Abstract
Introduction
Bevacizumab improves survival in patients with advanced non-small cell lung cancer (NSCLC). This phase II clinical trial assessed the effects of the addition of bevacizumab to neoadjuvant chemotherapy in resectable non-squamous NSCLC.
Methods
Patients with resectable stage IB-IIIA non-squamous NSCLC were treated with bevacizumab followed by imaging 2 weeks later to assess single agent effect. They then received 2 cycles of bevacizumab with 4 cycles of cisplatin and docetaxel followed by surgical resection. Resected patients were eligible for adjuvant bevacizumab. The primary endpoint was the rate of pathological downstaging (decrease from pretreatment clinical stage to post-treatment pathological stage). Secondary endpoints included overall survival, safety and radiologic response.
Results
Fifty patients were enrolled. Thirty-four (68%) were clinical stage IIIA. All 3 doses of neoadjuvant bevacizumab were delivered to 40/50 patients. Six (12%) patients discontinued due to bevacizumab-related adverse events. The rate of downstaging (38%), response to chemotherapy (45%), and perioperative complications (12%) were comparable to historical data. No partial responses were observed to single-agent bevacizumab but 18% developed new intratumoral cavitation with a trend toward improved pathologic response (57% vs. 21%, p=0.07). A major pathologic response (≥90% treatment effect) was associated with survival at 3 years (100% vs. 49%, p=0.01). No patients with KRAS-mutant NSCLC (0/10) had a pathologic response as compared with 11/31 with wild-type KRAS.
Conclusions
While preoperative bevacizumab plus chemotherapy was feasible, it did not improve downstaging in unselected patients. New cavitation after single-agent bevacizumab is a potential biomarker. Alternative strategies are needed for KRAS-mutant tumors.
Introduction
Patients with stage IB-IIIA non-small cell lung cancers (NSCLC) are potentially curable with a multi-modality approach, however 5-year survival rates remain disappointing: 67% for stage I, 54% for stage II and 40% for stage III.1 The use of neoadjuvant therapy followed by surgery has the advantage of allowing administration of 90% of planned cisplatin-based chemotherapy compared with 50% of planned treatment in the post-operative setting without any increase in surgical risk.2 This approach provides earlier systemic therapy for the treatment of micrometastatic disease and allows for an assessment of treatment efficacy in each individual patient. Ineffective therapies can be stopped and alternative drugs substituted. Neoadjuvant therapy also allows for an in vivo evaluation of new therapeutic approaches, providing critical information for drug development as response to therapy can be assessed both radiographically and correlatively in post-treatment pathologic specimens.
While disease free and overall survivals remain the gold-standard for evaluation of perioperative interventions, surrogate endpoints can provide an earlier estimation of the effectiveness of new therapies. In particular, the downstaging from pretreatment to posttreatment stage and pathological response to neoadjuvant chemotherapy have consistently correlated with survival in neoadjuvant clinical trials.3-7
Bevacizumab is a recombinant humanized monoclonal antibody that binds to vascular endothelial growth factor A. In combination with chemotherapy in stage IV non-squamous NSCLC, bevacizumab improved objective the response rate from 15 to 35% and overall survival from 10 to 12 months.8 We sought to evaluate whether perioperative bevacizumab would be of benefit to patients with resectable stage IB-IIIA non-squamous NSCLC.
The primary endpoint of this study was to determine whether the addition of bevacizumab to neoadjuvant chemotherapy improved downstaging from clinical to pathological stage compared to historical controls.9 The secondary endpoints included safety, evaluation of pathological and radiological response, time to progression (TTP), recurrence free (RFS) and overall survival (OS) of all patients and of the subset with stage IIIA disease.
