Significance
This research provides the earliest direct chemical evidence for the production of alcoholic beverage pulque in Mesoamerica, based on organic residues recovered from pottery vessels from Teotihuacan. A novel bacterial lipid biomarker approach is reported, which provides a new means of documenting the consumption of bacterially fermented alcoholic beverages in antiquity worldwide. At Teotihuacan, we have evidence that pulque was stored in distinctive amphorae vessels sealed with pine resin, as well as in other, less specialized vessels. Direct evidence of pulque production provides new insights into how the nutritional requirements of Teotihuacanos were sustained in a region in which the diet was largely based on plants and crop failures, due to drought and frost damage, which resulted in frequent shortfalls in staples.
Keywords: Mexico, pine resins, hopanes
Abstract
Although in modern societies fermented beverages are associated with socializing, celebration, and ritual, in ancient times they were also importa`nt sources of essential nutrients and potable water. In Mesoamerica, pulque, an alcoholic beverage produced from the fermented sap of several species of maguey plants (Agavaceae; Fig. 1) is hypothesized to have been used as a dietary supplement and risk-buffering food in ancient Teotihuacan (150 B.C. to A.D. 650). Although direct archaeological evidence of pulque production is lacking, organic residue analysis of pottery vessels offers a new avenue of investigation. However, the chemical components of alcoholic beverages are water-soluble, greatly limiting their survival over archaeological timescales compared with hydrophobic lipids widely preserved in food residues. Hence, we apply a novel lipid biomarker approach that considers detection of bacteriohopanoids derived from the ethanol-producing bacterium Zymomonas mobilis for identifying pulque production/consumption in pottery vessels. Gas chromatography–mass spectrometry selected ion monitoring (m/z 191) of lipid extracts of >300 potsherds revealed characteristic bacteriohopanoid distributions in a subset of 14 potsherds. This hopanoid biomarker approach offers a new means of identifying commonly occurring bacterially fermented alcoholic beverages worldwide, including palm wine, beer, cider, perry, and other plant sap- or fruit-derived beverages [Swings J, De Ley J (1977) Bacteriol Rev 41(1):1–46].
Teotihuacan, located in the semiarid highlands of Central Mexico, is one of the largest urban centers of prehistory. Founded around 150 B.C., the city grew very rapidly, in part by absorbing a high proportion of the regional population. The city eventually covered around 20 km2 and may have reached a population level of 100,000 inhabitants. Teotihuacan was the capital of a state that controlled the Basin of Mexico, and probably much of the adjacent parts of the Central Mexican Highlands, and maintained extensive trade relationships that in specific places and times involved military incursion. It survived as an important economic, political, and cultural power in Mesoamerica until roughly A.D. 650 (1, 2).
High altitude, low rainfall, and limited groundwater resources make most of the Teotihuacan Valley a high-risk area for cultivating maize, which was nevertheless a key crop for traditional farmers in the region and was thought to have been important in the diet of residents of ancient Teotihuacan (3–5). Although maize is a food of high caloric value, it contains only low concentrations of several important micronutrients and essential amino acids (e.g., B-complex vitamins, ascorbic acid, iron, calcium, lysine, tryptophan, methionine, and isoleucine) (2, 6). However, a maize-based diet supplemented with beans, which are especially rich in protein, can provide a reasonably balanced diet; although if the components are not consumed in the correct proportions, serious nutritional deficiencies occur (3, 6, 7). Indeed, human osteoarchaeological investigations provide clear evidence of nutritional stress within urban Teotihuacan, especially among lower-status households (8).
