Abstract
Tumor necrosis factor (TNF)-α promotes tumor development under chronic inflammation. Because TNF also activates caspase-8, selective inhibition of TNF-induced extrinsic apoptosis would be required for inflammation-associated tumor growth. In a mouse model of inflammation-associated colon carcinogenesis, we found nuclear expression of β-catenin in tumors of wild-type, but not mutant, mice that were made resistant to TNF-induced apoptosis by a germline mutation blocking caspase cleavage of the retinoblastoma (RB) protein, despite similar frequencies of β-catenin exon-3 mutations in these two genetic backgrounds. TNF-induced apoptosis was also attenuated in human colon cancer cell lines with genetically activated β-catenin. However, we found that HCT116 cells, which contain an activated allele of β-catenin but do not express nuclear β-catenin, were sensitive to TNF-induced apoptosis. In HCT116 cells, TNF stimulated efficient RB cleavage that preceded chromatin condensation. In contrast, TNF did not induce RB cleavage in colon cancer cells expressing nuclear β-catenin and these cells could be sensitized to basal and/or TNF-induced apoptosis by the knockdown of β-catenin or RB. In the apoptosis-resistant colon cancer cells, knockdown of β-catenin led to a reduction in the RB protein without affecting RB mRNA. Furthermore, ectopic expression of the caspase-resistant, but not the wild-type, RB re-established resistance to TNF-induced caspase activation in colon cancer cells without β-catenin. Together, these results suggest that nuclear β-catenin–dependent RB stabilization suppresses TNF-induced apoptosis in caspase-8–positive colon cancer cells.
Introduction
Inflammation is a complex physiologic response induced by infections and injuries to eliminate damaged cells and stimulate tissue repair. While the inflammatory response is essential to health, chronic inflammation has been recognized as a major risk factor for cancer (1). In mouse models of inflammation-associated cancer, TNF and its downstream inflammatory effector, NF-κB, have been shown to play key roles in tumorigenesis (1). Interestingly, TNF receptor 1 (TNFR1) and its signaling complex of TRADD, RIPK1, TRAF2, and cIAP not only activate NF-κB but also activate caspase-8 through the adaptor FADD (2). While caspase-8 and FADD are essential to the induction of extrinsic apoptosis downstream of TNFR1, studies of caspase-8 and FADD knockout mice have shown that these two proteins also play a critical role in cell survival (3, 4). Recent studies have uncovered the mechanism underlying caspase-8–dependent survival, which involves the suppression of necrosis (5). The current data support a model where assembly of a heterodimeric complex of caspase-8 and FLIP through activated FADD leads to caspase-8 activation without self-cleavage and this caspase-8-FLIP complex cleaves CLYD, RIPK1, and RIPK3 to inhibit necrosis (necroptosis) in lymphocytes and intestinal epithelial cells (5–8). On the other hand, formation of a homo-oligomeric complex of caspase-8 and FADD is required for self-cleavage and the apoptotic activation of caspase-8 (5, 9). The discovery of the antinecrosis function of caspase-8 provides an explanation for the continued expression of caspase-8 in the majority of cancer cells. However, inflammation-associated tumor development would require mechanisms that inactivate TNF-induced apoptosis in caspase-8–positive cancer cells.
The canonical Wnt/β-catenin signaling cascade plays a crucial role in the intestinal crypt proliferation and homeostasis (10, 11). From a comprehensive genetic and epigenetic analysis of 276 human colorectal cancer (CRC) samples, it has been determined that the canonical Wnt pathway is constitutively activated in more than 90% of human CRCs through mutational activation of CTNNB1 (β-catenin) or inactivation of DKK, APC, AXIN2 (12). While activated β-catenin can enter the nucleus to regulate gene expression, its nuclear translocation and accumulation require additional factors, for example, Ahi1 or FOXM, and is not fully understood (13–15). Although the Wnt pathway is activated by genetic and epigenetic alterations in more than 90% of human colorectal cancer (12), nuclear β-catenin expression has been detected in only 47% of 742 sporadic human colon cancer tissue samples (16). These findings suggest that nuclear expression of β-catenin may require additional selective pressure beyond activation of the Wnt pathway in colon cancer cells.
