Significance
In vitro and in vivo data indicate that hypersialylated tumor cells can engage Siglec-9 on myelomonocytic cells and influence the outcome of the interaction, depending on the stage of tumor growth and the microenvironment. On one hand, engagement of Siglec-9 or Siglec-E by tumor-associated ligands inhibited immunosurveillance and tumor cell killing during establishment of autologous tumors and new metastatic foci. On the other hand, inhibition of tumor-associated macrophages through Siglec-9 led to M1 polarization and reduced growth-promoting inflammation within the tumor microenvironment. This demonstrates a previously unidentified dualistic function of Siglec-9 during cancer progression. A functional polymorphism of Siglec-9 correlated with altered survival of lung cancer patients, suggesting that Siglec-9 might be therapeutically targeted.
Keywords: hypersialylation, tumor-associated macrophages, tumor-associated neutrophils, immune evasion, tumor-associated inflammation
Abstract
Certain pathogenic bacteria are known to modulate the innate immune response by decorating themselves with sialic acids, which can engage the myelomonocytic lineage inhibitory receptor Siglec-9, thereby evading immunosurveillance. We hypothesized that the well-known up-regulation of sialoglycoconjugates by tumors might similarly modulate interactions with innate immune cells. Supporting this hypothesis, Siglec-9–expressing myelomonocytic cells found in human tumor samples were accompanied by a strong up-regulation of Siglec-9 ligands. Blockade of Siglec-9 enhanced neutrophil activity against tumor cells in vitro. To investigate the function of inhibitory myelomonocytic Siglecs in vivo we studied mouse Siglec-E, the murine functional equivalent of Siglec-9. Siglec-E–deficient mice showed increased in vivo killing of tumor cells, and this effect was reversed by transgenic Siglec-9 expression in myelomonocytic cells. Siglec-E–deficient mice also showed enhanced immunosurveillance of autologous tumors. However, once tumors were established, they grew faster in Siglec-E–deficient mice. In keeping with this, Siglec-E–deficient macrophages showed a propensity toward a tumor-promoting M2 polarization, indicating a secondary role of CD33-related Siglecs in limiting cancer-promoting inflammation and tumor growth. Thus, we define a previously unidentified impact of inhibitory myelomonocytic Siglecs in cancer biology, with distinct roles that reflect the dual function of myelomonocytic cells in cancer progression. In keeping with this, a human polymorphism that reduced Siglec-9 binding to carcinomas was associated with improved early survival in non–small-cell lung cancer patients, which suggests that Siglec-9 might be therapeutically targeted within the right time frame and stage of disease.
Bacterial pathogens use several means to evade the innate immune system, including secretion of toxins that kill leukocytes (1) or up-regulation of host self-associated molecular patterns, such as sialic acids (Sias) (2). Sias on bacteria engage receptors like myelomonocytic Siglec-9, resulting in inhibition of antibacterial responses in vitro and in vivo (3–5). Siglecs are Sia-binding Ig-like proteins prominently expressed on leukocytes (6–9). They are divided into two groups based on homology and conservation (6). Siglecs-1, -2, -4, and -15 have mammalian orthologs, whereas the CD33-related Siglecs (CD33rSiglecs) undergo rapid evolution due to selection pressure by pathogens (6, 9). Thus, there are sometimes no clear orthologs of CD33rSiglecs, but there are functionally equivalent homologs. For example, human Siglec-9 and mouse Siglec-E are found on cells of the myelomonocytic lineage and have similar functional properties. However, Siglec-9 expression in humans is broader and also found on NK cells and certain subtypes of T cells (6). As mentioned before, most such CD33rSiglecs transmit inhibitory signals into immune cells, thus functioning as receptors for self-associated glycans that help to keep resting immune cells in a quiescent state (2).
Elimination of early tumor cells by the immune system, including NK cells, lymphocytes, and myelomonocytic cells, is mediated by a process called cancer immunosurveillance (10). During malignant progression, tumor cells can escape immunosurveillance (10). Importantly, in tumors that have escaped, immune cells can instead facilitate cancer progression via chronic inflammation (11–13). Myelomonocytic cells, including neutrophils and monocytes/macrophages, can be either antitumor or protumor mediators, depending on the phase of cancer progression and microenvironment. Whereas polarization to M1 macrophages enhances tumor cell clearance, polarization to an M2 phenotype supports cancer progression (12, 14). Similarly, neutrophils can have either tumor-arresting or tumor-promoting functions (15–19).
One classic hallmark of malignant transformation is the up-regulation of sialylation (20–22). The resulting hypersialylated tumor cells can signal within the tumor microenvironment via interaction with Sia-binding lectins, such as selectins, expressed on leukocytes, platelets, and endothelial cells during cancer progression (23). Moreover, tumors formed in immune-deficient mice tend to have lower sialylation compared with those from WT mice, suggesting that immunoediting selects for increased tumor sialylation (24). Hypersialylation of tumor cells can also inhibit complement activation via factor H recruitment (25–27). However, the effect on myelomonocytic innate immune cells has not been fully studied. Whereas interactions of CD33rSiglecs and up-regulation of tumor-associated Siglec ligands were very recently analyzed in NK cell-mediated killing (28, 29), the role of Siglec-9 on myelomonocytic cells has not yet been tested in vivo or in cancer patients.
We hypothesized that hypersialylated tumor cells inhibit the innate immune system by engaging the predominant Siglec on myelomonocytic cells, Siglec-9, similar to what was recently shown for pathogenic bacteria, such as group B Streptococci, in vitro (4) and in a mouse model in vivo by taking advantage of Siglec-E–deficient mice (5). We therefore analyzed the role of myelomonocytic Siglecs during cancer progression in different mouse and in vitro models. Our results support a paradigm whereby tumor cell ligands for Siglec-9 exert dualistic effects on innate immune cells of the myelomonocytic lineage, depending on the phase of cancer progression and the corresponding microenvironment.
