze task versions and tested on the switch to response-based/procedural (B) or place-based/declarative version (C) (*P < 0.05).
Remarkably, we did not observe enhanced learning by the Foxp2hum/hum mice in the response-based task or the place-based task. The Foxp2hum/hum and WT mice learned both tasks equally rapidly (response-based, n = 7–8 per genotype, RMA: F1,13 = 0.43, PGT = 0.53; F4.6,13 = 0.56, Pday*GT = 0.72; place-based, n = 19–20 per genotype, RMA: F1,37 = 0.45, PGT = 0.51; F6.2,37 = 0.83, Pday*GT = 0.55; Fig. S4). Thus, Foxp2hum/hum mice did not learn faster when the mice were required to use only place-based learning or only response-based learning to solve the task, despite exhibiting accelerated learning when both strategies could be used.
Prompted by this finding, we tested whether the enhanced performance of the Foxp2hum/hum mice resulted from an altered interaction between the two learning systems, attenuating the declarative and favoring the procedural system. We required mice that previously had acquired both tasks without significant difference in performance to shift from place-based learning to response-based learning. We expected to find a difference only during the first days after the task switch, when the two learning systems would likely be in direct competition with each other. To control for general effects on memory or behavioral flexibility, we additionally tested the mice on the opposite direction of transition, measuring learning speeds during the first days after a shift from response-based to place-based learning.
For the transition from place-based to response-based learning, the Foxp2hum/hum mice switched significantly more rapidly (n = 7–8 per genotype; RMA: F1,13 = 5.68, PGT = 0.03; Fig. 2B and Table S3). By contrast, their learning rates did not differ from those of their WT littermates after the opposite, response-to-place transition conditions (n = 7–8 per genotype, RMA: F1,13 = 0.19, PGT = 0.67; Fig. 2C). These findings suggest that it is specifically the transition from declarative/place-based learning to procedural/response-based learning that is enhanced by the introduction of the humanized form of Foxp2, and not either one of these learning systems alone. The findings further suggest that the competitive interaction between these systems could be lessened in mice with humanized Foxp2, therefore facilitating the transition from declarative to procedural learning that is proposed to occur during striatum-dependent habit learning (18, 20–22).
By contrast, we did not detect differences between Foxp2hum/hum mice and their WT siblings in either of these learning systems when they were tested individually. The two genotypes exhibited equivalent procedural/response-based learning as assessed with the accelerating rotarod protocol, the tilted running wheel test, the T-maze protocol in which extramaze cues had been removed, and the procedural/response-based version of the cross-maze task. We also did not observe a difference in the place-based learning of the Foxp2hum/hum mice, which we tested in the declarative/place-based version of the cross-maze task. Only when both learning systems could be engaged in parallel and could interact during the early acquisition phase of learning, as in the T-maze task with extramaze cues, did the humanized Foxp2 mice exhibit more efficient learning. By challenging this interaction between the learning systems with the abrupt shift from declarative/place-based to procedural/response-based learning in the cross-maze task, we found that the more rapid learning in the humanized Foxp2 mice could reflect a faster transition from declarative to response learning.
We next tested the possibility that such a change in learning dynamics could reflect differential effects of the Foxp2 humanization on the dorsomedial and dorsolateral striatum, nodes in circuits that differently support these learning forms.
Differential Effects of Humanized Foxp2 on mRNA Expression Profiles in the Dorsomedial and Dorsolateral Striatum.
To test the possibility that humanized Foxp2 might influence the dorsomedial and the dorsolateral striatum differently, we isolated striatal samples from each subregion by laser capture microdissection in adult Foxp2hum/hum mice and WT littermates (n = 11–12 per genotype) and obtained profiles of mRNA expression with >20 million RNA-Sequencing (Seq) reads per sample. We found many differences between the mRNAs in the two regions [5,895 of 25,259 detected genes with a false discovery rate (FDR) < 0.05; Ppermutations < 0.001], but no single gene differed between genotypes (no genes with an FDR < 0.1; Ppermutations = 0.17). This result indicated that the introduction of humanized Foxp2 does not produce massive changes in the expression profile of striatal cells at the level of single genes.
