Significance
The roles of disease and species hybridization in maintaining biodiversity are of wide interest, yet are rarely studied simultaneously in wild populations. Using genomic analysis of beak and feather disease virus in an avian ring-species complex, Platycercus elegans, to our knowledge we find viral phylogenetic structure analogous to Mayr’s ring-species hypothesis for the first time in any pathogen. Across 8 y, the host’s viral prevalence and infection load was lower in hybrid birds and in phenotypically intermediate subspecies. Viral genetic variation did not explain host prevalence or infection load, supporting conclusions that the evolved host response is more important. We show how host–species complexes and viral genomic analyses can provide insight into maintenance of biodiversity.
Keywords: psittacine circovirus, host–pathogen coevolution, heterosis, sympatric speciation, crimson rosella
Abstract
Pathogens have been hypothesized to play a major role in host diversity and speciation. Susceptibility of hybrid hosts to pathogens is thought to be a common phenomenon that could promote host population divergence and subsequently speciation. However, few studies have tested for pathogen infection across animal hybrid zones while testing for codivergence of the pathogens in the hybridizing host complex. Over 8 y, we studied natural infection by a rapidly evolving single-strand DNA virus, beak and feather diseases virus (BFDV), which infects parrots, exploiting a host-ring species complex (Platycercus elegans) in Australia. We found that host subspecies and their hybrids varied strikingly in both BFDV prevalence and load: both hybrid and phenotypically intermediate subspecies had lower prevalence and load compared with parental subspecies, while controlling for host age, sex, longitude and latitude, as well as temporal effects. We sequenced viral isolates throughout the range, which revealed patterns of genomic variation analogous to Mayr’s ring-species hypothesis, to our knowledge for the first time in any host–pathogen system. Viral phylogeny, geographic location, intraspecific host density, and parrot community diversity and composition did not explain the differences in BFDV prevalence or load between subpopulations. Overall, our analyses suggest that functional host responses to infection, or force of infection, differ between subspecies and hybrids. Our findings highlight the role of host hybridization and clines in altering host–pathogen interactions, dynamics that can have important implications for models of speciation with gene flow, and offer insights into how pathogens may adapt to diverging host populations.
A long-standing puzzle in evolutionary ecology concerns the processes that promote speciation, and particularly the factors that favor or constrain genetic divergence in the absence of physical barriers to gene flow (1, 2). Coevolution between pathogens and their hosts is considered a fundamental interaction that influences microevolutionary changes in both the host and pathogen, and could potentially mediate gene flow between populations and consequently speciation (3, 4). Parasites have the potential to influence incipient speciation of their hosts by differentially influencing the fitness of diverging or intermediate host lineages, and thus the genetic exchange between host populations (4–6). Conversely, differing selection pressures exerted by host populations may lead to specialization and subsequent speciation of their parasites, especially if transmission between host populations is limited (4). Excellent opportunities to study such phenomena are provided by clinal and hybridizing populations, which offer natural laboratories in which to investigate population divergence and the early stages of speciation (2, 7). Parasitism may either promote or penalize hybridization, depending on a range of host, parasite, or environmental factors (8). Currently, our understanding of how host–parasite coevolution proceeds in diverging or hybridizing populations and its role in speciation is limited, in large part because of the small number of studies that examine variation in both hosts and parasites over sufficient spatial or temporal scales, or in hybridizing communities (8, 9).
To date, studies of host–parasite interactions in hybridizing species have been overwhelmingly focused on plants (8–10). Moulia and Joly (9) identified only eight animal hybridization models where parasitism has been studied under natural conditions. Overall, both plant and animal studies suggest that higher parasite loads in hybrids compared with parental forms is the norm (8, 9), suggesting that hybrids are typically more susceptible to parasites compared with their parental species, and may therefore restrict gene flow between parental populations. For example, a hybrid population between two subspecies of house mice (Mus domesticus) were found to have higher helminth loads (6, 9, 11), suggesting that parasites could be selecting against hybridization. A similar but more complex pattern was found in hybridogenetic water frogs (Rana lessonae and Rana esculenta). Joly et al. (12) reported a higher load of lung flukes in hybrids, but this pattern varied depending on the particular parasite being tested, as this study also demonstrated that parental frogs had higher loads of lung roundworms. A separate study supporting this claim on the same system reported no differences in prevalence in loads of a nematode or two trematode species between hybrid and parental frogs (13). Baird et al. (14) recently found, in the same house mouse system mentioned above, that hybrids between two subspecies have lower helminth loads, the opposite pattern to what was previously found. This finding questions whether parasitic selection against hybrids in this system is consistent enough to prevent gene flow between the parental subspecies. Furthermore, doubt has been raised over whether helminth parasites have a fitness cost on hybrid mice (15). These studies are indicative of a dynamic interaction between hosts and parasites. Most studies have attempted to explain differences in infection levels across diverging host populations in terms of host genes or environmental variation (8). However, in general exogenous selection from environmental variation and differences in host architecture arising from hybridization have not provided satisfactory explanations for the infection scenarios observed (8). Recent explanations for discrepancies between studies have invoked the possibility of Red Queen dynamics leading to dynamic infection scenarios in hybridizing communities over space or time (8, 13, 15), although the empirical data required to fully test this has been inadequate both in spatial and temporal terms (8). Notably, few field or laboratory studies of hybrid parasitism have examined genetic variation in the parasite (8, 15; but see ref. 16). Experimental infections using different house mouse strains have demonstrated that host genotype affects host–protozoa interactions, but these experiments only used a single parasite strain. This is potentially a significant shortcoming, because parasites can evolve faster than their hosts (17) and host populations may be subject to specific parasite variants early in the process of divergence, potentially leading to variation in virulence when transmitted to a different population. Thus, parasite divergence may play a crucial role in host divergence and incipient speciation of their hosts.