Results
Ligands for Inhibitory Myelomonocytic Siglec-9 are Up-Regulated in Human Carcinomas.
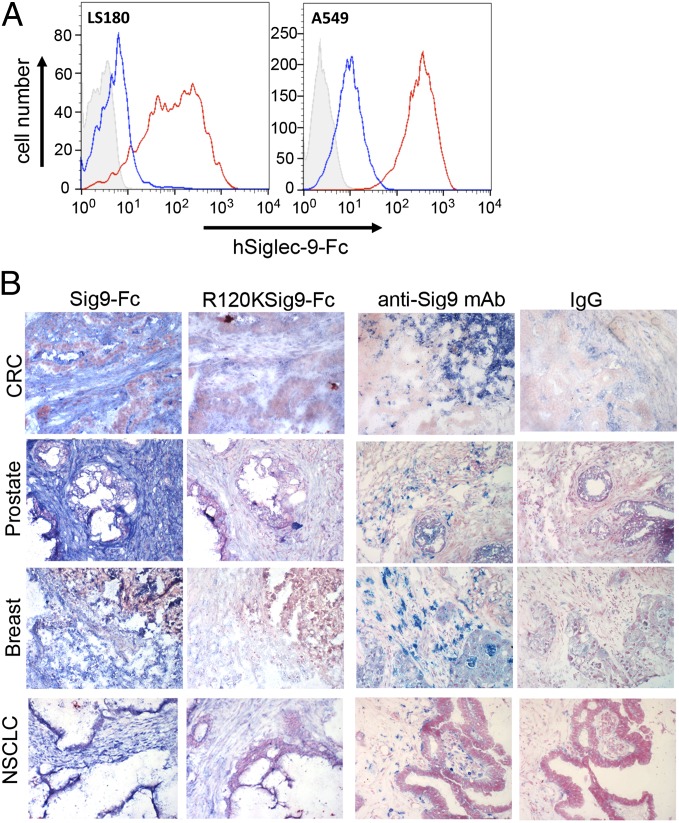
We hypothesized that the known increase in tumor-cell-surface–sialylated glycans can act as ligands for inhibitory Siglecs on myelomonocytic cells. To probe for such ligands, we analyzed the binding of a recombinant soluble Fc chimeric protein of Siglec-9 (Siglec-9–Fc) to human carcinoma cell lines. To determine if binding was specifically dependent on Sias, we used an R120K mutant of Siglec-9–Fc that is specifically devoid of the ability to bind Sia-containing ligands (30) or in situ ligand modification via periodate oxidation of the diol at position C8-C9 of the Sia side chain (31). Using these approaches, we observed strong Sia-dependent binding of Siglec-9–Fc to carcinoma cell lines tested (Fig. 1A and SI Appendix, Fig. S1A). We also tested for ligands of Siglec-5 and Siglec-7. Siglec-7–Fc also bound in a Sia-dependent manner to some carcinoma cell lines (SI Appendix, Fig. S1B).
Fig. 1.
Ligands for Siglec-9 are up-regulated in human carcinomas. (A) LS180 (colorectal cancer, CRC) and A549 (NSCLC) cell lines were probed for Siglec-9 ligands using hSiglec-9–Fc chimera (red line) against control (R120K, blue line) or second reagent alone (solid gray). Histograms are shown. (B) Expression of Siglec-9 ligands and Siglec-9 receptor in human carcinomas, CRC, prostate, breast, and NSCLC cancer using Siglec-9–Fc chimera or anti–Siglec-9 antibody, respectively. R120K–Siglec-9–Fc chimera and mouse IgG isotype were negative controls. (Magnification, 200×.)
We further analyzed the binding of Siglec-9–Fc as well as the infiltration of Siglec-9–positive immune cells in histological sections of human cancer samples. Similar positive results were obtained in colorectal, breast, ovarian, prostate, and non–small-cell lung cancers (Fig. 1B and SI Appendix, Fig. S1 C–G). There was also a strong expression of Siglec-9 ligands detected by Siglec-9–Fc staining in the tumor stroma. In comparison, normal tissue from the ovary or colon did not show an accumulation of stromal ligands (SI Appendix, Fig. S1 H and I). Most of the tumor-infiltrating myelomonocytic cells in primary tumors from patients with colorectal cancer were Siglec-9–positive (SI Appendix, Fig. S1J).
Ligands for Siglec-9 on Tumor Cells Inhibit Neutrophil Activation.