We did detect a significant effect of humanized Foxp2 at the level of functional gene categories, in particular, a down-regulation of genes in the dorsomedial striatum (1,485 of 3,930 categories at an FDR < 0.05; Ppermutations = 0.013; Dataset S1). The most significant category affected was “signaling,” and the strongest enrichment was found for “neurotransmitter transporter activity” and many categories involved in synaptic regulatory processes (Fig. S5 and Dataset S1). Effects in the dorsolateral striatum were often smaller and nonsignificant (914 of 3,930 categories at an FDR < 0.05; Ppermutations = 0.08). Thus, we detected differential effects of humanized Foxp2 on genes involved in synaptic regulatory processes in the two striatal regions. These subtle molecular effects could reflect important physiological alterations, if present in a subset of cells or if produced by differential inputs to the two striatal districts.
Humanized Foxp2 Influences Dopamine Levels Differently in the Dorsomedial and Dorsolateral Striatum.
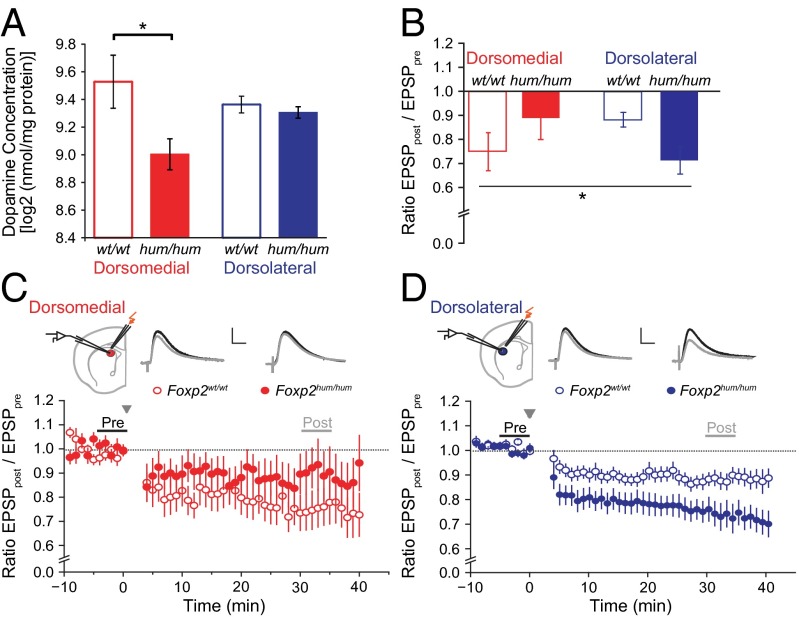
To explore such potential physiological consequences of the Foxp2 humanization, we next analyzed striatal dopamine levels, which are known to be related to learning and to be reduced in striatal samples spanning the dorsomedial and dorsolateral regions in Foxp2hum/hum mice (4). Dopamine levels in the dorsomedial striatum of the Foxp2hum/hum mice were reduced to 70% of those found in WT control mice (n = 10–22 per genotype; t test, t30 = 3.7; PGT = 0.001), whereas dopamine levels in the dorsolateral striatum were similar in the two genotypes (n = 9–22 per genotype; t test, t29 = 0.7; PGT = 0.5). Thus, humanized Foxp2 influences dopamine levels differently in the sensorimotor and associative regions of the dorsal striatum, reducing them dorsomedially (RMA, F1,29 = 5.73, PGT*region = 0.02; Fig. 3A).
Fig. 3.
Foxp2hum/hum mice exhibit differential effects of dopamine levels and synaptic plasticity in the dorsomedial and the dorsolateral striatum. (A) Average (±SEM) concentrations of dopamine in dorsomedial (red) and dorsolateral (blue) striatal biopsies of Foxp2hum/hum mice (hum/hum) relative to WT (wt/wt) levels (*P < 0.05). (B) Averaged excitatory postsynaptic responses (±SEM) in dorsomedial and dorsolateral MSNs in mutant and WT mice 30–40 min after HFS to induce LTD, normalized to baseline levels (*P < 0.05). (C and D) Recording location, representative traces, and time course of LTD induction (post; mean amplitudes ± SEM), normalized to baseline levels (pre) and after stimulation in the dorsomedial (C) and dorsolateral (D) striatum. (Scale bars: 2 mV and 10 ms.)