Materials and Methods
Study Design
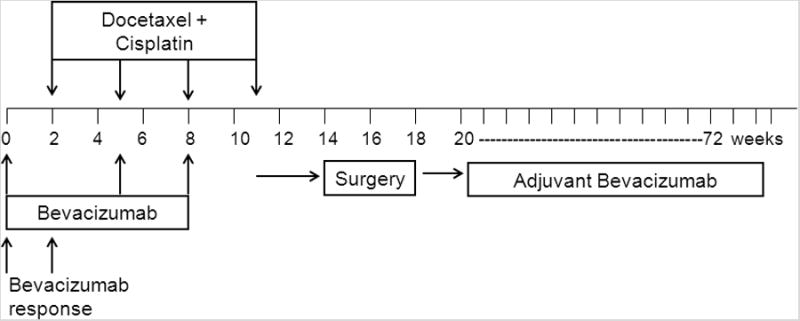
This was a single institution phase II study. The schema is presented in Figure 1. The primary endpoint was the rate of downstaging in patients with clinical stage IB-IIIA non-squamous NSCLC treated with neoadjuvant docetaxel and cisplatin chemotherapy in combination with bevacizumab. Downstaging was defined as any decrease in the final pathologic stage when compared with the clinical stage (before neoadjuvant therapy). Secondary endpoints included safety, radiologic response to single-agent bevacizumab, radiologic response to chemotherapy, TTP and OS from the start of chemotherapy, and RFS from the date of complete resection. The survival endpoints included analysis in all patients and in the subset with stage IIIA disease. This protocol was approved by the institutional review board and all patients signed informed consent.
Figure 1. Treatment Schema.
Patients
Eligible patients had pathologically confirmed non-squamous NSCLC of clinical stages IB -IIIA (T1-3N0-2M0) by American Joint Committee on Cancer Staging 6th edition. Pretreatment evaluation included chest CT, PET scan, brain MRI, and pathologic mediastinal staging (mediastinoscopy or endobronchial ultrasound) if clinically indicated. Patients were required to have a Karnofsky Performance Status of ≥70%, adequate organ function, and to be deemed resectable by a thoracic surgeon. Patients were ineligible if they had large central tumors, were receiving anticoagulation or had a history of hemoptysis, stroke, or myocardial infarction within the past year, uncontrolled hypertension, non-healing wound/ulcer/fracture, hearing loss, or peripheral neuropathy > grade 1.
Preoperative Treatment
The first dose of bevacizumab (15 mg/kg) was administered 2 weeks before cisplatin and docetaxel to allow assessment of the radiographic effects of bevacizumab alone. Patients received 4 cycles of docetaxel (75 mg/m2) and cisplatin (75 mg/m2) intravenously every 21 days. Docetaxel, cisplatin and bevacizumab were administered on the same day cycles 2 and 3. Bevacizumab was omitted from cycle 4 (Figure 1). Pegfilgrastim was administered prophylactically at the discretion of the investigator. Toxicities were graded using National Cancer Institute Common Toxicity Criteria, version 3.0.
Surgery and Analyses
Patients were re-evaluated for surgery by clinical examination, chest CT, PET scan, pulmonary function tests, and brain MRI. Radiographic response was assessed using RECIST.10 Surgical exploration, resection and mediastinal lymph node dissection occurred 3 to 8 weeks after chemotherapy and ≥6 weeks after the last bevacizumab. The surgical specimens were reviewed by one thoracic pathologist (WDT). Tumors were representatively sampled with one section per centimeter diameter of the tumor, examined by light microscopy for histologic diagnosis and the amount of treatment effect including necrosis, fibrosis and inflammation. The treatment effect was semi-quantitatively estimated in 10 percent increments. Molecular analyses were performed for mutations in EGFR and KRAS per standard methods.11, 12
Post-operative Treatment and Follow-up
Adjuvant bevacizumab (15 mg/kg) was administered intravenously starting 42 to 56 day postoperatively and continued every 21 days for 1 year (18 cycles). If post-operative radiotherapy was indicated based on N2 nodal involvement or a positive resection margin, bevacizumab was delayed until 28 to 52 days following the completion of radiation. No other chemotherapy was given postoperatively.
Patients were followed for disease recurrence with history, physical examination, and CT scans of the chest and upper abdomen every 4 months for 2 years, every 6 months for the third year, and annually thereafter.
Outcome Analysis
The primary endpoint was an improvement in downstaging from the published rate of 33%9 to the target rate of 50%. A sample size of 50 patients was chosen to allow distinction between 33% and 50% downstaging rates with one-sided type I and type II error rates of 11.6% and 10.2%, respectively. With these parameters, the trial would be considered successful if at least 21/50 patients were downstaged. Patients who were not resected were counted as not downstaged. Early stopping rules were in effect in the case that excessive toxicity was observed.
