Significance
Not all possible biological shapes are actually seen in nature. Despite much experimental work, the developmental basis explaining the presence or absence of certain biological shapes remains poorly understood. We studied Drosophila melanogaster development using the sex comb, a group of modified bristles exhibiting spectacular morphological diversity among Drosophila species. We provide several lines of evidence suggesting that increasing D. melanogaster sex comb length produces a mechanical blockage, affecting comb shape and position. We infer that simple physical principles acting on tissues can influence the direction of evolution, and comparative studies of other fly species are consistent with this hypothesis. This work highlights the fundamental role of development for understanding biodiversity and evolution.
Abstract
In spite of the diversity of possible biological forms observed in nature, a limited range of morphospace is frequently occupied for a given trait. Several mechanisms have been proposed to explain this bias in the distribution of phenotypes including selection, drift, and developmental constraints. Despite extensive work on phenotypic bias, the underlying developmental mechanisms explaining why particular regions of morphological space remain unoccupied are poorly understood. To address this issue, we studied the sex comb, a group of modified bristles used in courtship that shows marked morphological diversity among Drosophila species. In many Drosophila species including Drosophila melanogaster, the sex comb rotates 90° to a vertical position during development. Here we analyze the effect of changing D. melanogaster sex comb length on the process of rotation. We find that artificial selection changes the number of bristles per comb without a proportional change in the space available for rotation. As a result, when increasing sex comb length, rather than displaying a similar straight vertical shape observed in other Drosophila species, long sex combs bend because rotation is blocked by a neighboring row of bristles. Our results show ways in which morphologies that would be favored by natural selection are apparently impossible to achieve developmentally. These findings highlight the potential role of development in modifying selectable variation in the evolution of Drosophila sex comb length.
Despite the immense diversity of plausible biological forms, only a restricted range of morphospace is occupied in nature (1–4). This bias in the distribution of phenotypes is explained by the interplay between different mechanisms including selection, drift, and developmental constraints (2–4). An extreme position suggests that natural selection is the key player responsible for the distribution of observed phenotypes. An alternative view holds that nonadaptive factors, such as developmental constraints, may account for the empty regions of morphospace (2–4). A developmental constraint can be defined as a bias on phenotypic variability, which can be caused by “the structure, character, composition, or dynamics of the developmental system” (5). A suggested example of a developmental constraint is the absence of even numbered segments in centipedes of the order Geophilomorpha (6, 7).
In the last decade, this debate has experienced a transition from theoretical and philosophical considerations to empirical approaches (8, 9). In particular, artificial selection experiments have demonstrated that many morphological traits respond to selection (10, 11). For example, artificial selection in the butterfly, Bicyclus anynana, have shown that the size of eyespots and wings displays a rapid and pronounced response (10, 11). In contrast, a developmental constraint has been inferred when selection fails to uncouple traits as in the failure to robustly uncouple changes in the diameter of the black and gold eyespot rings in B. anynana (8, 12). Although comparative studies have proposed a compelling list of potential developmental constraints, there is little experimental evidence demonstrating mechanisms underlying their existence (5, 6, 9). Furthermore, while there may have been a tendency in the past to consider a dichotomy between selection and constraint, the adaptation process is currently thought to emerge from the interplay of both (3, 7, 13–15), and attention is shifting to identifying mechanisms underlying putative constraints (2, 3, 15, 16).
To explore how changes in developmental processes produce different arrays of phenotypes that are subjected to natural selection (2, 3), three main approaches are desirable: detailed descriptive embryology, experimental manipulation, and comparative morphological studies within and between species (2, 13). Here we study the male sex comb, a secondary sexual trait in the model system Drosophila melanogaster, which provides the unusual opportunity for the combined use of these three approaches.
The sex comb is a male-specific group of bristles located on the foreleg of many species in the family Drosophilidae (17–19). Sex combs are used in stereotypical behaviors during courtship (17, 20), and removing the sex comb results in a reduction in the frequency of successful mating in a number of species (17, 20). In addition to the sex comb, the first tarsal segment has other bristle rows including the transverse rows (TR; Fig. 1). TRs are thought to be used as eye-cleaning utensils and are highly conserved across Drosophila species (17, 21). In many species, including D. melanogaster, the sex comb rotates from a transverse to a vertical position, while the TRs remain transverse during development. Given the proximity of the sex comb and the distal TR, there is limited space for the rotation to occur (Fig. 1), and often, as the rotating sex comb approaches the distal TR, this TR is bent upward as if it has been moved by the comb. In this study, we provide different types of evidence suggesting that this spatial limitation may have played an important role in determining sex comb length in evolution.
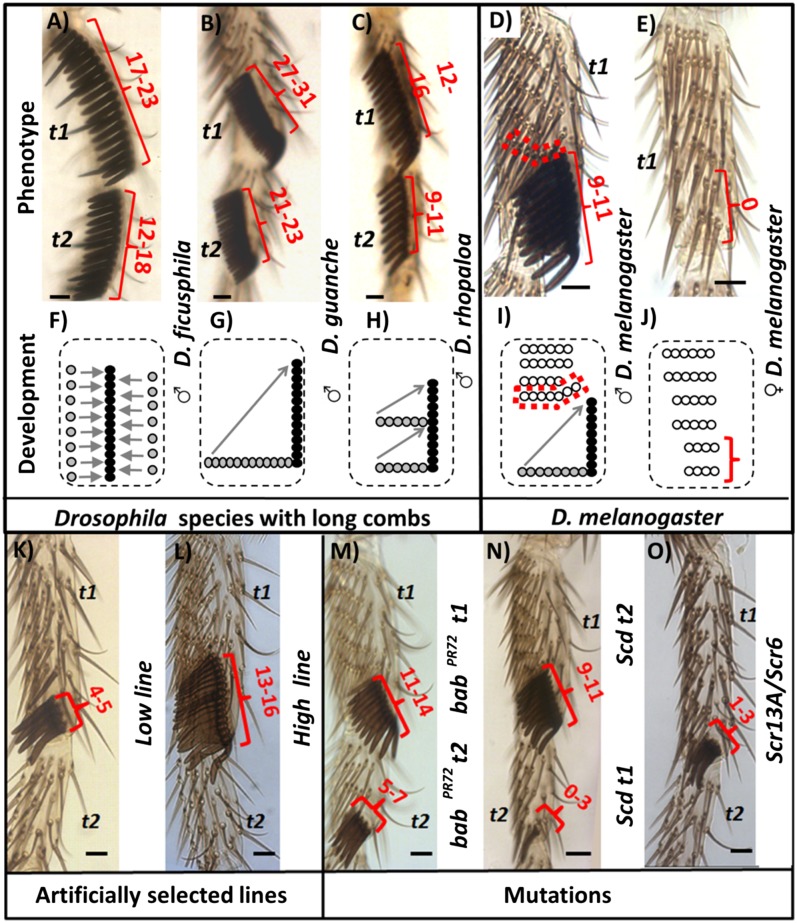
Fig. 1.