Humanized Foxp2 Influences Induction of LTD Differently in the Dorsomedial and Dorsolateral Striatum.
To explore potential electrophysiological effects of the Foxp2 humanization, we measured in acute brain slices the induction of dopamine-dependent long-term depression (LTD) after high-frequency stimulation (HFS) in medium spiny neurons (MSNs) located in the dorsolateral and dorsomedial striatum (n = 9–19 cells per genotype and striatal region). In the Foxp2hum/hum mice, LTD in the dorsolateral striatum was stronger than that in WT controls (Fig. 3D), in accordance with previous results (4, 5). However, in the dorsomedial striatum, LTD tended to be weaker in the Foxp2hum/hum mice relative to that in WT controls (Fig. 3C), indicating again the presence of a region-specific effect of humanized Foxp2 (n = 9–19; ANOVA, F1,52 = 5.9, PGT*region = 0.02; Fig. 3B).
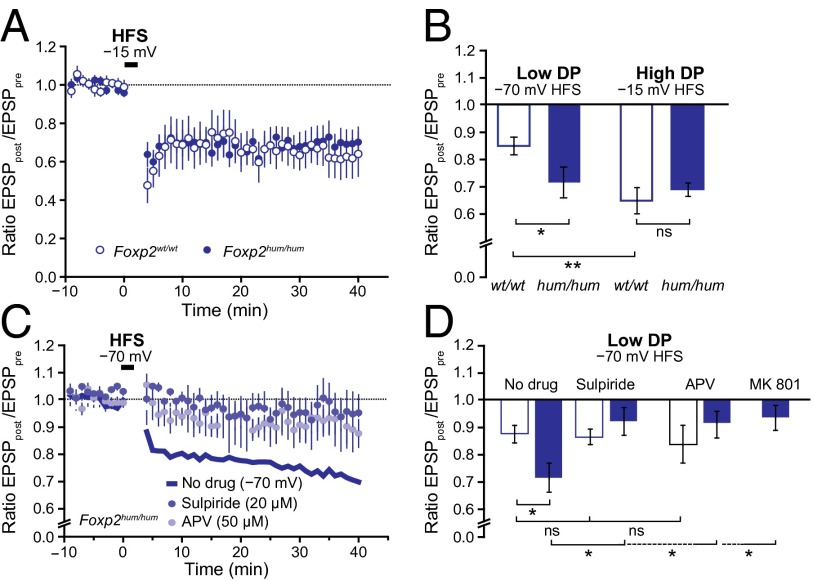
To determine the mechanistic basis of the stronger LTD in the dorsolateral striatum of the Foxp2hum/hum mice, we first compared our protocol, involving a modest −70-mV depolarization during induction, vs. the commonly used HFS-LTD protocol in which stronger depolarization to −15 mV (37) favors the activation of voltage-gated calcium channels (38, 39). When we used the strong depolarization, the genotype difference disappeared. We also observed robust LTD in WT mice (n = 7–17 per LTD protocol; ANOVA, F1,22 = 10.1, P = 0.004; Fig. 4 A and B), and the magnitude of this LTD was similar to that in the Foxp2hum/hum mice (n = 7–8 per genotype; ANOVA, F1,13 = 0.28, P = 0.6). This result indicates that LTD is more readily inducible in MSNs of the dorsolateral striatum of the Foxp2hum/hum mice and requires less depolarization than LTD in the corresponding region of the WT.
Fig. 4.
