Abstract
Prostate cancer is considered a disease of older men, but today over 10% of new diagnoses occur in U.S. men ≤ 55 years. Early onset prostate cancer, i.e., diagnosed at ≤55 years, differs from prostate cancer in older men in several ways. Among men diagnosed with high grade and stage prostate cancer, men with early onset prostate cancer are more likely to die of their cancer, with higher cause-specific mortality than all others except those diagnosed over age 80. This suggests that important biological differences may exist in early onset disease compared to late onset disease. Furthermore, early onset prostate cancer has been shown to have a more significant genetic component indicating that this group may benefit more than most from evaluation of genetic risk. Clinically, although the majority of cases ≤ 55 years are diagnosed with low risk disease, their extended life expectancy exposes them to long-term risk of disease progression resulting in death from prostate cancer, but also to prolonged impact from treatment-related morbidities. These patients pose unique challenges and opportunities for both the research and clinical communities. We therefore suggest that early onset prostate cancer is a distinct phenotype, from both an etiologic and clinical perspective, that deserves further attention.
Incidence and mortality in young men with prostate cancer
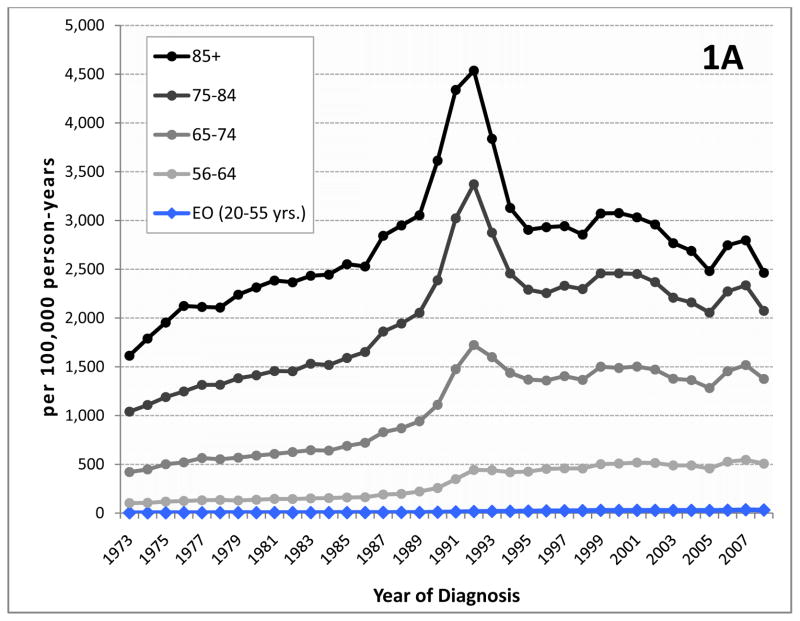
Prostate cancer is the most common non-cutaneous malignancy diagnosed in US men. Approximately 10% of the 241,740 men diagnosed with prostate cancer in 2012 represent early-onset prostate cancer defined herein as men diagnosed at 55 years of age or younger1, 2. There has been an increase in prostate cancer incidence since prostate cancer screening with serum prostate-specific antigen (PSA) was introduced. Specifically, the incidence of prostate cancer in young men increased by 5.7-fold (95% CI 5.0, 6.7) between 1986 and 2008 from 5.6 to 32 cases per 100,000 person years2. Over the same period, the rising incidence across all ages(from 119 to 163 cases per 100,000) made prostate cancer the most commonly diagnosed non-cutaneous cancer in men3, but the disproportionate burden of the increase among younger men is not well appreciated. Although the median age of prostate cancer diagnosis has shifted toward younger ages, decreasing from 72 years in 1986 to 67 years in 20092, this does not account for the steep rise seen in the rate of early onset prostate cancer. During the last two decades, this group of young men has experienced a greater increase in incidence than any other age group (Figure 1). This development may be an especially important concern given existing data suggesting that survival in this cancer varies by age at diagnosis.
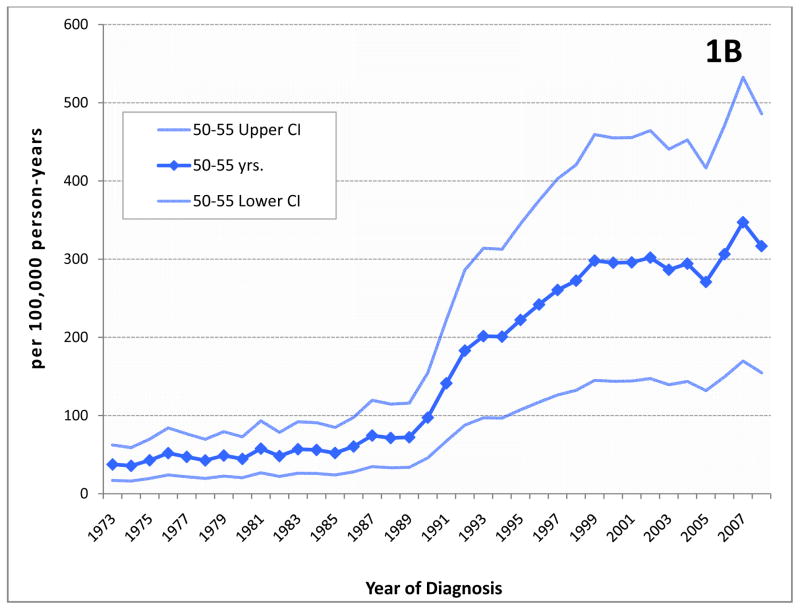
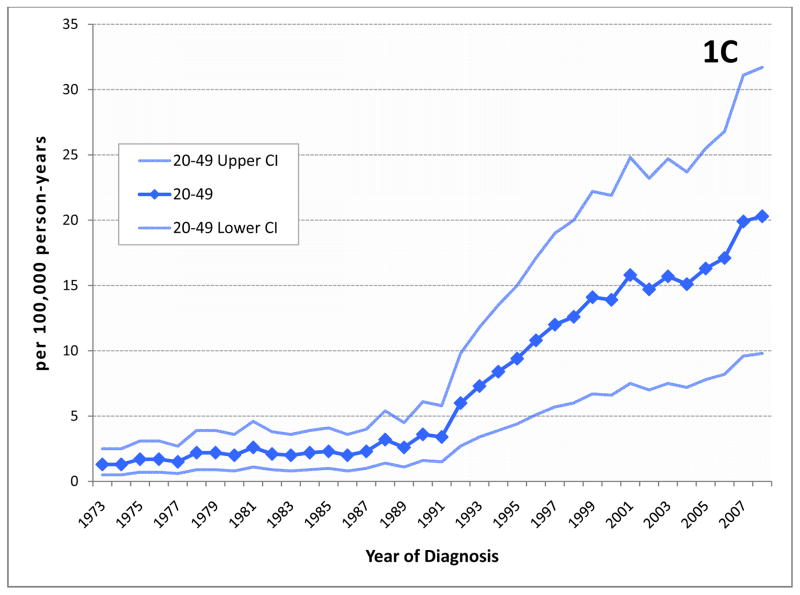
Figure 1.
