Significance
This work demonstrates that specific peripheral clocks play unique and discrete roles in specific aspects of reproductive biology. Our use of a cell-specific conditional knockout model, in coordination with ovary transplant technology, permits examination of a peripheral clock without the impacts of off-target deletions that might indirectly impact reproductive function. In this case, we show that the molecular circadian clock, found in ovarian steroidogenic cells, is crucial for normal female reproduction, specifically embryonic implantation. The observation that implantation can be rescued by a single ovary with normal molecular clock machinery [i.e., brain muscle arnt-like 1 (BMAL1)] may provide direction for clinical intervention strategies when aberrant circadian oscillations are influencing fertility.
Keywords: ovary, circadian rhythm, fertility, steroidogenesis
Abstract
The circadian clock plays a significant role in many aspects of female reproductive biology, including estrous cycling, ovulation, embryonic implantation, onset of puberty, and parturition. In an effort to link cell-specific circadian clocks to their specific roles in female reproduction, we used the promoter that controls expression of Steroidogenic Factor-1 (SF1) to drive Cre-recombinase–mediated deletion of the brain muscle arnt-like 1 (Bmal1) gene, known to encode an essential component of the circadian clock (SF1-Bmal1−/−). The resultant SF1-Bmal1−/− females display embryonic implantation failure, which is rescued by progesterone supplementation, or bilateral or unilateral transplantation of wild-type ovaries into SF1-Bmal1−/− dams. The observation that the central clock, and many other peripheral clocks, are fully functional in this model allows the assignment of the implantation phenotype to the clock in ovarian steroidogenic cells and distinguishes it from more general circadian related systemic pathology (e.g., early onset arthropathy, premature aging, ovulation, late onset of puberty, and abnormal estrous cycle). Our ovarian transcriptome analysis reveals that deletion of ovarian Bmal1 disrupts expression of transcripts associated with the circadian machinery and also genes critical for regulation of progesterone production, such as steroidogenic acute regulatory factor (Star). Overall, these data provide a powerful model to probe the interlocking and synergistic network of the circadian clock and reproductive systems.
Disruption of circadian rhythms has been implicated in a variety of human health conditions, including cancer, diabetes, and infertility (1–5). Previous work has shown that the brain muscle arnt-like 1 (BMAL1) protein (also known as MOP3 or ARNTL), in a dimeric complex with the CLOCK protein, is an essential component of the “molecular clock” that controls circadian driven gene expression (6, 7). In an effort to understand the relationship between the molecular clock and human disease, we have developed a model of cell-specific circadian disruption, whereby the Bmal1 locus in the mouse is conditionally deleted by using cell-specific recombinases (8, 9). In this study, we set out to identify those cellular sites where the mammalian circadian clock is essential for female reproduction.
The importance of circadian rhythms in female reproduction is demonstrated by epidemiological evidence indicating that “shift work” in women is associated with menstrual irregularities and reproductive difficulties (4, 5). Genetic studies from animal models also support this relationship, as mutations in core components of the circadian clock have deleterious effects on a spectrum of reproductive measures. For example, global Bmal1−/− female mice display irregular estrous cycles, late onset of puberty, absence of proestrous luteinizing hormone surges, and implantation failure (10–12). Similarly, ClockΔ19 mutants display many of the above phenotypes in addition to higher rates of fetal reabsorption (13–15). Finally, as female Per1−/− and Per2−/− mice age, they bear smaller litters compared with their wild-type controls (16). Taken in sum, these observations suggest that disruption of molecular clocks within mammalian cells may have a significant influence on many aspects of fertility.
Given the complexity of female reproduction (11), we set out to determine whether specific cellular clocks in peripheral tissues play specific roles in specific aspects of reproductive biology. Evidence for the reproductive importance of the central clock residing within the suprachiasmatic nucleus (SCN) comes from observations from SCN ablation studies that reveal the role of this central clock in regulating estrous cycles and ovulation in female rats (17, 18). Evidence for the importance of peripheral clocks is supported by conditional Bmal1 mutants, and include the observations that deletion of Bmal1 in the pituitary gonadotropes results in a greater variance in estrous cycle length (12) and that deletion of Bmal1 in the myometrium results in abnormal timing of parturition (19). In contrast to global Bmal1−/− females, both of these peripheral Bmal1 deleted mutants still give rise to a high frequency of viable offspring. These observations suggest that additional peripheral clocks exist that are essential for normal reproduction, especially implantation.
Given the potential for conditional mouse models of Bmal1 to help us understand the relative roles of various peripheral clocks in reproduction, we first set out to understand the role of the molecular clock in the steroidogenic cells of the ovary. To this end, we used the Cre transgene driven by the promoter of steroidogenic factor 1 (i.e., Cresf1) (20), which deletes Bmal1 in steroidogenic cells of the gonads and adrenal glands and gonadotrophic cells of the pituitary (SF1-Cre). To isolate the effects of our Cresf1 deletion to the ovary, we used ovary transplantation.
Results
Conditional Bmal1 Knockout Mice.
The Bmal1fx allele was generated by inserting lox-P sites to flank the basic helix–loop–helix encoding exon of murine Bmal1 (Fig. S1A) (8, 9). To disrupt the circadian clock in SF1-Cre cells, mice harboring the Bmal1fx allele were bred to mice expressing a Cre transgene driven by the promoter of the Nr5a1 gene, which controls expression of the SF1 protein (this transgene is hereafter designated Cresf1) (20). In these studies, mice homozygous for the “floxed” allele (fx) and hemizygous for Cre were used as the experimental group (i.e., Bmal1fx/fxCresf1, designated as SF1-Bmal1−/−). Littermates that were negative for the Cre transgene (i.e., Bmal1fx/fx, or Bmal1+/+) or littermates that were positive for Cre while harboring a wild-type allele (i.e., Bmal1fx/+Cresf1) were used as control groups. To examine the specificity of excision mediated by Cresf1, we analyzed DNA from various tissues for the presence of the Bmal1fx-excised and Bmal1fx-unexcised alleles. As shown in Fig. S1B, the Cresf1 transgene leads to excision of the Bmal1fxallele in brain, pituitary, adrenal gland, and ovaries, but not in liver, muscle, uterus, and oviduct.