The enhanced LTD in dorsolateral MSNs of Foxp2hum/hum mice is specific for LTD induction under low depolarization (DP) conditions and depends on D2Rs and NMDA channels. (A) HFS (gray arrow) with depolarization to −15 mV instead of weaker depolarization of −70 mV (Fig. 3 B–D) induced comparable LTD in control and mutant mice during the 40 min post-HFS period. (B) Changing from HFS with weak depolarization (−70 mV) to high depolarization conditions (−15 mV) enhanced mean LTD levels measured at 30–40 min after the HFS in WT but not in mutant mice. Error bars indicate SEM (*P < 0.05 and **P < 0.005). ns, not significant. (C) Readily inducible LTD under low depolarization conditions in dorsolateral MSNs of Foxp2hum/hum mice is abolished by the D2R antagonist sulpiride or by external application of the NMDA receptors antagonist APV. Shown are excitatory postsynaptic potential amplitudes (post), normalized to the mean baseline levels (pre), after HFS in the low depolarization condition of −70 mV (gray arrow) in the presence of sulpiride (20 μM) or APV (50 µM). (D) In mutant mice, the readily inducible LTD measured 30–40 min after HFS under low depolarization conditions can be reversed to WT levels by blocking D2Rs by sulpiride or NMDA receptors with extracellular APV or intracellular MK801 (1 mM; electrode solution). Recordings in the presence of sulpiride, APV, or MK801 were not different from control recordings obtained without HFS stimulation (n = 5–17; ANOVA, P = 0.32–0.98; Fig. S6A). Error bars indicate SEM (*P < 0.05). ns, not significant.
We next tested whether the readily inducible LTD in Foxp2hum/hum mice is based on the dopamine D2 receptor (D2R)-dependent striatal mechanism that has been consistently described for LTD in WT mice (38, 40). Applying the D2R antagonist sulpiride to the slice bath eliminated LTD induction in the Foxp2hum/hum mice (n = 6–19 per treatment; ANOVA, F1,22 = 5.5, P = 0.03; Fig. 4 C and D), suggesting that the effect of humanized Foxp2 on striatal LTD depends on D2R-associated mechanisms.
We tested the alternative possibility that the LTD difference could be the result of a confounding effect of long-term potentiation (LTP) present only in WT mice. LTP in striatal MSNs is considered to be mediated by NMDA receptors and is consistently reported to be blocked by APV (38, 41). Therefore, we antagonized NMDA receptors by adding extracellular APV (50 µM) to the bath solution under the modest −70-mV depolarization conditions. The responses in the dorsolateral striatum were not lowered by APV application in the WT mice, excluding the possibility of a confounding LTP effect (n = 5–17; ANOVA, F1,20 = 0.32, P = 0.58; Fig. 4 C and D). By contrast, in the dorsolateral striatum of the Foxp2hum/hum mice, NMDA receptor inhibition abolished the readily inducible, weak-depolarization LTD, so that the response in humanized mice was no longer distinguishable from WT (n = 10–17 per genotype and treatment; ANOVA, F1,25 = 0.42, P = 0.52; Fig. 4 C and D).
To determine whether this extracellular NMDA receptor blockade in the Foxp2hum/hum mice resulted from effects at the presynaptic level or from the actions of postsynaptic receptors on the MSNs themselves, we added the NMDA channel blocker MK801 (1 mM) to the intracellular solution. This treatment blocked the readily inducible LTD in humanized MSNs (n = 5–19 per treatment; F1,22 = 4.3, P = 0.04; Fig. 4D), suggesting that, under low-depolarization conditions, postsynaptic NMDA receptor activation accounts for LTD induction in the Foxp2hum/hum mice. Our findings thus implicate the humanized form of Foxp2 in enhancing a mechanism of LTD induction in the dorsolateral striatum by means of postsynaptic NMDA receptors. At present, we do not assume a specific increase in NMDA receptors to be responsible for this increased modulation, as the ratio of NMDA to AMPA currents remains unaltered in the Foxp2hum/hum mice (Fig. S6B).
Discussion
Our findings suggest a striking selectivity in the effects of humanized Foxp2 on behavioral learning dynamics as well as on striatal dopamine levels, gene expression levels, and synaptic plasticity. Based on our experimental findings, we suggest as a working hypothesis that humanized Foxp2 differentially influences the functional contributions of the associative and sensorimotor striatum to learning dynamics (Fig. S7). In this view, the Foxp2hum/hum mice exhibited an altered interaction between the declarative and procedural learning strategies, favoring the procedural system when both learning systems were engaged as indicated by their more rapid transition toward procedural behavior in the cue-enriched conditional T-maze task and in the place-to-response switching cross-maze task. This condition would contrast with WT conditions, in which the declarative system is thought to dominate and render the naturally occurring transition toward the procedural learning system less than maximally efficient (17, 19, 25).