We have previously shown that retinoblastoma (RB) is cleaved by caspase at a C-terminal site, DEAD886G887, to generate 2 fragments—ΔRB (1-886) and C42 (887-928), which are unstable and further degraded in apoptotic cells (17, 18). We created an Rb-MI allele in the mouse genome to encode a caspase-resistant RB-MI protein (DEAA886E887) and have shown that intestinal epithelial cells in the Rb-MI mice are protected from inflammation-induced apoptosis (19–21). We have shown that Rb-MI promotes colon tumor development in a p53-null genetic background (19). We therefore subjected Rb-MI mice to an inflammation-associated colon carcinogenesis protocol. We show here that colonic tumors in the Rb-wt mice acquire resistance to TNF-induced apoptosis, which is a normal phenotype of the Rb-MI colonic epithelial cells. In this inflammation-associated colon carcinogenesis model, β-catenin is consistently activated by exon-3 mutations and expressed in the nucleus of colon tumor cells (22, 23). We found exon-3 mutations in the Rb-wt and Rb-MI colon tumors; however, nuclear expression of β-catenin was mostly absent from the Rb-MI tumor cells. These observations led us to investigate how nuclear β-catenin expression might affect RB stability and TNF-induced apoptosis in colon cancer cells.
Materials and Methods
Antibodies
Anti-β-catenin (610153), anti-FADD (556402), and anti-RIPK1 (610458) were from BD Biosciences. Anti-caspase-8 (#9746), anti-cleaved caspase-8 (#9496), anti-cleaved caspase-3 (#9664), anti-PARP1 (#9542), anti-FLIP (#3210), anti-cIAP (#4952), anti-XIAP (#2042), and horseradish peroxidase (HRP)-conjugated secondary antibodies were from Cell Signaling Technology. Anti-RB (RB-C) (ab1119) and anti-TNFRI (ab19139) were from Abcam. Anti-GAPDH (MAB374) and anti-active-β-catenin(05-665) were from Millipore. Anti-TRADD (sc-7868) and anti-IKB-α (SC-371) were from Santa Cruz Biotechnology. Anti-RB (851) was raised against the C-terminal fragment (residues 768–928) of RB.
Mice
Construction of the mouse Rb-MI allele and genotyping were described previously (20). All animal studies were conducted under protocols approved by the University of California at San Diego (La Jolla, CA) Institutional Animal Care and Use Committee. For the induction of colon tumors, 6- to 7-week-old male mice were injected intraperitoneally (i.p.) once with 12.5 mg/kg azoxymethane (AOM; Sigma Aldrich) and a week later were fed dextran sulfate sodium (DSS) salt (3%, 36–50 kDa, ICN Biomedical) in the drinking water for 7 days and euthanized 21 weeks after AOM injection. Tumor-bearing mice were i.p. injected with murine recombinant TNF-α (PeproTech; 1 μg/mouse in PBS containing 2% FBS) or with PBS containing 2% FBS and euthanized 24 hours later. For the ulceration study, male mice at 6 weeks of age injected with AOM and then treated with DSS were euthanized 24 hours after the completion of DSS treatment, and the colonic tissues were collected for hematoxylin and eosin (H&E) staining.
Immunohistochemistry and terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling assay
Immunohistochemistry was carried out with DAKO LSAB + System-HRP according to manufacturer’s protocol. Terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling assay (TUNEL) with the TACS XL In Situ Apoptosis Detection Kit was conducted according to the manufacturer’s instructions (R&D Systems).
Cell culture
The LIM cell lines were maintained as previously described (24). Other cell lines were from American Type Culture Collection and maintained accordingly.
RNA interference
siRNAs targeting β-catenin (s438) were from Ambion. The lentiviral shRNA plasmids were from Sigma-Aldrich: TRCN0000003846 (β-catenin shRNA-A), TRCN0000-003845 (β-catenin shRNA-B), TRCN0000010419 (RB #1), TRCN0000010418 (RB #2), and TRCN0000040167 (shRB). The lentiviral β-catenin short hairpin RNA (shRNA) plasmid and packaging plasmids were transfected into 293FT cells using GeneTran (Biomiga) to produce lentiviral particles.