Variation in sex comb tooth number and development. (A–C) Examples of Drosophilidae forelegs with long combs. (D and E) D. melanogaster forelegs. (F–J) Schematics of foreleg development of the top Drosophila legs. (K–O) Examples of D. melanogaster perturbations in sex comb tooth number. (A–C and F–H) Drosophila species with long sex combs achieve vertical orientation by different mechanisms: (i) Teeth initially form in a vertical orientation (F) (e.g., D. ficusphila t1–t2); (ii) rotation of a long row (G) (e.g., D. guanche t1–t2 and D. rhopaloa t1); (iii) rotation of multiple small rows and posterior fusion into a long sex comb (H) (e.g., D. rhopaloa t2). (D, E, I, and J) In D. melanogaster, the male sex comb rotates from a horizontal to a vertical position (diagrammed in D), while TRs remain horizontal. The only exception is the most distal transverse row (red dotted box in D and I), which bends proximally close to the top part of the sex comb. In contrast, the female rows of bristles homologous to the sex comb remain static during development (brackets in E and J). In order to study the phenotypic and developmental effect of changing the number of sex comb teeth, this trait was perturbed using artificial selection (K and L), mutants (M–O), and UAS-Scr RNAi transgenic lines (Materials and Methods). Gray circles represent sex comb teeth in the initial position and black circles represent sex comb teeth in the final position. Empty circles represent the TR bristles. Gray arrows indicate movement of individual tooth or sex comb rows. Red brackets indicate sex combs or homologous female bristles. Red numbers represent the range of sex comb teeth in each line. babPR72, bric à bracPR72; scd, sex combs distal; Scr, sex combs reduced; t1 and t2, first and second tarsal segment, respectively. Distal is down and posterior is to the right. Scale bar: 20 µm.
As with many secondary sexual traits, over a relatively short timescale, sex combs have evolved great pattern diversity between species including different numbers of bristles per comb and various degrees of bristle pigmentation (17, 18, 22, 23). In particular, sex comb length displays a high level of variation across Drosophila species, and has increased and decreased repeatedly during evolution (17, 18, 22, 23) (Supporting Information, Fig. S1) (Any mention in this paper regarding sex comb length strictly refers to bristle number per comb). Similar to the butterfly, B. anynana (10, 11), artificial selection experiments have demonstrated segregating variation for modifying sex comb tooth number in D. melanogaster (24). After short-term selection, ∼20 generations, it is possible to increase the number of sex comb teeth from 9 to 11 teeth up to 18 teeth (24).
This finding might suggest that strong selection on sex comb tooth number may be sufficient to explain the changes in sex comb length during evolution. However, developmental studies in other Drosophila species show that the evolution of long sex combs can be more complicated than expected. Long vertical sex combs have evolved multiple times in evolution using different developmental mechanisms (refs. 22, 25; Fig. 1 A–C). Why do long sex combs display such dramatic developmental modifications if selection is able to rapidly change sex comb length in a few generations?
Here we show that selection for increasing sex comb length intensifies the space conflict between the distal TR and the sex comb, and these different developmental mechanisms provide the opportunity to increase space for rotation in novel ways. To do so, we changed sex comb length in D. melanogaster using both artificial selection and mutational perturbations. We found that despite the variation in bristle number, elongated sex combs result in atypical shapes and incomplete rotation indicating that they do not have enough physical space for rotation during development. Analyzing representative Drosophila species, we show similar atypical phenotypes in long rotating sex combs as well as multiple morphological changes, which allow the formation of relatively straight long sex combs. This study shows the important role of developmental mechanisms in achieving a presumably adaptive phenotype, a vertical sex comb.
Results
Atypical Sex Comb and Distal Transverse Row Shapes Associated with Increase in Sex Comb Bristle Number.
In D. melanogaster, the presumptive sex comb bristles rotate from a horizontal to a vertical position early in pupal development (Fig. 1 D, I). To test whether changing tooth number has an effect on the shape of the sex comb, we used mutants that perturb the development of the first and second tarsal segments (t1 and t2), artificially selected lines, and transgenic tools to alter gene expression in the sex comb region (lines studied and their respective abbreviations are summarized in Fig. 1 K–O). The analysis of light microscopy images of adult legs showed that although sex combs with small to moderate numbers of teeth are straight, possessing a higher number of sex comb teeth than wild type results in sex combs that are frequently bent or separated into two distinct sex combs (Fig. 2 A and B).
Fig. 2.
Atypical shapes in long D. melanogaster sex combs and distal transverse rows. (A and B) Photographs of sex combs of different tooth number. (A) Sex comb tooth number equal to or lower than wild type. (B) Sex comb tooth number greater than wild type. (C) Frequency of the most common atypical bent phenotype, cane-shaped structure (white asterisk in B) in the genotypes scd t1, babPR72 t1, and High line. (D) Shape of distal TRs (indicated by dashed lines). (A) Wild type and lines with a low number of sex comb teeth display a straight shape (blue dotted lines). (B) In contrast, lines with long sex combs display multiple atypical phenotypes in their sex comb shape (red dotted line). Similarly, the distal TR of long sex combs displays an atypical upward bending (D). (N = 20 in each line). Scale bar = 20 µm.