Incidence of prostate cancer by age group.(A) The age-adjusted incidence of prostate cancer increased dramatically during the late 1980’s and early 1990’s as a result of screening with serum PSA. Since then, incidence has decreased or stabilized among most age groups. Although prostate cancer in men aged ≤55 (early onset prostate cancer or EO) represents only a portion of all men diagnosed with this disease, incidence in this group continues to rise. (B) Incidence in men 50–55 years at diagnosis, among whom screening was commonly recommended by most medical organizations until recently, may have stabilized to some extent, however, (C) among younger men who are not expected to have been screened with PSA, incidence rates continue to rise. (Note the different scale for each chart.)
BOX.
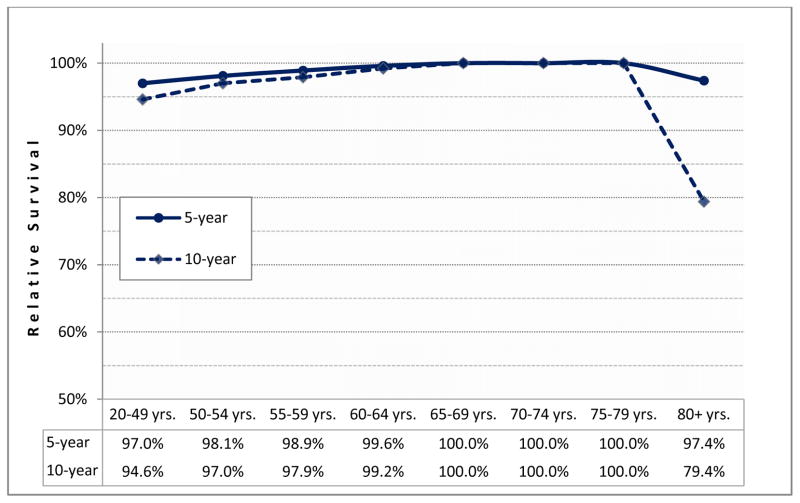
Relative survival: compares the observed proportion of survivors among men diagnosed with prostate cancer to the expected proportion of survivors in a similarly aged group of men of the same race over the same period. It is an effective measure of cancer survival in the absence of other competing causes of death.(Figure 2)
Figure 2. Relative survival of prostate cancer cases by age at diagnosis (1994–2008).
The survival of men diagnosed with prostate cancer is compared to the survival of men with similar demographic characteristics from the US population using SEER data4. A reduction in relative survival from 100% demonstrates the impact of death due to prostate cancer. Among men diagnosed between 1994–2008, those diagnosed with prostate cancer before age 55 have worse 5- and 10-year survival than all other men except the elderly (80+ years at diagnosis).
The majority of men diagnosed with prostate cancer today have a favorable prognosis, with the most recent estimates for five- and ten-year relative survival being 100.0% and 98.7%, respectively, based on data from men diagnosed between 1994–2009 in seventeen Surveillance, Epidemiology, and End Results (SEER) registries4. These estimates reflect the fact that the majority of prostate cancers are identified early in the natural history of the tumor when they can be treated with curative intent and advanced stage disease makes up only a small proportion of total diagnoses. Moreover, younger men with prostate cancer may also have fewer comorbid conditions that might complicate treatment choice or response5 and may also be more likely to receive aggressive treatment compared to older cases6, 7.
Consistent with this, several clinical studies report no significant difference in survival across age groups8–11 or an improved prognosis in the youngest men12–18 after radical prostatectomy19, 20, brachytherapy21–23 or radiation therapy24–26. However, other studies, based on cancer registry or other data point to consistently lower survival for the youngest patients27, 28, worse than for all other age groups except men diagnosed at over 80 years. Among men living in the US, data from the SEER cancer registries, representing 28% of the population, reveal that recently diagnosed (1994–2008) men between 20–54 years have a 5-year relative survival of 98.0% whereas the survival of men diagnosed during the same period but aged 55–79 years was 100.0%4. This is not a novel observation. The notion that early age at onset is associated with worse prognosis was common among physicians in the pre-PSA era. It was also the subject of several early European and U.S. studies8, 9, 12, 13, 27, 29–33, including large population-based cancer registry cohorts in the U.S.31, Sweden8, and Scotland27.
Merrill and Bird examined 5-year survival among men 40 years and older diagnosed between 1973–1997 and observed the strongest evidence for poor survival in the youngest age group (<50 years) among men diagnosed with advanced or unknown stage or grade prostate cancers31. Lin and colleagues considered 318,774 men diagnosed at 35–74 years between 1988–2003 from SEER data. They found that men diagnosed at ≤55 years were more likely to have lower grade cancers. Conversely, among men with high Gleason grade or locally advanced cancer at diagnosis, younger men had particularly poor prognoses34. Specifically, men diagnosed with stage IV cancer and aged 35–44 years had an approximately 1.5-fold greater risk of dying of their cancer compared to men aged 65–74 years. Similarly, men diagnosed with high-grade tumors (Gleason grade 8–10) and aged 35–44 years were 1.4 times as likely to die of their prostate cancer compared to men aged 65–74 years at diagnosis. Young men diagnosed with both high grade and stage IV disease also had an increased risk of prostate cancer-specific death compared to the oldest men, although this was not statistically significant (HR=1.24, 95% CI 0.86–1.78), however only a small number of cases had these features. Both studies suggest that the poor prognosis seen for men with early onset prostate cancer was driven by men diagnosed with severe disease, i.e., advanced stage or grade at diagnosis. A European study with cases diagnosed between 1966–1976 found similar results27.