At Teotihuacan, mural paintings (depicting maguey plants and scenes of possible pulque consumption) and artifactual remains (amphorae resembling vessels used to contain pulque during the later Aztec period and modern times, and types of stone endscrapers thought to have been used in maguey sap extraction) have led to the suggestion that pulque might also have been important there (9). The maguey (Fig. 1A) withstands frost and drought that seriously affect less-hardy plants such as maize, with its sap (Fig. 1B) providing significant calories at times of shortfall in other crops (10). Because of its high viscosity (Fig. 1C), conferred by dextran-producing bacteria, consuming pulque at such times would have naturally helped satiate sensations of hunger. Pulque would also have helped alleviate more chronic nutritional deficiencies. Apart from the ethanol content of ca. 4.5% and pH of 3–4, pulque contains concentrations of most macro/micronutrients, which, together with lactic acid bacteria, confer probiotic properties (11–13). In addition, the presence of phytase mediates dephosphorylation of plant phytates (myo-inositol hexakisphosphate), an indigestible form of phosphorus occurring in high abundance in grains, thereby increasing the bioavailability of iron and zinc, the most deficient minerals in maize (14). Furthermore, the high concentration of ascorbic acid in pulque enhances the absorption of iron, and possibly zinc, into the digestive system (14).
Fig. 1.
(A) Maguey plant (Agave salmiana), (B) maguey sap (aguamiel) pooled within a maguey in the process of sap extraction, and (C) man transferring pulque to a 2.5-L container for sale in Apan, Mexico.
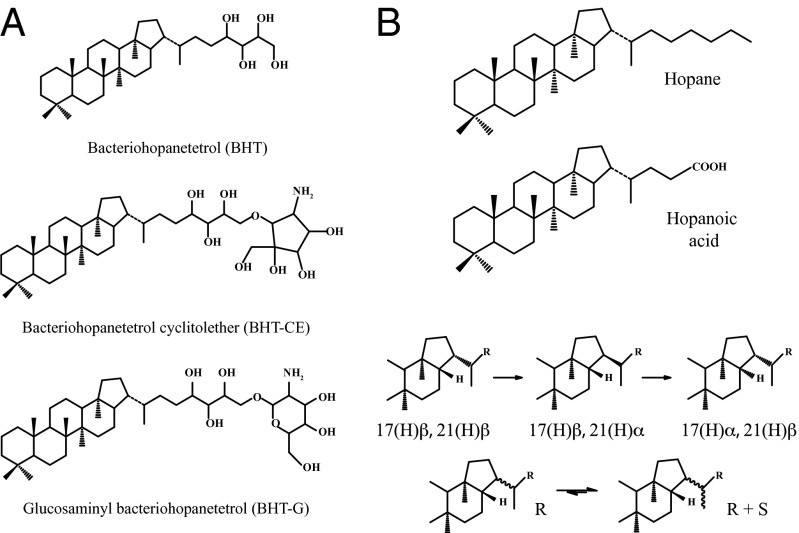
Despite the significant challenges involved in identifying organic residues from alcoholic beverages in the archaeological record (15), the fermentation process in pulque production offers a potentially specific means of detecting this activity. The major bacterium involved in pulque fermentation is Zymomonas mobilis, which, together with yeast, produces alcohol (11, 12, 16). To be able to resist ethanol stress and low pH, Z. mobilis has evolved a membrane containing the highest concentration of hopanoids currently known in bacteria (i.e., ca. 30 mg g−1 dry cell mass), mainly as tetrahydroxybacteriohopane and its ether and glycosidic derivatives (16) (Fig. 2A). Hopanoids are extensively used as biomarkers in studies of extinct and extant bacteria in sediments (17–19); however, the use of hopanoids as biomarkers of alcoholic beverage production in archaeology is untested until now.
Fig. 2.
(A) Major complex hopanoids in ethanol producer bacterium Z. mobilis (16). (B) Possible diagenetic products and transformations of complex hopanoids (17).