We studied geographic variation in the prevalence, infection load, and genetic variation of a virus (beak and feather disease virus, BFDV) infecting a parrot species complex (crimson rosella, Platycercus elegans). The P. elegans complex is a long-postulated example of a “circular overlap” or “ring species,” of which only about 25 have been proposed worldwide (2, 18–20), because it features clinally diverging populations with ongoing gene flow (21, 22) in an approximate horse shoe-shaped distribution, which culminate in a zone of overlap between the most divergent taxa (terminal forms). Such species complexes offer powerful and unique insights into coevolution of traits, population divergence, and speciation (e.g., refs. 20, 23, and 24), but surprisingly, the opportunity presented by such systems has not yet been used to understand host–parasite interactions (20). BFDV occurs in many wild and captive parrot populations worldwide, with the potential to cause high mortality (25, 26). Accordingly, it is considered a significant conservation threat and has been implicated in parrot declines in Australia and globally (27–30). BFDV possesses a single-stranded DNA genome of ∼2,000 nucleotides (31). Like most small single-stranded DNA and RNA viruses, BFDV shows high levels of genetic variation and recombination (27, 32, 33), and evolves rapidly in novel conditions (34), with multiple variant infections present in individual animals (29). This parrot–virus system is thus an excellent candidate to study how pathogens interact with diverging and hybridizing hosts.
We investigated the prevalence and infection load of BFDV over 8 y across a 1,200-km-wide study area, which included the three main host subspecies (Platycercus elegans elegans, Platycercus elegans flaveoulus, Platycercus elegans adelaidae), and a zone of hybridization (Western Slopes or WS hybrid) where the most phenotypically distinct host subspecies overlap (Fig. 1). In this way we could determine the role of host factors (subspecies, sex, age) and ecology (host population density, host community diversity, and composition, temporal, and spatial location) on both viral prevalence and viral load. We also sequenced the virus throughout the host range to determine how it differs in response to host divergence and hybridization, and how it may differ phylogenetically from BFDV virus in other host species. We used these data to test whether BFDV phylogeography supports the hypothesis that P. elegans is a ring species.
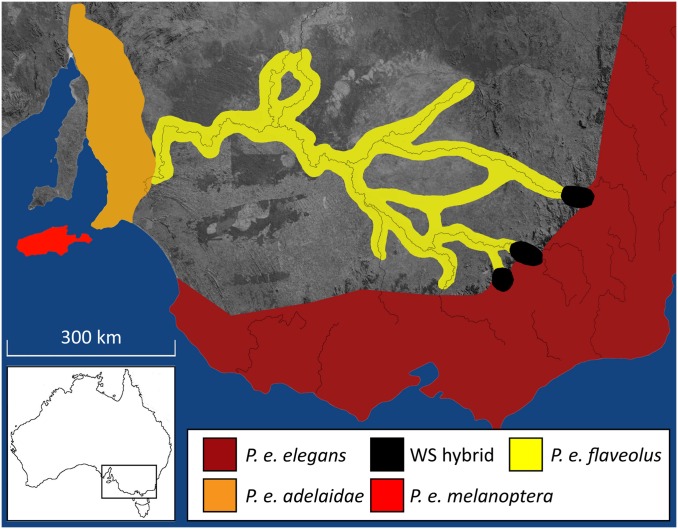
Fig. 1.
Map of Platycercus elegans geographic distribution in south eastern Australia. Colors indicate the approximate range of each subspecies based on observational data from The New Atlas of Australian Birds (53); P. e. melanoptera was not used in this study.
Results
BFDV Prevalence, Infection Load, and Seroprevalence.
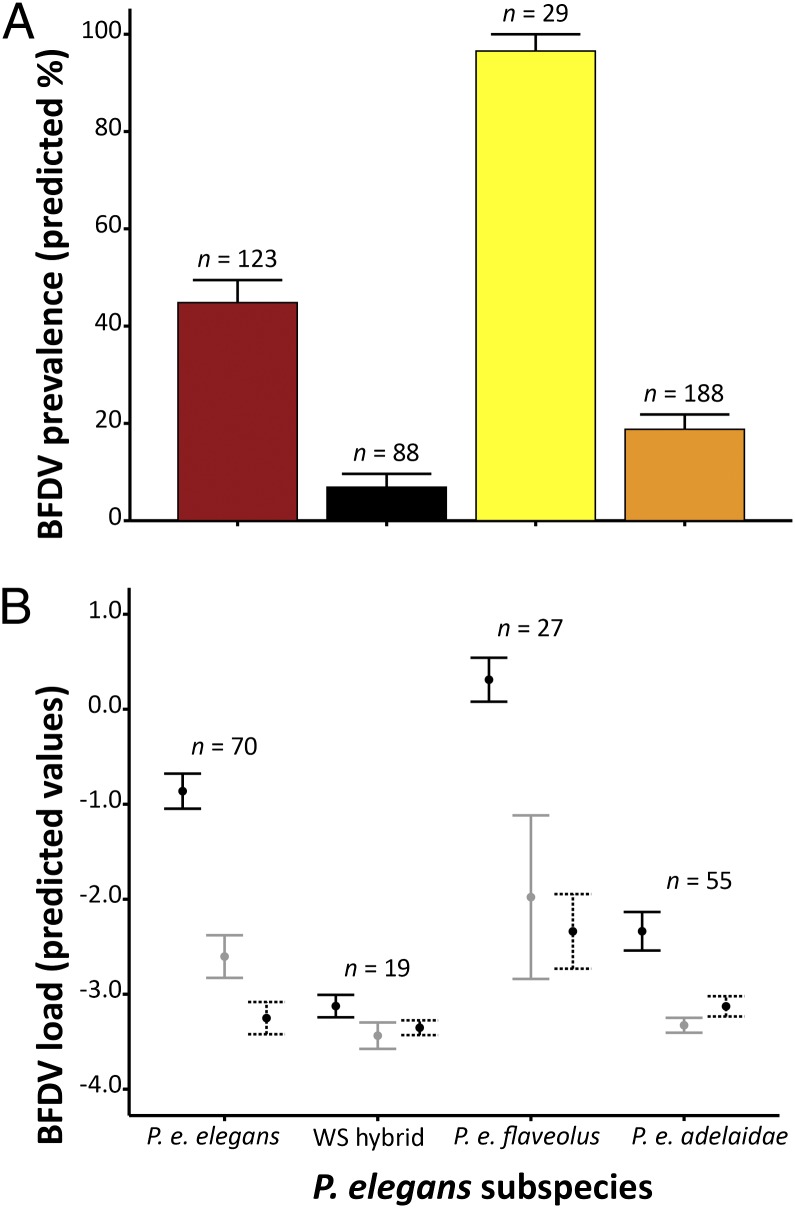
Although we detected BFDV in all subspecies of P. elegans, both prevalence (χ2 = 63.1, df = 3, P < 0.001) and load (Kruskal–Wallis: H = 28.13, df = 3, P < 0.001) varied significantly between the different host subspecies. The phenotypically most distinct subspecies, P. e. elegans (crimson rosella) and P. e. flaveolus (yellow rosella), had the highest BFDV prevalence and load, whereas the phenotypically and geographically intermediate forms (P. e. adelaidae and WS hybrids) had a lower prevalence and load (Fig. 2). Model selection revealed a single best model (wi > 0.9, where wi is weight) predicting prevalence [k = 17, corrected Akaike Information Criterion (AICc) = 1890.205, wi = 0.999], which comprised the terms subspecies, date, host sex, and an interaction between subspecies and date. The null model, containing DNA source (fixed effect), year (random effect), longitude and latitude (fixed effects) received much less support (ΔAICc = 107.73, wi = < 0.0001, evidence ratio = 2.48 × 1023) than the most plausible model, and was ranked 12 of 64 models compared.