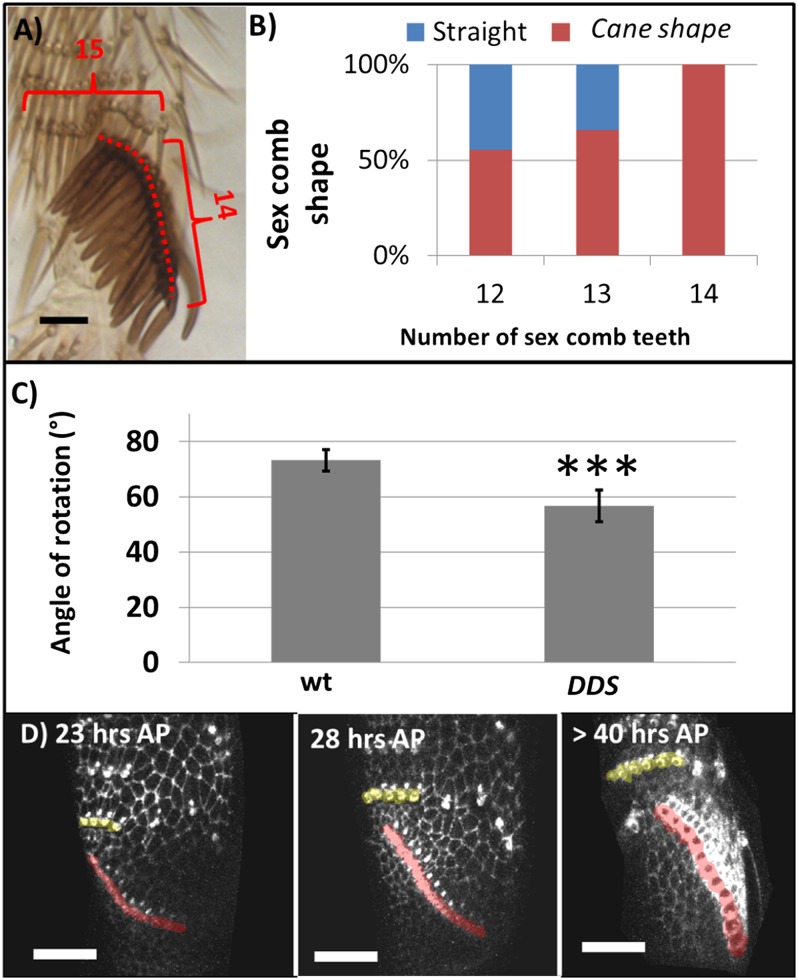
In order to confirm whether there is a relationship between length and shape of the sex combs, we quantified how frequently the top (proximal) part of the sex comb bends and forms a cane-shaped structure in the adult (Fig. 2B). When the angle between three adjacent teeth was higher than 30°, the sex combs were considered to have a cane-shaped structure (Fig. S2 C–E). This quantification confirmed that small sex combs (<6 teeth in Low line, scd t2, babPR72 t2; Scr6/Scr13A, rnGAL4; UAS Scr RNAi at 25°; N = 20 per line) and wild-type sex combs (N = 20) are straight (99.5%; Fig. 2A). In contrast, this cane shape was always observed in the line scd t1, babPR72 t1, and High line (Fig. 2 B and C). In the three lines, increasing the number of sex comb teeth also increases the frequency of this cane shape, but the relationship between these two parameters varies between strains. For example, in the High line more sex comb teeth are necessary to cause a cane shape than in scd t1 (Fig. 2C). This variability in frequency suggests that in addition to sex comb length, other factors may result in this cane-shaped structure.
A good candidate to limit the shape of the sex comb resulting in the cane shape is the closest neighboring row of bristles to the sex comb, the most distal TR (Fig. 1). In adult flies, depending on the sex comb length, this row of bristles may bend proximally. The longer the sex comb, the higher the degree of bending (Fig. 2 C and D and Fig. S3 A and B). We hypothesized that the space available for long sex combs to rotate is not enough, and as a result, the distal TR is displaced upward. In addition, this spatial limitation could also explain the atypical phenotypes observed in sex comb shapes. In order to test this hypothesis, we studied the development of the rotation process in D. melanogaster lines with different sex comb lengths.
Developmental Basis of Rotation in D. Melanogaster Lines with Different Sex Comb Lengths.
To test whether long sex combs have enough space to rotate, we measured the distance between the developing sex comb and the distal TR before rotation takes place. We studied the following lines: the male wild type, the mutants babPR72 t1, and scd t1, as well as the lineages artificially selected for high and low numbers of sex comb teeth. By introgressing the ubiDEcadherin::GFP into the lines, we were able to use fluorescence to label cell boundaries between bristles and epithelium cells during the rotation and measure the distance between these two bristle rows at 12–16 h after pupariation (AP). Two-dimensional projections of confocal stacks revealed that the distance between the developing sex comb and distal TR is not significantly different between the different lines studied [Fig. 3A; Tukey’s honestly significantly different (HSD), F6,60 = 0.66, P = 0.65]. As a result, although the sex comb can easily change its number of bristles per row, the proximal space to rotate remains the same, independent of sex comb length.
Fig. 3.
Developmental basis of rotation obstruction in long D. melanogaster sex combs. (A) Average distance between the sex comb and distal TR before rotation in six genotypes (N = 10 per strain). (B) The 2D projections of different stages of the rotation process in D. melanogaster lines with different sex comb length (developing sex comb cells shaded in red, distal TR cells shaded in yellow). DDS, double directional selected line for high number of bristles in the sex comb and distal TR; AP, after pupariation. Error bars correspond to standard deviation. Scale bar = 20 µm.