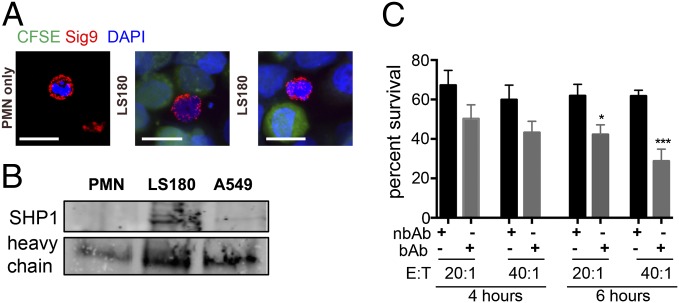
To analyze Siglec–tumor interactions, we incubated human neutrophils with Siglec-9-ligand–positive carcinoma cells and analyzed the distribution of Siglec-9 on the neutrophil surface. Siglec-9 was found to be circumferentially present but accumulated at contact points with the carcinoma cells, suggesting Siglec-9 interactions with ligands (Fig. 2A and SI Appendix, Fig. S2A), whereas other glycosylated neutrophil proteins, including CD45, did not cluster (SI Appendix, Fig. S2B). We further found enhanced recruitment of Src homology region 2 domain containing phosphatase-1 (SHP1) to Siglec-9 only in the presence of LS180 or A549 tumor cells, indicating phosphorylation of the intracellular domain of Siglec-9 and a transmission of intracellular inhibitory signals (Fig. 2B). Neutrophil activation was analyzed by measurement of extracellular reactive oxygen species (ROS), when neutrophils were in contact with tumor cells. We found lower levels of ROS being produced within the tumor–neutrophil coculture compared with neutrophils alone, which were presumably slightly activated due to the isolation process (SI Appendix, Fig. S2C). Moreover, blocking neutrophil Siglec-9 with an antibody specific for the Sia-binding domain (clone 191240) gave enhanced neutrophil activation compared with an antibody that binds outside of the sialic acid-binding area (clone E10-286, SI Appendix, Fig. S2C). Finally, we observed increased tumor cell killing and apoptosis (increased cleaved caspase 3 levels) in tumor cells coincubated with neutrophils with blocked Siglec-9, in comparison with control IgG or nonblocking antibody (Fig. 2C and SI Appendix, Fig. S2D). These findings demonstrate that neutrophil activation can be inhibited by interaction of sialylated tumor-associated ligands with Siglec-9 and that blocking of engagement of Siglec-9 by Sia-dependent ligands restores neutrophil activation against tumor cells.
Fig. 2.
Binding of tumor cell surface ligands to Siglec-9 inhibits neutrophil activation. (A) Staining of Siglec-9 on polymorphonuclear leukocytes [neutrophils, polymorphonuclear cells (PMN)] before or after coincubation with LS180 tumor cells [carboxyfluorescein succinimidyl ester (CSFE), green]. (Scale bar, 20 μm.) (B) Immunoprecipitation of Siglec-9 and immunostaining for SHP1 from coincubation of neutrophils with LS180 or A549 cells. The heavy chain of the anti–Siglec-9 antibody was used as a loading control. (C) CFSE-labeled LS180 after 4 and 6 h coculture with neutrophils at different effector to target (E:T) ratios. Survival was measured by counting fluorescent tumor cells and normalized to the number of tumor cells not coincubated with neutrophils (n = 3). **P < 0.01.
Engagement of Murine Siglec-E Inhibits Tumoricidal Neutrophil Activity in Vivo.
We next asked if the presence of Siglec ligands on tumor cells and within the microenvironment could influence neutrophil-mediated tumor cell killing in vivo. The main CD33rSiglec on myelomonocytic cells and the functionally equivalent homolog of Siglec-9 in mice is Siglec-E (although compared with human Siglec-9 there is no Siglec-E expression on NK cells or T cells in mice) (32). To study the function of inhibitory Siglec-E, we used Siglec-E null (SigE−/−) mice, together with syngeneic murine tumor cell lines MC38 and LLC, which both bind to chimeric Siglec-E–Fc (SI Appendix, Fig. S3 A and B).
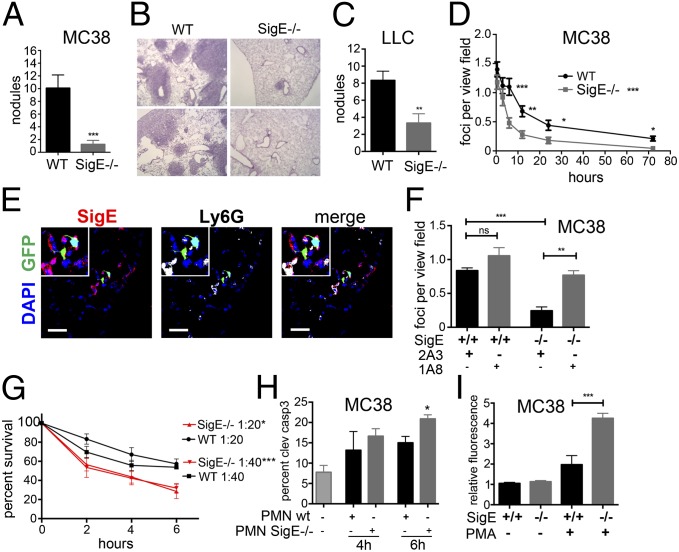
Although the impact of neutrophils on circulating tumor cells is debated (16, 17), recent evidence suggests that neutrophils can play a role in restricting lung colonization (18). Thus, we reasoned that i.v. injected MC38-GFP cells could be analyzed for their immediate interaction with neutrophils in the pulmonary vasculature. Compared with WT controls, SigE−/− mice had a markedly lower incidence of lung nodules and GFP-positive MC38 tumor cells 21 d after i.v. injection, suggesting a reduced survival of i.v. injected MC38 cells in SigE−/− mice (Fig. 3 A and B and SI Appendix, Fig. S3C). This observation was repeated with syngeneic LLC carcinoma cells (Fig. 3C). We then analyzed the number of GFP-positive foci over time. A decrease in the number of GFP-positive tumor cell foci was observed in SigE−/− mice as early as 3 h after i.v. injection, suggesting an early enhanced immunosurveillance by SigE−/− cells (Fig. 3D). We further observed a strong physical association of Siglec-E–positive neutrophils with MC38-GFP cells 3 h after i.v. injection (Fig. 3E and SI Appendix Fig. S3 D and E). No difference in recruitment of NK cells or T cells was observed between SigE−/− mice and WT controls (SI Appendix, Fig. S3 F–I). Depletion of neutrophils before administration of MC38-GFP cells led to a significant increase in GFP-positive foci 6 h after i.v. injection of MC38-GFP cells in SigE−/− mice (Fig. 3F) and to a significant increase in the number of tumor nodules at 21 d (SI Appendix, Fig. S3J). In contrast, depletion of monocytes/macrophages by clodronate liposomes had no effect on the number of GFP-positive foci 6 h after i.v. injection of MC38-GFP cells (SI Appendix, Fig. S3K).