Many contemporary clinical studies that considered survival (or surrogate markers of survival such as biochemical recurrence) after treatment with curative intent focused on men with organ-confined or early stage disease who were eligible for such therapies19–26, 32. As a result, these studies would probably not have included younger men with high-grade or metastatic tumors34. Therefore, most clinical studies of prostate cancer that have compared outcomes between younger and older cases have underrepresented or excluded the very early onset cases with the worst prognoses. Studies that evaluated prostate cancer relative survival by age across all disease stages and grades have uncovered the observation that early onset cases may have worse relative survival than all others except the elderly, i.e., those diagnosed at greater than 80 years of age.
Modeling of Early Onset Prostate Cancer
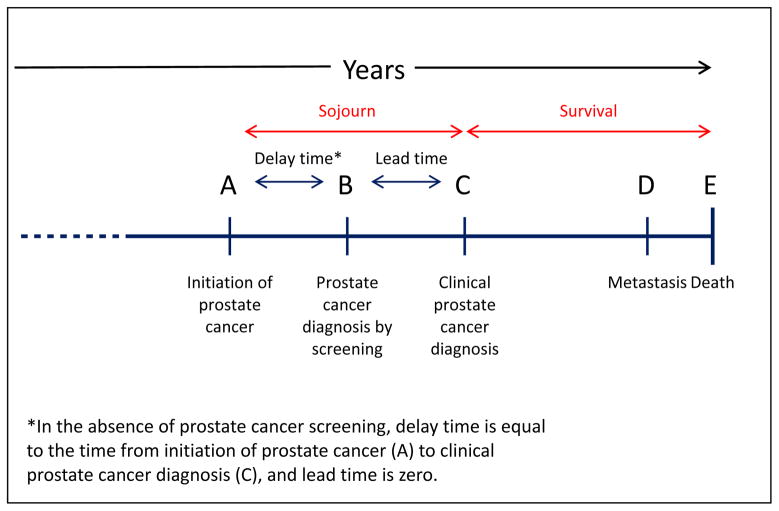
The unexpectedly poor prognosis of advanced early onset prostate cancer lends support to the idea that a novel clinical subtype may exist in the subset of men with early onset prostate cancer. The diagnosis of prostate cancer at a young age naturally selects for more rapidly growing or aggressive tumors. Figure 3 demonstrates the natural history of prostate cancer from initiation to detection, either by screening in asymptomatic men or by clinical symptoms, and eventually metastasis and death. In young cases, the period available for tumor growth between onset and detection by screening or symptoms is necessarily compressed compared to older men. For example, a tumor that begins to grow at 40 years of age and is then diagnosed through either prostate cancer screening or as a result of symptoms will have progressed to a detectable state in a single year if diagnosed in a patient at age 41. By comparison, if a different man has a tumor that also begins to grow at age 40 and is diagnosed at age 68, this cancer will have taken three decades to attain detection. Although the shortened sojourn time for prostate cancer in young men does not preclude the existence of rapidly growing tumors in older men, but it does suggest that the most aggressive tumors will more commonly occur in early onset prostate cancer.
Figure 3. Natural history of prostate cancer.
This figure illustrates the course of prostate cancer from initiation (A), to diagnosis by screening, (B), to diagnosis based upon clincial symptoms (C), to clinically detectable metastatic disease (D), and finally to death from prostate cancer (E). The “sojourn” time is the time between cancer initiation (A) and clinical detection (C). Survival is the time between cancer diagnosis (C) and death form the disease (E).
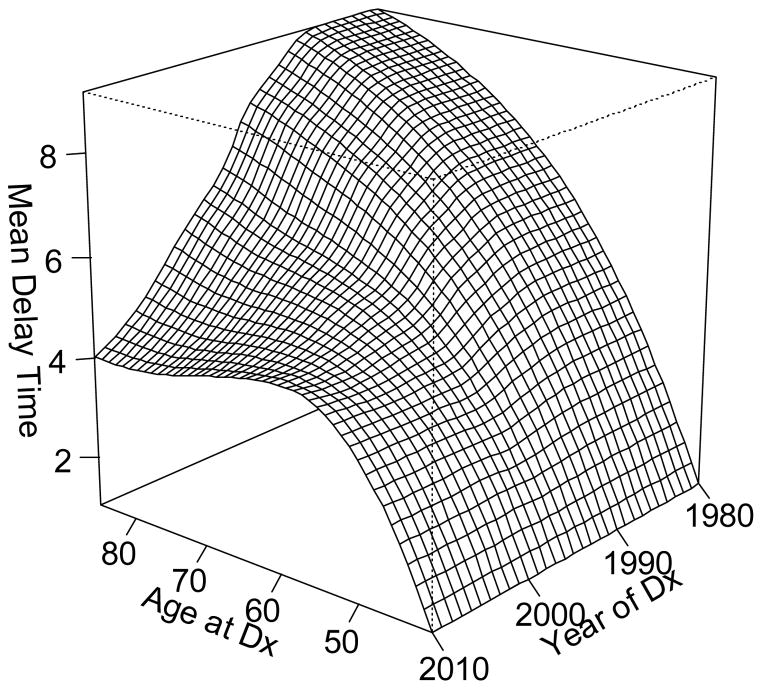
In prostate cancer, the prevalence of prostate cancer screening is a key determinant of the duration of the delay time, or the time between tumor initiation and diagnosis. A statistical model developed for the National Cancer Institute’s Cancer Intervention and Surveillance modeling Network (CISNET)35 provides a quantitative measure of the relation between U.S. screening patterns and prostate cancer incidence36, 37. The model estimates the average delay time and the results are shown by age and calendar period in Figure 4. In older men, the delay time can reach ten years duration, but men diagnosed prior to age 50 tend to have delay times under two years, with progressively shorter delays between tumor onset and clinical detection at the youngest ages. In the late eighties, a sharp decline in the estimated duration of delay time followed the introduction of PSA screening, as screening advanced the diagnosis of prostate cancer to an earlier point in its natural history. The shortening of the latency period was experienced only by men over 55 years of age at diagnosis and had increasingly greater impact with advancing age, so that the greatest shortening occurred in the oldest men. The differential effect of PSA screening on delay time across age groups may be partially related to length bias. Length bias refers to the greater probability for screening to identify tumors with longer latency, which have a greater opportunity for detection38. As a consequence, screen-detected tumors are predisposed to slow growing or indolent cancers. The effect of length bias is especially pronounced in early onset prostate cancer with its already fore shortened latency period. At its extreme, the fastest growing tumors in younger men, with the briefest available window for detection before symptoms appear, may be missed entirely by screening. With this context, it is not surprising that the more rapidly growing tumors selected for by a younger age at prostate cancer diagnosis would tend to be associated with the worst prognoses.