To examine if the space between the presumptive sex comb and distal TR constitutes a limitation for sex comb length, we examined the rotation process in pupal D. melanogaster sex combs with different lengths. In favor of the obstruction hypothesis we found that when sex comb or TR length increases, the distal TR is initially straight, but as the sex comb rotation proceeds, the TR bristles closest to the sex combs are pushed proximally to different degrees (Fig. 3B and Movies S1–S2). When the rotation is complete, developing sex comb shape is straight only in wild type and lines with lower sex comb lengths. However, as shown in Fig. 2, the frequency of bent, cane-shaped sex combs correlates with an increase in the number of sex comb teeth (Fig. 3B). In the next section, we show that the length of the distal TR also displays atypical phenotypes when sex comb length is increased, and that the interactions between sex comb and distal TR length are important for understanding the atypical sex comb shapes.
Relationship Between Numbers of Bristles in Sex Combs and Distal Transverse Rows.
As the sex comb is a modified TR (17), it has been suggested that the same underlying mechanism is responsible for regulating the number of bristles in the sex comb and each of the TRs (21, 26). For example, reducing the expression of the gene Scr results in lower numbers of bristles per row in both the sex comb and TR (26). We test whether this potential correlation is a secondary effect of the genetic perturbations, or a property of the leg bristle pattern.
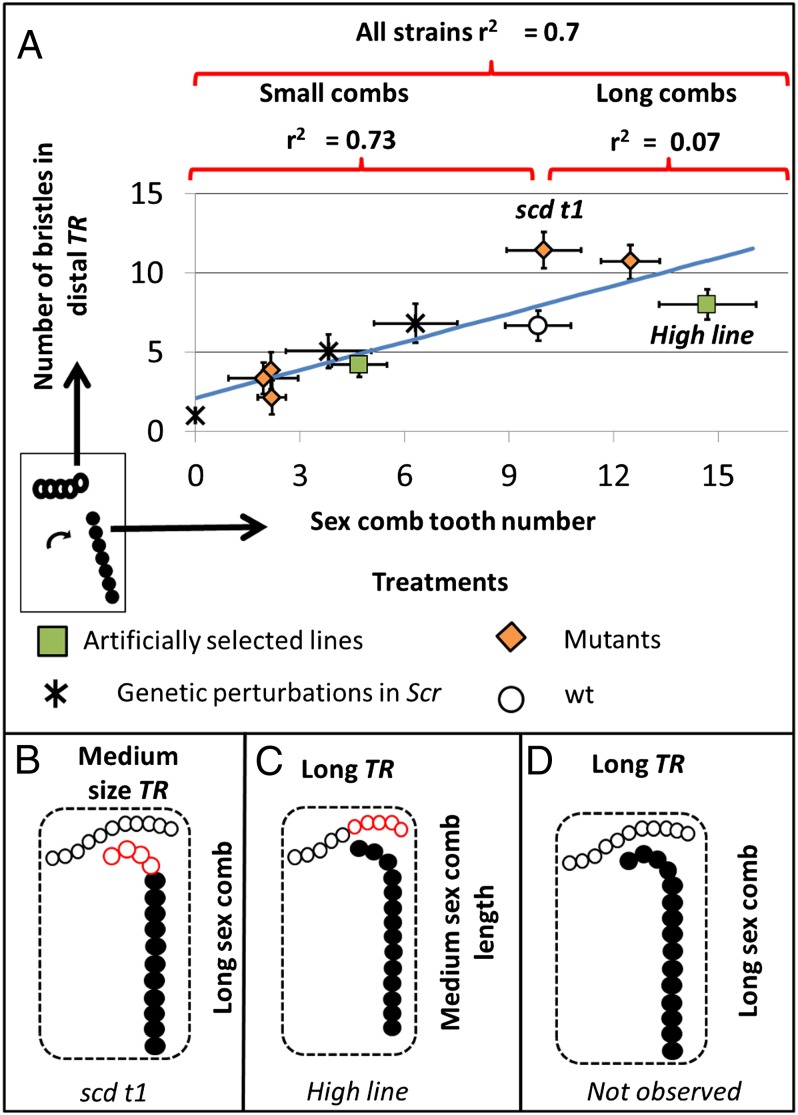
Light microscopy images of adult legs demonstrated that regardless of the method used to modify sex comb length, there was a positive correlation between the number of sex comb teeth and the number of bristles in the most distal TR (artificially selected lines and wild type, r = 0.846, P < 0 .001, N = 60; mutants, r = 0.777, P < 0.001, N = 100; UAS-Scr RNAi, r = 0.8668, P < .001, N = 60; three treatments together, r = 0.70, P < 0.001, N = 220). Similarly, when analyzing the data based on sex comb length rather than the type of perturbation, the positive correlation is still seen for small sex combs (r = 0.73, P < .001, N = 140) (Fig. 4). However, when the number of sex comb teeth is equal to or greater than that found in wild type, a much lower correlation was found (r = 0.07, P < 0.001, N = 80); (Fig. 4). Interestingly, we never observed a leg in which a long sex comb is accompanied by a long TR. In the High line, although sex combs are long, the number of bristles in the distal TR is similar to wild type, suggesting that selection had at least partially decoupled bristle numbers in the two rows. In contrast, in the mutant scd t1, the most distal TR is longer than it is in wild type, but the sex comb displays a wild type number of bristles implying that mutations can also decouple the relationship between sex comb and TR length (Fig. 4).
Fig. 4.