Fig. 3.
Deficiency of Siglec-E enhances neutrophil activation and tumor cell killing in vivo. (A) Macroscopic lung nodules in the left lobe of SigE−/− and WT mice i.v. injected with MC38-GFP cells after 21 d (n = 9–10). (B) H&E of lung sections from SigE−/− and WT. (200×) (C) Number of lung nodules in SigE−/− or WT 21 d after i.v. injection of LLC cells (n = 7–8). (D) Survival of MC38-GFP cells in lungs of SigE−/− and WT from GFP-positive foci per view field (400×) (n = 3 mice per time point). Analyzed by two-way ANOVA, post hoc Bonferroni correction for overall effect, and Fisher’s least significant difference test for single time points. (E) Immunofluorescence image of MC38-GFP cells 3 h after i.v. injection in lungs of WT mice. (Scale bar, 20 μm.) (F) Depletion of neutrophils in SigE−/− and WT mice with anti-Ly6G antibody (1A8). GFP-positive foci in lungs counted 6 h postinjection (control, IgG 2A3; n = 4–5). (G) Survival of MC38-GFP by counting fluorescent cells after coculture with bone-marrow–derived neutrophils (BMDN) from WT and SigE−/− mice (n = 3). (H) Measurement of cleaved caspase 3 after coincubation with BMDNs from WT and SigE−/− mice (n = 3). (I) Extracellular ROS by BMDNs from SigE−/− or WT mice and cocultured with MC38 cells in the presence of PMA (1 μg/mL, n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001.
To further analyze the interaction of tumor cells and SigE−/− neutrophils, we performed coculture of SigE−/− or WT bone-marrow–derived neutrophils with MC38 and LLC cells and found decreased survival of tumor cells in the presence of SigE−/− neutrophils (Fig. 3 G and H and SI Appendix, Fig. S3L). Preactivation of neutrophils with phorbol 12-myristate 13-acetate (PMA) led to a higher production of ROS in SigE−/− neutrophils (Fig. 3I). We also found an increased expression of apoptosis inducing TNF-related apoptosis inducing ligand (TRAIL) and FasL on the surface of SigE−/− neutrophils after coculture with MC38 cells (SI Appendix, Fig. S3 M and N), indicating that the observed apoptosis was rather induced through cell–cell signaling via TRAIL and FasL, and not ROS.
Absence of Myelomonocytic Siglecs Enhances Cancer Immunosurveillance in a Carcinogen-Induced Tumor Model.
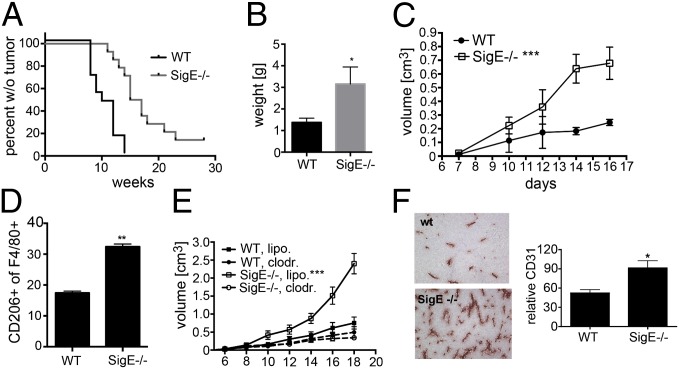
We next wanted to test if this Siglec-ligand interaction could influence tumor immunosurveillance in an autologous tumor model using s.c. injection of the carcinogen 3-methylcholanthrene (MCA). The resulting sarcomas indeed appeared later in SigE−/− mice compared with WT littermate control mice (Fig. 4A). A reduced dose of MCA also showed a delayed tumor development in SigE−/− mice, although the difference was not as strong (SI Appendix, Fig. S4A). Surprisingly, although the tumors developed later in SigE−/− mice, once they appeared they started to grow faster, and average tumors after 18 wk were larger in the SigE−/− mice compared with WT controls (Fig. 4B). We analyzed the infiltration of neutrophils and macrophages and macrophage polarization in the s.c. MCA tumors by flow cytometry, finding a significant increase of polarization toward M2 in SigE−/− mice (SI Appendix, Fig. S4B). Although Siglec-E–Fc chimeras strongly bound to MCA sarcoma cells, there was no difference between genotypes (SI Appendix, Fig. S4C), suggesting that engagement of Siglec-E is not the only factor that selects for hypersialylation in this model.
Fig. 4.
M2-polarized macrophages support tumor growth in Siglec-E–deficient mice. (A) Appearance of tumors in SigE−/− and WT mice after s.c. injection of 100 μg MCA (n = 10). Analyzed by Logrank test. (B) Mass of developed tumors at 18 wk after injection of 100 μg MCA (n = 5–6). *P < 0.05. (C) Growth curve of s.c. injected MC38 cells (n = 6–8) into littermate WT or SigE−/− mice. (D) Flow cytometry, CD11b+ F4/80+ CD206+ M2-polarized macrophages in s.c. MC38 tumors at day 18. (E) Growth curve of s.c. MC38 tumors in WT or SigE−/− mice treated with clodoronate liposomes versus control liposomes 8 d later (n = 8–9). (F) MC38 tumors from SigE−/− and WT mice stained for CD31. Analyzed using ImageJ software, n = 5. *P < 0.05, **P < 0.01, and ***P < 0.001.