In this study we evaluated overall survival (OS) and time to progression (TTP) in all patients, from the start of neoadjuvant chemotherapy and recurrence free survival (RFS) in patients who underwent surgical resection, from the date of surgery. Patients were followed until death in OS analyses; until time to documented progression in the TTP analysis; and until death or disease recurrence, whichever came first, in the RFS analysis. Patients who did not experience the event of interest were censored at the time of the last available follow-up. All time-to-event outcomes were estimated using Kaplan-Meier method. Planned subgroup analyses were restricted to the subset with clinical Stage IIIA disease. Clinical outcomes following surgery were compared by downstaging, nodal downstaging (in patients with clinical N1 or N2 disease), pathological response and radiological response using non-parametric log-rank test. Analyses based on intratumoral cavitation and KRAS status were unplanned and exploratory. Two-by-two comparisons were performed using Fisher exact test. Statistical analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC) and R (version 2.14.1; R Development Core Team, 2011).
Results
Patient Characteristics
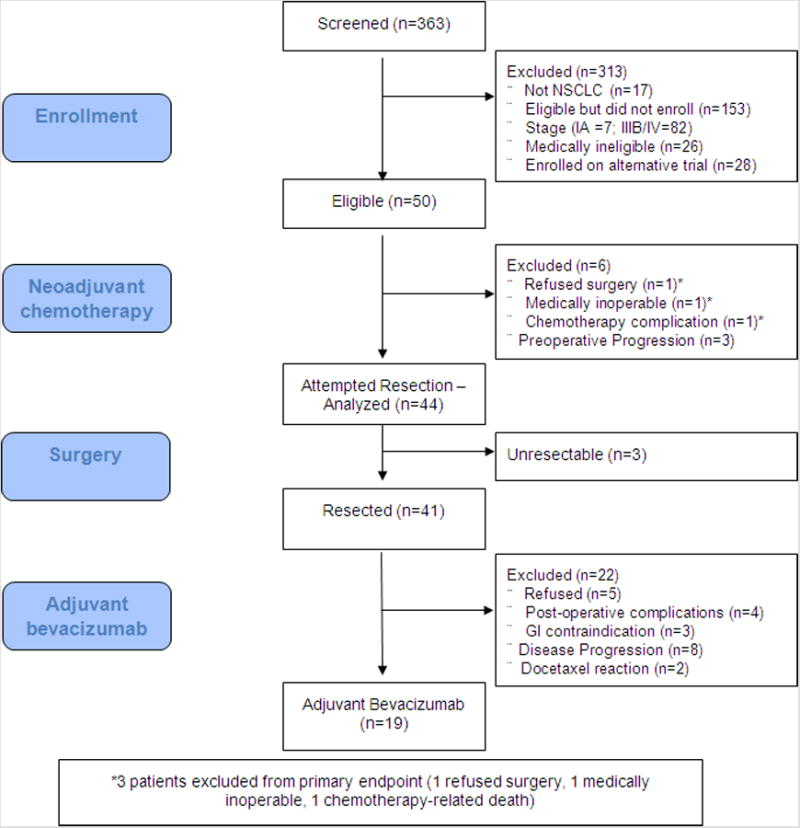
50 patients were enrolled between August 2005 and April 2011. Baseline patient characteristics are summarized in Table 1. The flow of patients screened, treated and analyzed is presented in Figure 2.