Materials and Methods
As part of broader paleodietary and cultural investigations at ancient Teotihuacan, organic residue analyses were performed on a wide range of systematically sampled archaeological potsherds from three localities within the city and immediate periphery: La Ventilla (n = 148), San José 520 (n = 73), and site 15:N1E6 (n = 92). Selected sherds dated to the Tlamimilolpa and Xolalpan phases (ca. A.D. 200–550). Absorbed lipids extractions were performed on 313 potsherds of various ware types and forms: ollas (n = 144), craters (n = 83), bowls (n = 24), and amphorae (n = 62), utilitarian cooking vessels that are more likely to have been used in the processing of food, and that thus have higher concentrations of organic residues. Briefly, ca. 1–3 g were sampled, and surfaces were cleaned with a modeling drill to remove any exogenous lipids. The potsherds were then ground to powder, an internal standard (n-tetratricontane, 2 μg g−1) was added, and acidified methanol solution (5 mL H2SO4/MeOH, 2% vol/vol, 70 °C, 1 h) was added (20). The lipids were then extracted from the aqueous phase with hexane (4 × 3 mL). The solvent was evaporated under a gentle stream of nitrogen to obtain the total lipid extract (TLE). Aliquots of the TLE were derivatized using 20 µL N,O-bis(trimethylsilyl)trifluoroacetamide, at 70 °C, for 1 h and submitted to gas chromatography (GC) and GC–mass spectrometry (GC-MS) analyses.
For the bacteriohopanepolyols aging experiment, ∼20 g of alumina and crushed replica pot were saturated with ca. 30 mL pulque and then incubated at 100 °C for 2 wk. This experiment was performed in duplicate with experimental blanks of alumina and replica pot. After artificial aging, the lipid extraction was carried out as described earlier.
All TLEs were initially screened in a Hewlett-Packard 5890 Series II GC. Helium was used as carrier gas, and a flame ionization detector was used to monitor column effluent. The data were acquired and analyzed with Clarity software. The diluted TLE samples (1 µL) were injected into a fused silica capillary column (50 m × 0.32 mm i.d.) coated with a dimethyl polysiloxane stationary phase (J&W Scientific; CP-Sil 5 CB, 0.1-µm film thickness). The temperature program was as follows: initial temperature was held at 50 °C for 2 min, followed by an increase to 300 °C (10 min) at a rate of 10 °C min−1. Peaks were identified by comparison of retention times with those of an external standard. Quantification was achieved by the internal standards method.
GC-MS analyses were performed using a ThermoFinnigan trace mass spectrometer. The sample (1 µL) was introduced using a programmed temperature vaporizing injector set to splitless mode onto a polydimethylsiloxane column (Phenomenex, ZB-1, 60 m × 0.32 mm i.d., 0-1 µm film thickness). The MS was operated in electron ionization mode at 70 eV with a GC interface temperature of 300 °C and a source temperature of 200 °C. The emission current was 150 µA, and data acquisition between m/z 50–650 at 1.3 scans per second. The temperature program was as follows: initial temperature held at 50 °C for 2 min, followed by an increase to 300 °C (10 min) at a rate of 10 °C min−1. Lipid extracts were analyzed using the MS in total ion current and selected ion monitoring modes acquiring at m/z 105, 191, and 523.6 Daltons at 0.12 seconds per scan to check for the presence of ω-(o-alkylphenyl)alkanoic acids, hopanes, and n-dotriacontanol, respectively. To improve chromatographic peak resolution in resin and hopane-containing extracts and pulque aging, experiment samples were rerun using the following temperature program: initial temperature 50 °C for 2 min, followed by an increase to 270 °C (10 min) at a rate of 10 °C min−1, and then from 270 °C to 300 °C at 6 °C min−1 (10 min). The MS was operated in total ion current and selected ion monitoring modes acquiring at m/z 191 Daltons at 0.12 seconds per scan. The acquisition and analysis of the data were carried out using XCalibur software.
Principal components analysis was used to investigate patterns in the variability of different biomarkers present in the lipid extracts recovered from the archaeological potsherds from Teotihuacan. The input variables are based on the presence and absence of peaks indicative of 75 biomarkers. XLSTAT 2014.3.01 was used for the analysis.
Results and Discussion
GC screening of the total lipid extracts showed that ca. 70% of the potsherds contained detectable lipids, although concentrations were low (mean, 16 µg g−1 for sherds containing lipids; maximum, 53 µg g−1). The low lipid concentrations reflect the paucity of animal products and reliance on plants in the diet. Lipids were observed in 63% of the ollas, 78% of the craters, 85% of the bowls, and 65% of the amphorae.