How this behavioral change in the Foxp2hum/hum mice is brought about is not clear. However, the modest effects of humanized Foxp2 on gene expression patterns suggest that generalized molecular or cellular reconfigurations of striatal MSNs are not involved. The region-specific effects of humanized Foxp2 on dopamine content and synaptic plasticity could reflect mechanisms directly related to the behavioral effects, given the differential function of the dorsomedial and dorsolateral striatum in place-based/declarative and response-based/procedural forms of learning. Our electrophysiological recordings indicate a region-specific enhancement of readily inducible LTD in the Foxp2hum/hum mice. This form of LTD followed the D2R-dependent mechanism identified for classical strong-depolarization induction protocols (40), but required the activation of NMDA receptors. Such a mechanism has been described in other brain regions (42), but, in the striatum, has been linked mainly, but not exclusively, to the induction of LTP, not LTD (38, 40, 41, 43, 44). Given that the unaltered ratio between NMDA and AMPA currents indicated no increase in NMDA receptors of Foxp2hum/hum mice, and that dopamine is critical for striatal synaptic plasticity, one alternative is that an altered dopamine-dependent modulation of NMDA receptors could be responsible for the humanized effect we observed in these mice (45–47).
The contrasting effects in the dorsomedial and dorsolateral striatum of Foxp2hum/hum mice are striking given that different regional brain-imaging activation patterns have been reported for what are considered as homologous striatal districts in humans lacking one functional copy of FOXP2 (28, 29). How these findings relate to the effect of humanized version of Foxp2 in shaping the development of a human brain to enable traits such as language and speech acquisition is unknown. The relation between declarative and procedural learning strategies and language learning is itself unclear (10–12). One possibility raised by our findings is that efficient proceduralization might accelerate probabilistic learning of language features (10) by chunking single speech and language-related actions into sequences, a chunking function that has been suggested to be a core property of the striatum in experimental work (48, 49). If so, such a process could free up declarative capacities by implementing procedural components at earlier time points. Our findings prompt the intriguing speculation that the humanization of this gene imparted a facilitated ability to use procedural forms of learning and therefore to shift more rapidly from declarative to procedural forms of learning, a change that could have been important for the emergence of proficient language and speech.
Materials and Methods
Additional description of study materials and methods is provided in SI Materials and Methods.
Animals.
A total of 303 Foxp2hum/hum mice [5H10 line (4); 1.8–15.2 mo; postnatal day (P)21–P53 for electrophysiological experiments] and WT littermates (160 for behavioral tests, 23 for gene expression assays, 32 for dopamine measurements, and 88 for electrophysiology experiments) were used, and they were balanced for genotype and sex in each experiment. Behavioral procedures were approved by the Committee on Animal Care at the Massachusetts Institute of Technology, and other procedures were in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986 and guidelines of the Max Planck Institute for Evolutionary Anthropology and federal regulations of Saxony, Germany.
Behavioral Experiments.
Rotarod and tilted running wheel experiments were conducted as previously described (31). For the maze experiments, mice were food-restricted and were habituated to apparatus and reward (chocolate milk). They were then trained on a T-maze (40 trials each day) to obtain reward on the correct goal arm as instructed by tactile conditional cues (rough or smooth floor surface) or on a cross maze (10 trials each day) to go to a specific goal (place-based version) or to make a particular turn (response-based version) to receive reward. Statistical analysis was performed by using RMA and generalized linear mixed models (SI Materials and Methods).
Laser Capture Microdissection and RNA Sequencing.
The dorsomedial and dorsolateral striatum of adult mice was dissected from brain slices by using a laser microscope (P.A.L.M. System; Zeiss). Twenty-five nanograms total RNA were used to construct barcoded mRNA-Seq libraries that were sequenced on a Genome Analyzer IIx platform as described earlier (50). Gene expression analysis was performed by the multifactor model of the R package for differential expression analysis for sequence count data (51). Effects of humanized Foxp2 were summarized by the π-value that multiplies the magnitude and significance of genotype effect (52). This ranking was used for the Wilcoxon rank test implemented in FUNC (https://func.eva.mpg.de/) (53) to identify enriched Gene Ontology categories. Permutations of genotype labels were used to assess global significance (Ppermutations).
Dopamine Content.