Failure to find D. melanogaster legs with a simultaneous increase in the sex comb and distal transverse row lengths. (A) Variation in correlation between numbers of bristles in the distal TR and sex comb tooth number. (B–D) Schematics of legs with different sex comb and distal TR length. (A) Correlation coefficient between sex comb and distal TR length is reduced by a factor of 10 with increasing sex comb tooth number. (B and C) High line has a shorter distal TR than expected (red circles) for its average sex comb tooth number and the Scd t1 has a longer one. The combination of long sex combs and transverse rows (D) has not yet been seen. Brackets indicate strains taken into account in calculating the correlation coefficient above it. (N = 20 per strain). Error bars correspond to standard deviation. Black circles represent sex comb teeth and empty circles represent the TR bristles.
Effect of Simultaneous Directional Selection for High Bristle Number in the Sex Comb and Distal Transverse Row on Sex Comb Shape and Degree of Rotation.
A possible explanation for the absence of both high sex comb tooth and TR bristle number in the scd t1 mutants, as well as in the High line and babPR72 t1 lines, is that we did not study enough lines, and this phenotype is relatively easy to obtain. Another explanation is that the combination of these two traits intensifies the atypical phenotypes previously described, and as a result, a long distal TR and sex comb is avoided by natural selection. To test this hypothesis, we crossed the High line and the scd mutant, artificially selecting for both traits, a long sex comb and long distal TR. After six generations, this double directional selected line (DDS) displays a rapid response in both traits, increasing the sex comb tooth number up to 14 teeth, and the most distal TR up to 15 bristles (Fig. 5 A and B). The analysis of adult legs showed that when these two traits are observed, the cane-shaped structure is more frequent than in the previous lines studied (Fig. 5 A and B). We also found that the cane shaped is more abrupt than in other long combs, and the angle of rotation is significantly lower (Tukey’s test, HSD, F6,171 = 15.65, P < 0.001; Fig. 5C).
Fig. 5.
Line artificially selected for both a long sex comb and distal transverse row. (A) Sex comb exposed to DDS. (B) Frequency distribution between straight and cane-shaped sex comb in DDS line. (N = 20). (C) Average degree of rotation in wild type and DDS line. Wild type degree of rotation is significantly higher than DDS. (D) The 2D projections of different stages of rotation of DDS (developing sex comb cells shaded in red, distal TR cells shaded in yellow). Red brackets enclose bristle rows with bristle numbers indicated. Error bars correspond to standard deviation. Statistically significant differences indicated by asterisks (***P < 0.01). (N = 20 per treatment).
Time-lapse movies of the DDS line showed that although initially the rotation seems to occur normally (Movie S3), as development proceeds the top part of the sex comb is not able to complete the rotation (Fig. 5D and Movie S4). In addition, consistent with our hypothesis that the atypical sex comb shapes resulted from mechanical obstruction, there was no indication that the cellular changes accompanying rotation in the DDS line differed from those of wild type.
Sex Comb and Distal Transverse Row Length across Drosophila Species.
As previously shown in Fig. 1, long Drosophila sex combs achieve their final orientation in different ways including rotation of a single long comb, rotation and posterior fusion of multiple small combs, and appearance of sex comb bristles already in a vertical position (25). We investigated whether the atypical phenotypes observed when increasing sex comb length in D. melanogaster are also seen in other Drosophila species, and whether there is a possible connection between the diversity in sex comb development and the length of TRs. To do so, we studied cultures of several Drosophila species with different sex comb lengths and determined whether previous or new atypical phenotypes are observed in adult sex combs and/or the most distal TR.
Images of adult legs show that Drosophila species lacking sex combs, or those with long vertical sex combs that do not rotate during development, never show a bend in the most distal TR (Fig. S4). In contrast, in species with medium and long sex combs, it is possible to observe the same TR bending described in D. melanogaster (Fig. 6 A and B). Consistent with our obstruction hypothesis, species with long sex combs display many morphological modifications, which seem to provide more space for rotation (Figs. 6 and 7). For example, Drosophila species with long rotating sex combs have a lower number of TRs, and this can provide more space for the sex comb to rotate (Fig. 6C). Except for the modifications in the distal TR, other morphological changes that could potentially provide more space were never observed in D. melanogaster lines with different sex comb tooth number. These include altering the spacing between TRs and the relationship between the number of TRs and the t1 length (Figs. S5 and S6, respectively).
Fig. 6.
Distal transverse row as a barrier to rotation of long sex combs in nonmodel Drosophila species. (A and B) Long rotating sex combs from different species display similar atypical phenotypes to those observed in D. melanogaster lines with an increase in sex comb length. (C) Differences in number of TRs between males and females across Drosophila species. In other Drosophila species, long rotating sex combs have fewer numbers of TRs, which can increase the space to rotate. Comparison of the number of TRs between males (N = 10) and females (N = 10) of different Drosophila species. Dotted lines represent atypical phenotypes. Red brackets enclose bristle rows and indicate atypical phenotype. Yellow curved arrow shows displacements of distal TR. Black numbers in B indicate number of bristles in distal TR. Scale bar = 20 µm.
Fig. 7.
Tarsal segment schematics describing potential ways to increase space for rotation and avoid obstruction on rotation. Morphological analyses in several Drosophila species suggest that various developmental modifications can potentially increase space available for rotation (red polygons), but only some of them are observed in long D. melanogaster sex combs (gray boxes). Gray circles represent sex comb teeth in the initial position and black circles represent sex comb teeth in the final position. Empty circles represent the TR bristles. Gray arrows indicate movement of individual tooth or sex comb rows. Yellow curved arrow shows displacement of distal TR.
Discussion
The Distal Transverse Row as a Barrier to the Rotation of Long D. melanogaster Sex Combs.
Several lines of evidence suggest that the distal TR acts as a barrier blocking the rotation of long D. melanogaster sex combs. First, a previous investigation of the cellular basis of the sex comb rotation showed that row of bristles acted as a barrier (27). TR bristles, like sex comb teeth, emerge as noncontiguous proneural clusters, which proceed to join to each other forming rows during development. After formation, these rows become barriers preventing epithelial cells from moving between bristles during the process of cell rearrangement associated with rotation (27). As a result, when the sex comb and/or the distal TR length increases, the distal TR appears to prevent the proximal part of the sex comb from completing the rotation. The mechanical basis for incomplete rotation remains to be analyzed, although we described this barrier at the epithelial level, future studies can show that this barrier goes deeper. As bristle cells project underneath the epithelium and are attached to the peripheral neural system (28).