Fig. 2.
(A) Model-predicted prevalence (%) of BFDV infection between the two terminal subspecies, P. e. elegans (crimson rosella) and P. e. flaveolus (yellow rosella), and the significantly less infected, P. e. adelaidae and WS hybrid. (B) Model-predicted mean BFDV infection load (log10 gene expression) of infected subspecies and age classes (solid: subadult; shaded: young adult; dotted: old adult). Bars represent 1 SE. Sample sizes of each subspecies (n) in both A and B are included above the bars.
We identified a single best model that predicts BFDV load (k = 27, AICc = 579.077, wi = 0.970). This model included the predictors subspecies, host age, host sex, and an interaction between subspecies × age. In line with the results for prevalence, P. e. elegans and P. e. flaveolus had higher BFDV load than P. e. adelaidae and WS hybrids (Fig. 2B). Subadults (1–2 y of age) showed a higher BFDV load than adults (young adults 2–5 y and old adults >5 y) in both P. e. elegans and P. e. flaveolus (Fig. 2B). Females had lower BFDV load than males. The null model for BFDV load (as above) was less likely than the most plausible models (ΔAICc = 109.16, wi = <0.0001, evidence ratio = 5.06 × 1023). When considering BFDV load of blood and tissue samples separately, we found no difference in the overall results (most plausible model: subspecies + host age + host sex + subspecies × host age) (SI Results and Table S1).
Difference in BFDV prevalence and load at sampling locations was not correlated with the geographic distance between sampling locations (prevalence: Mantel R = −0.125, P = 0.301, load: Mantel R = −0.177, P = 0.211). To further exclude the possibility that BFDV varies according to sampling location, we partitioned our data to include only a 90-km transect encompassing two separate WS hybrid populations (Moyhu/Edi and Bonegilla/Tangambalanga) and two nearby P. e. elegans sampling sites (Stanley, 35 km from Bonegilla/Tangambalanga, and Myrrhee, 15 km from Moyhu/Edi). WS hybrids had a significantly lower prevalence (χ2 = 35.82, df = 3, P < 0.001) and load (Kruskal–Wallis: H = 15.57, df = 3, P = 0.001) despite the close proximity of the subpopulations in these areas. BFDV infection was also not correlated with community diversity (prevalence: Spearman’s r = −0.449, P = 0.264, load: Spearman’s r = −0.289, P = 0.485), or two measures of community composition: Sørensen’s similarity index (prevalence: Mantel R = −0.027, P = 0.188, load: Mantel R = −0.027, P = 0.372) and β-diversity (prevalence: Spearman’s r = 0.449, P = 0.264, load: Spearman’s r = 0.289, P = 0.488). Prevalence at sampling locations was not correlated with P. elegans density (Spearman’s r = −0.419, P = 0.301) but was negatively correlated with load (Spearman’s r = −0.714, P = 0.047). However, this was not significant when using a measure of density that took account of subspecies limits (Spearman’s r = −0.488, P = 0.153).
One individual from P. e. elegans had antibodies for BFDV, although it was a weak signal (antibody titer 1:20). This individual was a subadult and was PCR-positive for BFDV. All other individuals were negative for antibodies or had levels below the detection limit of this test (antibody titer <1:20).
Phylogenetic Inference and Recombination.