Siglec Engagement Inhibits M2 Polarization and Tumor Growth.
The enhanced growth of established MCA tumors in SigE−/− mice prompted us to further investigate the role of Siglec-E on tumor growth and on tumor-infiltrating macrophages. We studied s.c. administered syngeneic MC38, LLC, and B16F1 cells and found that tumors grew faster in SigE−/− than in WT mice (Fig. 4C and SI Appendix, Fig. S4 D and E). There was an up-regulation of infiltration of Siglec-E–positive cells in tumors over time in WT mice, indicating an important role of inflammation-supported tumor growth by Siglec-E–positive cells (SI Appendix, Fig. S4F). Overall there was no significant difference in leukocyte infiltration (cells positive for panleukocyte marker CD45) into the tumors from either genotype (SI Appendix, Fig. S4G). Most of the Siglec-E–positive cells in s.c. MC38 tumors in WT mice were macrophages (SI Appendix, Fig. S4H). Analysis of subpopulations of tumor-infiltrating leukocytes showed an increased infiltration of tumor growth-promoting CD11b+ (Mac-1, marker for myelomonocytic cells), F4/80+ (marker for mouse macrophages), and CD206+ (macrophage mannose receptor, marker for M2 polarization) macrophages in SigE−/− mice (Fig. 4D). Depletion of macrophages by clodronate liposomes at phases when the difference between the two genotypes usually appeared reduced the enhanced tumor growth in SigE−/− mice (Fig. 4E), whereas in accordance with low numbers of infiltrating neutrophils into s.c. MC38 tumors (SI Appendix, Fig. S4I), depletion of neutrophils with the neutrophil-specific anti-Ly6G antibody 1A8 had no effect (SI Appendix, Fig. S4J). In keeping with the proangiogenic function of M2 macrophages, increased angiogenesis was also found in s.c. tumors of SigE−/− mice (Fig. 4F).
To further analyze the function of Siglec-E on macrophage polarization, we studied resident peritoneal macrophages, which express not high, but detectable levels of Siglec-E (SI Appendix, Fig. S5A). Bone-marrow–derived macrophages were not used, because they express minimal amounts of Siglec-E (SI Appendix, Fig. S5A, 32). When cocultured with MC38 tumor cells for 2 d, peritoneal macrophages from Siglec-E–deficient mice showed more CD206 and less CCR2 (marker for M1 polarization) expression than macrophages from WT control mice (SI Appendix, Fig. S5 B and C), suggesting a skewing of tumor-associated SigE−/− macrophages to an M2 phenotype. Macrophages not coincubated with tumor cells showed similar amounts of M1 and M2 markers (SI Appendix, Fig. S5 B and C). To investigate the impact of Siglec-9 tumor cell interactions on human macrophage polarization, we differentiated monocytes from human peripheral blood into macrophages. As published previously (6–8), we found multiple inhibitory CD33rSiglecs on such macrophages (SI Appendix, Fig. S6A). Functional blocking of macrophage Siglec-9 with an antibody that blocks Sia recognition increased the number of CD11b+ CD206+ positive macrophages (M2) in tumor cocultures. However, the addition of the antibody alone did not change the expression level of CD206 (SI Appendix, Fig. S6 B and C). This finding shows that sialylated ligands on tumor cells mediate the effect observed. Further studies are more difficult, due to the redundancy of inhibitory Siglecs on human monocytes and macrophages.
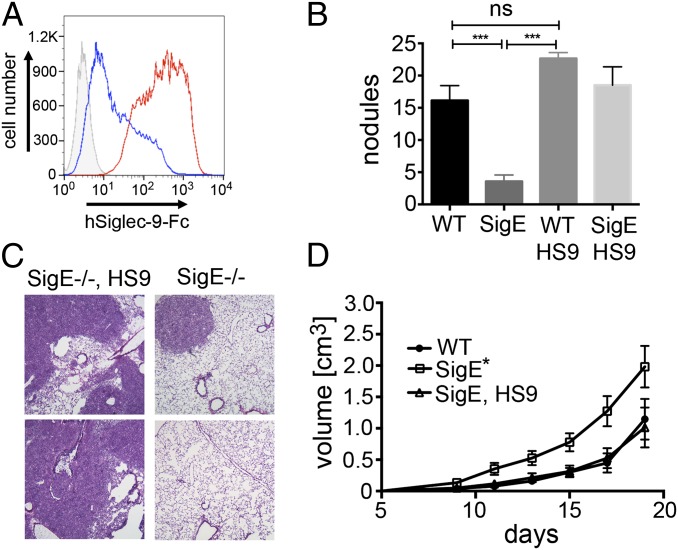
Transgenic Expression of Siglec-9 on Myelomonocytic Cells Reverses Effects of Siglec-E Deficiency.