The obstruction between bristle rows can explain different atypical phenotypes described in the sex comb and TR. For example, when sex combs display a wild type or lower number of sex comb teeth, there is enough space for this row of bristles to change in position (Fig. 3A), so the degree of rotation is normal and the TR is straight or slightly pushed in an upward direction (Fig. 2D). However, with increasing sex comb length, the most distal TR blocks the trajectory of sex comb rotation. As a result, the distal TR is pushed proximally and the sex combs bend, reducing the degree of rotation (Fig. 8). Further evidence for the importance of the TR as a barrier to rotation is that sex comb length alone is not sufficient to predict the likelihood of a cane-shaped sex comb. Instead, knowledge of the length of both sex comb and distal TR is required. For example, long sex combs (<15 teeth in High line) rarely display a cane shape because they are accompanied by a relatively small distal TR (8 bristles). In contrast, medium-size sex combs (11 teeth in scd t1) frequently display a cane shape because the distal TR is longer than in other lines (11 bristles; Fig. 2C). The synergistic action between the sex comb and distal TR length was confirmed by artificially selecting for an increase in both traits. As expected, the DDS line displays a higher frequency of cane-shaped combs and lower degree of rotation (Fig. 5C) than either of the stocks with long sex combs, High line and babPR72 t1.
Fig. 8.
Distal transverse row acts as a barrier to sex comb rotation across Drosophila species. (A–D) Schematic of D. melanogaster lines with different sex comb and transverse row length. (E–G) Modifications to developmental mechanisms can avoid mechanical obstruction on rotation. (A–D) In D. melanogaster, the distal TR can block the trajectory of the sex comb rotation. Increasing the length of the distal TR and sex comb produces an upward bending of the distal TR (black arrows) and downward bend of the sex comb (red arrows). (E–G) Schematics of different Drosophila sex comb developmental mechanisms used to achieve the vertical position. However, in other Drosophila species, this obstruction between bristles can be avoided in different ways such as reducing the number of TRs (E), transforming distal TRs into a rotating sex comb (F), or producing sex combs already in a vertical position (G). Gray circles represent sex comb position in initial stages, while black circles indicate sex combs at final stages of development. Empty circles represent the TR bristles. Black dotted lines indicate trajectory of rotation or individual sex comb teeth.
Transverse Rows as a Barrier for Rotation During Sex Comb Evolution.
Our data suggest that similar to D. melanogaster, in other Drosophila species with rotating sex combs, the distal TR acts as a barrier to rotation. The first indication of the existence of this obstruction is that the same atypical phenotypes observed in D. melanogaster sex comb and distal TR are also observed in rotating sex combs found in other Drosophila species (Fig. 6 A and B). For example, the small length and the displacement of the most distal TR as well as the separation and bending of the sex comb can be seen in D. guanche and D. rhopaloa in the t1 (Fig. 6 A and B). Another indication of the importance of the distal TR during sex comb evolution is that long rotating sex combs display a smaller number of TRs than other Drosophila species, presumably providing additional space for rotation (Fig. 7). In contrast, in nonrotating sex combs with a vertical orientation, the number can vary.
The variation in mechanisms producing a long vertical sex comb demonstrates the flexibility of development to circumvent potential spatial barriers. For example, in species with long rotating sex combs, such as D. guanche, the sex comb is able to rotate without hindrance because two TRs are absent (Figs. 6C and 8E). In some species, multiple small sex combs rotate and fuse such as in the t2 of D. rhopaloa (22) and Lordiphosa magnipectinata (25), potential obstacles are not only absent, but are replaced by additional sex combs (Fig. 8F). Finally, nonrotating vertical sex combs derived from longitudinal row bristles suggest an alternative developmental strategy to permit large combs with apparently fewer spatial limitations than those encountered when modifying transverse row bristles into combs (22, 25) (Fig. 8G). As a result, the TRs can vary in number, filling the entire tarsal segment (D. nikananu) or being located only in the top region of the tarsal segment (D. ficusphila t1).
Evolutionary Implications.
Evolution through natural selection requires selectable heritable variation that is expressed during development (2, 4, 29). In order to modify development, we used genetic perturbations to study the arrays of possibilities observed when changing sex comb length. By reducing tooth number, we were able to reproduce sex comb phenotypes observed in other Drosophila species including degree of rotation, sex comb length, and shape. However, increasing tooth number over a threshold, in addition to producing an array of phenotypic possibilities, development may also limit the phenotypic variation (Fig. 5). D. melanogaster sex combs are no longer straight when both distal TR and sex comb lengths are increased (Fig. 5).
A detailed developmental analysis showed that either short-term selection or mutations can easily increase sex comb tooth number. However, due to D. melanogaster developmental–structural considerations, the space available for the rotation is not increased proportionally. For example, as the spacing between bristle rows is uncoupled from bristle row length, the coupling between the length of the sex comb and distal TR prevents the rotating comb from moving past this TR. Taken together, these data suggest that developmental processes are highly integrated in ways that make predicting phenotypic outcomes of genetic changes extremely difficult. In addition, the mechanical aspects of development represented by the forces involved in comb rotation produced emergent phenotypes such as cane-shaped sex combs and bending of the TRs. Only through developmental analysis can we tease out these different mechanistic components and understand how development produces variation that is fodder for selection.