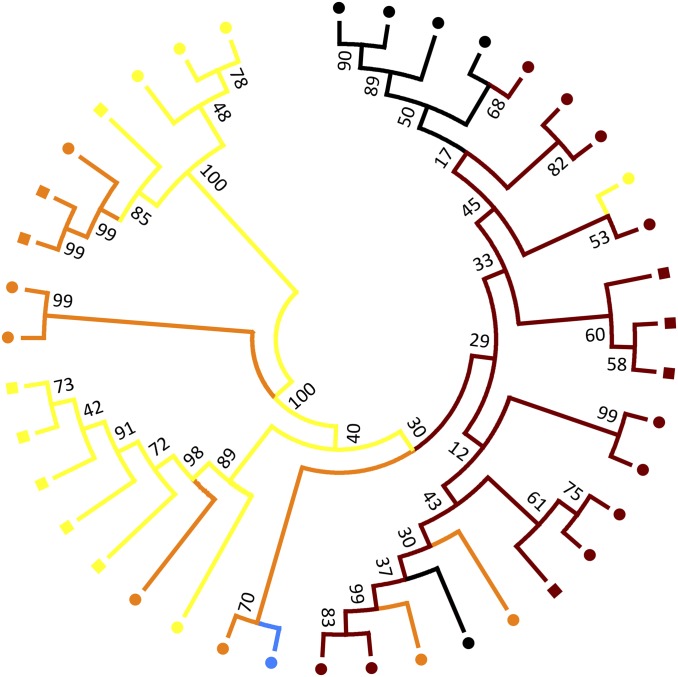
Bayesian phylogenetic inference of all known endemic BFDV sequences shows clear structuring consistent with both host species and host subfamily [association index and (AI) and parsimony score (PS) statistics; see Table S2, and for Bayesian phylogenetic tree see Fig. S1]. Fig. S1 shows that P. elegans BFDV isolates branch out from all other Australian endemic species and that they show a common ancestor with BFDV isolates from Platycercus eximius that were introduced into New Zealand (35). One exception is the presence of a BFDV isolated from Calyptorhynchus lathami, an endangered species that was in captivity. Significant association of subspecies was also found when analyzing BFDV sequences isolated from P. elegans (Table S2). Phylogenetic inference of the 1,629-nt partial BFDV region showed five groups (Fig. 3), with the largest representing all P. e. elegans, some P. e. adelaidae, P. e. flaveolus, and WS populations, and two representing different populations of P. e. flaveolus with some isolates from P. e. adelaidae in each. Within the first and larger group there exists some structure, with hybrids generally clustering together within the distal group. P. e. adelaidae and Platycercus elegans nigrescens formed minor groups within the phylogeny. The capsid ORF (Fig. S2A) was similar to the partial BFDV tree, with only minor changes in topology. There is little support for phylogenetic separation within the partial replication-associated protein ORF sequence, although some topological structure exists, depicting four main groups (Fig. S2B). Noncoding region sequences again show clustering of subspecies, but weaker support for phylogenetic separation (Fig. S2C).
Fig. 3.
Maximum-likelihood phylogenetic tree based on an ∼1,629-nt region of the BFDV genome (support values represent a percentage from 1,000 bootstrap replicates). Colors indicate P. elegans subspecies (see Fig. 1), and shapes indicate evidence of recombination (square = recombination detected, circle = no recombination detected).
We found evidence of recombination in 12 sequences (30%) in our dataset. These recombination events occurred in all subspecies except the WS hybrid (Fig. 3 and Fig. S2); see Table S3 for recombination breakpoints and detection methods. Recombination events did not qualitatively alter phylogenetic inference. This observation was based on comparing trees with and without sequences containing evidence of recombination (Fig. 3 and Fig. S3, respectively).
Isolation-by-Distance and Selection.
We found evidence for weak but significant isolation-by-distance (IBD) in all coding regions of BFDV (Fig. S4). Using the shortest geographic distance between virus sampling locations within the host range, the capsid ORF showed a clear pattern of IBD (Mantel R = 0.407, P = 0.001). Similarly, the partial genome (Mantel R = 0.267, P = 0.001) and replication-associated protein ORF (Mantel R = 0.099, P = 0.001) both showed significant IBD as well, although the effect sizes were smaller. When using the geographic distances corrected to assume no viral gene flow across the WS hybrid zone, these patterns were the same for the capsid ORF (Mantel R = 0.394, P = 0.001), the partial genome sequences (Mantel R = 0.247, P = 0.001), and the replication-associated protein ORF (Mantel R = 0.088, P = 0.001), with similar effect sizes.
Tajima’s neutrality test supported neutral selection in the capsid ORF (Tajima’s D = −0.87, P = 0.2). Splitting subspecies into separate populations supported the same conclusion (Table S4). The dn/ds ratio for the capsid ORF within P. e. adelaidae and P. e. elegans had evidence for positive selection dn/ds > 1 (Table S4). In contrast, the dn/ds ratio for the capsid ORF in all subspecies, P. e. flaveolus, and WS hybrid populations separately was <1, indicating negative selection. Neutrality was rejected for replication-associated protein ORF (Tajima’s D = −1.5, P = 0.05), implying positive selection. Splitting subspecies into separate populations supported neutrality (Table S4). The dn/ds ratios for the replication-associated protein ORF indicated slight purifying selection in all subspecies, except the P. e. elegans population, where dn/ds was >1, indicating positive selection (Table S4).
Discussion
We tested the role of host factors in viral prevalence and load in a hypothesized ring-species host. Our data showed that subspecies was the most important predictor of prevalence and load, with WS hybrids and the phenotypically intermediate subspecies P. e. adelaidae having much lower prevalence and load than the terminal subspecies (P. e. elegans and P. e. flaveolus). Phylogenetic analyses of the virus, geographic location, intraspecific host density, or host community diversity and composition did not explain these striking differences. Our results therefore provide support for differences in susceptibility, mortality, or force of infection between subpopulations. Host–parasite associations are considered responsible for much of the genetic diversity present in wild populations (36), and we propose that BFDV may contribute to the maintenance of diversity in the P. elegans species complex, which has been a long-standing puzzle (1, 2).
Why Is Hybridization Associated with Lower Infection?