SF1-Bmal1−/− Females Display Behaviors Consistent with a Normally Functioning Central Circadian Clock.
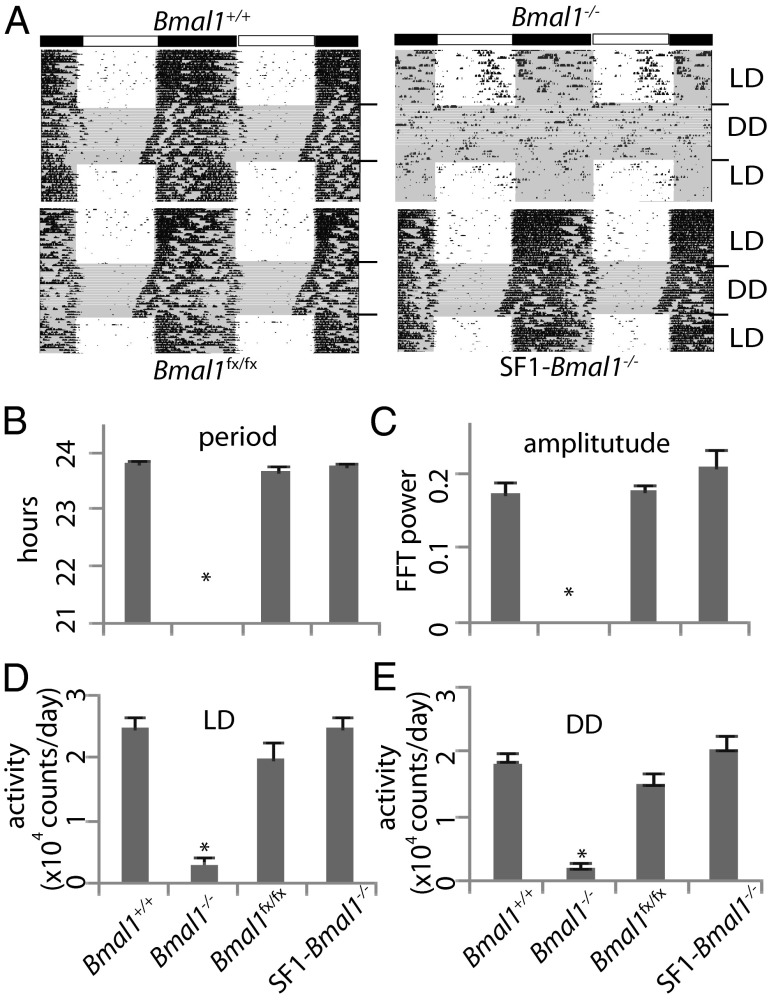
Wheel running analysis of SF1-Bmal1−/− females revealed normal circadian rhythms of locomotor activity, with no significant differences from controls for free running period, circadian rhythm amplitude, or activity levels (Fig. 1). Consistent with previous reports (6), global Bmal1−/− females showed a significantly lower circadian rhythm amplitude and a significantly lower activity level in both light-dark situations and constant darkness (P < 0.05). Further evidence of normal circadian rhythms of behavior in SF1-Bmal1−/− females came from studies of feeding behavior, where control females and SF1-Bmal1−/− females displayed normal circadian feeding rhythms, consuming more food in dark phase than in light phase (Fig. S2A). In contrast, global Bmal1−/− females consumed similar amounts of food in light phase and dark phase (P > 0.05). Taken in sum, these analyses are consistent with the idea that SF1-Bmal1−/− females have normal circadian rhythms of behavior due to a normally functioning central clock within the SCN (21).
Fig. 1.
Effect of Bmal1 deletion on wheel running activity. (A) Representative activity records of individual Bmal1+/+, Bmal1−/−, Bmal1fx/fx, and SF1-Bmal1−/− females are presented in double-plotted format. The bar above shows the light-dark cycle. DD, constant darkness; LD, light-dark. Gray shading indicates constant darkness in the actograms. (B–E) Effects of genotype on free running period (B), amplitude of circadian rhythm (C), and activity level in LD (D) and DD (E). *P < 0.05, by one-way ANOVA. Genotypes are indicated at the bottom of B–E.
SF1-Bmal1−/− Females Lack Arthropathy and Early Aging Phenotypes.
Global deletion of Bmal1 leads to an early onset arthropathy and premature aging phenotypes such as body weight reduction and organ shrinkage (22, 23). Unlike global Bmal1−/− mice, the hind limbs of 6-mo-old SF1-Bmal1−/− mice, stained with alizarin red (to detect calcification of ligaments and tendons; ref. 22), exhibited no inappropriate ossification (Fig. S2B). Additional evidence of normal aging includes the observation that, unlike Bmal1−/− animals, SF1-Bmal1−/− mice appeared outwardly healthy and displayed normal body weights as late as 300 d of age (Fig. S2C). Furthermore, SF1-Bmal1−/− females also display normal weights of whole body, ovaries, uterus, spleen, heart, kidney, and liver at 100 and 150 d (Fig. S2 D–J).
SF1-Bmal1−/− Females Are Unable to Deliver Offspring Despite Normal Onset of Puberty and Normal Estrous Cycles.