To further examine the function of inhibitory Siglecs on myelomonocytic cells, we transgenically expressed human Siglec-9 under the LysM promoter, which expresses Siglec-9 exclusively in myelomonocytic cells (33), and bred these mice into the SigE−/− background (SI Appendix, Fig. S7A). Siglec-9 was expressed in up to 95% of myelomonocytic cells in peripheral blood as determined by flow cytometry (SI Appendix, Fig. S7B). Siglec-9–Fc protein also bound to MC38 cells in a Sia-dependent manner (Fig. 5A). Transgenic mice that express human Siglec-9 instead of Siglec-E showed a strong increase in the ability to form lung nodules, comparable to that seen in WT mice (Fig. 5 B and C). The replacement of Siglec-E by expression of Siglec-9 in LysM+ myelomonocytic cells also led to reversal of s.c. MC38 tumor growth to the slow-growing WT level, thus confirming that inhibitory Siglecs in myelomonocytic cells can decrease tumor growth in the s.c. model (Fig. 5D). Myelomonocytic cells (CD11b+) that infiltrated tumors in Siglec-9 transgenic mice were Siglec-9–positive (SI Appendix, Fig. S7C). We also observed a reduction in M2-polarized macrophages (F4/80+ CD206+) from mice with transgenic expression of Siglec-9 in LysM-positive cells compared with SigE−/− mice (SI Appendix, Fig. S7 D and E). Although we found more macrophages infiltrating the s.c. MC38 tumors in SigE−/− (F4/80+ of CD45+), no significant differences of the percentage of leukocytes (CD45+) and neutrophils (Ly6G+) were seen between genotypes (SI Appendix, Fig. S7 F–H).
Fig. 5.
Expression of human Siglec-9 in myelomonocytic cells reverses the phenotype of Siglec-E–deficient mice. (A) Flow cytometry, Siglec-9–Fc chimera binding to MC38 cells (red). IO4− was used as a control (blue, secondary reagent: gray). (B) Tumor nodules in the left lobe 21 d after i.v. injection of MC38 cells into SigE−/−, WT, and SigE−/−HS9 mice (n = 6–9; HS9, expression of human Siglec-9 under in cells expressing LysM-Cre). (C) H&E images (200×) of tumors from SigE−/− and SigE−/−HS9 mice. (D) Growth curves of s.c. MC38 tumors in SigE−/−, SigE−/−HS9, and WT mice (n = 7–8) *P < 0.05, ***P < 0.001.
Naturally Occurring Siglec-9 Polymorphism Influences Early Survival of Patients with Non–Small-Cell Lung Cancer.
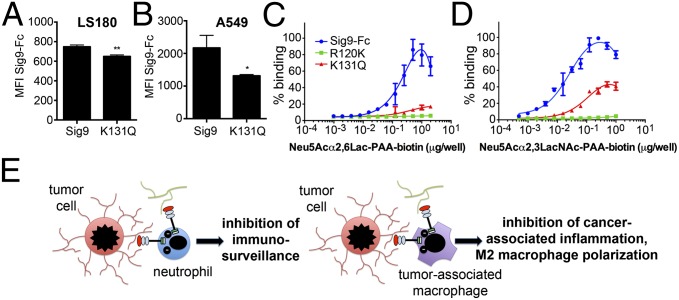
Because we found a significant role of myelomonocytic Siglecs in cancer initiation and progression, we wondered if there is a functional importance in cancer patients. Human Siglec-9 has a known K131Q (A391C) polymorphism (rs16988910) that reduces binding to Sia-containing ligands (34). We used this chimeric Fc protein to ask whether this polymorphism reduced binding to carcinoma cell lines. Some cell lines showed a complete abrogation to the point seen with the synthetic R120K mutant, and others showed a reduced binding (Fig. 6 A and B and SI Appendix, Fig. S8). Binding of α2–6-sialyl-lactose-polyacrylamide or α2–3-sialyl-N-acetyl-lactosamine-polyacrylamide to K131Q–Siglec-9–Fc was clearly reduced compared with Siglec-9–Fc (Fig. 6 C and D). Accordingly, analysis on a sialoglycan microarray showed a reduced binding of K131Q–Siglec-9–Fc protein to all Siglec-9 target structures (SI Appendix, Figs. S9 and S10).
Fig. 6.
Siglec-9 polymorphism reduces binding to tumor-associated ligands. (A) Flow cytometry, mean fluorescence intensity (MFI) of Siglec-9–Fc chimera or K131Q–Siglec–Fc chimera binding to LS180 and (B) A549 cell lines (n = 6). *P < 0.05, **P ;< 0.01. (C and D), Binding of Neu5Acα2–6-Lactose-polyacrylamide (C) or Neu5Acα2–3-N-actyl-lactosamine-polyacrylamide (D) to different Siglec-9–Fc chimeras. (E) Summary of findings. Inhibition of immunosurveillance by myelomonocytic cells (Left) or inhibition of M2 polarization (Right) via CD33r inhibitory Siglecs.
To investigate the role of this polymorphism in patients we analyzed patients with non–small-cell lung cancer (NSCLC). We studied both cancer risk and survival in a group of NSCLC patients and controls with African ancestry, because the allele is only present in this population (for baseline characteristics of studied subjects, see SI Appendix, Tables S1 and S2). Although risk of NSCLC was not significantly associated with rs16988910 genotype variation after adjustment for covariates, early survival (<2 y) was significantly improved in minor allele carriers (A/C or C/C) compared with A/A cases, adjusting for age, sex, cigarette pack years, and stage at diagnosis [hazard ratio, HR(95% confidence interval, CI) = 0.66(0.44, 0.97), P = 0.035; Table 1]. After 2 y the difference was lost, and overall survival was not significantly different among rs16988910 genotypes (Table 1). We also noted that patients with the A391C (K131Q) polymorphism had a significantly increased risk of emphysema compared with patients with the major allele only after adjusting for covariates [odds ratio, OR(95% CI) = 1.49(1.05, 2.11), P value = 0.027; SI Appendix, Table S3], presumably due to a loss of inhibition of neutrophil cells in lung tissue and increased production or release of proteases. Overall, our data indicate that diminished recognition of sialoglycan ligands by Siglec-9 in humans is associated with better initial control of tumor growth, an effect that is lost once the tumor progresses.
Table 1.