Tissue samples from 1 mm cryocut slabs of the dorsomedial and the dorsolateral striatum were homogenized, and their protein content was measured. Dopamine was detected at an electrode potential of 0.8 V. Statistical analyses were performed on log2-transformed dopamine amounts per milligram of protein normalized per region, sex, and batch.
In Situ Electrophysiology.
Coronal striatal slices (250 µm) were prepared from P21–P53 mice, and responses of MSNs to stimulation of cortical afferents (0.33–0.2 Hz) were measured during periods before (15 min) and after (40 min) a tetanic HFS (4 × 100 Hz, at −70 or at −15 mV) in the presence of the GABA(A) receptor blocker SR95531 (GABAzine) by using a whole-cell patch-clamp setup. We applied one- and two-way ANOVAs to test region- and genotype-specific effects.
Supplementary Material
Acknowledgments
We thank H. F. Hall for his expert help with experimental equipment manufacture and setup; H. Jenny, D. Hu, and C. Ye for help with behavioral experiments; J. P. De Bono for assistance with the running wheel experiments; I. Bliesener and V. Wiebe for technical support; A. McWhinnie for help with graphics; N. Cohen for reading the manuscript; and K. S. Smith and Y. Kubota for help with editing the manuscript. This work was funded by the Nancy Lurie Marks Family Foundation, the Simons Foundation Autism Research Initiative Grant137593, National Institutes of Health Grant R01 MH060379, the Wellcome Trust Grants 075491/Z/04 and 080971, the Fondation pour la Recherche Médicale, and the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
Database deposition: Sequences have been deposited in the Gene Expression Omnibus (GEO), (accession no. GSE60659).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414542111/-/DCSupplemental.
References
- 1.Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6(2):131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- 2.Enard W, et al. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418(6900):869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- 3.Enard W. FOXP2 and the role of cortico-basal ganglia circuits in speech and language evolution. Curr Opin Neurobiol. 2011;21(3):415–424. doi: 10.1016/j.conb.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Enard W, et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137(5):961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Reimers-Kipping S, Hevers W, Pääbo S, Enard W. Humanized Foxp2 specifically affects cortico-basal ganglia circuits. Neuroscience. 2011;175:75–84. doi: 10.1016/j.neuroscience.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 7.Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: Converging mechanisms in birdsong and human speech. Nat Rev Neurosci. 2010;11(11):747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- 8.Friederici AD. What’s in control of language? Nat Neurosci. 2006;9(8):991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- 9.Nicolson RI, Fawcett AJ. Procedural learning difficulties: Reuniting the developmental disorders? Trends Neurosci. 2007;30(4):135–141. doi: 10.1016/j.tins.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Romberg AR, Saffran JR. Statistical learning and language acquisition. Wiley Interdiscip Rev Cogn Sci. 2010;1(6):906–914. doi: 10.1002/wcs.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teichmann M, Dupoux E, Cesaro P, Bachoud-Lévi AC. The role of the striatum in sentence processing: Evidence from a priming study in early stages of Huntington’s disease. Neuropsychologia. 2008;46(1):174–185. doi: 10.1016/j.neuropsychologia.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 2004;92(1-2):231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection. Memory Systems of the Brain. New York: Oxford Univ Press; 2001. [Google Scholar]
- 14.Moussa R, Poucet B, Amalric M, Sargolini F. Contributions of dorsal striatal subregions to spatial alternation behavior. Learn Mem. 2011;18(7):444–451. doi: 10.1101/lm.2123811. [DOI] [PubMed] [Google Scholar]
- 15.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 16.Tolman EC, Ritchie BF, Kalish D. Studies in spatial learning; response learning vs. place learning by the non-correction method. J Exp Psychol. 1947;37(4):285–292. doi: 10.1037/h0057434. [DOI] [PubMed] [Google Scholar]
- 17.White NM. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav Brain Res. 2009;199(1):3–23. doi: 10.1016/j.bbr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146(1):122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- 19.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 20.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 21.Yin HH, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12(3):333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorn CA, Atallah H, Howe M, Graybiel AM. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron. 2010;66(5):781–795. doi: 10.1016/j.neuron.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagher A, Owen AM, Boecker H, Brooks DJ. The role of the striatum and hippocampus in planning: A PET activation study in Parkinson's disease. Brain. 2001;124(Pt 5):1020–1032. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- 24.Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson’s disease. Behav Neurosci. 2004;118(2):438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- 25.Chang Q, Gold PE. Inactivation of dorsolateral striatum impairs acquisition of response learning in cue-deficient, but not cue-available, conditions. Behav Neurosci. 2004;118(2):383–388. doi: 10.1037/0735-7044.118.2.383. [DOI] [PubMed] [Google Scholar]