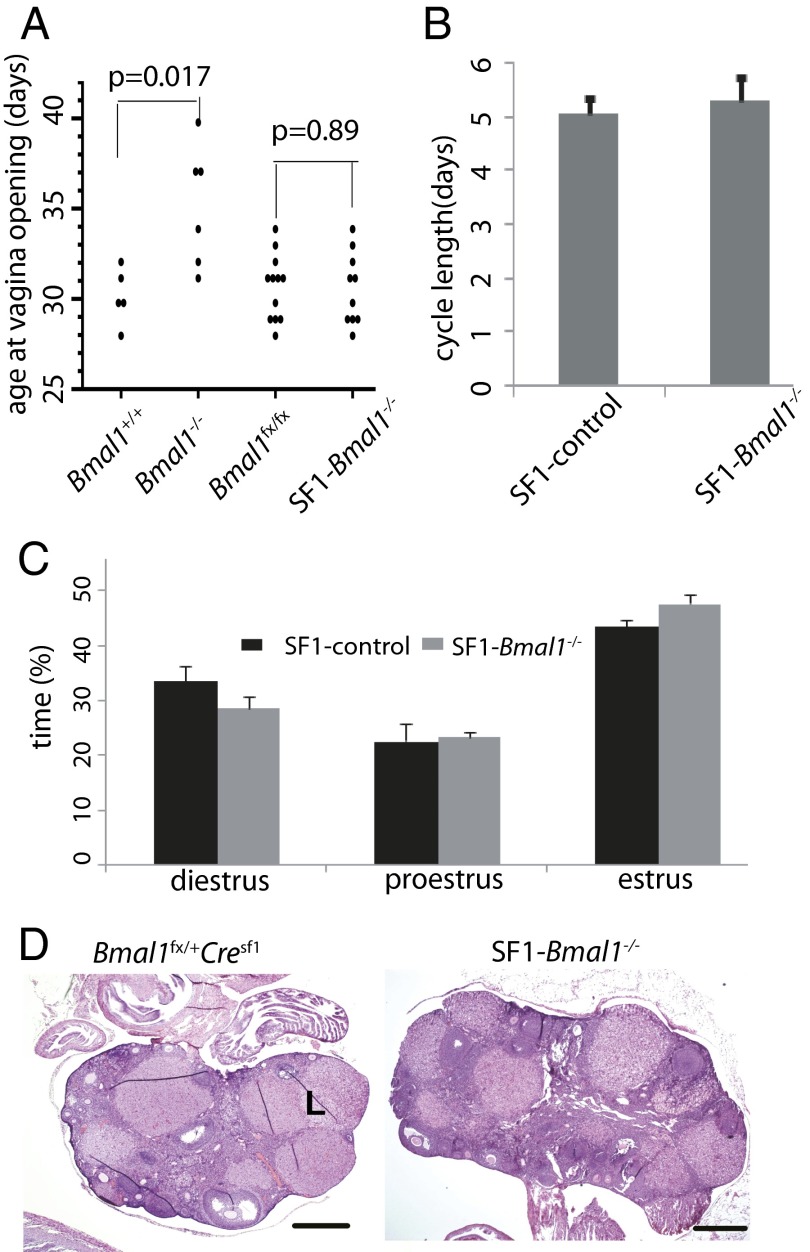
Consistent with a previous report (11), we observed that Bmal1−/− females exhibit a 5-d delay in a classical marker of female puberty, vaginal opening, than do control mice (Fig. 2A). Importantly, this delay in vaginal opening is not observed in SF1-Bmal1−/− females, indicating normal onset of puberty in these mice. Unlike global Bmal1−/− females (11), the SF1-Bmal1−/− females also exhibited normal estrous cycling that was indistinguishable from wild-type controls (Fig. 2 B and C and Fig. S3A). No significant difference was found in estrous cycle length or proportion of each stage between control and SF1-Bmal1−/− mice (P > 0.05, Wilcoxon rank sum test).
Fig. 2.
Effect of Bmal1 deletion on the onset of vaginal opening, estrous cycle, and ovary histology. (A) Age at vaginal opening. Each point represents an individual animal. P values (Wilcoxon rank sum test) for comparison of SF1-Bmal1−/− (n = 10) vs. Bmal1fx/fx (n = 12) and Bmal1−/− females (n = 6) vs. Bmal1+/+ (n = 5) are shown. (B) Estrous cycle length (mean ± SEM) of SF1-control (four Bmal1fx/fx and one Bmal1fx/+Cresf1) and SF1-Bmal1−/− females (n = 6). (C) Proportion of time (mean ± SEM) spent in each estrous stage in control (n = 5) and SF1-Bmal1−/− females (n = 6). (D) Representative histology sections of ovaries from control and SF1-Bmal1−/− females at 3.5 dpc. L, corpus luteum. (Scale bars: 0.5 mm.)
To further assess female reproductive potential, females were mated to proven wild-type fertile males. Females were scored for ability to deliver pups over a 3-mo period. As shown in Table S1, ∼95% of control females gave birth to pups. In contrast, none of the Bmal1−/− (0/13) or SF1-Bmal1−/−(0/12) females delivered pups, although copulation plugs appeared regularly every 6–8 d in SF1-Bmal1−/− females but not in global Bmal1−/− females. In contrast to the control females, SF1-Bmal1−/− displayed wide vaginal openings and cornified cells in vaginal smears at approximately 6.5 days postcoitum (dpc), characteristic of early pregnancy loss and reentry into estrus (Fig. S3B).
SF1-Bmal1−/− Females Display Implantation Failure.
To understand reproductive failure in the SF1-Bmal1−/− females, we paired females with wild-type fertile males and examined them for evidence of mating, ovulation, and implantation. As shown in Table 1, SF1-Bmal1−/− females exhibited rates of copulation and frequency of ovulation that were indistinguishable from controls. Normal ovulation was also demonstrated by histological analysis of the SF1-Bmal1−/− ovaries at 3.5 dpc, showing that SF1-Bmal1−/− females displayed similar numbers of corpora lutea (4.6 ± 0.5 per section, n = 13) compared with wild-type controls (5.0 ± 0.7 per section, n = 6) (Fig. 2D). In contrast, the global Bmal1−/− displayed a slightly lower rate of ovulation than SF1-Bmal1−/− (Table 1; P = 0.1, χ2 test). Although it did not reach our level of statistical significance, this observation is consistent with previous reports that ovulation in Bmal1−/− females is slightly affected (12). However, by 6.5 dpc, 16 of 17 SF1-Bmal1−/− females did not exhibit normal implantation sites (Fig. S3C) (P < 0.001). The implantation sites observed in the remaining SF1-Bmal1−/− female at 6.5 dpc were less developed than those of wild-type uteri, and no embryos were found upon histological examination. In a second experiment, we extended the observation period to 10.5 dpc and found no implantation sites in SF1-Bmal1−/− females (n = 4). These data lead us to conclude that SF1-Bmal1−/− females are unable to deliver offspring because they fail to support embryonic implantation.
Table 1.