GC-MS of lipid extracts revealed that these potsherds included mainly fatty acid, n-alkanes, and n-alkanols. The distributions of long-chain fatty acids (C14:0–C34:0), n-alkanes (C23:0–C33:0), and n-alkanols (C16:0–C34:0) confirmed an origin in plant oils or waxes (15) (Fig. 3A). In most extracts of this type, the biomarker of maize (C32 long-chain n-alkanol) was identified (21), which is consistent with the paleobotanical record of the region (3–5).
Fig. 3.
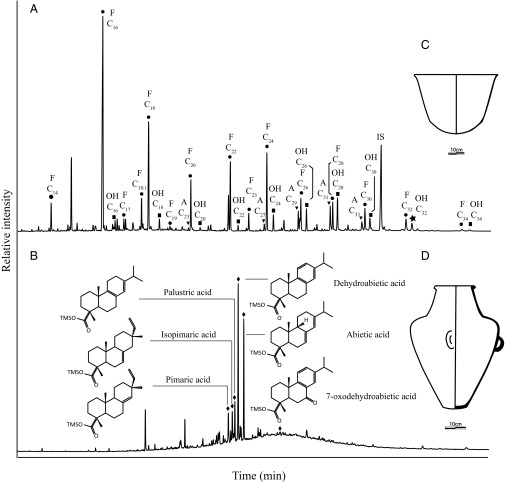
Partial gas chromatograms of total lipid extracts from pottery from the locality of La Ventilla. (A) Crater: characteristic distribution of plant waxes (LV-058). (B) Amphora: abietic acid derivatives (LV-014). Drawings of representative (C) crater and (D) amphora vessels. A, n-alkane; F, fatty acid methyl ester; OH, n-alkanol; OH C32, biomarker of maize (21); IS, internal standard. Lipid extracts were run in different GC temperature programs to improve chromatographic peak resolution.
Significantly, an unusual lipid distribution (Fig. 3B) was observed in 14 potsherds from La Ventilla (a locality containing both high- and low-status residential contexts) (22), 10 of which were from amphorae and suspected for formal reasons to have been used in pulque production and/or transportation (Table 1). The main components of these potsherds displayed base peaks and molecular ions (M+.) at m/z 73, 374 (C23H38O2Si); 241, 374 (C23H38O2Si); 241, 374 (C23H38O2Si); 239, 372 (C23H36O2Si); 256, 374 (C23H38O2Si); and 253, 386 (C23H34O3Si), respectively. Comparisons with reference mass spectra indicated they were diterpenoid compounds, the major constituents and transformation products of coniferous resins (23): pimaric, isopiramric, palustric, dehydroabietic, abietic, and 7-oxodehyrdoabietic acids [as trimethylsilyl (TMS) derivatives; Fig. 3B]. Archaeological and ethnographic examples of postfiring treatment of pottery vessels with tree resins have been associated with waterproofing unglazed ceramic vessels to improve storage of liquids (24). The finding of coniferous resin only in these potsherds (despite intensive searching of the extracts in the entire assemblage) pointed to a specialized function. The low abundance of aromatic diterpenoid components suggests the resin was applied in a relatively fresh form, rather than being a pyrolytic product. The latter also rules out an origin for the diterpenoids in wood smoke condensate from heating over a fire (23, 25, 26).
Table 1.