Table 1. Patient Characteristics.
| Characteristic | N (%) |
|---|---|
|
| |
| Age – median (range) | 61 (45-77) |
|
| |
| Male | 20 (40) |
| Female | 30 (60) |
|
| |
| Smoking History | |
| Never | 2 (4) |
| Former | 27 (54) |
| Current | 21 (42) |
|
| |
| Pack years – median (range) | 36 (0-150) |
|
| |
| Clinical Stage | |
| IB | 6 (12) |
| IIA | 5 (10) |
| IIB | 5 (10) |
| IIIA | 34 (68) |
|
| |
| Mediastinal Staging | |
| Mediastinoscopy | 15 (30) |
| Endobronchial Ultrasound | 5 (10) |
|
| |
| Histology | |
| Adenocarcinoma | 45 (90) |
| Large cell neuroendocrine carcinoma | 4 (8) |
| Adenosquamous carcinoma | 1 (2) |
|
| |
| Molecular Profile | |
| EGFR mutation | 4 (8) |
| KRAS mutation | 13 (26) |
| EGFR/KRAS wild-type | 30 (60) |
| Insufficient for molecular analysis | 3 (6) |
Figure 2. Flow of patients.
Primary Endpoint – Downstaging
The primary endpoint of the study was downstaging. Considering unresected patients as treatment failures, 19/50 patients enrolled were downstaged, this rate of 38% (95%CI: 25% - 53%) did not meet the primary endpoint of the study. Downstaging from clinical to pathological stage included 14 patients with stage IIIA (pathologic 5 IA, 4 IB, 3 IIA, and 2 IIB), 1 patient with stage IIB (with complete pathologic response to Stage 0), 2 patients with IIA (pathologic stage IA and IB), and 2 patients with stage IB (pathologic stage IA).
Chemotherapy Compliance and Toxicity
Chemotherapy cycles delivered and reasons for drug discontinuation are listed in Table 2. Of the 42 patients who completed all 4 cycles of chemotherapy, 32 received full dose treatment. Ten patients required dose reductions for febrile neutropenia (2), neutropenia without fever (3), fatigue (3), mucositis/colitis (2).
Table 2. Chemotherapy Delivery.
| Drug Cycles | N | Dose-limiting Event (N) |
|---|---|---|
|
| ||
| Bevacizumab | ||
| 3 | 40 | Diverticulitis with perforation(1) |
| 2 | 2 | Hemoptysis(1), hypertension(1) |
| 1 | 8 | Hemoptysis(3), Cavitary PNA(1), docetaxel reaction (2), sepsis/death(1), sepsis/pneumonitis(1) |
| Docetaxel/Cisplatin | ||
| 4 | 42 | |
| 3 | 3 | Transaminitis(1), fatigue(1) |
| 2 | 0 | |
| 1 | 5 | Cavitary PNA(1), docetaxel reaction(2), sepsis/death(1), sepsis/pneumonitis(1) |
| Adjuvant bevacizumab | ||
| 18 | 9 | |
| 17 | 2 | Patient preference(2) |
| 14 | 1 | Hemoptysis |
| 13 | 2 | Fatigue(1), withdrew consent(1) |
| 10 | 1 | EKG changes |
| 4-7 | 3 | Progression of Disease(3) |
| 1 | 1 | Hypertensive urgency |
Thirty-one patients received prophylactic pegfilgrastim. There was 1 treatment-related death with the first cycle of chemotherapy attributed to neutropenic sepsis in a patient who did not receive prophylactic pegfilgrastim. Grade 3 or 4 neutropenia occurred in 15 (30%) patients, and there were 4 (8%) cases of febrile neutropenia. One patient had a cavitary pneumonia. Anemia and thrombocytopenia were uncommon with 12% and 8% of patients experiencing grade 3/4 toxicities, respectively. There was 1 patient with transient grade 4 hepatotoxicity. There were 2 grade 2 allergic reactions to docetaxel. Thirty-one (62%) patients had grade 1 or 2 fatigue or malaise, and 11 (22%) experienced grade 3 fatigue. The toxicities in Table 2 potentially attributable to neoadjuvant bevacizumab totaled 16% with 4 cases of grade 1 or 2 hemoptysis, 1 grade 3 hypertension, 1 grade 4 colitis with perforation (after 3rd dose), 1 cavitary pneumonia, and the single death from neutropenic sepsis.