Effect of Bmal1 deletion on parameters in early pregnancy
| Genotype | Bmal1+/+ | Bmal1−/− | SF1-control | SF1-Bmal1−/− |
| % copulation (n) | 88 (49) | 81 (53) | 90 (71) | 93 (69) |
| % ovulation (n) | 80 (15) | 67 (27) | 83 (41) | 83 (42) |
| % implantation (6.5 dpc) (n) | 86 (28) | 0 (16)* | 83 (23) | 6 (17)* |
| % implantation (10.5 dpc) (n) | — | — | — | 0 (4) |
The females were paired with proven males. The copulation rate was calculated as the number of females with vaginal plugs divided by the total number of females examined. The ovulation rate was calculated as the number of females with embryos at 3.5 dpc divided by the number of plugged females examined. The implantation rate was calculated as the number of females with detectable decidual swellings divided by the total number of 6.5 dpc or 10.5 dpc plugged females examined. Numbers in parentheses = number of mice tested. SF1-control includes Bmal1fx/+Cresf1 and Bmal1fx/fx females.
Significantly different between mutant and controls (P < 0.001, χ2 test).
Gene Expression Analysis Provides Evidence of Impaired Ovarian Steroid Biosynthesis in SF1-Bmal1−/− Females.
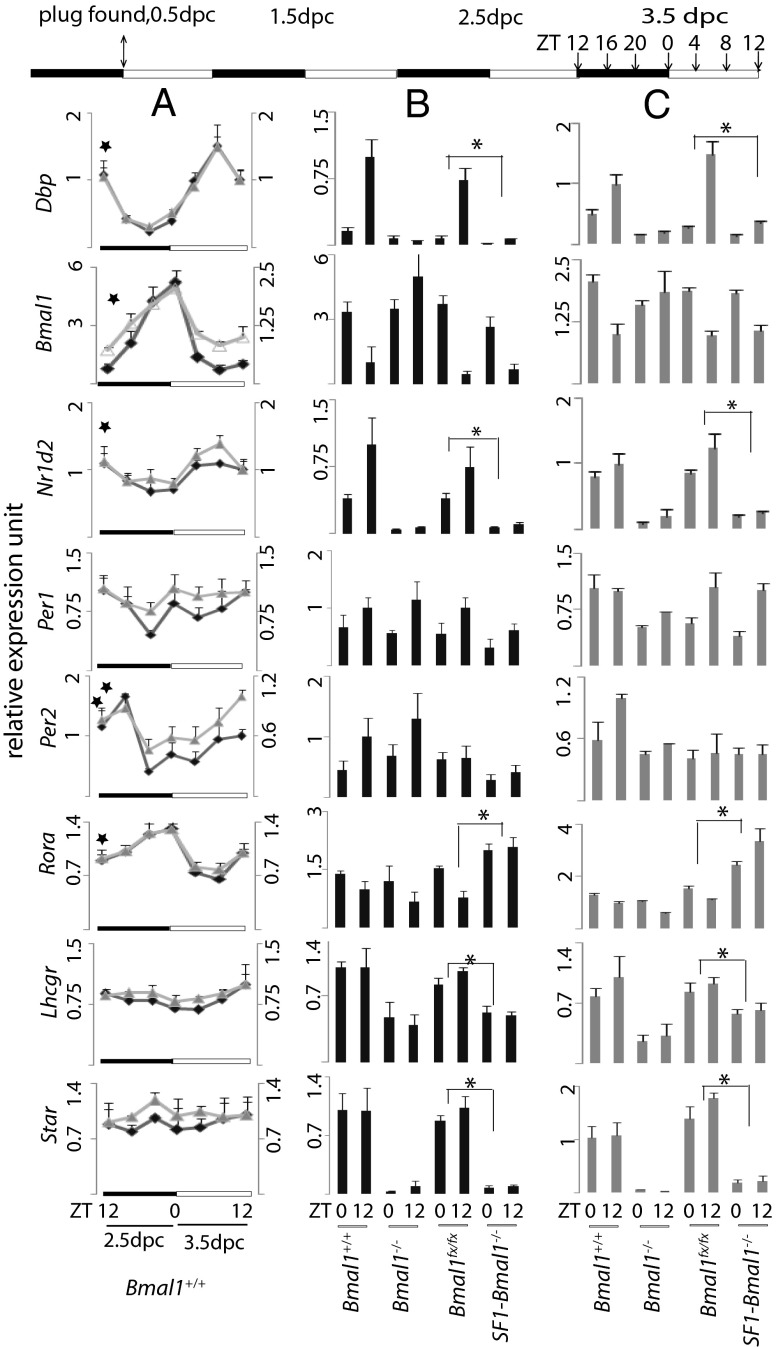
To explore the mechanisms underlying implantation failure in SF1-Bmal1−/− females, we performed gene expression analysis to profile mRNA expression in ovaries before implantation (Fig. 3). Both microarray and supporting quantitative PCR (qPCR) analyses indicated normal functioning of molecular clocks in wild-type ovaries based on the observation that classical clock outputs such as Dbp, Bmal1, Nr1d2, Rora, and Per2 mRNAs displayed rhythmic expression in wild-type ovaries from ZT12, 2.5 dpc, to ZT12, 3.5 dpc [cosine wave-optimization algorithm (COSOPT), pMMC-β<0.05] (24). When pathway analysis (25) was used to examine the pathways of differentially expressed genes between SF1-Bmal1−/− and Bmal1fx/fx based on the microarray data, the top 10 most significant processes included “lipid biosynthetic and metabolic processes,” “steroid metabolic process,” “cholesterol biosynthetic process,” and “circadian rhythm” (Table S2).
Fig. 3.
Expression of Dbp, Bmal1, Nr1d2, Per1, Per2, Rora, Lhcgr, and Star mRNAs in ovaries. Values are expressed as mean ± SEM, setting mean expression value of Bmal1+/+ at ZT12 on 3.5 dpc to 1. Ovaries were harvested every 4 h from 2.5 to 3.5 dpc as shown on the timeline at the top. (A) Gene expression assay on C57BL/6J females obtained from JAX (n = 3 per time point) by microarray (∆, right axis) and qPCR (♦, left axis). ★, pMMC-β<0.05 in COSOPT. (B) qPCR gene expression assay on mice of the indicated genotype bred in our mouse facility (n ≥ 4 per time point⋅genotype−1). (C) Microarray gene expression assay on C57/B6 females (same animals as A), Bmal1−/−(n = 3 per time point), Bmal1fx/fx and SF1-Bmal1−/− (n = 4 per time point⋅genotype−1).*P < 0.05 (two-way ANOVA between Bmal1fx/fx and SF1-Bmal1−/−).