RNA preparation and quantitative real-time PCR
Total RNA was isolated from cells using the RNeasy Kit (QIAGEN) and reverse transcribed with the ABI revere transcription kit. Quantitative real-time polymerase chain reaction (Q-RT-PCR) was carried out using 7900HT Fast Real-Time PCR System (ABI) with primers: 5′GATTGATTCGA-AATCTTGCCCT3′ and 5′CTGATGTGCACGAACA-AGCA3′ for β-catenin; 5′GCAGTATGCTTCCACCAG-GC3′ and 5′AATCCGTAAGGGTGAACTAGGAAAC3′ for RB.
Coimmunoprecipitation
Cells were collected in PBS with 10% glycerol, lysed in lysis buffer [10 mmol/L Tris-HCl, 200 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.5 mmol/L dithiothreitol (DTT), 0.5% NP-40, 0.125% sodium deoxycholate, 0.05%SDS, and 20% glycerol] supplemented with 5 μmol/L ethidium bromide and protease inhibitors (Roche). The lysates were sonicated, treated with RNase (100 μg/mL) and DNase (100 μg/mL) for 30 minutes on ice, sonicated again, and then centrifuged at 14,000 rpm at 4°C for 30 minutes. Primary antibody (2 μg/mL) was added to 500 μg of total cell lysate and incubated overnight at 4°C, followed by adding 15 μL of protein A/G agarose for 2 hours. Immunoprecipitated proteins were analyzed by Western blotting (Supplementary Fig. S6).
Statistical analysis
GraphPad Prism programs were used to analyze the data and plot curves. Data are represented as mean and SD. Two-tailed unpaired t test was used to determine statistical significance of the differences between data sets. P < 0.05 was considered as statistically significant.
Results
Inflammation-associated colonic tumors acquire resistance to TNF-induced apoptosis
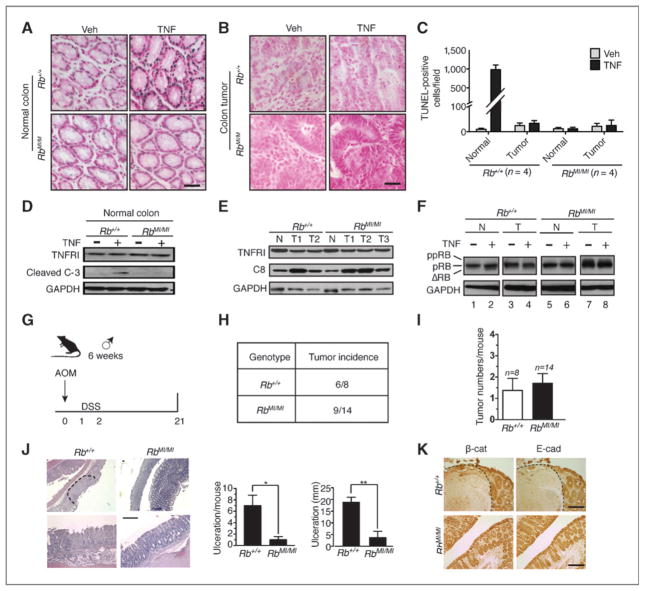
In Rb-MI mice, intestinal epithelial cells are resistant to apoptosis induced by bacterial lipopolysaccharide (LPS) or DSS (19–21). We show here that colonic epithelial apoptosis induced by TNF was also suppressed in the Rb-MI mice (Fig. 1A, C, D), despite wild-type levels of TNFR1 and pro-caspase-8 expression (Fig. 1E) and wild type-levels of splenic apoptosis (Supplementary Fig. S1). We then placed the Rb-wt and Rb-MI mice on the AOM/DSS carcinogenesis protocol (Fig. 1G) to induce colon tumors (25) and treated tumor-bearing mice with TNF. We found that colonic tumors in the Rb-wt mice became resistant to TNF-induced apoptosis (Fig. 1B and C), and showed reduced RB cleavage response to TNF (Fig. 1F), although these apoptosis-resistant Rb-wt tumors continued to express TNFR1 and caspase-8 (Fig. 1E).