Subset of potsherds shown by GC-MS and GC-MS selected ion monitoring to contain diterpenoids diagnostic of pine resin and bacteriohopane proxies of Z. mobilis
| Sample | Phase | Ware | Form | Vessel part | Provenience | Lipid concentration, μg g−1 |
| LV-001 | Tlamimilolpa | Granular | Amphora | Neck | Frente 5 | 21 |
| LV-002 | Tlamimilolpa | Granular | Amphora | Neck | Frente 5 | 17 |
| LV-003 | Tlamimilolpa | Granular | Amphora | Body | Frente 5 | 12 |
| LV-009 | Tlamimilolpa | Granular | Amphora | Body | Frente 5 | 9 |
| LV-010 | Tlamimilolpa | Granular | Amphora | Body | Frente 5 | 6 |
| LV-011 | Tlamimilolpa | Granular | Amphora | Body | Frente 5 | 17 |
| LV-012 | Tlamimilolpa | Granular | Amphora | Body | Frente 5 | 11 |
| LV-013 | Tlamimilolpa | Burnished | Olla | Body | Frente 5 | 14 |
| LV-014 | Tlamimilolpa | Granular | Amphora | Neck | Frente 5 | 22 |
| LV-020 | Tlamimilolpa | Burnished | Olla | Body | Frente 5 | 30 |
| LV-082 | Xolalpan | SMO* | Amphora | Body | Frente 5 | 10 |
| LV-100 | Xolalpan | SMO | Amphora | Body/base | Frente 5 | 14 |
| LV-138 | Tlamimilolpa | Burnished | Olla | Neck/body | Southern Area, PGC† | 7 |
| LV-150 | Tlamimilolpa | Burnished | Olla | — | Southern Area, PGC | 17 |
All 14 cases come from La Ventilla excavation materials.
San Martín Orange.
Patio of the Glyphs Compound.
Further inspection of the full-scan GC-MS data from the lipid extracts of potsherds containing the coniferous resin biomarker derivatives revealed a further series of compounds characterized by a base peak at m/z 191 and molecular ions (M+.): 370 (C27H46), 398 (C29H50), 412 (C30H52), 426 (C31H54), 440 (C32H56), 454 (C33H58), and 468 (C34H60) corresponding to hopanes 17(H)α,21(H)β C27, C29, C30, C31(S,R), C32(S,R), C33(S,R), and C34(S,R), respectively (17) (Fig. 4A). Remarkably, these hopanes were only detectable in potsherds from resin-containing vessels, despite extensive searches, including using GC-MS-selected ion monitoring (m/z 191), which increases detection limits by several orders of magnitude. The absence of these characteristic hopanoids biomarkers from the vast majority of the assemblage, and their co-occurrence specifically in the resin-treated potsherds, excludes exogenous, pre- or postexcavation, contamination as a possible source.
Fig. 4.
Biomarker distributions observed in potsherds from Teotihuacan, projected as scores on the first two components from principal components analysis.
Principal components analysis was carried out to characterize major patterning in the occurrence of biomarkers within the entire pottery assemblage. Fig. 4 shows the projection of the data onto the first two principal components, which capture just over 40% of the variance in the original data. Two distinct sets of scores on principal component 2 (PC2) divide the pottery into well-separated groups. Group 4 (in the upper part of the plot) is composed entirely of the 14 sherds containing abietic acid derivatives and hopanes. The rest of the samples show roughly the same range of scores on PC2 but can be subdivided into three less salient groups by scores on PC1. Group 1 (right) corresponds to potsherds showing the most complex lipid distributions, dominated by fatty acids, n-alkanols, n-alkanes, hydroxyacids, campestanol, and/or sitostanol. Group 2 (middle) is formed by samples showing fatty acids, n-alkanols, hydroxyacids, plant sterols, plant triterpenoids, and/or ω-(o-alkylphenyl)alkanoic acids; the lipid distributions of these potsherds are similar to, but simpler than, those in Group 1. Finally, Group 3 (left) corresponds to pottery samples with no appreciable lipids. In summary, PC1 is a dimension that reflects variation in the complexity of lipid composition profiles, whereas PC2 simply isolates cases associated with residues of tree resin and fermentation-derived hopanes from all others.
The detection of the hopanoid biomarker proxies of Z. mobilis in vessels likely used to contain pulque is consistent with the complex biohopanoids it produces, having been transformed during vessel use and burial via microbial action, or possibly abiologically, via reactions catalyzed by clay minerals and possibly involving heating in the past (27). The principal transformations of the complex biohopanoids include defunctionalization and isomerization (17, 18, 27). Such reactions give rise to C32 hopanoic acid or the parent hydrocarbons, hopanes (up to C35; Fig. 2B) (17, 27). In early diagenesis, the biological conformation 17(H)β,21(H)β generally remains, subsequently isomerizing to the thermodynamically more stable 17(H)α,21(H)β configuration; changes in stereochemistry at C-22(R) can also occur, producing a mixture of C-22(R) and 22(S) isomers (17, 18, 27) (Fig. 2B). In general, these processes are thought to occur over extended periods; however, laboratory experiments on steroids and studies involving recent sediments suggest the latter transformations can occur more rapidly, being microbially mediated or acid-catalyzed (18, 19, 27).