Radiographic Responses
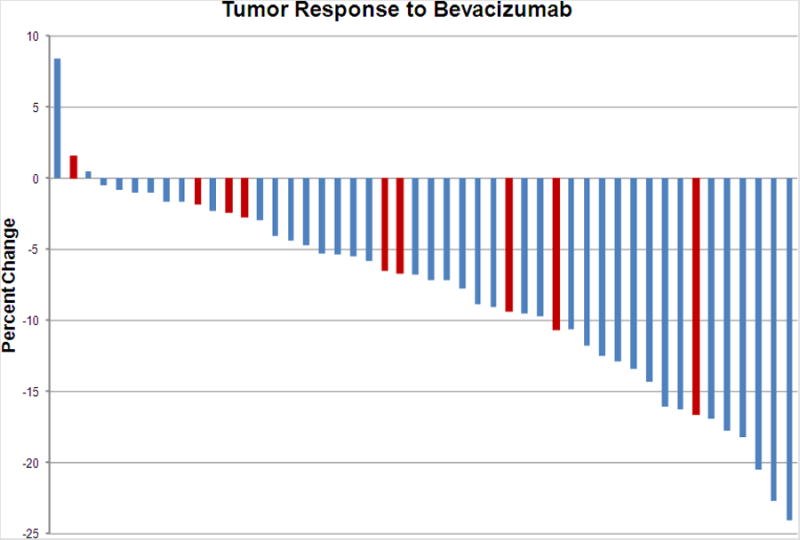
The CT responses measured 2 weeks after single agent bevacizumab are depicted in Figure 3. The median regression seen was -7% (range +8 to -24%). There were no partial responses. Nine patients (shown in red in Figure 3) developed cavitation after a single dose of bevacizumab.
Figure 3. Percent change in tumor burden 2 weeks after bevacizumab per RECIST v1.1 criteria. Patients with new intra-tumoral cavitation shown in red.
Forty-four patients had CT scans of the chest with measurable disease both pre-treatment and after completion of neoadjuvant chemotherapy; 20 had a partial response, for an objective response rate of 45% (95% CI: 30% to 61%).
Surgery and post-operative complications
Forty-four of 50 patients had surgery. Patients did not have surgery because of death due to chemotherapy toxicity (1), development of metastatic disease (3), inadequate pulmonary reserve to tolerate resection (1) and withdrawal of consent (1). Of the 44 patients, 3 were found to have unresectable disease. Forty-one patients were resected with 4 (8%) pneumonectomies (2 right and 2 left), 3 (6%) bilobectomies, 32 (64%) lobectomies, and 2 (4%) wedge resections. Resection was complete (R0) in 36 patients (82% of those explored, 72% of those enrolled) and incomplete in 5 (3 R1 and 2 R2 resections).
There were no perioperative deaths. Immediate post-operative complications included single cases of gastrointestinal bleed, volvulus, bronchopleural fistula, and empyema, all grade 3. Late post-operative complications included 1 patient with a bronchopleural fistula and 1 with an infected mesh. Empyema and infected mesh were felt to be unrelated to bevacizumab, therefore, perioperative complications at least potentially attributable to preoperative bevacizumab among patients with attempted resection totaled 9% (4/44) (95% CI: 11%-35%).
Adjuvant therapy and complications
Thirteen of the 41 patients who had surgical resection received post-operative radiation therapy (PORT). Nine of these patients had involved N2 nodes and 6 had positive resection margins.
Thirty-nine patients were eligible to receive adjuvant bevacizumab as outlined in Figure 2. Nineteen of 39 patients received post-operative bevacizumab. The median number of cycles was 17 (range 1-18). Toxicities and reasons for discontinuation of adjuvant bevacizumab are summarized in Table 2.
Clinical Outcomes
With a median follow-up of 29 months at the time of analysis, 25/50 patients suffered disease progression. In patients who underwent resection, all observed deaths were in patients with recurrent disease. The median OS following start of neoadjuvant therapy has not been reached for the entire cohort, and the 3-year OS was 64% (95%CI: 51% to 79%). Median TTP following start of chemotherapy was 49 months (95% CI: 18 to not reached), with 53% (95%CI: 40% to 70%) of patients progression free at 3 years. In patients with clinical Stage IIIA disease (n=34), the median OS was 37 months (95% CI: 14 to not reached), the 3-year OS was 52% (95% CI: 38% to 72%), the median TTP was 20 months (95% CI: 8 to not reached) and 42% (95% CI: 28% to 63%) were progression free at 3 years.