Mortality risk associated with rs16988910 in NSCLC patients, dichotomized by follow-up time
| Follow-up interval | HR (95% CI) | P value | Test of proportional hazards (P value) |
| < 2 y | 0.66 (0.44, 0.97) | 0.035 | 0.209 |
| ≥ 2 y | 0.97 (0.64, 1.46) | 0.888 | 0.286 |
Adjusted for age, sex, cigarette pack years, and stage.
Discussion
Immune cells can either inhibit or support cancer, depending on the microenvironment and phase of disease progression (10, 13). The impact of innate immune cells also depends on the mouse model used. Here we describe a previously unidentified dualistic function of inhibitory CD33rSiglecs on myelomonocytic cells in modulating cancer progression, depending on the microenvironment and model used (Fig. 6E). Similar to certain pathogenic bacteria that subvert innate immunity by engaging inhibitory CD33rSiglecs (3–5), we show that the sialic acid-dependent binding of tumor-associated ligands to Siglec-9 and -E can inhibit neutrophils and increase lung colonization in an experimental metastasis assay. As previously mentioned, prior work has shown that neutrophils can either exert protumor or antitumor activity (35). Although antitumor neutrophils were previously implicated in limiting cancer progression, including dissemination to the lung (15, 18), other studies also suggested a supporting role in organ colonization (16, 17, 19). Thus, the function of neutrophils during experimental organ colonization and cancer progression may depend on the exact context and microenvironment as well as the model used. Nevertheless, our data show that inhibitory Siglecs expressed on such cells can modulate the final outcome.
In contrast to the facilitating effects of Siglec-E/-9 on lung colonization in the i.v. model and on the appearance of s.c. MCA tumors, inhibitory Siglecs are shown to be involved in restricting the polarization of macrophages toward a tumor-promoting M2 phenotype during s.c. tumor growth. This suggests that Sia-dependent ligands can inhibit tumor-associated inflammation, which would support tumor growth (Fig. 6E). Tumor-promoting macrophages can support cancer progression by suppressing antitumor immune responses and inducing angiogenesis (36–38). We show here in a model of s.c. tumor growth that Siglec-E deficiency leads to increased presence of M2 macrophages, which in turn enhanced the growth of tumors. Depletion of macrophages reversed the effect seen in SigE−/− mice (Fig. 4). One possible explanation for the expansion of M2 macrophages in our s.c. tumor models is that ligands of Siglec-E could directly inhibit the formation of tumor-promoting M2 macrophages and reprogram them toward an antitumor phenotype. In support of this hypothesis, in vitro coculture experiments show an up-regulation of M2 markers when Siglecs were absent or blocked by antibodies. However, the increased number of macrophages found in s.c. tumors of SigE−/− mice also suggests that recruitment or local proliferation could also be involved in vivo.
The dualistic effect of myelomonocytic Siglecs on cancer progression might be a matter of kinetics: neutrophils are recruited first and disappear over time, whereas macrophages infiltrate and accumulate later. Early analysis of s.c. tumors after injection of tumor cells is difficult, but in the i.v. model, the tumor must escape neutrophils in the blood. Thus, to survive neutrophil-mediated destruction (in metastasis or during early tumorigenesis in the MCA model), tumors are selected to up-regulate Siglec-E ligands. At later time points, Siglec-E ligands could induce M1 macrophages, but possibly because the tumor is already at a large size, this does not lead to rejection. Moreover, there is precedence for Siglecs to have different activities in different cell types. For example, Siglec-8 induces apoptosis in eosinophils but modifies Fc receptor signaling without inducing apoptosis in mast cells (39). Siglec-7 can promote expression of inflammatory cytokines in monocytes but not in NK or T cells (40). Our findings with Siglec-9 could be related to complex evolutionary pressures on this molecule (9).
Other recent analysis of Siglec-9 in cancer focused on activation of tumor cells via Siglec-9 ligands, such as mucin 1 (41, 42). Previous analysis also identified Siglec-7 and -9 ligands present in colorectal cancer and also found a role for those Siglecs in the generation of tumor-associated macrophages (43). Moreover, inhibitory Siglecs, including Siglec-7 and, in a minor fraction, Siglec-9, are expressed on NK cells (6, 44). In this regard, in a recent study, Hudak et al. (29) introduced synthetic sialoglycoconjugates into the surface of cell lines, and the resulting increased sialylation lead to an inhibition of NK cell activation against these cell lines in vitro. Another recent paper (28) shows that interactions between Siglec-7/9 receptors and native tumor cell ligands can influence NK cell-dependent tumor immunosurveillance. This study nicely complements our current work and emphasizes that NK cell–Siglec interactions might play a role in the human situation (mice do not have a major expression of Siglecs on NK cells). Moreover, Siglec-9 is also expressed on a subgroup of CD8 T cells (45), although at a low level (46).
We present here for the first time to our knowledge combined in vitro and in vivo data that myelomonocytic CD33rSiglecs are involved in modulating cancer progression in a dualistic fashion. An association with survival of NSCLC patients corroborates these findings and suggests that blocking Siglec-9 at the right time points during treatment could be a potential therapeutic approach.
Methods
Murine Models.
SigE−/− mice were described previously (32) and provided by Paul Crocker, College of Life Sciences, University of Dundee, Dundee, United Kingdom. The cDNA of human Siglec-9 were cloned into a vector that expresses a loxP-flanked GFP under the minimal chicken beta-actin (CAG) promoter with a subsequent stop codon upstream and electroporated into S129/Sv embryonic stem cells. Mice were backcrossed into a SigE−/− C57BL/6 background for >10 generations and crossed with SigE−/− LysM-Cre–expressing mice. Upon expression of Cre recombinase under the promoter of LysM in myelomoncytic cells the GFP and the stop codon were removed, and human Siglec-9 was expressed. All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee of University of California, San Diego.