Figure 1.
Inflammation-induced colonic tumors acquire resistance to TNF-induced apoptosis. A to C, TUNEL staining (blue) of normal (A) and tumor (B) colonic tissue sections and quantification (C) from the indicated number (n) of tumor-bearing mouse treated with PBS (Veh) or murine TNF (1 μg per mouse) for 24 hours. Nuclei were counterstained with TACS Fast Red. Scale bar, 50 μm. D, TNFR1 and cleaved caspase-3 expression in normal colonic tissue extracts from the indicated mice after TNF treatment. E, TNFR1 and caspase-8 expression in normal (N) or tumor (T) colonic tissues from the indicated mice. F, TNF-induced RB cleavage in normal (N) and tumor (T) colonic tissues from the indicated mice. G, summary of the AOM/DSS colon carcinogenesis protocol. Only male mice were used in this study and the protocol began with mice at 6 weeks of age. H, tumor incidence of AOM/DSS-treated Rb-wt and Rb-MI mice. I, number of tumors per mouse, n, number of mice. J, DSS-induced colonic ulceration was suppressed in Rb-MI mice. Area of ulceration is denoted by dashed line in sections stained with H&E. Images from 2 different magnifications are shown. Scale bar, 100 μm. Histograms show the number and size distribution of ulceration. Ulceration diameter was determined using ImageJ software.*, P < 0.05; **, P < 0.01. K, immunohistochemical staining for E-cadherin and β-catenin in colonic tissues of AOM/DSS-treated Rb-wt (+/+) and Rb-MI (MI/MI) mice at 24 hours after the cessation of DSS feeding. Note that E-cadherin and β-catenin were not detected in tissues that expanded in areas of ulceration. Scale bar, 100 μm.
We have previously shown that Rb-MI stimulates spontaneous colon cancer development in the p53-null background (19). However, Rb-MI did not promote colonic tumor development in the AOM/DSS carcinogenesis protocol (Fig. 1H and I; Supplementary Fig. S2). The tumor incidences (Fig. 1H and I), histologic features (Supplementary Fig. S2A), tumor size distributions (Supplementary Fig. S2B), and tumor expression of proliferation markers [proliferating cell nuclear antigen (PCNA), Ki67, cyclin D1, and c-Myc; Supplementary Fig. S2C–S2F] were found to be similar in the Rb-wt and Rb-MI mice. A possible explanation for why Rb-MI did not promote tumor development in the AOM/DSS carcinogenesis protocol could be because Rb-MI suppressed DSS-induced tissue damage (Fig. 1J). Associated with DSS-induced ulceration and tissue damage was an expansion of stromal tissues, which could be distinguished from the epithelial tissues by the lack of cell surface expression of E-cadherin and β-catenin (Fig. 1K). This injury-associated tissue expansion was observed in the Rb-wt mice but diminished in the Rb-MI mice (Fig. 1K). Therefore, the Rb-MI–mediated epithelial survival might have a double-edged effect—it reduced apoptosis but it also reduced tissue regeneration; as a result, on balance, Rb-MI did not affect the tumor outcome. It is also possible that an alternative apoptosis suppression mechanism is readily activated by the AOM/DSS treatment; as a result, the antiapoptosis function of Rb-MI became irrelevant in this colon carcinogenesis model.