Among the 41 patients who underwent resection, the median follow-up after surgery was 29 months, during which 14 patients died. The median RFS was 54 months (95% CI: 24 to not reached), median OS was not reached, and 3-year OS was 62% (95% CI: 48% to 80%). Among patients with stage IIIA, the median RFS was 30 months (95%CI: 9 to not reached), median OS was 35 months (95% CI: 18 to not reached) and 3-year OS was 50% (95% CI: 34% to 74%).
In resected patients, downstaging was not associated with improved OS following resection (3-yr OS = 70% vs. 56% in the group without downstaging, p=0.24) or RFS (3-yr RFS = 65% vs. 52% in the group without downstaging, p=0.52). Nodal downstaging was marginally associated with improved OS (3-yr OS = 66% vs. 37%, p=0.051) but did not increase RFS (3-yr RFS = 54% vs. 37%, p=0.40).
Exploratory Outcomes
Among the 41 resected patients, 11 (27%, 95% CI: 14% - 43%) had ≥90% pathologic response. Pathologic response did correlate with outcomes. In the 11/41 (27%) with ≥90% versus those with <90% treatment effect, the 3-year RFS was 91% vs. 48%, p=0.024 and OS 100% vs. 49%, p=0.011. These outcomes remained significant when adjusted for clinical stage (p=0.035 and 0.018, respectively).
Intratumoral cavitation after 1 dose of bevacizumab was associated with a trend toward improved pathologic response, whereas KRAS mutation predicted lack of response, as outlined in Table 3.
Table 3. Exploratory Analyses (only resected patients included).
| Outcome | |||
|---|---|---|---|
|
| |||
| Cavitation (N=7) | No cavitation (N=34) | p-value | |
|
| |||
| Downstaged | 5 (71%) | 14 (41%) | |
| Not downstaged | 2 (29%) | 20 (59%) | 0.22 |
|
| |||
| Nodal down-staged | 5 (83%) | 12 (46%) | |
| Not downstaged | 1 (17%) | 14 (54%) | 0.18 |
|
| |||
| Path response ≥90% | 4 (57%) | 7 (21%) | |
| <90% | 3 (43%) | 27 (79%) | 0.069 |
|
| |||
| 3-yr RFS all | 63% | 53% | 0.43 |
| 3-yr RFS IIIA | 57% | 39% | 0.32 |
|
| |||
| 3-yr OS all | 60% | 60% | 0.83 |
| 3-yr OS IIIA | 57% | 44% | 0.48 |
|
| |||
| KRAS-mutant (N=10) | KRAS wild-type (N=31) | ||
|
| |||
| Downstaged | 5 (50%) | 14 (45%) | |
| Not downstaged | 5 (50%) | 17 (55%) | 1.0 |
|
| |||
| Nodal down-staged | 5 (63%) | 12 (50%) | |
| Not downstaged | 3 (37%) | 12 (50%) | 0.69 |
|
| |||
| Path response ≥90% | 0 (0%) | 11 (35%) | |
| <90% | 10 (100%) | 20 (65%) | 0.039 |
|
| |||
| 3-yr RFS all | 35% | 65% | 0.14 |
| 3-yr RFS IIIA | 17% | 56% | 0.096 |
|
| |||
| 3-yr OS all | 33% | 72% | 0.036 |
| 3-yr OS IIIA | 17% | 61% | 0.068 |
Discussion
This study represents the first published trial evaluating the addition of neoadjuvant bevacizumab to chemotherapy as part of a combined modality approach to the treatment of patients with resectable stage IB-IIIA NSCLC. Randomized multi-modality trials have demonstrated a 10-30% survival advantage with the use of adjuvant or neoadjuvant chemotherapy over surgery alone.2, 13-19 The debate remains as to whether neoadjuvant or adjuvant chemotherapy is the best approach, with advantages and disadvantages to each. Adjuvant treatment has the advantage of providing clinicians with a pathologic stage to guide postoperative therapy recommendations. While upfront surgery guarantees adequate tissue for molecular studies, current targeted therapies (such as erlotinib in EGFR-mutant NSCLC) remain investigational in the adjuvant setting and are being studied only after completion of standard therapies. The main disadvantage of adjuvant chemotherapy is the difficulty of drug delivery postoperatively.2 For clinical investigation, administration of neoadjuvant chemotherapy allows for an in vivo assessment of treatment response radiologically and pathologically, and the use of surrogate endpoints such as downstaging and pathologic response.