Perhaps the most parsimonious hypothesis explaining low BFDV prevalence and load in WS hybrids is that they are more resistant to BFDV infection. Our data suggest that subadult birds have a significantly higher BFDV load than the two adult classes, but this occurs to a lesser degree in WS hybrids and P. e. adelaidae. Higher resistance in the latter two populations could explain the reduced load and faster rate to baseline load levels that appear consistent across all subspecies in the old adult age class. Reduced pathogen infection among hybrid populations compared with parental species has rarely been identified in both plant and animal systems, but is particularly rare in animal models (8–10). Investigations of animal hybrid systems that have found hybrid resistance patterns (reduced parasite prevalence and load) (9, 12, 14) have typically been contradicted in subsequent studies where hybrids were found to have a higher prevalence than the parental species (8). This inconsistency has been hypothesized to result from frequency-dependant selection (8, 14). However, our data suggest that the lower BFDV prevalence and load in WS hybrids and the subspecies P. e. adelaidae are temporally consistent, at least over the 8 y of this study. This hybrid-resistance scenario is dependent on the assumption that lower prevalence and load represents higher resistance; however, other explanations are possible. The lower prevalence and load in WS hybrid and P. e. adelaidae could alternatively be explained by a lower susceptibility, which does not necessarily depend on factors that influence resistance. The host community diversity or composition, or the density of P. elegans populations, can be hypothesized to contribute to variation in exposure and the force of infection. However, analyses of these factors indicated that they did not account for our findings concerning prevalence and load. Our assessment of BFDV seroprevalence, using the most sensitive assay available, to determine if there was variation in BFDV exposure (i.e., higher seroprevalence would suggest higher exposure) revealed surprisingly that all except one individual were negative for antibodies. However, this result is consistent with equal exposure among all subspecies and hybrid populations. Antibody levels that are absent or below the detection limit may also arise from high tolerance to BFDV in P. elegans. Higher resistance, and subsequently lower susceptibility in hybrids that is constant, could indicate a lower selective pressure and could favor hybridization between P. e. elegans and P. e. flaveolus. This hybrid advantage could potentially explain the maintenance of the WS hybrid population and therefore the maintenance of a genetic bridge, preventing complete speciation between P. e. elegans and P. e. flaveolus.
An alternate hypothesis to explain our results is that WS hybrids and P. e. adelaidae are more susceptible to BFDV. Many studies conclude that a hybrid susceptibility model is appropriate based on the observation that hybrids have a higher parasite prevalence and load (reviewed in ref. 8). However, lower parasite prevalence and load within a population may also indicate susceptibility by representing a higher mortality of infected individuals in that population. Therefore, it is possible that lower BFDV prevalence/load indicates higher mortality in WS hybrids and P. e. adelaidae and that a higher BFDV prevalence/load represents tolerance in P. e. elegans and P. e. flaveolus. In this scenario, BFDV could potentially be promoting speciation between P. e. elegans and P. e. flaveolus. One mechanism that could explain hybrid susceptibility is through genetic admixture between P. e. elegans and P. e. flaveolus, which could lead to a breakdown of coadapted gene complexes. This situation arises when parental populations diverge sufficiently enough that, during secondary contact, alleles that have evolved with one genome are less functional with another (9, 14). Alternatively, genetic admixture between P. e. elegans and P. e. flaveolus is likely to produce rare host variants that are less susceptible to pathogens, which are adapted to common host genotypes, as a Red Queen model might predict (8). This situation could also result in more genetically diverse populations with a greater number of heterozygous individuals. It is generally accepted that more genetically diverse individuals and populations have a lower prevalence and load than more inbred populations (reviewed in ref. 4). This genetic mechanism could explain a hybrid-resistant model, as suggested above and in studies that have measured intermediate or higher immunity in avian hybrid systems (37, 38).
Viral Phylogenetic Structure.
Hosts exert selective pressure on their pathogens and in some cases this can lead to pathogen specialization and perhaps even speciation of the pathogen (4). There is substantial evidence in our data to suggest that BFDV is genotypically associated with host species and that BFDV sequences isolated within P. elegans are specific to this species, although there does appear to be evidence for isolate spill-over. The most notable example is C. lathami, a captive endangered cockatoo species that was likely exposed to wild P. elegans. Similar BFDV spill-over and host-switch events have been shown recently for the endangered orange-bellied parrot (Neophema chrysogaster) (28, 29). Our results showed that the partial BFDV genome had phylogenetic structure consistent with the host from which it was sampled with five groups, two of which represent different P. e. flaveolus sampling locations (Fig. 3). The larger group in Fig. 3 contains all BFDV sequences from P. e. elegans but also BFDV samples from the WS hybrids. This finding was surprising, as we might expect that BFDV in a host hybrid zone would be intermediate between P. e. elegans and P. e. flaveolus, particularly as the host mitochondrial data suggest that WS hybrids cluster with both parental species (21). However, this finding is consistent with the host microsatellite data, which show phenotypic WS hybrids clustering with P. e. elegans (21). P. e. adelaidae subspecies were found to have BFDV sequences that occurred in all groups in Fig. 3. This pattern is consistent with an intermediate population separating the terminal host subspecies, but is also consistent with the host’s mitochondrial data (21). However, the host’s microsatellite data indicate that there is a genetic discontinuity between P. e. adelaidae and P. e. flaveolus birds. Therefore, the microsatellite data are inconsistent with P. e. adelaidae BFDV data that suggest gene flow between P. e. flaveolus and P. e. adelaidae BFDV sequences. The nucleotide region that encodes the capsid protein had a similar phylogeography as the partial genome mentioned above (Fig. S2A). However, there is little support for all groups to be considered separate clades. BFDV capsid sequences from P. e. elegans seem to be closely related to one BFDV population from P. e. flaveolus (Fig. S2A) (1), a result likely to be explained by evidence for recombination in those BFDV sequences from P. e. flaveolus. This evidence for gene flow between isolates and the relatively conserved nature of the replication-associated protein region may explain the lack of phylogenetic separation in this coding region. Overall, BFDV in this system appears to have coevolved with the terminal subspecies, but only limited specialization has occurred, allowing the intermediate subspecies (P. e. adelaidae) to be infected. Across hybrid zones parasite isolates have been found to specialize on parental species, leaving F1 hybrids unaffected (16). However, our results are more consistent with one other study that found that only one parental parasite species was able to infect the hybrid population (39).
Evidence for the Ring-Species Concept Within P. elegans.