As previously suggested, across Drosophila species, changes in the developmental mechanisms responsible for producing a long vertical sex comb can provide new opportunities in which the obstruction on rotation is not present anymore (Fig. 8). For example, many of the species with long, vertical sex combs do not utilize rotation at all. Instead, their sex combs coopt bristles from longitudinal rows (Fig. 8). On the other hand, in species with long, rotating combs, the space available for rotation is increased (Figs. 6 and 7). Further experiments are necessary to test whether such modifications can also be produced by short-term selection. However, potential developmental issues may arise when selecting on multiple traits that increase the space for rotation. For example, although increasing the size of the tarsal segment would, in theory, provide more space for rotation, D. melanogaster lines with longer tarsal segments develop additional TRs instead of providing more space for the rotation (Fig. S6). This illustrates the complex interplay between selection and developmental processes during adaptation.
Although this work focuses on describing a mechanical obstruction when increasing sex comb length, this integrative framework could also be useful to develop a better understanding of how sex combs are lost or reduced in length between closely related clades (Fig. S1). In artificial selection experiments in D. melanogaster, small sex combs occurred more rapidly than longer sex combs (24). Based on these experiments, we would predict that small rotating sex combs can be produced faster than long rotating sex combs in evolution. However, a detailed developmental analysis can complement those selection experiments and provide a mechanistic perspective of such changes. For example, future developmental studies can explain why teeth are removed from the top rather than bottom of the sex comb and why short-term selection is not able to remove the two most distal sex comb teeth (24).
This work shows that inclusion of developmental approaches in evolutionary studies can be a powerful tool to understand the process of adaptation. Changing sex comb length shows that rather than a dichotomy between selection and developmental constraints, they are indivisible (3, 13, 14, 30), and the study of developmental mechanisms provides a basis for understanding how selection occurs (2, 3, 16). In addition, this work demonstrates that the mechanical properties of tissues may provide the context in which selection occurs and that simple physical principles acting on the mechanical interactions in cells and tissues can produce phenotypic variation that may influence the direction of evolution.
Materials and Methods
Fly Strains.
Flies were reared on yeast–cornmeal–molasses medium at 25 °C. Lines for high and low numbers of sex comb teeth were developed by artificial selection for 24 generations following the protocol described by ref. 24. The line artificially selected for high number of bristles in the sex comb and distal TR were developed through the introgression of the High line into the scd mutant for six generations. In each generation from the pooled progeny of the previous generation five scd males were selected that had the longest sex comb and distal TR. Each of these flies was put into a different vial and crossed with five to seven females of the High line.
Three mutants were studied: babPR72 (31), scd (BDSC #5070), Scr13A/Scr6 (32). The UAS GAL4 system was used to perturb sex comb development. The driver chosen was rnGAL4-5 (BDSC #7405), which is expressed in the distal region of tarsal segments 1, 2, 3, and 4 in all legs. As a responder, UAS Scr RNAi was used (BDSC #3883). The rnGAL4 X UAS Scr RNAi crosses were grown at three different temperatures: 18°, 25°, and 28 °C. The introgression of the fluorescent marker required several generations of backcrossing between the ubi-DE::cadGFP and the artificially selected lines. Standard genetic crosses were sufficient to introgress the fluorescent marker into the babPR72 and scd mutants.
Data Collection.
For image acquisition, adult legs were dissected, and mounted on cover slips 22X22 mm No 1 (VWR), viewed with an Olympus BX41M and imaged with a Cool Snap camera U-CMAD (Photometrics) following protocols previously described (12, 24). Angles were measured using the angle tool of Image J software (http://rsb.info.nih.gov/ij). To study the developmental basis of the rotation, pupal legs were mounted in halocarbon oil (series 700; Halocarbon Products) on a coverslip (Sigma) and imaged with a laser 510 scanning confocal microscope (ZEISS) at 25 °C with a 40× objective, using LSM Browser software (ZEISS). The time interval between the acquisition of z stacks was 30 min.
Morphological Measurements in D. melanogaster Lines and Drosophila Species with Different Sex Comb Length.
Forelegs of at least 20 individuals were used for each of the following D. melanogaster lines: wild type, Low and High line, and the first and second tarsal segment sex combs of the mutants babPR72 and scd. The following measurements were carried out: sex comb shape, displacement of most distal TR, degree of rotation, number bristles per TRs, distance between TRs, size of tarsal segment, and number of TRs per tarsal segment as shown in Fig. S2.
In order to compare TR number across Drosophila species, 10 males and females per species were studied. The following species were analyzed: D. willistoni, D. virilis, D. mojavensis, D. guanche, D. rhopaloa, D. nikananu, D. ficusphila, and D. serrata as indicated in Fig. S2. In addition to those species, D. erecta, D. yakuba, and D. persimilis were measured to study the size of the tarsal segment, distance between TRs, the degree of displacement, and length of distal TR.
Statistical Analyses.
ANOVA was used as an omnibus test while post hoc comparisons were performed using Tukey’s HSD test. For all the comparisons, the statistical package Sigma Plot11.0 (Scientific Computing) was used. The calculation of correlation coefficients and chi-square tests were performed in Excel (Microsoft). Errors bars in the figures represent ± 1 standard deviation.
Supplementary Material
Acknowledgments
Ian Dworkin and two anonymous reviewers provided valuable comments to improve the manuscript. We thank the Bloomington Drosophila Stock Center and the Artyom Kopp and Anthony Percival-Smith laboratories for Drosophila strains.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 14011.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322342111/-/DCSupplemental.