Figure 4.
Mean delay time (years) in prostate cancer detection in the US population by age and calendar time of diagnosis (Dx).
BOX.
Length Bias: the tendency for screening to preferentially detect slow-growing tumors over fast-growing tumors with shorter pre-clinical asymptomatic phases; this can lead to systematic errors in the interpretation of data and particularly the spurious appearance of improved outcomes even in the absence of treatment.
Genetic Focus in Early Onset Prostate Cancer
The three recognized risk factors for prostate cancer are increasing age, African American race39, 40, and a family history. A positive family history of prostate cancer is associated with a two- to three-fold greater risk, with additional increases for multiple affected relatives41 and younger ages at diagnosis42. The effect of family history on prostate cancer risk is not uniform across ages. In the Health Professionals Follow-up Study, having a positive family history of prostate cancer was associated with a greater increase in risk among men under 65 years(RR=2.3; 95% CI 2.0–2.6) than among their counterparts aged 65 years and over (RR=1.7; 95% CI 1.5–1.9)43. Other studies have reported similar findings44. This increased impact in younger men follows directly from the pre-PSA era estimate by Carter et al.45 that hereditary prostate cancer accounts for a greater proportion of prostate cancer in early onset cases (men ≤55 years at diagnosis) than it does in men diagnosed at older ages. It also parallels other malignancies, such as breast, colorectal, and endometrial cancers, where earlier age at cancer diagnosis is more likely to represent a hereditary presentation of a tumor46–48. Above and beyond family history of prostate cancer, men diagnosed with early onset prostate cancer are also more likely to carry a greater number of genetic variants that have been associated with increased risk of prostate cancer in GWA studies49. Lange et al.49 documented a statistically significant difference between men diagnosed with early onset prostate cancer (n=12.4)and men diagnosed at older ages (n=11.9, p=1.7x10−5). This was part of a significant overall trend toward increasing cumulative number of risk alleles with decreasing age at diagnosis (p=4.4x10−5). This trend has been confirmed50. The increased genetic burden in younger cases is also consistent with the shorter period available for younger men to accrue other exposures that might influence their risk of prostate cancer (e.g., infectious agents51, dietary52 or lifestyle patterns53).
The identification of genes involved in prostate cancer development and susceptibility has proven challenging54. An ongoing issue remains in that the existing loci together explain only a small proportion of the familial clustering observed, leaving open the question of additional prostate cancer genetic variants that could explain the ‘missing’ heritability55. Rare variants with low to moderate penetrance are likely candidates because they are known to exist, but current study designs are poorly suited to identifying them56. The availability of next generation sequencing technology may provide an approach to uncovering these variants57. Indeed, recent next generation sequencing data reveal the widespread existence of rare variants in the human population, with rare variants appearing more likely than common ones to affect the function of proteins58–60. Although most studies of prostate cancer genetics have typically included only a small proportion of men ≤ 55 years, the available evidence suggests that focusing efforts on these early onset cases, representing a population enriched for genetic susceptibility to prostate cancer, should provide the best opportunity for successfully identifying new variants. The potential value of this approach is illustrated by the recent discovery from our research team of a rare non-synonymous single-nucleotide polymorphism (SNP) in HOXB13(G84E or rs138213197), a transcription factor in early prostate development and differentiation61 that has been implicated in androgen-independent prostate cancer cell growth62. Although the G84E allele was observed in unrelated prostate cancer cases, including those diagnosed at later age (minor allele frequency; MAF 0.6%), it was identified as a result of its significant enrichment in men with early onset prostate cancer (MAF 2.2%), and particularly among early onset cases with a family history of prostate cancer (MAF3.1%)61. The variant was subsequently shown to be associated with both hereditary and sporadic prostate cancer in many independent study populations.63–70 Continued focus on early onset prostate cancer cases may provide an opportunity to identify novel genetic loci associated with increased risk for this disease.
Genetic Risk Profiling in Early Onset Prostate Cancer
The discovery of almost 70 genetic variants from GWAS as well as the HOXB13 Gly84Glu variant has generated interest in the possibility of personalized genetic testing to identify individuals at higher risk of prostate cancer who may benefit from increased surveillance71–85. However, the clinical utility of this approach has been inhibited by the modest effect size of individual polymorphisms, which are typically on the order of ~1.2–fold greater for carriers of a cancer risk allele compared to non-carriers. One approach to overcoming this limitation was to consider cumulative genetic burden as acount of risk alleles carried by each man. Generally, this tends to strengthen the association between genetic burden and prostate cancer risk, although the magnitude of risk associated with each aggregate score can vary widely across study populations. Increasing the number of variants, as when additional novel risk alleles such as HOXB13 G84E are identified, is also expected to improve the performance of a genetic risk test. For example, in a Swedish case-control study, inclusion of additional SNPs to a genetic risk test did not appreciably improve the positive predictive value (PPV) of the test, (i.e., the accuracy of a positive test) but did increase the test sensitivity (i.e., the ability of the test to correctly classify men diagnosed with prostate cancer)81. Even when as many as 25 common prostate cancer genetic markers were considered in a large case-control study50, the area-under-the-curve (AUC) only improved from 0.526 (family history alone) to 0.642 (family history + SNPs). For comparison, the AUC for a single PSA test at >0.70 in a meta-analysis of 23 studies86 yet considerable controversy exists over the public health utility of this test. Rare alleles may be associated with increased penetrance (e.g HOXB13 G84E), however they will affect only a small proportion of the population at risk and this limitation will be compounded when multiple rare alleles are considered simultaneously. Currently, no current genetic risk model is likely to qualify as a suitable discriminative test for prostate cancer.