- 26.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Miyachi S, Hikosaka O, Miyashita K, Kárádi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115(1):1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- 28.Liégeois F, et al. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003;6(11):1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- 29.Vargha-Khadem F, et al. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci USA. 1998;95(21):12695–12700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.French CA, et al. An aetiological Foxp2 mutation causes aberrant striatal activity and alters plasticity during skill learning. Mol Psychiatry. 2012;17(11):1077–1085. doi: 10.1038/mp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groszer M, et al. Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008;18(5):354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorn CA, Graybiel AM. Differential entrainment and learning-related dynamics of spike and local field potential activity in the sensorimotor and associative striatum. J Neurosci. 2014;34(8):2845–2859. doi: 10.1523/JNEUROSCI.1782-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middei S, Restivo L, Sgobio C, Passino E, Ammassari-Teule M. Reversible inactivation of hippocampus and dorsolateral striatum in C57BL/6 and DBA/2 inbred mice failed to show interaction between memory systems in these genotypes. Behav Brain Res. 2004;154(2):527–534. doi: 10.1016/j.bbr.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Passino E, Middei S, Restivo L, Bertaina-Anglade V, Ammassari-Teule M. Genetic approach to variability of memory systems: Analysis of place vs. response learning and fos-related expression in hippocampal and striatal areas of C57BL/6 and DBA/2 mice. Hippocampus. 2002;12(1):63–75. doi: 10.1002/hipo.10007. [DOI] [PubMed] [Google Scholar]
- 35.Pittenger C, et al. Impaired bidirectional synaptic plasticity and procedural memory formation in striatum-specific cAMP response element-binding protein-deficient mice. J Neurosci. 2006;26(10):2808–2813. doi: 10.1523/JNEUROSCI.5406-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolman EC, Ritchie BF, Kalish D. Studies in spatial learning; place learning versus response learning. J Exp Psychol. 1946;36:221–229. doi: 10.1037/h0060262. [DOI] [PubMed] [Google Scholar]
- 37.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5(5):446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 38.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: Physiological and pharmacological characterization. J Neurosci. 1992;12(11):4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA. 1997;94(6):2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25(45):10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84(3):1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- 42.Jurado S, Biou V, Malenka RC. A calcineurin/AKAP complex is required for NMDA receptor-dependent long-term depression. Nat Neurosci. 2010;13(9):1053–1055. doi: 10.1038/nn.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28(10):2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer JP, Murphy KP. Bi-directional changes in synaptic plasticity induced at corticostriatal synapses in vitro. Exp Brain Res. 2000;135(4):497–503. doi: 10.1007/s002210000523. [DOI] [PubMed] [Google Scholar]
- 45.Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79(1):82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- 46.Sarantis K, Antoniou K, Matsokis N, Angelatou F. Exposure to novel environment is characterized by an interaction of D1/NMDA receptors underlined by phosphorylation of the NMDA and AMPA receptor subunits and activation of ERK1/2 signaling, leading to epigenetic changes and gene expression in rat hippocampus. Neurochem Int. 2012;60(1):55–67. doi: 10.1016/j.neuint.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18(24):10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70(1-2):119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 49.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286(5445):1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- 50.Wunderlich S, et al. Primate iPS cells as tools for evolutionary analyses. Stem Cell Res (Amst) 2014;12(3):622–629. doi: 10.1016/j.scr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao Y, et al. A novel significance score for gene selection and ranking. Bioinformatics. 2014;30(6):801–807. doi: 10.1093/bioinformatics/btr671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prüfer K, et al. FUNC: A package for detecting significant associations between gene sets and ontological annotations. BMC Bioinformatics. 2007;8:41. doi: 10.1186/1471-2105-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.