Nuclear β-catenin is mostly absent from colonic tumors of Rb-MI mice
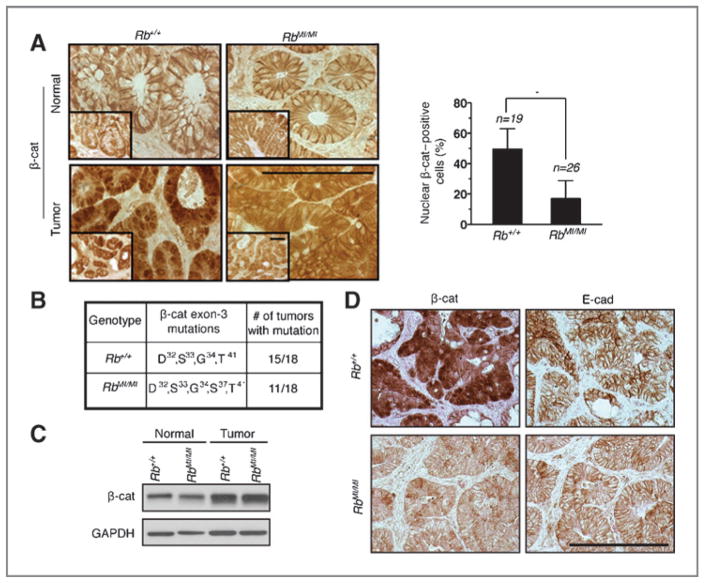
Previous studies have shown that β-catenin exon-3 mutations that interfere with phosphorylation by GSK3β are frequently found in AOM/DSS–induced colon tumors (23, 26, 27). Consistently, we found similar frequency of β-catenin exon-3 mutations (Fig. 2B), higher levels of β-catenin protein (Fig. 2C), and similar expression of E-cadherin (Fig. 2D) in the Rb-wt and the Rb-MI colonic tumors. The expression of cyclin D1 and c-Myc, 2 established β-catenin target genes, was similarly upregulated in the Rb-wt and the Rb-MI colonic tumors (Supplementary Fig. S2E and S2F). Microarray-based gene expression analysis further confirmed similar expression profiles for the Rb-wt and the Rb-MI colonic tumors (not shown). However, immunohistochemical analysis of tumor sections showed that the majority of Rb-wt tumor cells (in 19 adenomas examined) expressed nuclear β-catenin, whereas less than 20% of Rb-MI tumor cells (in 26 adenomas examined) expressed nuclear β-catenin (Fig. 2A). These results suggest that nuclear expression of exon-3–mutated β-catenin is not necessary for its transactivation function in the AOM/DSS-induced colonic tumors. Instead, nuclear expression of β-catenin appeared to be a tumor phenotype in the Rb-wt mice that involved factors other than exon-3 mutation or E-cadherin expression. While those other factors were not identified by this study, our data showed that nuclear expression of β-catenin was mostly absent from AOM/DSS–induced colon tumors of the Rb-MI mice. This observation suggests that nuclear expression of β-catenin might suppress TNF-induced apoptosis, and thus the selection for this cancer phenotype could be bypassed in the Rb-MI mice.
Figure 2.