Although the finding of hopanes is entirely consistent with those from sedimentary materials, we performed an artificial diagenetic (aging) experiment by saturating alumina and replica pottery fabric with pulque and incubating at 100 °C for 2 wk. The aim was to assess whether the metals present in the ceramic fabric (e.g., aluminum) were able to mediate the proposed transformations, specifically the dehydration of the bacteriohopanpolyols, a commonly used strategy in organic synthesis (28). After artificial aging, the hydrocarbon fraction was extracted and assessed by GC-MS. The distribution obtained showed a remarkable congruence with the hopane distributions observed in the archaeological potsherds (Fig. 5 A–D), confirming the ceramic matrix is able to catalyze the diagenetic transformations of bacteriohopanoids during burial. As we have seen with other lipid transformations, heating of pulque vessels during their use could conceivably contribute to the formation of the hopanes observed in the archaeological pottery. The Franciscan friar Sahagún records boiling as part of pulque production during the Aztec period (29), and various early colonial accounts mention a kind of pulque made by fermenting maguey syrup, a concentrate made by boiling ordinary maguey sap (30). Depending on when it was carried out, however, boiling would seem to hinder normal fermentation and is not part of the process recorded in major ethnographies describing pulque making in modern Central Mexico (31, 32).
Fig. 5.

GC-MS selected ion monitoring (m/z 191) showing hopane distributions (C29–C34) in (A) amphora from La Ventilla (LV-100), (B) replica pot and (C) alumina saturated with pulque after incubation, and (D) alumina (blank). Chromatographic peak denoted by a filled star corresponds to diploptene, another bacteriohopanoid present in pulque; however, it is highly susceptible to degradation and is thus absent in the archaeological amphorae.
These findings provide compelling evidence for the use of ceramic vessels to contain pulque in the locality of La Ventilla around A.D. 200–550, at the height of Teotihuacan’s growth and power (1). The vessel forms from which as “hopane-bearing” potsherds derived, that is, amphorae and ollas, would have been ideal for processing/storing liquids, with the presence of pine resin being entirely consistent with postfiring waterproofing. Given the presence of hopanes, bacterial markers of pulque, exclusively in this group of vessels, there is little doubt that pulque was produced at Teotihuacan. Only a more comprehensive survey of absorbed organic residues of pottery samples, coupled with systematic analysis of the spatial and temporal distribution of relevant artifact classes (particularly specialized ceramic vessels such as amphorae and stone tools used in sap extraction) will ultimately establish the level and intensity of pulque production within the ancient city. Notwithstanding this, our results are a critical first step, providing the first direct chemical evidence to the authors’ knowledge for the production of an alcoholic beverage in Prehispanic Mesoamerica and a foundation for fresh insights into how the nutritional needs of a major prehistoric city were met in an environmental zone in which critical shortfalls in other food crops were probably all too common.
Supplementary Material
Acknowledgments
We thank Erika Carrillo Ruíz (Zona de Monumentos Arqueológicos de Teotihuacan, Instituto Nacional de Antropología e Historia (INAH) for her assistance in preparing the pottery samples and Drs. Helen Talbot and Julianne Bischoff (University of Newcastle) for performing the bacteriohopanepolyol analysis of pulque. We thank the Mexican Consejo Nacional de Ciencia y Tecnología for the PhD studentship (to M.C.-A.); the UK Natural Environment Research Council for the mass spectrometry facilities at the University of Bristol; and the Consejo de Arqueología of the Instituto Nacional de Antropología e Historia of Mexico for granting permits for excavation at La Ventilla, San José 520, and 15:N1E6, and for the export of samples to the United Kingdom. Funding for archaeological projects at these localities was provided by INAH, the Foundation for the Advancement of Mesoamerican Studies, the Wenner-Gren Foundation, Arizona State University, and Stanford University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.E.S. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408339111/-/DCSupplemental.