References
- 1.Muller GB, Wagner GP. Novelty in evolution - Restructuring the concept. Annu Rev Ecol Syst. 1991;22:229–256. [Google Scholar]
- 2.Olson ME. The developmental renaissance in adaptationism. Trends Ecol Evol. 2012;27(5):278–287. doi: 10.1016/j.tree.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Salazar-Ciudad I. Developmental constraints vs. variational properties: How pattern formation can help to understand evolution and development. J Exp Zoolog B Mol Dev Evol. 2006;306(2):107–125. doi: 10.1002/jez.b.21078. [DOI] [PubMed] [Google Scholar]
- 4.Fusco G. How many processes are responsible for phenotypic evolution? Evol Dev. 2001;3(4):279–286. doi: 10.1046/j.1525-142x.2001.003004279.x. [DOI] [PubMed] [Google Scholar]
- 5.Maynard Smith J, et al. Developmental constraints and evolution. Q Rev Biol. 1985;60:265–287. [Google Scholar]
- 6.Chipman AD, Arthur W, Akam M. A double segment periodicity underlies segment generation in centipede development. Curr Biol. 2004;14(14):1250–1255. doi: 10.1016/j.cub.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Arthur W. Developmental constraint and natural selection. Evol Dev. 2003;5(2):117–118. doi: 10.1046/j.1525-142x.2003.03017.x. [DOI] [PubMed] [Google Scholar]
- 8.Klingenberg CP. Evolution and development of shape: Integrating quantitative approaches. Nat Rev Genet. 2010;11(9):623–635. doi: 10.1038/nrg2829. [DOI] [PubMed] [Google Scholar]
- 9.Brakefield PM. Evo-devo and constraints on selection. Trends Ecol Evol. 2006;21(7):362–368. doi: 10.1016/j.tree.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Frankino WA, Zwaan BJ, Stern DL, Brakefield PM. Natural selection and developmental constraints in the evolution of allometries. Science. 2005;307:718–720. doi: 10.1126/science.1105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beldade P, Koops K, Brakefield PM. Developmental constraints versus flexibility in morphological evolution. Nature. 2002;416(6883):844–847. doi: 10.1038/416844a. [DOI] [PubMed] [Google Scholar]
- 12.Allen CE, Beldade P, Zwaan BJ, Brakefield PM. Differences in the selection response of serially repeated color pattern characters: Standing variation, development, and evolution. BMC Evol Biol. 2008;8:94. doi: 10.1186/1471-2148-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberch P, Gale E. A developmental analysis of an evolutionary trend : Digital reduction in amphibians. Evolution (N Y) 1985;39:8–23. doi: 10.1111/j.1558-5646.1985.tb04076.x. [DOI] [PubMed] [Google Scholar]
- 14.De Bakker MAG, et al. Digit loss in archosaur evolution and the interplay between selection and constraints. Nature. 2013;500:445–8. doi: 10.1038/nature12336. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh KD, et al. Developmental bias in the evolution of phalanges. Proc Natl Acad Sci USA. 2013;110(45):18190–18195. doi: 10.1073/pnas.1315213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Futuyma DJ. Evolutionary constraint and ecological consequences. Evolution. 2010;64(7):1865–1884. doi: 10.1111/j.1558-5646.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 17.Kopp A. Drosophila sex combs as a model of evolutionary innovations. Evol Dev. 2011;13(6):504–522. doi: 10.1111/j.1525-142X.2011.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atallah J, Liu NH, Dennis P, Hon A, Larsen EW. Developmental constraints and convergent evolution in Drosophila sex comb formation. Evol Dev. 2009;11(2):205–218. doi: 10.1111/j.1525-142X.2009.00320.x. [DOI] [PubMed] [Google Scholar]
- 19.True JR. Combing evolution. Evol Dev. 2008;10(4):400–402. doi: 10.1111/j.1525-142X.2008.00250.x. [DOI] [PubMed] [Google Scholar]
- 20.Ng CS, Kopp A. Sex combs are important for male mating success in Drosophila melanogaster. Behav Genet. 2008;38(2):195–201. doi: 10.1007/s10519-008-9190-7. [DOI] [PubMed] [Google Scholar]
- 21.Hannah-Alava A. Developmental genetics of the posterior legs in Drosophila melanogaster. Genetics. 1958;43(5):878–905. doi: 10.1093/genetics/43.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka K, Barmina O, Kopp A. Distinct developmental mechanisms underlie the evolutionary diversification of Drosophila sex combs. Proc Natl Acad Sci USA. 2009;106(12):4764–4769. doi: 10.1073/pnas.0807875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barmina O, Gonzalo M, McIntyre LM, Kopp A. Sex- and segment-specific modulation of gene expression profiles in Drosophila. Dev Biol. 2005;288(2):528–544. doi: 10.1016/j.ydbio.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 24.Ahuja A, Singh RS. Variation and evolution of male sex combs in Drosophila: Nature of selection response and theories of genetic variation for sexual traits. Genetics. 2008;179(1):503–509. doi: 10.1534/genetics.107.086363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atallah J, Watabe H, Kopp A. Many ways to make a novel structure: A new mode of sex comb development in Drosophilidae. Evol Dev. 2012;14(6):476–483. doi: 10.1111/ede.12001. [DOI] [PubMed] [Google Scholar]
- 26.Held L. How does Scr cause first legs to deviate from second legs? Drosoph Inf Serv. 2011;93:132–146. [Google Scholar]
- 27.Atallah J, et al. Cell dynamics and developmental bias in the ontogeny of a complex sexually dimorphic trait in Drosophila melanogaster. Evol Dev. 2009;11(2):191–204. doi: 10.1111/j.1525-142X.2009.00319.x. [DOI] [PubMed] [Google Scholar]
- 28.Held L. Imaginal Discs: The Genetic and Cellular Logic of Pattern Formation. New York: Cambridge Univ Press; 2002. [Google Scholar]
- 29.Darwin CR. 1872. On the Origin of Species by Means of Natural Selection (New York: P.F. Collier & Son, London: John Murray) 6th Ed.
- 30.Arthur W. 2011. Evolutionary Developmental Biology : Developmental Bias and Constraint. eLS, 10.1002/9780470015902.a0001066.pub.
- 31.Godt D, Couderc J, Cramton S, Laski F. Pattern formation in the limbs of Drosophila: bric à brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119(3):799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- 32.Sivanantharajah L, Percival-Smith A. Analysis of the Sequence and Phenotype of Drosophila Sex combs reduced Alleles Reveals Potential Functions of Conserved Protein Motifs of the Sex combs reduced Protein. Genetics. 2009;182(1):191–205. doi: 10.1534/genetics.109.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.