In this trial, we added bevacizumab to neoadjuvant chemotherapy in patients with resectable NSCLC in an attempt to improve the rate of downstaging.9 With 38% of patients showing an improvement from initial clinical to final pathological stage, this study failed to meet its primary endpoint (an increase from the reported 33%9 to a goal of 50%). From the start of chemotherapy, the median survival of patients with clinical stage IIIA NSCLC was 37 months which is comparable to previously published neoadjuvant trials.4
This study has many limitations. With concerns for bevacizumab-related toxicities, this study had many strict eligibility criteria. As a single institution study, these criteria slowed the anticipated rate of accrual. Stict eligibility criteria also raise questions about potential confounders when compared to historical controls, as patients with many common comorbid illnesses were not included. Finally and perhaps the largest limitation was the selection of the primary endpoint in the absence of uniform mediastinal staging. While the data for downstaging were compelling and allowed for inclusion of lymph node negative patients who would otherwise be appropriate for neoadjuvant chemotherapy, the lack of uniform pathological (mediastinal) staging on all patients may bias the results. Alternative endpoints, such as pathologic response, may have better correlated with long-term outcomes such as recurrence free and overall survivals and been equally as reliable regardless of pretreatment stage. Despite these limitations and failure to meet the primary endpoint, this study has many informative observations.
Neoadjuvant bevacizumab was generally well tolerated. Uncomplicated hemoptysis was the most common reason for bevacizumab discontinuation and there was no evidence of increased chemotherapy toxicity due to the combination. There was one grade 4 diverticulitis with perforation and one death from infection in the setting of treatment-related neutropenia. Bevacizumab has been associated with diverticulitis and shown to increase chemotherapy induced neutropenia.8 The early stopping criteria were not met. Perioperative complications potentially attributable to bevacizumab included gastrointestinal bleeding and bronchopleural fistulization. While the gastrointestinal complication rate is higher than expected after standard neoadjuvant chemotherapy without bevacizumab followed by thoracic surgery, the overall perioperative morbidity was comparable to what has been published.20 After induction treatment and surgery, the delivery of bevacizumab was not feasible with approximately half of eligible patients (19/39) receiving any adjuvant therapy (Figure 2).
In patients with clinical stage IIIA (N2), both clinical and nodal downstaging have been shown to be pathological correlates of clinical outcomes.3-5 In this trial, 82% (41/50) of enrolled patients were resected after neoadjuvant chemotherapy. There was no association between downstaging or nodal downstaging with survival. Alternative surrogate endpoints include pathologic response, defined as treatment effect of more than 90%. Junker, et al. described pathological response as predictive of outcomes after multimodality therapy, with patients with <10% viable tumor cells remaining experiencing the best outcomes.21 In a more recent publication of 192 patients with NSCLC who received neoadjuvant chemotherapy, the long term OS and RFS of the 19% of patients with tumors demonstrating ≥90% pathological response were significantly different than those with <90% response.22 While our pathological response data must be interpreted cautiously as an unplanned and exploratory analysis, the 27% of resected patients who had tumors with ≥90% pathologic response had a 3-year recurrence free survival of 91% vs. 48% in those with < 90% pathologic response.
This trial would suggest patients without a major pathologic response are at high risk for recurrence and may be ideal candidates for additional therapy, potentially as informed by an analysis of their tumor tissue. It is intriguing to note that in the 10 patients with KRAS-mutant lung cancer who underwent resection, there were no major pathological responses. These patients may be appropriate for studies of targeted neoadjuvant or alternative adjuvant approaches.