References
- 1.Cowgill GL. Teotihuacan as an Urban Place. In: Mastache AG, Cobean RH, García Cook A, Hirth KG, editors. El Urbanismo en Mesoamérica (Urbanism in Mesoamerica) Vol 2. University Park, PA: Pennsylvania State University; 2008. pp. 85–112. [Google Scholar]
- 2.Sanders WT, Parsons JR, Santley RS. The Basin of Mexico. Ecological Processes in the Evolution of a Civilization. 1st Ed. New York: Academic Press; 1979. pp. 233–236. [Google Scholar]
- 3.Santley RS, Rose EK. Diet, nutrition and population dynamics in the Basin of Mexico. World Archaeol. 1979;11(2):185–207. doi: 10.1080/00438243.1979.9979760. [DOI] [PubMed] [Google Scholar]
- 4.McClung de Tapia E. 1987. Patrones de subsistencia urbana en Teotihuacan. Teotihuacan: Nuevos Datos, Nuevas Síntesis, Nuevos Problemas, eds McClung de Tapia E, Rattray EC (Instituto de Investigaciones Antropológicas, Universidad Nacional Autónoma de México, Mexico D.F., Mexico), pp 57–74.
- 5.McClung de Tapia E, Martínez Yrñizar D, Ibarra Morales E, Adriano Morán C. Los Orígenes Prehispánicos de una Tradición Alimentaria en la Cuenca de México. Ann Antropol. 2014;48(1):97–121. [Google Scholar]
- 6. FAO (1992) Maize in Human Nutrition. FAO Food and Nutrition Series, No. 25 (Food and Agriculture Organization of the United Nations). Available at: www.fao.org/docrep/t0395e/t0395e00.HTM. Accessed August 1, 2014.
- 7.Sotelo A, Sousa H, Sánchez M. Comparative study of the chemical composition of wild and cultivated beans (Phaseolus vulgaris) Plant Foods Hum Nutr. 1995;47(2):93–100. doi: 10.1007/BF01089257. [DOI] [PubMed] [Google Scholar]
- 8.Storey R, Márquez-Morfín L, Núñez LF. Teotihuacan Neighborhoods and the Health of Residents: The Risks of Preindustrial Urban Living. In: Arnauld MC, Manzanilla L, Smith ME, editors. The Neighborhood as a Social and Spatial Unit in Mesoamerican Cities. Tucson, AZ: Univ of Arizona Press; 2012. pp. 117–131. [Google Scholar]
- 9.Sheehy JJ. 2001. Aguamiel and pulque: Modeling perishable goods production in classic Teotihuacan. Fleeting Identities: Perishable Material Culture in Archaeological Research, ed Drooker PB (Center for Archaeological Investigations, Southern Illinois University at Carbondale, Carbondale, IL), Occasional Paper Vol 28, pp 254–272.