Despite the current limitations of genetic testing for prostate cancer overall, there is data to suggest that genetic risk prediction may be more useful younger compared to older men. Using data from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium (BPC3), Lindstrom et al.50 reported that the performance of an aggregate genetic risk score, based on 25 common prostate cancer genetic markers, was improved by testing younger men. The discriminative ability of the 25-SNP model increased significantly with decreasing age (p=0.009), with better performance in men ≤ 60 years compared to older age groups. This was true both for a model with SNPs alone and for one with SNPs and family history of prostate cancer in any first-degree relative. This result has important implications for the targeted application of genetic risk stratification among younger men as a means to identify those who may potentially benefit from early detection strategies and/or chemoprevention.
Challenges and Opportunities in Early Onset Prostate Cancer
BOX.
“IS CURE NECESSARY IN THOSE FOR WHOM IT IS POSSIBLE, AND IS CURE POSSIBLE IN THOSE FOR WHOM IT IS NECESSARY?” - Willet Whitmore87
Although many men are diagnosed with prostate cancer today, not all of them will benefit from treatment. The PIVOT trial is the largest trial conducted to date to evaluate the effectiveness of surgery vs. observation for men with localized prostate cancer. The investigators reported no effect of radical prostatectomy on all-causemortality (HR=0.88; 95% CI 0.71–1.08) or prostate cancer-specific mortality (HR=0.63; 95% CI 0.36–1.09) after a median follow-up of 10 years88. It is worth noting that although no difference was observed in the results of the trial by age at diagnosis, only 75 out of a total of 731 participants were under 60 years, including four men under age 50. Since the median age at diagnosis for prostate cancer is 68 years and the 15-year relative survival for prostate cancer is 77%34, it is possible that many of the men in the trial might have succumbed to other causes before they could experience a death due to their malignancy. Younger men, on the other hand, are less likely to die of other causes. The question therefore remains whether the lack of survival benefit observed for radical prostatectomy would hold true if a greater number of early onset prostate cancer cases had been included.
The sobering result of the PIVOT trial adds to other evidence that serves to raise compelling concerns about the ability to distinguish prostate tumors that will lead to significant illness and premature death from those that will not89, 90. Among men in the U.S., there is a considerable gap between the 17% lifetime risk of developing prostate cancer (1 in 6) and the 2.8% lifetime risk of dying from it (1 in 36). Clinical focus must be redirected toward patients more likely to have clinically significant tumors that will benefit from treatment, but this goal can only be realized through the development of a test capable of identifying men at risk for developing clinically significant prostate cancer. Unfortunately, only a few genetic variants have been(modestly) associated with more aggressive prostate cancer despite efforts by some researchers to identify these markers91. The possible existence of a particularly aggressive clinical subtype within early onset prostate cancer may provide a unique opportunity to investigate the existence of genetic susceptibility specifically to aggressive, rapidly progressing prostate cancer. The increased role of genetic risk in early onset prostate cancer may enhance the ability to address the most crucial challenge in prostate cancer today. Interestingly, although such a result would benefit all men, those men at risk of advanced (high stage or grade) early onset prostate cancer, who are among the least likely to benefit from traditional screening with PSA (See Length bias), would potentially experience the greatest benefit.
At present, the majority of men with early onset prostate cancer are diagnosed with moderately differentiated, organ-confined disease4. In a large national disease registry called Cancer of the Prostate Strategic Urologic Research Endeavor or CaPSURE, over 90% of men with organ-confined prostate cancer opted for curative treatment92. Furthermore, review of treatment choices reveals a clear preference for radical prostatectomy in men younger than age 65 (79%) compared to men older than 65 (37%). Studies on treatment decisions in younger patients also suggest that different priorities and concerns may exist compared to older men. For example, among men diagnosed with Gleason grade 6 prostate cancer, younger men placed a greater importance on sexual function after treatment than urinary function93. In an older group of prostate cancer patients with an average age of approximately 65 years, fear of incontinence was cited as a greater concern than impotence in their treatment decisions94.
Conclusion
Several features of early onset prostate cancer present unique opportunities for prostate cancer genetic and clinical research. Inherited prostate cancer susceptibility plays a greater role in prostate cancer diagnosed in younger men than in older men and many indicate a richer group of cases for cancer susceptibility gene discovery. In addition, prostate cancer in the youngest group of men may also lead to further understanding and identification of the role of otherwise rare alleles, such as HOXB13 mutations and possibly others with pleiotropic phenotypes that may contribute more to the development of disease in younger men. Finally, because the majority of men diagnosed at younger ages tend to have lower grade, organ-confined tumors, they are likely to represent a unique group of cancer survivors who will experience any morbidities that result from the treatment of their cancer for a longer period of time than their older peers. Taken together, early onset prostate cancer is a unique clinical entity which is rising in incidence in the US. Since early onset prostate cancer is enriched for genetic compared to environmental risk factors, future research focusing on these uncommon cases has the opportunity to identify additional risk alleles that may be help improve our understanding the etiology of this cancer.
Key Points.
The incidence of prostate among the youngest group of at-risk men has increased sharply over the last two decades, making early onset prostate cancer an important emerging issue for public health.
Increased screening activity in young men 55 years of age and under may account for some, but not all of the increase in early onset cases.
Young men diagnosed with advanced higher grade prostate cancer may have a distinct clinicopathologic form of prostate cancer with more aggressive progression to disease-specific death than similar stage and grade prostate cancer in their older peers.
Men diagnosed with early onset prostate cancer are likely to have greater genetic risk of prostate cancer than older cases, making this group an especially rich resource for investigating genetic susceptibility to prostate cancer.
Acknowledgments
Grant sponsor: NIH; Grant numbers: RO1 CA79596, RO1 CA136621, SPORE P50 CA69568:CISNET U01 CA157224.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2009. 2012 (Vintage 2009 Populations), . based on November 2011 SEER data submission, posted to the SEER web site, April 2012. [serial online] Available from URL: http://seer.cancer.gov/csr/1975_2009_pops09/</csr/1975_2009_pops09/
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.SEER17 Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER 17 Regs Reserach Data + Hurricane Impacted Louisiana Cases 2010.