Given the paucity of single agent bevacizumab data in NSCLC we administered bevacizumab alone followed by repeat imaging 2 weeks later. This short interval was chosen as to not substantially delay the cytotoxic chemotherapy administration. We found tumor regression with single agent bevacizumab and tumor cavitation in 18% of patients. Fifty-seven percent of patients with tumor cavitation had a major pathologic response compared to 21% of patients without cavitation. Given the small sample size these data were not significant but are nevertheless intriguing. Cavitation has been described with bevacizumab and a new set of response criteria for radiographic assessment to account for the development of cavitation have been proposed23 - however the association with pathologic response has not been previously reported. Identification of a predictor of intratumoral cavitation could allow us to contemplate a biomarker for angiogenesis inhibitors which to date has not been well established.
In the era of personalized therapies for the treatment of NSCLC, neoadjuvant approaches permit individual patient assessment of treatment efficacy. Future trials will incorporate targeted therapies as part of a neoadjuvant strategy based on molecular profile, with the goal of improving pathologic response beyond that currently achievable with unselected standard cytotoxic chemotherapy. Adaptive strategies to improve outcomes in those who are unlikely to respond (lack of cavitation with bevacizumab or KRAS mutation) or those who do fail to respond to neoadjuvant therapy (lack of pathologic response) should be considered.
Acknowledgments
Support: This trial was supported by Genentech
This study was sponsored by a research grant from Genentech.
Footnotes
This trial has been previously presented in part at the ASCO Annual Meeting 2007, 2008, 2009 and at the IASLC World Conference on Lung Cancer 2011.
Disclosures: Drs. Kris, Riely and Krug have received consulting fees from Genentech/Roche.
References
- 1.Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5:220–228. doi: 10.1097/JTO.0b013e3181c814e7. [DOI] [PubMed] [Google Scholar]
- 2.Felip E, Massuti B, Alonso G, et al. Surgery Alone, or Surgery Followed by Adjuvant Paclitaxel/Carboplatin (PC) or Preoperative PC Followed by Surgery, in Early Stage Non-Small Cell Lung Cancer: Results of the Multicenter, Randomized, Phase III NATCH Trial. J Clin Oncol. 2009;(27):CRA7500. [Google Scholar]
- 3.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003;21:1752–1759. doi: 10.1200/JCO.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 4.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94:1099–1106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dooms C, Verbeken E, Stroobants S, et al. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128–1134. doi: 10.1200/JCO.2007.13.9550. [DOI] [PubMed] [Google Scholar]
- 6.Mouillet G, Monnet E, Milleron B, et al. Pathologic Complete Response to Preoperative Chemotherapy Predicts Cure in Early-Stage Non–Small-Cell Lung Cancer: Combined Analysis of Two IFCT Randomized Trials. Journal of Thoracic Oncology. 2012;7:841–849. doi: 10.1097/JTO.0b013e31824c7d92. doi:810.1097/JTO.1090b1013e31824c31827d31892. [DOI] [PubMed] [Google Scholar]
- 7.Pataer A, Kalhor N, Correa AM, et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer After Neoadjuvant Chemotherapy. J Thorac Oncol. 2012 doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 9.Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol. 2002;20:1989–1995. doi: 10.1200/JCO.2002.08.092. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Arcila M, Lau C, Nafa K, et al. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. J Mol Diagn. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 14.Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 15.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 16.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 17.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 18.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 19.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 20.Barnett SA, Rusch VW, Zheng J, et al. Contemporary results of surgical resection of non-small cell lung cancer after induction therapy: a review of 549 consecutive cases. J Thorac Oncol. 2011;6:1530–1536. doi: 10.1097/JTO.0b013e318228a0d8. arn. [DOI] [PubMed] [Google Scholar]
- 21.Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest. 2001;120:1584–1591. doi: 10.1378/chest.120.5.1584. [DOI] [PubMed] [Google Scholar]
- 22.Pataer A, Kalhor N, Correa AM, et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer After Neoadjuvant Chemotherapy. J Thorac Oncol. 2012;7:825–832. doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Lee KS, Hwang HS, et al. Molecularly targeted therapy using bevacizumab for non-small cell lung cancer: a pilot study for the new CT response criteria. Korean journal of radiology : official journal of the Korean Radiological Society. 2010;11:618–626. doi: 10.3348/kjr.2010.11.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]