- 10.Evans ST. The Productivity of Maguey Terrace Agriculture in Central Mexico during the Aztec Period. Lat Am Antiq. 1990;1(2):117–132. [Google Scholar]
- 11.Escalante A, et al. Characterization of bacterial diversity in pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol Lett. 2004;235(2):273–279. doi: 10.1016/j.femsle.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 12.Escalante A, et al. Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int J Food Microbiol. 2008;124(2):126–134. doi: 10.1016/j.ijfoodmicro.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Lappe-Oliveras P, et al. Yeasts associated with the production of Mexican alcoholic nondistilled and distilled Agave beverages. FEMS Yeast Res. 2008;8(7):1037–1052. doi: 10.1111/j.1567-1364.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- 14.Tovar LR, Olivos M, Gutierrez ME. Pulque, an alcoholic drink from rural Mexico, contains phytase. Its in vitro effects on corn tortilla. Plant Foods Hum Nutr. 2008;63(4):189–194. doi: 10.1007/s11130-008-0089-5. [DOI] [PubMed] [Google Scholar]
- 15.Evershed RP. Organic residue analysis in archaeology: The archaeological biomarker revolution. Archaeometry. 2008;50(6):895–924. [Google Scholar]
- 16.Hermans MA, Neuss B, Sahm H. Content and composition of hopanoids in Zymomonas mobilis under various growth conditions. J Bacteriol. 1991;173(17):5592–5595. doi: 10.1128/jb.173.17.5592-5595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ourisson G, Albrecht P. Hopanoids. 1. Geohopanoids: The most abundant natural products on Earth? Acc Chem Res. 1992;25(9):398–402. [Google Scholar]
- 18.Quirk MM, Wardroper AMK, Wheatley RE, Maxwell JR. Extended hopanoids in peat environments. Chem Geol. 1984;42(1-4):25–43. doi: 10.1016/0009-2541(84)90003-2. [DOI] [PubMed] [Google Scholar]
- 19.Rohmer M, Dastillung M, Ourisson G. Hopanoids from C30 to C35 in recent muds. Naturwissenschaften. 1980;67(9):456–458. [Google Scholar]
- 20.Correa-Ascencio M, Evershed RP. High throughput screening of organic residues in archaeological potsherds using direct acidified methanol extraction. Analytical Methods. 2014;6:1330–1340. [Google Scholar]
- 21.Reber EA, Dudd DN, van der Merwe NJ, Evershed RP. Direct detection of maize in pottery residues via compound specific stable carbon isotope analysis. Antiquity. 2003;78(301):682–691. [Google Scholar]
- 22.Cabrera-Castro R, Gómez-Chávez S. La Ventilla: A Model for a Barrio in the Urban Structure of Teotihuacan. In: Mastache AG, Cobean RH, García Cook A, Hirth KG, editors. El Urbanismo en Mesoamérica (Urbanism in Mesoamerica) Vol 2. University Park, PA: Pennsylvania State University; 2008. pp. 37–83. [Google Scholar]
- 23.Evershed RP, Jerman K, Eglinton G. Pine wood origin for pitch from the Mary Rose. Nature. 1985;314(6011):528–530. [Google Scholar]
- 24.Stern B, Heron C, Tellefsen T, Serpico M. New investigations into the Uluburun resin cargo. J Archaeol Sci. 2008;35(8):2188–2203. [Google Scholar]
- 25.Regert M. Investigating the history of prehistoric glues by gas chromatography-mass spectrometry. J Sep Sci. 2004;27(3):244–254. doi: 10.1002/jssc.200301608. [DOI] [PubMed] [Google Scholar]
- 26.Scalarone D, Lazzari M, Chiantore O. Ageing behaviour and pyrolytic characterisation of diterpenic resins used as art materials: Colophony and Venice turpentine. J Anal Appl Pyrolysis. 2002;64(2):345–361. [Google Scholar]
- 27.Brassell SC, et al. Molecular Changes in Sediment Lipids as Indicators of Systematic Early Diagenesis. Philos Trans R Soc Lond A. 1985;315(1531):57–75. [Google Scholar]
- 28.Pinnes H, Manassen J. The Mechanims of Dehydration of Alcohols over Alumina Catalysts. In: Eley D, Pines H, Weiz PB, editors. Advances in catalysis. Vol 16. New York: Academic Press; 1966. pp. 49–90. [Google Scholar]
- 29. de Sahagún B (1961) The People, Florentine Codex: General History of the Things of New Spain, trans Anderson AJO and Dibble CE (The School of American Research, Santa Fe, NM; University of Utah Press, Salt Lake City), Vol 10, p 74.
- 30.Bruman HJ. Alcohol in Ancient Mexico. Salt Lake City: University of Utah Press; 2000. p. 76. [Google Scholar]
- 31.Parsons JR, Parsons MH. 1990. Maguey Utilization in Highland Central Mexico. An Archaeological Ethnography. (Museum of Anthropology, University of Michigan, Ann Arbor), pp 279–284.
- 32.Fournier García P. Los Hñähñü del Valle del Mezquital: Maguey, Pulque y Alfarería. Mexico D.F., Mexico: Instituto Nacional de Antropología e Historia, Escuela Nacional de Antropología e Historia; 2007. [Google Scholar]