Additional support for disruption of the circadian clock and steroid biosynthesis in ovaries of SF1-Bmal1−/− mice came from two observations. First, microarray and qPCR revealed that three of the most sensitive outputs of the clock, Dbp, Nr1d2, and Rora, were significantly dysregulated in both Bmal1−/−or SF1-Bmal1−/− ovaries at ZT0 and ZT12 (Fig. 3 and Table S2). Although a transcript from the excised Bmal1fx allele is generated, genomic analysis indicates no functional protein product is produced from the excised allele (6, 8, 9). Second, microarray demonstrated that the top three most down-regulated (based on fold change) transcripts in the ovaries of SF1-Bmal1−/− mice were the products of the Aldob, Star, and Ms4a10 loci (20-, 10-, and ninefold down-regulated, respectively). The Star gene product, steroidodogenic acute regulatory protein, is a rate limiting enzyme in steroidogenesis. The ∼10-fold down-regulation of Star mRNA in SF1-Bmal1−/− ovaries recapitulates what has been reported for ovaries from mice with a global deletion of Bmal1 (10, 11), which we also reproduce here (Fig. 3). Further examination of the gene list also revealed a down-regulation of the Lhcgr gene product (Fig. 3; P < 0.05, two-way ANOVA). This locus encodes the receptor for luteinizing hormone and is thought to play a significant role in Star expression (26).
Transplantation of Wild-Type Ovaries into SF1-Bmal1−/− Females Rescues Implantation Failure.
To isolate the effects of our SF1 deletion to the ovarian compartment, as opposed to the adrenal or pituitary, we performed ovary transplantation experiments (Table 2). First, we performed bilateral replacement of SF1-Bmal1−/− ovaries with Bmal1fx/fx ovaries. We observed this transplantation led to a 100% rescue (n = 12) of implantation and resulted in a normal number of live births. In the reverse transplantation, we replaced the ovaries of Bmal1fx/fx females with SF1-Bmal1−/− ovaries. Despite the regular appearance of copulation plugs in all 11 dams, only 4 of 11 recipients gave birth to pups.
Table 2.
Effect of ovary transplantation on fertility outcome
| Donor ovary genotype | Recipient genotype | Ovary transplant | % of recipients with successful parturition (n) | P value |
| Bmal1fx/fx | SF1-Bmal1−/− | Bilateral | 100 (12) | 9e−4* |
| SF1-Bmal1−/− | Bmal1fx/fx | Bilateral | 36 (11) | |
| Bmal1+/+ | SF1-Bmal1−/− | Unilateral | 100 (3) |
Donor and recipient genotypes are indicated. Numbers in parentheses are number of mice transplanted.
The ratio of successful parturition is significantly higher in recipients with wild type ovaries than SF1-Bmal1−/− ovaries (χ2 test).
To evaluate the relative contributions of the ovary and hypothalamus-pituitary axis and test the hypothesis that a wild-type ovary could compensate for an SF1-Bmal1−/− ovary, we used unilateral transplantation. To this end, a single ovary was removed from an SF1-Bmal1−/− female and replaced with a wild-type (i.e., Bmal1+/+) ovary, so that the recipient SF1-Bmal1−/− females carried one wild-type ovary and one SF1-Bmal1−/− ovary. All three of these unilaterally transplanted SF1-Bmal1−/− recipient females were mated to Bmal1+/+ males and all three gave birth to offspring. Furthermore, all recipients produced pups carrying Bmal1fx allele (70% of a total of 37 pups detected), indicating that (i) the SF1-Bmal1−/− ovaries in SF1-Bmal1−/− females are capable of producing viable oocytes resulting in offspring in the presence of a normal ovary and (ii) a functioning BMAL1 protein is only required in one ovary to allow implantation.
Effects of Progesterone or Prolactin Supplementation on Implantation in SF1-Bmal1−/− Females.
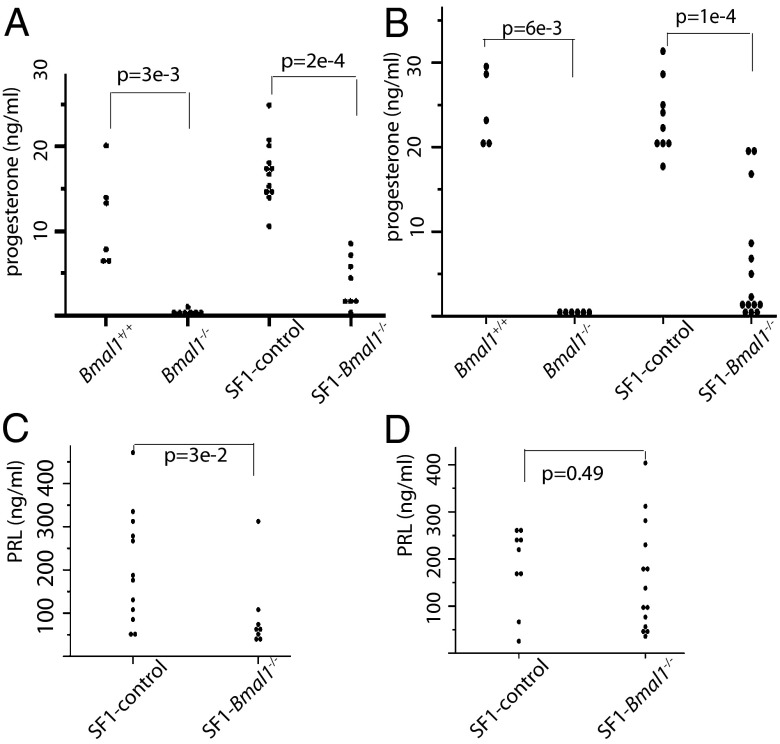
Given that progesterone secretion by the corpus luteum is essential for implantation and maintenance of pregnancy (27, 28), we examined the levels of this hormone in SF1-Bmal1−/− mice. We observed that both Bmal1−/− and SF1-Bmal1−/− females had significantly reduced serum progesterone levels compared with controls at both 3.5 dpc and 6.5 dpc (Fig. 4 A and B, respectively). Interestingly, at both times, progesterone was still detectable in SF1-Bmal1−/− mice, suggesting incomplete excision within the ovarian compartment of Bmal1 using SF1-Cre. We also tested progesterone levels in the bilaterally transplanted females on 3.5 dpc (Fig. S3D). Progesterone levels cosegregated with the genotype of the donor ovaries, with recipients receiving SF1-Bmal1−/− ovaries displaying lower progesterone level than those who received Bmal1fx/fx ovaries (P < 0.05).