As highlighted in recent studies (18, 21, 24), ring species provide powerful insights into speciation because we may infer from spatial variation in populations how divergence can proceed over time, and because they show how divergence and reproductive isolation can occur despite gene flow. Ring species feature two distinct and nonrecombining “terminal” populations that meet upon secondary contact, yet are connected through a series of intermediate populations with ongoing gene flow encircling unsuitable habitat (2, 18). As the ancestral populations expand around the unsuitable habitat, divergence around the ring is thought to arise, at least in part, because of IBD (24). However, ring species are exceptionally rare and—moreover to our knowledge—have not been studied with respect to their pathogens. The ring-species model has formerly only been applied to animals and plants and its study has presented numerous challenges (18). P. elegans has long been held to be a possible example of a ring species, but conclusive evidence has remained elusive (2, 19, 21). Genomic analyses of highly diverse and rapidly evolving pathogens, such as viruses, can offer unique inferences about host population history (40–42). Our findings show how this can offer a novel perspective on the long-standing evolutionary puzzle of ring species.
The ring species model invokes three key predictions (2, 18, 21). First, a genetic discontinuity is predicted where the terminal forms meet. In line with this prediction, we did not find any evidence for recombination within the BFDV sequences from the WS host populations at the east of the ring, although the sample size was small. This lack of recombination and the fact that BFDV sequences from WS hybrid hosts are among the most genetically dissimilar to P. e. flaveolus BFDV sequences (Fig. 3 and Figs. S1 and S4) suggests that there is BFDV genetic discontinuity across the host hybrid zone. Second, the model predicts that the distinct terminal forms are connected by a series of intermediate populations, with gene flow encircling unsuitable habitat. Our BFDV phylogenies, in conjunction with the tip-association test, revealed that BFDV isolates from P. e. flaveolus and P. e. elegans form separate clades. In geographically and phenotypically intermediate P. e. adelaidae hosts in the western end of the ring, virus variants representing all main clades were observed (Fig. 3). Third, a pattern of IBD around the ring, from one terminal form to the other, is predicted. We found significant IBD within the BFDV genome. However, IBD did not account for the majority of genetic divergence in BFDV, suggesting other processes, such as selection, may also contribute substantially to BFDV genetic variation. Although tests of selection suggested that much of the genetic variation in BFDV is neutral, some evidence for positive selection was found in the replication-associated coding region; this region also showed the weakest IBD (Mantel R = 0.099). Weak patterns of IBD may be caused by recent range expansion (24, 43), which could arise if ancestral BFDV originated in species other than P. elegans (as supported by our phylogeny) (Fig. S1) and crossed to P. elegans relatively recently.
Overall, our data suggest that gradual evolutionary changes around a large geographic barrier may have accumulated and resulted in genetic discontinuity of BFDV at the terminal ends of the distribution. As such, these patterns in BFDV are analogous to the key predictions outlined under the ring-species concept, and thus support the notion that divergence in the face of gene flow, to the extent that recombination may be reduced or eliminated, is possible in such viruses. We suggest that our data represent a worthy first step in identifying whether quasi-species–like variation in BFDV in P. elegans offers, to our knowledge, the first known example of a pathogen analog of a ring species. Further work will be required to test predictions arising from this conclusion, and could offer important advances in our understanding of the role of host–pathogen interactions in speciation.
Conclusions
We show that a phenotypically intermediate subspecies (P. e. adelaidae) and hybrid populations (WS) have much lower BFDV prevalence and load than their parental subspecies (P. e. elegans and P. e. flaveolus). We found no evidence that the results were explained by potential geographic confounds, P. elegans population density, or host community diversity and composition. Our phylogenetic analyses of BFDV sequence variation provides empirical support for the ring-species concept, and thereby demonstrates a novel approach to testing ring-species predictions in hosts and pathogens. Hybrid zones and ring-species complexes are natural laboratories that have greatly enhanced our understanding of speciation processes. We hypothesize that BFDV infection in P. elegans gives rise to differential selective pressures with implications for the maintenance of host diversity, and potentially host speciation. Further study of such systems should provide powerful new insights into how hosts and parasites diverge and coevolve.
Materials and Methods
Study Species and Sampling.
This work was approved by Deakin University Animal Ethics Committee. We studied the crimson rosella (P. elegans) species complex, which consists of several geographically continuous but phenotypically divergent subspecies encircling unsuitable habitat (Fig. 1). This species complex contains three distinct subspecies (Adelaide, P. e. adelaidae; crimson, P. e. elegans; and yellow P. e. flaveolus; the latter two subspecies culminating in three zones of overlap, Western Slopes hybrid) (Fig. 1) (21). To sample BFDV, we collected whole blood or muscle tissue samples between the years 2004 and 2011. Birds were caught at the nest or in walk-in traps. For detailed sampling locations (Table S5), primers (Table S6), and techniques, see SI Materials and Methods.
BFDV Seroprevalence, PCR Detection, and Sequencing.
DNA was extracted using a standard ammonium acetate method and birds were sexed following ref. 44. Samples from 406 individuals were screened for BFDV using a probe based quantitative real-time PCR (qRT-PCR) developed and tested in this species. Seroprevalence was assayed using hemagglutination inhibition as described in ref. 45. See SI Materials and Methods for a detailed description of these techniques.
Phylogenetic Inference, Recombination, and Selection.
Maximum-likelihood phylogenies of each sequence subset [Capsid ORF, noncoding region, partial replication-associated protein ORF, and all regions concatenated (partial BFDV genome)] were inferred using MEGA (46). The program BaTS (Bayesian Tip-association Significance testing) was used to test for a host species association among the phylogenetic tips using all known BFDV sequences from endemic Australian host species but also among P. elegans subspecies in a separate analysis (47). MrBayes was used to produce a posterior set of trees to be used in BaTS (48). Recombination was tested using RDP 4.16 (49). DnaSP v5 was used to test for selection (50). We used the Mantel test function in the Genalex 6.5 add-in for Microsoft Excel 2007 (51) to test for IBD (SI Materials and Methods).
Statistical Analyses.