- 5.Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106:462–469. doi: 10.1111/j.1464-410X.2010.09334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alibhai SM, Krahn MD, Cohen MM, Fleshner NE, Tomlinson GA, Naglie G. Is there age bias in the treatment of localized prostate carcinoma? Cancer. 2004;100:72–81. doi: 10.1002/cncr.11884. [DOI] [PubMed] [Google Scholar]
- 7.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 8.Gronberg H, Damber JE, Jonsson H, Lenner P. Patient age as a prognostic factor in prostate cancer. J Urol. 1994;152:892–895. doi: 10.1016/s0022-5347(17)32601-0. [DOI] [PubMed] [Google Scholar]
- 9.Harrison GS. The prognosis of prostatic cancer in the younger man. British Journal of Urology. 1983;55:315–320. doi: 10.1111/j.1464-410x.1983.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 10.Benson MC, Kaplan SA, Olsson CA. Prostate cancer in men less than 45 years old: influence of stage, grade and therapy. J Urol. 1987;137:888–890. doi: 10.1016/s0022-5347(17)44285-6. [DOI] [PubMed] [Google Scholar]
- 11.Byar DP, Mostofi FK. Cancer of the prostate in men less than 50 years old: an analysis of 51 cases. Journal of Urology. 1969;102:726–733. doi: 10.1016/s0022-5347(17)62240-7. [DOI] [PubMed] [Google Scholar]
- 12.Huben R, Natarajan N, Pontes E, Mettlin C, Smart CR, Murphy GP. Carcinoma of prostate in men less than fifty years old. Data from American College of Surgeons' National Survey. Urology. 1982;20:585–588. doi: 10.1016/0090-4295(82)90304-1. [DOI] [PubMed] [Google Scholar]
- 13.Silber I, McGavran MH. Adenocarcinoma of the prostate in men less than 56 years old: a study of 65 cases. Journal of Urology. 1971;105:283–285. doi: 10.1016/s0022-5347(17)61510-6. [DOI] [PubMed] [Google Scholar]
- 14.Smedley HM, Sinnott M, Freedman LS, Macaskill P, Naylor CP, Pillers EM. Age and survival in prostatic carcinoma. Br J Urol. 1983;55:529–533. doi: 10.1111/j.1464-410x.1983.tb03363.x. [DOI] [PubMed] [Google Scholar]
- 15.Riopel MA, Polascik TJ, Partin AW, Sauvageot J, Walsh PC, Epstein JI. Radical prostatectomy in men less than 50 years old. Urol Oncol. 1995;1:80–83. doi: 10.1016/1078-1439(95)00010-f. [DOI] [PubMed] [Google Scholar]
- 16.Carter HB, Epstein JI, Partin AW. Influence of age and prostate-specific antigen on the chance of curable prostate cancer among men with nonpalpable disease. Urology. 1999;53:126–130. doi: 10.1016/s0090-4295(98)00466-x. [DOI] [PubMed] [Google Scholar]
- 17.Ruska KM, Partin AW, Epstein JI, Kahane H. Adenocarcinoma of the prostate in men younger than 40 years of age: diagnosis and treatment with emphasis on radical prostatectomy findings. Urology. 1999;53:1179–1183. doi: 10.1016/s0090-4295(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith CV, Bauer JJ, Connelly RR, et al. Prostate cancer in men age 50 years or younger: a review of the Department of Defense Center for Prostate Disease Research multicenter prostate cancer database. J Urol. 2000;164:1964–1967. doi: 10.1016/s0022-5347(05)66929-7. [DOI] [PubMed] [Google Scholar]
- 19.Khan MA, Han M, Partin AW, Epstein JI, Walsh PC. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology. 2003;62:86–91. doi: 10.1016/s0090-4295(03)00404-7. discussion 91-82. [DOI] [PubMed] [Google Scholar]
- 20.Magheli A, Rais-Bahrami S, Humphreys EB, Peck HJ, Trock BJ, Gonzalgo ML. Impact of patient age on biochemical recurrence rates following radical prostatectomy. J Urol. 2007;178:1933–1937. doi: 10.1016/j.juro.2007.07.016. discussion 1937–1938. [DOI] [PubMed] [Google Scholar]
- 21.Burri RJ, Ho AY, Forsythe K, Cesaretti JA, Stone NN, Stock RG. Young men have equivalent biochemical outcomes compared with older men after treatment with brachytherapy for prostate cancer. International journal of radiation oncology, biology, physics. 2010;77:1315–1321. doi: 10.1016/j.ijrobp.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Merrick GS, Wallner KE, Butler WM, et al. Brachytherapy in men aged < or = 54 years with clinically localized prostate cancer. BJU Int. 2006;98:324–328. doi: 10.1111/j.1464-410X.2006.06248.x. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro EY, Rais-Bahrami S, Morgenstern C, Napolitano B, Richstone L, Potters L. Long-term outcomes in younger men following permanent prostate brachytherapy. J Urol. 2009;181:1665–1671. doi: 10.1016/j.juro.2008.11.122. discussion 1671. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TD, Poortmans PM, van der Hulst M, et al. The curative role of radiotherapy in adenocarcinoma of the prostate in patients under 55 years of age: a rare cancer network retrospective study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2005;77:286–289. doi: 10.1016/j.radonc.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Rossi CJ, Jr, Slater JD, Yonemoto LT, et al. Influence of patient age on biochemical freedom from disease in patients undergoing conformal proton radiotherapy of organ-confined prostate cancer. Urology. 2004;64:729–732. doi: 10.1016/j.urology.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone PA, Riffenburgh RH, Moul JW, et al. Effect of age on biochemical disease-free outcome in patients with T1-T3 prostate cancer treated with definitive radiotherapy in an equal-access health care system: a radiation oncology report of the Department of Defense Center for Prostate Disease Research. Int J Radiat Oncol Biol Phys. 2003;55:964–969. doi: 10.1016/s0360-3016(02)04283-9. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JM, Kemp IW, Stein GJ. Cancer of the prostate. Do younger men have a poorer survival rate? Br J Urol. 1984;56:391–396. doi: 10.1111/j.1464-410x.1984.tb05828.x. [DOI] [PubMed] [Google Scholar]
- 28.Tjaden HB, Culp DA, Flocks RH. Clinical adenocarcinoma of the prostate in patients under 50 years of age. Journal of Urology. 1965;93:618–621. doi: 10.1016/S0022-5347(17)63840-0. [DOI] [PubMed] [Google Scholar]
- 29.Bratt O, Kristoffersson U, Olsson H, Lundgren R. Clinical course of early onset prostate cancer with special reference to family history as a prognostic factor. European Urology. 1998;34:19–24. doi: 10.1159/000019672. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DE, Lanieri JP, Ayala AG. Prostatic adenocarcinoma occurring in men under 50 years of age. J Surg Oncol. 1972;4:207–216. doi: 10.1002/jso.2930040305. [DOI] [PubMed] [Google Scholar]