Fig. 4.
Effects of Bmal1 deletion on serum progesterone and PRL levels. Each point represents a single animal. Progesterone levels in Bmal1−/− and SF1-Bmal1−/− females at 3.5 dpc (A) and 6.5 dpc (B) and PRL levels in SF1-Bmal1−/− females at 3.5 dpc (C) and 6.5 dpc (D) are shown. SF1-control includes Bmal1fx/fx and Bmal1fx/+Cresf1 females. P values, by Wilcoxon sum test, are indicated.
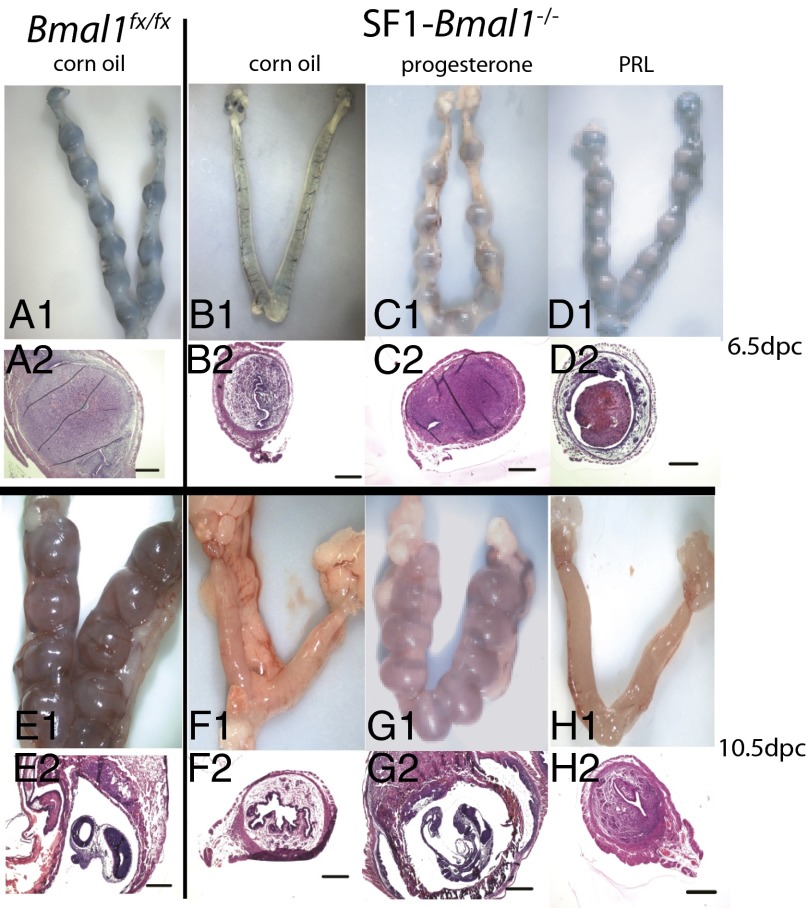
To determine whether progesterone biosynthesis was related to implantation failure in SF1-Bmal1−/− females, we asked whether progesterone supplementation could rescue implantation. As shown in Table 3 and Fig. 5, on 6.5 dpc, five of eight SF1-Bmal1−/− females supplemented with progesterone exhibited successful implantation (Fig. 5C, 1 and 2) (P < 0.001 vs. SF1-Bmal1−/− females with vehicle control), with similar numbers of implantation sites compared with controls (P > 0.2 vs. wild-type females) (Table 3 and Fig. 5A, 1 and 2). None of nine vehicle-treated SF1-Bmal1−/− females (Fig. 5B, 1 and 2) showed evidence of implantation at 6.5 dpc. To test whether progesterone-rescued pregnancies could be sustained beyond 6.5 dpc, we injected five females with progesterone until 10.5 dpc. Of these five females, three displayed histologically normal embryos (Table S3 and Fig. 5G, 1 and 2), with numbers of implantation sites and overall embryo development similar to that of wild-type controls (Fig. 5E, 1 and 2).
Table 3.
Effect of progesterone or PRL on implantation sites in SF1-Bmal1−/− females at 6.5 dpc
| Genotype | Treatment | % with IM. (n) | I.S./mouse* |
| SF1-control | Corn oil | 91 (11) | 9.4 ± 0.4 |
| SF1-control | Progesterone | 75 (12) | 8.9 ± 0.4 |
| SF1-Bmal1−/− | Corn oil | 0 (9)† | 0 |
| SF1-Bmal1−/− | Progesterone | 62.5 (8)‡ | 7.2 ± 1.2 |
| SF1-control | Saline | 91 (11) | 8.8 ± 0.33 |
| SF1-control | PRL | 87.5 (8) | 9.7 ± 0.43 |
| SF1-Bmal1−/− | Saline | 0 (6)§ | 0 |
| SF1-Bmal1−/− | PRL | 44 (9)¶,|| | 8 ± 0.91 |
Numbers in parentheses are number of mice tested. IM., implantation; I.S., implantation sites.
The number of implantation sites in mice with successful implantation. Values are mean ± SEM.
P < 0.005 vs. SF1-control (Bmal1fx/+Cresf1 and Bmal1fx/fx) mice treated with corn oil (χ2 test).
P < 0.005 vs. SF1-Bmal1−/− mice treated with corn oil (χ2 test).
P < 0.001 vs. SF1-control mice treated with saline (χ2 test).
P = 0.06 vs. SF1-Bmal1−/− mice treated with saline (χ2 test).
Chicago sky blue staining did not permeate the implantation sites.
Fig. 5.
Effects of progesterone or PRL treatment on implantation at 6.5 dpc or 10.5 dpc. Representative whole uteri (1) and H&E-stained sections (2) are shown. SF1-Bmal1−/− females (B–D and F–H) and wild-type controls (A and E) received corn oil (A, B, E, and F), progesterone (C and G), or PRL (D and H) until 6.5 dpc (A–D) or 10.5 dpc (E–H). (Scale bars: 0.5 mm.)