Using the statistics package SPSS 22 (IBM) we created a series of generalized linear mixed models (all subset approach) to analyze two response variables: (i) individual infection status (infected or not infected) and (ii) BFDV infection load. Fixed predictors included: (i) subspecies, (ii) age, (iii) Julian date, (iv) Julian date2, and (v) host sex. DNA source (blood/tissue), longitude and latitude were controlled for in all models as fixed effects. The corrected AIC was used to select the best-fitting models and calculate the weights (wi) for each model and predictor (52). In separate analyses we tested whether geographic location (0.5 × 0.5 decimal degree grid squares), P. elegans population density, or the community diversity (species richness) and composition (β-diversity) of potential hosts (Psittaciformes species), were predictors of prevalence and load (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank all the landowners who allowed us on to their properties and many field work volunteers; Darren Martin and Ben Longdon for phylogenetic advice; Steve Cooper plus staff at the Australian Animal Health Laboratory for helpful comments; and two anonymous reviewers for greatly improving this manuscript. This study was supported in part by the Biotechnology and Biological Sciences Research Council, Leverhulme Trust, Birdlife Australia, Holsworth Wildlife Research Endowment, Deakin University’s Centre for Integrative Ecology, and the Metabolic Research Unit.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KJ953846–KJ953885).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403255111/-/DCSupplemental.
References
- 1.Mayr E. Systematics and the Origin of Species, from the Viewpoint of a Zoologist. New York: Columbia Univ Press; 1942. [Google Scholar]
- 2.Mayr E. Animal species, evolution, and geographic isolation—Reply. Syst Zool. 1963;12(4):204–206. [Google Scholar]
- 3.Haldane JBS. Disease and evolution. Ric Sci. 1949;19:68–76. [Google Scholar]
- 4.Schmid-Hempel P. Evolutionary Parasitology. New York: Oxford Univ Press; 2011. [Google Scholar]
- 5.Fritz RS. Resistance of hybrid plants to herbivores: Genes, environment, or both? Ecology. 1999;80(2):382–391. [Google Scholar]
- 6.Sage RD, Heyneman D, Lim K-C, Wilson AC. Wormy mice in a hybrid zone. Nature. 1986;324(6092):60–63. doi: 10.1038/324060a0. [DOI] [PubMed] [Google Scholar]
- 7.Barton NH, Hewitt GM. Adaptation, speciation and hybrid zones. Nature. 1989;341(6242):497–503. doi: 10.1038/341497a0. [DOI] [PubMed] [Google Scholar]
- 8.Wolinska J, Lively CM, Spaak P. Parasites in hybridizing communities: The red queen again? Trends Parasitol. 2008;24(3):121–126. doi: 10.1016/j.pt.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Moulia C, Joly P. Parasitism and hybrid zones. In: Thomas F, Guégan J-F, Renaud F, editors. Ecology and Evolution of Parasitism. Oxford: Oxford Univ Press; 2009. pp. 69–82. [Google Scholar]
- 10.Fritz RS, Moulia C, Newcombe G. Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu Rev Ecol Syst. 1999;30:565–591. [Google Scholar]
- 11.Moulia C, et al. Wormy mice in a hybrid zone: A genetic control of susceptibility to parasite infection. J Evol Biol. 1991;4(4):679–687. [Google Scholar]
- 12.Joly P, et al. Heterozygosity and parasite intensity: Lung parasites in the water frog hybridization complex. Parasitology. 2008;135(Pt 1):95–104. doi: 10.1017/S0031182007003599. [DOI] [PubMed] [Google Scholar]
- 13.Planade B, et al. Tracking a heterosis effect in the field: Tadpole resistance to parasites in the water frog hybridogenetic complex. Parasitology. 2009;136(9):1003–1013. doi: 10.1017/S0031182009006489. [DOI] [PubMed] [Google Scholar]
- 14.Baird SJE, et al. Where are the wormy mice? A reexamination of hybrid parasitism in the European house mouse hybrid zone. Evolution. 2012;66(9):2757–2772. doi: 10.1111/j.1558-5646.2012.01633.x. [DOI] [PubMed] [Google Scholar]
- 15.Gouy de Bellocq J, Ribas A, Baird SJE. New insights into parasitism in the house mouse hybrid zone. In: Macholán M, Baird SJE, Munclinger P, Piálek J, editors. Evolution of the House Mouse. Cambridge: Cambridge Univ Press; 2012. pp. 455–481. [Google Scholar]
- 16.Jackson JA, Tinsley RC. Parasite infectivity to hybridising host species: A link between hybrid resistance and allopolyploid speciation? Int J Parasitol. 2003;33(2):137–144. doi: 10.1016/s0020-7519(02)00255-2. [DOI] [PubMed] [Google Scholar]
- 17.Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: Patterns and determinants. Nat Rev Genet. 2008;9(4):267–276. doi: 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
- 18.Alcaide M, Scordato ESC, Price TD, Irwin DE. Genomic divergence in a ring species complex. Nature. 2014;511(7507):83–85. doi: 10.1038/nature13285. [DOI] [PubMed] [Google Scholar]
- 19.Cain AJ. A revision of Trichoglossus haematodus and of the Australian Platycercine parrots. Ibis. 1955;97(3):432–479. [Google Scholar]
- 20.Irwin DE, Bensch S, Price TD. Speciation in a ring. Nature. 2001;409(6818):333–337. doi: 10.1038/35053059. [DOI] [PubMed] [Google Scholar]
- 21.Joseph L, et al. Where and when does a ring start and end? Testing the ring-species hypothesis in a species complex of Australian parrots. Proc Biol Sci. 2008;275(1650):2431–2440. doi: 10.1098/rspb.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribot RFH, et al. Learned vocal variation is associated with abrupt cryptic genetic change in a parrot species complex. PLoS ONE. 2012;7(12):e50484. doi: 10.1371/journal.pone.0050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin DE. Song variation in an avian ring species. Evolution. 2000;54(3):998–1010. doi: 10.1111/j.0014-3820.2000.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 24.Irwin DE, Bensch S, Irwin JH, Price TD. Speciation by distance in a ring species. Science. 2005;307(5708):414–416. doi: 10.1126/science.1105201. [DOI] [PubMed] [Google Scholar]