- 31.Merrill RM, Bird JS. Effect of young age on prostate cancer survival: a population-based assessment (United States) Cancer causes & control : CCC. 2002;13:435–443. doi: 10.1023/a:1015764507609. [DOI] [PubMed] [Google Scholar]
- 32.Twiss C, Slova D, Lepor H. Outcomes for men younger than 50 years undergoing radical prostatectomy. Urology. 2005;66:141–146. doi: 10.1016/j.urology.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SE. Is Carcinoma of the Prostate Less Serious in Older Men? Journal of the American Geriatrics Society. 1965;13:791–798. doi: 10.1111/j.1532-5415.1965.tb02059.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer: a Population-based Cohort Study. Cancer. 2009;115:2863–2871. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CISNET. Cancer Intervention and Surveillance Modeling Network. [Google Scholar]
- 36.Tsodikov A, Szabo A, Wegelin J. A population model of prostate cancer incidence. Stat Med. 2006;25:2846–2866. doi: 10.1002/sim.2257. [DOI] [PubMed] [Google Scholar]
- 37.Chefo S, Tsodikov A. Stage-specific cancer incidence: an artificially mixed multinomial logit model. Stat Med. 2009;28:2054–2076. doi: 10.1002/sim.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelen M, Feinleib M. On the theory of screening for chronic diseases. Biometrika. 1969;53:601–614. [Google Scholar]
- 39.Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. based on November 2010 SEER data submission, posted to the SEER web site, 2011[serial online] Available from URL: http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 40.American Cancer Society. Cancer Facts & Figures. 2011 Available from URL: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf.
- 41.Zeegers MP, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: a meta-analysis. Cancer. 2003;97:1894–1903. doi: 10.1002/cncr.11262. [DOI] [PubMed] [Google Scholar]
- 42.Brandt A, Bermejo JL, Sundquist J, Hemminki K. Age-specific risk of incident prostate cancer and risk of death from prostate cancer defined by the number of affected family members. Eur Urol. 2010;58:275–280. doi: 10.1016/j.eururo.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Chen YC, Page JH, Chen R, Giovannucci E. Family history of prostate and breast cancer and the risk of prostate cancer in the PSA era. Prostate. 2008;68:1582–1591. doi: 10.1002/pros.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kicinski M, Vangronsveld J, Nawrot TS. An epidemiological reappraisal of the familial aggregation of prostate cancer: a meta-analysis. PLoS One [serial online] 2011;6:e27130. doi: 10.1371/journal.pone.0027130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter BS, Beaty TH, Steinberg GD, Childs B, Walsh PC. Mendelian inheritance of familial prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch HT, Guirgis H, Brodkey F, et al. Early age of onset in familial breast cancer. Genetic and cancer control implications. Arch Surg. 1976;111:126–131. doi: 10.1001/archsurg.1976.01360200032006. [DOI] [PubMed] [Google Scholar]
- 47.Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet. 1999;36:801–818. [PMC free article] [PubMed] [Google Scholar]
- 48.Vasen HF, Watson P, Mecklin JP, et al. The epidemiology of endometrial cancer in hereditary nonpolyposis colorectal cancer. Anticancer Res. 1994;14:1675–1678. [PubMed] [Google Scholar]
- 49.Lange EM, Salinas CA, Zuhlke KA, et al. The Prostate. 2011. Early onset prostate cancer has a significant genetic component. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindstrom S, Schumacher FR, Cox D, et al. Common genetic variants in prostate cancer risk prediction--results from the NCI Breast and Prostate Cancer Cohort Consortium (BPC3) Cancer Epidemiol Biomarkers Prev. 2012;21:437–444. doi: 10.1158/1055-9965.EPI-11-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dennis LK, Dawson DV. Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology. 2002;13:72–79. doi: 10.1097/00001648-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Rohrmann S, Platz EA, Kavanaugh CJ, Thuita L, Hoffman SC, Helzlsouer KJ. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control. 2007;18:41–50. doi: 10.1007/s10552-006-0082-y. [DOI] [PubMed] [Google Scholar]
- 53.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 54.Ostrander EA, Johannesson B. Prostate cancer susceptibility loci: finding the genes. Adv Exp Med Biol. 2008;617:179–190. doi: 10.1007/978-0-387-69080-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeggini E, Rayner W, Morris AP, et al. An evaluation of HapMap sample size and tagging SNP performance in large-scale empirical and simulated data sets. Nat Genet. 2005;37:1320–1322. doi: 10.1038/ng1670. [DOI] [PubMed] [Google Scholar]
- 57.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nature reviews Genetics. 2010;11:415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 58.Nelson MR, Wegmann D, Ehm MG, et al. An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science. 2012;337:100–104. doi: 10.1126/science.1217876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tennessen JA, Bigham AW, O'Connor TD, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337:64–69. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keinan A, Clark AG. Recent explosive human population growth has resulted in an excess of rare genetic variants. Science. 2012;336:740–743. doi: 10.1126/science.1217283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999;41:203–207. doi: 10.1002/(sici)1097-0045(19991101)41:3<203::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 62.Kim YR, Oh KJ, Park RY, et al. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol Cancer. 2010;9:124. doi: 10.1186/1476-4598-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laitinen VH, Wahlfors T, Saaristo L, et al. HOXB13 G84E mutation in Finland: population-based analysis of prostate, breast, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22:452–460. doi: 10.1158/1055-9965.EPI-12-1000-T. [DOI] [PubMed] [Google Scholar]
- 64.Chen Z, Greenwood C, Isaacs WB, et al. The G84E mutation of HOXB13 is associated with increased risk for prostate cancer: results from the REDUCE trial. Carcinogenesis. 2013;34:1260–1264. doi: 10.1093/carcin/bgt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kluzniak W, Wokolorczyk D, Kashyap A, et al. The G84E mutation in the HOXB13 gene is associated with an increased risk of prostate cancer in Poland. Prostate. 2013;73:542–548. doi: 10.1002/pros.22594. [DOI] [PubMed] [Google Scholar]