Analysis of A391C Polymorphism in Patients with NSCLC.
Data for phenotype–genotype analyses came from multiple case-control studies evaluating risk of lung cancer associated with genetic variability among individuals in Detroit (47). Population-based lung cancer cases were identified through the Metropolitan Detroit Cancer Surveillance System, a participant in National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program. DNA was acquired at the Karmanos Cancer Institute at Wayne State University, Detroit in accordance with institutional review board approval. Genomic DNA from patients with NSCLC (n = 332) and age-, race-, and sex-matched controls (n = 367) were genotyped for the A391C (rs16988910) polymorphism of Siglec-9 at the Applied Genomics Technology Center at the Karmanos Cancer Institute.
Supplementary Material
Acknowledgments
We thank Ismael Secundino for helpful discussions. We particularly thank Paul R. Crocker for providing the Siglec-E–deficient mouse. This work was supported by the Swiss National Science Foundation (to H.L.), by a Samuel and Ruth Engelberg Fellowship from the Cancer Research Institute (to O.M.T.P.), by Grant R01CA38701 from the National Institutes of Health (NIH) (to A. Varki), and by NIH Grants and Contracts N01PC35145, P30CA022453, R01CA14176, and R01060691 (to A.G.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409580111/-/DCSupplemental.
References
- 1.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3(12):948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21(9):1121–1124. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlin AF, et al. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med. 2009;206(8):1691–1699. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113(14):3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang YC, et al. Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog. 2014;10(1):e1003846. doi: 10.1371/journal.ppat.1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 7.von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann N Y Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crocker PR, McMillan SJ, Richards HE. CD33-related siglecs as potential modulators of inflammatory responses. Ann N Y Acad Sci. 2012;1253:102–111. doi: 10.1111/j.1749-6632.2011.06449.x. [DOI] [PubMed] [Google Scholar]
- 9.Padler-Karavani V, et al. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 2014;28(3):1280–1293. doi: 10.1096/fj.13-241497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Elinav E, et al. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 13.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science. 2013;339(6117):286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70(14):6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spicer JD, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–3927. doi: 10.1158/0008-5472.CAN-11-2393. [DOI] [PubMed] [Google Scholar]
- 18.Granot Z, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20(3):300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cools-Lartigue J, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36(3):322–335. doi: 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Varki A, Kannagi R, Toole BP. In: Essentials of Glycobiology. Varki A, et al., editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2009. pp. 617–632. [Google Scholar]
- 22.Fuster MM, Esko JD. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5(7):526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 23.Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20(3):169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Cohen M, et al. Sialylation of 3-methylcholanthrene-induced fibrosarcoma determines antitumor immune responses during immunoediting. J Immunol. 2010;185(10):5869–5878. doi: 10.4049/jimmunol.1001635. [DOI] [PubMed] [Google Scholar]
- 25.Shi WX, Chammas R, Varki NM, Powell L, Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem. 1996;271(49):31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 26.Fedarko NS, Fohr B, Robey PG, Young MF, Fisher LW. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J Biol Chem. 2000;275(22):16666–16672. doi: 10.1074/jbc.M001123200. [DOI] [PubMed] [Google Scholar]
- 27.Ajona D, et al. Expression of complement factor H by lung cancer cells: effects on the activation of the alternative pathway of complement. Cancer Res. 2004;64(17):6310–6318. doi: 10.1158/0008-5472.CAN-03-2328. [DOI] [PubMed] [Google Scholar]
- 28.Jandus C, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124(4):1810–1820. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol. 2014;10(1):69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J Biol Chem. 2000;275(29):22127–22135. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- 31.Gahmberg CG, Andersson LC. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977;252(16):5888–5894. [PubMed] [Google Scholar]
- 32.McMillan SJ, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling. Blood. 2013;121(11):2084–2094. doi: 10.1182/blood-2012-08-449983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 34.Cheong KA, et al. A novel function of Siglec-9 A391C polymorphism on T cell receptor signaling. Int Arch Allergy Immunol. 2011;154(2):111–118. doi: 10.1159/000320225. [DOI] [PubMed] [Google Scholar]
- 35.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23(3):141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 37.Sica A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 38.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39(3):317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varchetta S, et al. Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes. PLoS ONE. 2012;7(9):e45821. doi: 10.1371/journal.pone.0045821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabit I, et al. Binding of a sialic acid-recognizing lectin Siglec-9 modulates adhesion dynamics of cancer cells via calpain-mediated protein degradation. J Biol Chem. 2013;288(49):35417–35427. doi: 10.1074/jbc.M113.513192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanida S, et al. Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth. J Biol Chem. 2013;288(44):31842–31852. doi: 10.1074/jbc.M113.471318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazaki K, et al. Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and -9. J Immunol. 2012;188(9):4690–4700. doi: 10.4049/jimmunol.1100605. [DOI] [PubMed] [Google Scholar]
- 44.Angata T, Varki A. Siglec-7: A sialic acid-binding lectin of the immunoglobulin superfamily. Glycobiology. 2000;10(4):431–438. doi: 10.1093/glycob/10.4.431. [DOI] [PubMed] [Google Scholar]
- 45.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279(41):43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103(20):7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz AG, Cote ML, Wenzlaff AS, Land S, Amos CI. Racial differences in the association between SNPs on 15q25.1, smoking behavior, and risk of non-small cell lung cancer. J Thorac Oncol. 2009;4(10):1195–1201. doi: 10.1097/JTO.0b013e3181b244ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.