- 25.Raidal SR, McElnea CL, Cross GM. Seroprevalence of psittacine beak and feather disease in wild psittacine birds in New South Wales. Aust Vet J. 1993;70(4):137–139. doi: 10.1111/j.1751-0813.1993.tb06105.x. [DOI] [PubMed] [Google Scholar]
- 26.Rahaus M, Wolff MH. Psittacine beak and feather disease: A first survey of the distribution of beak and feather disease virus inside the population of captive psittacine birds in Germany. J Vet Med B Infect Dis Vet Public Health. 2003;50(8):368–371. doi: 10.1046/j.1439-0450.2003.00696.x. [DOI] [PubMed] [Google Scholar]
- 27.Heath L, et al. Evidence of unique genotypes of beak and feather disease virus in southern Africa. J Virol. 2004;78(17):9277–9284. doi: 10.1128/JVI.78.17.9277-9284.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, et al. Evidence of psittacine beak and feather disease virus spillover into wild critically endangered Orange-bellied Parrots (Neophema chrysogaster) J Wildl Dis. 2014;50(2):288–296. doi: 10.7589/2013-05-121. [DOI] [PubMed] [Google Scholar]
- 29.Sarker S, et al. Phylogeny of beak and feather disease virus in cockatoos demonstrates host generalism and multiple-variant infections within Psittaciformes. Virology. 2014;460-461(0):72–82. doi: 10.1016/j.virol.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Kundu S, et al. Tracking viral evolution during a disease outbreak: The rapid and complete selective sweep of a circovirus in the endangered Echo parakeet. J Virol. 2012;86(9):5221–5229. doi: 10.1128/JVI.06504-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassami MR, Ypelaar I, Berryman D, Wilcox GE, Raidal SR. Genetic diversity of beak and feather disease virus detected in psittacine species in Australia. Virology. 2001;279(2):392–400. doi: 10.1006/viro.2000.0847. [DOI] [PubMed] [Google Scholar]
- 32.Varsani A, Regnard GL, Bragg R, Hitzeroth II, Rybicki EP. Global genetic diversity and geographical and host-species distribution of beak and feather disease virus isolates. J Gen Virol. 2011;92(Pt 4):752–767. doi: 10.1099/vir.0.028126-0. [DOI] [PubMed] [Google Scholar]
- 33.Julian L, et al. Extensive recombination detected among beak and feather disease virus isolates from breeding facilities in Poland. J Gen Virol. 2013;94(Pt 5):1086–1095. doi: 10.1099/vir.0.050179-0. [DOI] [PubMed] [Google Scholar]
- 34.Domingo-Calap P, Sanjuán R. Experimental evolution of RNA versus DNA viruses. Evolution. 2011;65(10):2987–2994. doi: 10.1111/j.1558-5646.2011.01339.x. [DOI] [PubMed] [Google Scholar]
- 35.Massaro M, et al. Molecular characterisation of beak and feather disease virus (BFDV) in New Zealand and its implications for managing an infectious disease. Arch Virol. 2012;157(9):1651–1663. doi: 10.1007/s00705-012-1336-5. [DOI] [PubMed] [Google Scholar]
- 36.Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85(Pt 2):411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 37.Tompkins DM, Mitchell RA, Bryant DM. Hybridization increases measures of innate and cell-mediated immunity in an endangered bird species. J Anim Ecol. 2006;75(2):559–564. doi: 10.1111/j.1365-2656.2006.01076.x. [DOI] [PubMed] [Google Scholar]
- 38.Wiley C, Qvarnström A, Gustafsson L. Effects of hybridization on the immunity of collared Ficedula albicollis and pied flycatchers F. hypoleuca, and their infection by haemosporidians. J Avian Biol. 2009;40(4):352–357. [Google Scholar]
- 39.Heaney LR, Timm RM. Morphology, genetics, and ecology of pocket gophers (genus Geomys) in a narrow hybrid zone. Biol J Linn Soc Lond. 1985;25(4):301–317. [Google Scholar]
- 40.Falush D, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299(5612):1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 41.Biek R, Drummond AJ, Poss M. A virus reveals population structure and recent demographic history of its carnivore host. Science. 2006;311(5760):538–541. doi: 10.1126/science.1121360. [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto C, et al. Typing of urinary JC virus DNA offers a novel means of tracing human migrations. Proc Natl Acad Sci USA. 1997;94(17):9191–9196. doi: 10.1073/pnas.94.17.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slatkin M. Isolation by distance in equilibrium and non-equilibrium populations. Evolution. 1993;47(1):264–279. doi: 10.1111/j.1558-5646.1993.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 44.Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Mol Ecol. 1998;7(8):1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 45.Khalesi B, Bonne N, Stewart M, Sharp M, Raidal S. A comparison of haemagglutination, haemagglutination inhibition and PCR for the detection of psittacine beak and feather disease virus infection and a comparison of isolates obtained from loriids. J Gen Virol. 2005;86(Pt 11):3039–3046. doi: 10.1099/vir.0.81275-0. [DOI] [PubMed] [Google Scholar]
- 46.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker J, Rambaut A, Pybus OG. Correlating viral phenotypes with phylogeny: Accounting for phylogenetic uncertainty. Infect Genet Evol. 2008;8(3):239–246. doi: 10.1016/j.meegid.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin DP, et al. RDP3: A flexible and fast computer program for analyzing recombination. Bioinformatics. 2010;26(19):2462–2463. doi: 10.1093/bioinformatics/btq467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 51.Peakall R, Smouse P. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics. 2012;28(19):2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Symonds MRE, Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s Information Criterion. Behav Ecol Sociobiol. 2011;65(1):13–21. [Google Scholar]
- 53.Barrett G, Silcocks A, Barry S, Cunningham R, Poulter R. The New Atlas of Australian Birds. Australia: Royal Australasian Ornithologists Union, Hawthorn East, VIC; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.