Prolactin (PRL) is a pituitary hormone that also supports function of the corpus luteum during the periimplantation period (29). Because SF1-Bmal1−/− females showed lower PRL levels than wild-type controls on 3.5 dpc but not 6.5 dpc (Fig. 4 C and D), we evaluated the effects of supplemental PRL by using established protocols. PRL supplementation of SF1-Bmal1−/− females at 6.5 dpc resulted in small uterine swellings in four of nine females. Staining of these uteri revealed abnormal vasculature (Fig. 5D, 1), indicating decreased vascular permeability compared with wild-type or progesterone-rescued SF1-Bmal1−/− females (Fig. 5C, 1 and 2). Examination of histological sections confirmed that PRL supplementation increased uterine cell density, but decidual tissue (Fig. 5D, 2) was not as fully developed as wild-type or progesterone-rescued implantation sites (Fig. 5C, 2). None of the four SF1-Bmal1−/− females supplemented with PRL until 10.5 dpc displayed uterine swellings (Table S3 and Fig. 5H, 1 and 2). In summary, unlike progesterone, supplemental PRL only partially rescued implantation in SF1-Bmal1−/− females.
Gene Expression Studies in Pituitary.
Microarray analysis of pituitary gene expression at ZT0 and ZT12 indicated that Dbp and Bmal1 displayed antiphase expression pattern in control and SF1-Bmal1−/− pituitaries (Fig. S4 and Table S4), suggesting normal functioning of the molecular clock in this tissue (30). No significant gene expression differences were detected between control and SF1-Bmal1−/− pituitaries.
Discussion
The importance of the central clock in reproductive biology is supported by the observation that deletion of the central clock, through SCN lesions or global deletion of Bmal1, corresponds to defects in estrous cycling and ovulation (11, 17, 18). The SCN is also known to regulate PRL release and, thus, supports luteal maintenance of pregnancy (31, 32). Infertility in Bmal1−/− females has been attributed to many factors, including gonadotropin hormone release, oocyte production, corpus luteum growth and development, impaired steroidogenesis at undefined sites, and defects in embryo development and implantation (11). The majority of these events require complex intraorgan communication among the nervous system, multiple components of the endocrine reproductive axis, and their targets. Understanding the interplay between the central clock and peripheral clocks and their involvement in reproductive outcome is made difficult by the complicated interlocking nature of the reproductive and circadian clock systems (33). Our results demonstrate that the molecular clock within steroidogenic compartment of the ovary plays a crucial role in one aspect of fertility, implantation.
An advantage of the SF1-Bmal1−/− mouse model used here is that through cell-specific Cre-excision, deletion of a “steroidogenic cell clock” can occur without disruption of the biology driven by the central clock or many other peripheral clocks (Figs. 1 and 3 and Fig. S1). As evidence, we show that SF1-Bmal1−/− mice, with the exception of reproductive failure, are phenotypically similar to wild-type mice and do not display many of the significant pathologies described for the global Bmal1−/− mice that could indirectly influence fertility including early onset arthropathy (22), abnormal estrous cycle, late onset of puberty as defined by vaginal opening (Fig. 2 and Fig. S2) (11), and premature early aging as defined by organ shrinkage (23). Using the SF1-Bmal1−/− model, we show that deletion of Bmal1 in SF1-Cre cells leads to implantation failure associated with low progesterone levels (Fig. S3, Fig. 4, and Table 1). Thus, our model isolates the Bmal1−/− implantation failure to the steroidogenic compartments of the pituitary (i.e., gonadotrophs), adrenal, or ovary. Importantly, the implantation failure observed in this model recapitulates the progesterone-dependent implantation failure that is observed in global Bmal1−/− females (10).
By incorporating ovarian transplantation into this model system, we are able to isolate the BMAL1-dependent implantation failure to the ovarian steroidogenic compartment (Table 2). Moreover, we were able to use transplantation to demonstrate that the presence of a single wild-type ovary is sufficient to completely rescue implantation failure in SF1-Bmal1−/− females. This observation suggests that functioning of the gonadotropic or adrenal clocks in SF1-Bmal1−/− females is permissive for normal ovulation, fertilization, implantation, and parturition. Although the observation of complete implantation rescue after transplant of wild-type ovaries into SF1-Bmal1−/− mice implicates the ovarian clock, interpretation of the reverse transplantation experiment is complicated by the possibility that our surgical procedure results in the presence of enough residual ovarian tissue to support implantation. In support of this idea, publications from other laboratories suggest between 3 and 36% of litters obtained from such grafted animals may be derived from remaining ovarian host fragments that are difficult to remove during surgery (34, 35). Another possibility is that the wild-type hypothalamic-pituitary axis can support the transplanted SF1-Bmal1−/− ovaries. Nevertheless, the lower frequency of implantation suggests that this level of support is largely insufficient. Again, these results lead us to conclude that the ovarian clock is the primary determinant of implantation.
These data and those reported by others (10, 11) suggest that BMAL1 plays a role in the intrinsic molecular clock of ovarian steroidogenic cells, and that this clock plays an important role in the production of progesterone through the enzyme STAR. Importantly, the mechanism by which BMAL1 regulates Star is worthy of further investigation. Although Star expression has been reported to oscillate in F1 follicles of chicken ovaries or rat mature granulose cells (36, 37), we did not observe circadian oscillation of this mRNA in whole ovaries from 2.5 dpc to 3.5 dpc (Fig. 3). Moreover, although previous chromatin immunoprecipitation analysis in mouse adrenal indicates that Bmal1 binds to E-boxes in the Star promoter, other studies in liver have not shown evidence of direct interaction (38, 39). Although there is still much to be learned about the roles of the circadian clock in many aspects of fertility, these data support a model of female reproduction where regulation of progesterone by molecular clock machinery within steroidogenic cells of the ovary is a crucial factor in one critical aspect of fertility, implantation. Of equal importance is the conclusion that circadian clocks at additional sites appear to regulate other important aspects of fertility, such as onset of puberty, estrous cycling, and ovulation.