- 66.Stott-Miller M, Karyadi DM, Smith T, et al. HOXB13 mutations in a population-based, case-control study of prostate cancer. Prostate. 2013;73:634–641. doi: 10.1002/pros.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Witte JS, Mefford J, Plummer SJ, et al. HOXB13 mutation and prostate cancer: studies of siblings and aggressive disease. Cancer Epidemiol Biomarkers Prev. 2013;22:675–680. doi: 10.1158/1055-9965.EPI-12-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Lange EM, Lu L, et al. HOXB13 is a susceptibility gene for prostate cancer: results from the International Consortium for Prostate Cancer Genetics (ICPCG) Hum Genet. 2013;132:5–14. doi: 10.1007/s00439-012-1229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gudmundsson J, Sulem P, Gudbjartsson DF, et al. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nat Genet. 2012;44:1326–1329. doi: 10.1038/ng.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breyer JP, Avritt TG, McReynolds KM, Dupont WD, Smith JR. Confirmation of the HOXB13 G84E germline mutation in familial prostate cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:1348–1353. doi: 10.1158/1055-9965.EPI-12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng SL, Sun J, Wiklund F, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358:910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 72.Salinas CA, Koopmeiners JS, Kwon EM, et al. Clinical utility of five genetic variants for predicting prostate cancer risk and mortality. Prostate. 2009;69:363–372. doi: 10.1002/pros.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Helfand BT, Fought AJ, Loeb S, Meeks JJ, Kan D, Catalona WJ. Genetic prostate cancer risk assessment: common variants in 9 genomic regions are associated with cumulative risk. J Urol. 2010;184:501–505. doi: 10.1016/j.juro.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng SL, Sun J, Wiklund F, et al. Genetic variants and family history predict prostate cancer similar to prostate-specific antigen. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1105–1111. doi: 10.1158/1078-0432.CCR-08-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu J, Sun J, Kader AK, et al. Estimation of absolute risk for prostate cancer using genetic markers and family history. Prostate. 2009;69:1565–1572. doi: 10.1002/pros.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun J, Lange EM, Isaacs SD, et al. Chromosome 8q24 risk variants in hereditary and non-hereditary prostate cancer patients. Prostate. 2008;68:489–497. doi: 10.1002/pros.20695. [DOI] [PubMed] [Google Scholar]
- 77.Nam RK, Zhang WW, Trachtenberg J, et al. Utility of incorporating genetic variants for the early detection of prostate cancer. Clin Cancer Res. 2009;15:1787–1793. doi: 10.1158/1078-0432.CCR-08-1593. [DOI] [PubMed] [Google Scholar]
- 78.Beuten J, Gelfond JA, Franke JL, et al. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiology, Biomarkers and Prevention. 2009;18:1869–1880. doi: 10.1158/1055-9965.EPI-09-0076. [DOI] [PubMed] [Google Scholar]
- 79.Sun J, Chang BL, Isaacs SD, et al. Cumulative effect of five genetic variants on prostate cancer risk in multiple study populations. The Prostate. 2008;68:1257–1262. doi: 10.1002/pros.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penney KL, Salinas CA, Pomerantz M, et al. Evaluation of 8q24 and 17q Risk Loci and Prostate Cancer Mortality. Clin Cancer Res. 2009;15:3223–3230. doi: 10.1158/1078-0432.CCR-08-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun J, Kader AK, Hsu FC, et al. Inherited genetic markers discovered to date are able to identify a significant number of men at considerably elevated risk for prostate cancer. Prostate. 2011;71:421–430. doi: 10.1002/pros.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Helfand BT, Kan D, Modi P, Catalona WJ. Prostate cancer risk alleles significantly improve disease detection and are associated with aggressive features in patients with a "normal" prostate specific antigen and digital rectal examination. Prostate. 2011;71:394–402. doi: 10.1002/pros.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aly M, Wiklund F, Xu J, et al. Polygenic risk score improves prostate cancer risk prediction: results from the Stockholm-1 cohort study. Eur Urol. 2011;60:21–28. doi: 10.1016/j.eururo.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiklund FE, Adami HO, Zheng SL, et al. Established prostate cancer susceptibility variants are not associated with disease outcome. Cancer Epidemiol Biomarkers Prev. 2009;18:1659–1662. doi: 10.1158/1055-9965.EPI-08-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klein RJ, Hallden C, Gupta A, et al. Evaluation of multiple risk-associated single nucleotide polymorphisms versus prostate-specific antigen at baseline to predict prostate cancer in unscreened men. Eur Urol. 2012;61:471–477. doi: 10.1016/j.eururo.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schroder F, Kattan MW. The comparability of models for predicting the risk of a positive prostate biopsy with prostate-specific antigen alone: a systematic review. Eur Urol. 2008;54:274–290. doi: 10.1016/j.eururo.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 87.Montie JE, Smith JA. Whitmoreisms: memorable quotes from Willet F. Whitmore, Jr, M.D. Urology. 2004;63:207–209. doi: 10.1016/s0090-4295(03)00243-7. [DOI] [PubMed] [Google Scholar]
- 88.Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barry MJ, Mulley AJ., Jr Why are a high overdiagnosis probability and a long lead time for prostate cancer screening so important? J Natl Cancer Inst. 2009;101:362–363. doi: 10.1093/jnci/djp028. [DOI] [PubMed] [Google Scholar]
- 91.Lin DW, FitzGerald LM, Fu R, et al. Genetic variants in the LEPR, CRY1, RNASEL, IL4, and ARVCF genes are prognostic markers of prostate cancer-specific mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1928–1936. doi: 10.1158/1055-9965.EPI-11-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sidana A, Hernandez DJ, Feng Z, et al. Treatment decision-making for localized prostate cancer: what younger men choose and why. Prostate. 2012;72:58–64. doi: 10.1002/pros.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what's important. J Gen Intern Med. 2000;15:694–701. doi: 10.1046/j.1525-1497.2000.90842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]