Materials and Methods
Ovary transplantation was carried out bilaterally or unilaterally as indicated (40). Briefly, mice were anesthetized by inhalation through a nose cone of 2% (vol/vol) isoflurane (Halocarbon Products) mixed with oxygen using a V7276 anesthesia machine (Surgivet Veterinary Surgical Products). The mice received a s.c. injection of buprenophine (0.05–0.1 mg/kg, Sigma) and were placed on a heated disinfected pad. Recipient ovaries were surgically removed through a tiny incision of the bursa, and donor ovaries were inserted into the bursa of the recipient under a dissecting microscope. All females recovered normal movement 1 h after the surgery. Females were mated to wild-type fertile males 21 d after the surgery. For additional information, see SI Materials and Methods and Table S5.
Supplementary Material
Acknowledgments
This work was supported by The National Institute of Environmental Health Sciences Grant R37ES005703, University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520 (National Cancer Institute), and National Institutes of Health Training Grant T32ES007015-32 (to B.P.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE48758).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209249111/-/DCSupplemental.
References
- 1.Pukkala E, et al. Cancer incidence among Nordic airline cabin crew. Int J Cancer. 2012;131(12):2886–2897. doi: 10.1002/ijc.27551. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamble KL, Resuehr D, Johnson CH. Shift work and circadian dysregulation of reproduction. Front Endocrinol (Lausanne) 2013;4:92. doi: 10.3389/fendo.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int. 2002;23(6-7):703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- 6.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westgate EJ, et al. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117(16):2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BP, et al. The hepatocyte circadian clock controls acetaminophen bioactivation through cytochrome P450-oxidoreductase. Proc Natl Acad Sci USA submitted. 2014 doi: 10.1073/pnas.1421708111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1-/- mice. Endocrinology. 2009;150(4):1879–1885. doi: 10.1210/en.2008-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction. 2010;139(6):1077–1090. doi: 10.1530/REP-09-0523. [DOI] [PubMed] [Google Scholar]
- 12.Chu A, et al. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013;154(8):2924–2935. doi: 10.1210/en.2013-1080. [DOI] [PubMed] [Google Scholar]
- 13.Miller BH, et al. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennaway DJ, Boden MJ, Voultsios A. Reproductive performance in female Clock Delta19 mutant mice. Reprod Fertil Dev. 2004;16(8):801–810. doi: 10.1071/rd04023. [DOI] [PubMed] [Google Scholar]
- 15.Dolatshad H, et al. Developmental and reproductive performance in circadian mutant mice. Hum Reprod. 2006;21(1):68–79. doi: 10.1093/humrep/dei313. [DOI] [PubMed] [Google Scholar]
- 16.Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction. 2008;135(4):559–568. doi: 10.1530/REP-07-0434. [DOI] [PubMed] [Google Scholar]
- 17.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31(2):147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 18.Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci. 1977;198(1132):279–296. doi: 10.1098/rspb.1977.0098. [DOI] [PubMed] [Google Scholar]
- 19.Ratajczak CK, et al. Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod Fertil Dev. 2012;24(5):759–767. doi: 10.1071/RD11164. [DOI] [PubMed] [Google Scholar]
- 20.Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44(9):419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- 21.Sujino M, et al. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr Biol. 2003;13(8):664–668. doi: 10.1016/s0960-9822(03)00222-7. [DOI] [PubMed] [Google Scholar]
- 22.Bunger MK, et al. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41(3):122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- 23.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20(14):1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straume M. DNA microarray time series analysis: Automated statistical assessment of circadian rhythms in gene expression patterning. Methods Enzymol. 2004;383:149–166. doi: 10.1016/S0076-6879(04)83007-6. [DOI] [PubMed] [Google Scholar]
- 25.Huang DW, et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35(Web Server issue):W169–175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: Present and future perspectives. Mol Hum Reprod. 2009;15(6):321–333. doi: 10.1093/molehr/gap025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973;31:201–256. doi: 10.1016/s0083-6729(08)60999-1. [DOI] [PubMed] [Google Scholar]
- 28.Song H, Han K, Lim H. Progesterone supplementation extends uterine receptivity for blastocyst implantation in mice. Reproduction. 2007;133(2):487–493. doi: 10.1530/REP-06-0330. [DOI] [PubMed] [Google Scholar]
- 29.Bartke A. Reproduction of female dwarf mice treated with prolactin. J Reprod Fertil. 1966;11(2):203–206. doi: 10.1530/jrf.0.0110203. [DOI] [PubMed] [Google Scholar]
- 30.Ueda HR, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418(6897):534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 31.Poletini MO, Kennett JE, McKee DT, Freeman ME. Central clock regulates the cervically stimulated prolactin surges by modulation of dopamine and vasoactive intestinal polypeptide release in ovariectomized rats. Neuroendocrinology. 2010;91(2):179–188. doi: 10.1159/000254379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mai LM, Shieh KR, Pan JT. Circadian changes of serum prolactin levels and tuberoinfundibular dopaminergic neuron activities in ovariectomized rats treated with or without estrogen: The role of the suprachiasmatic nuclei. Neuroendocrinology. 1994;60(5):520–526. doi: 10.1159/000126789. [DOI] [PubMed] [Google Scholar]
- 33.Sellix MT. Clocks underneath: The role of peripheral clocks in the timing of female reproductive physiology. Front Endocrinol (Lausanne) 2013;4:91. doi: 10.3389/fendo.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sztein J, Sweet H, Farley J, Mobraaten L. Cryopreservation and orthotopic transplantation of mouse ovaries: New approach in gamete banking. Biol Reprod. 1998;58(4):1071–1074. doi: 10.1095/biolreprod58.4.1071. [DOI] [PubMed] [Google Scholar]
- 35.Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15(6):1300–1304. doi: 10.1093/humrep/15.6.1300. [DOI] [PubMed] [Google Scholar]
- 36.Nakao N, et al. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148(7):3031–3038. doi: 10.1210/en.2007-0044. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, et al. Downregulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol. 2013;304(12):C1131–C1140. doi: 10.1152/ajpcell.00008.2013. [DOI] [PubMed] [Google Scholar]
- 38.Son GH, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA. 2008;105(52):20970–20975. doi: 10.1073/pnas.0806962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hogan B, Beddington R, Costantini F, Lacy E (1994) Manipulating the Mouse Embryo: A Laboratory Manual. (Cold Spring Harbor Lab Press, Plainview, NY), 2nd Ed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.