Nuclear expression of β-catenin in colonic tumors of Rb-wt but not Rb-MI mice. Subcellular localization (A), exon-3 mutations (B) and levels of β-catenin in normal or tumor colonic tissues (C) from the indicated mice. Scale bar, 100 μm. n, number of tumors analyzed. *, P < 0.05. D, staining for β-catenin and E-cadherin in colonic tumor tissues of the indicated mice.
Resistance to TNF-induced apoptosis in human colon cancer cells with nuclear β-catenin
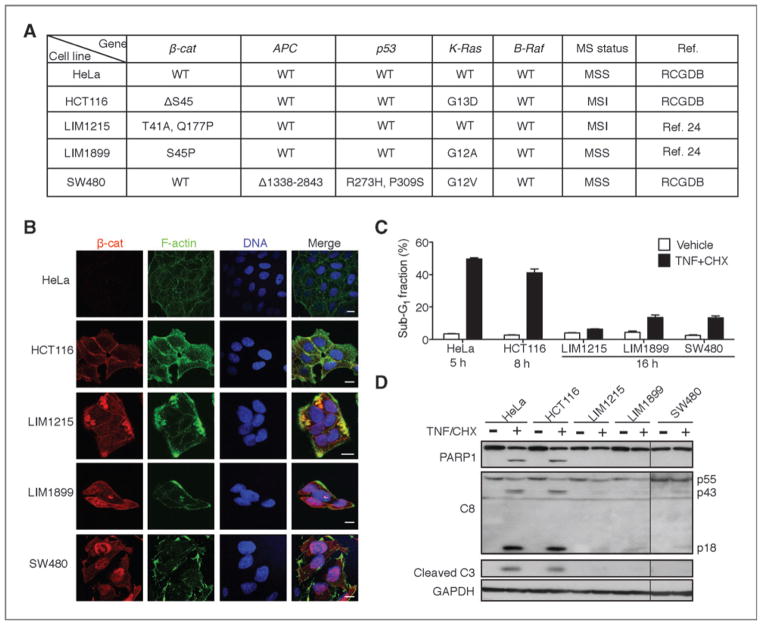
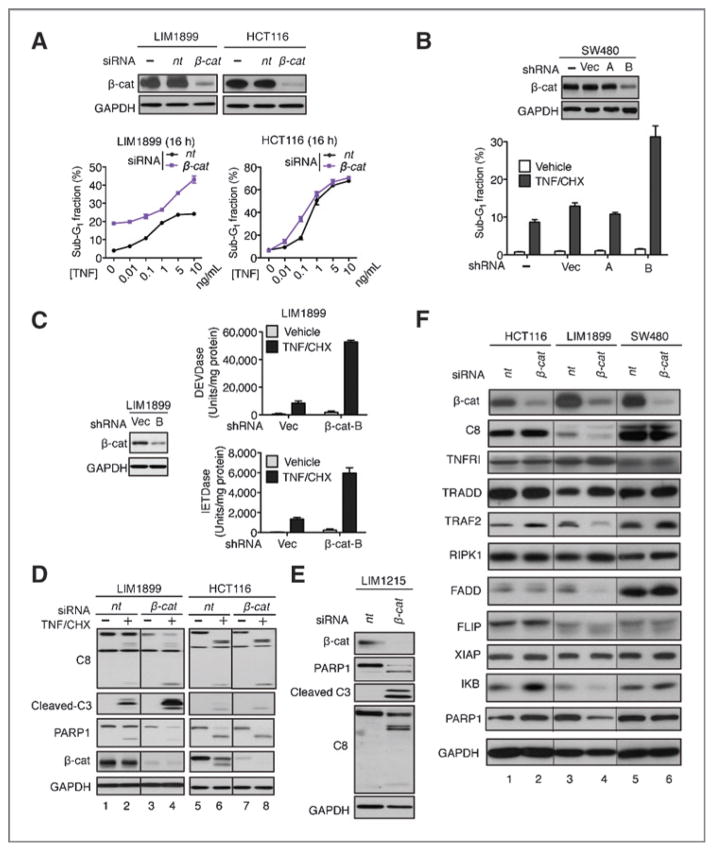
To test the hypothesis that nuclear expression of β-catenin, besides its mutational activation, is required to suppress apoptosis, we examined several human colon cancer cell lines containing CTNNB1 (β-catenin) or APC mutations (Fig. 3A) for β-catenin nuclear localization and response to TNF-induced apoptosis. Nuclear β-catenin was detected in LIM1215, LIM1899, and SW480 colon cancer cells but not in HCT116 cells (Fig. 3B), and the apoptotic response to TNF was significantly lower in cells expressing nuclear β-catenin (Fig. 3C and D). Previously published reports have established that the mutant β-catenin is constitutively active in HCT116 cells and can drive T-cell factor (TCF)-dependent transcription (28, 29); however, this activated β-catenin is not constitutively localized to the nucleus (Fig. 3B), and the non-nuclear localization of activated β-catenin correlated with sensitivity to TNF-induced apoptosis in HCT116 cells (Fig 3C and D).
Figure 3.
Human colon cancer cells with nuclear expression of β-catenin showed resistance to TNF-induced apoptosis. A, summary of known mutations in HeLa, HCT116, LIM1215, LIM1899, and SW480 cell lines. RCGDB, The Roche Cancer Genome Database. B, subcellular localization of β-catenin in colon cancer cell lines. Scale bar, 10 μm. C and D, colon cancer cells expressing nuclear β-catenin are resistant to TNF (10 ng/mL)/CHX (2.5 μg/mL)-induced apoptosis measured by sub-G1 (C) or caspase cleavage (D).
RB cleavage is more efficient and precedes chromatin condensation in apoptosis-sensitive colon cancer cells lacking nuclear β-catenin
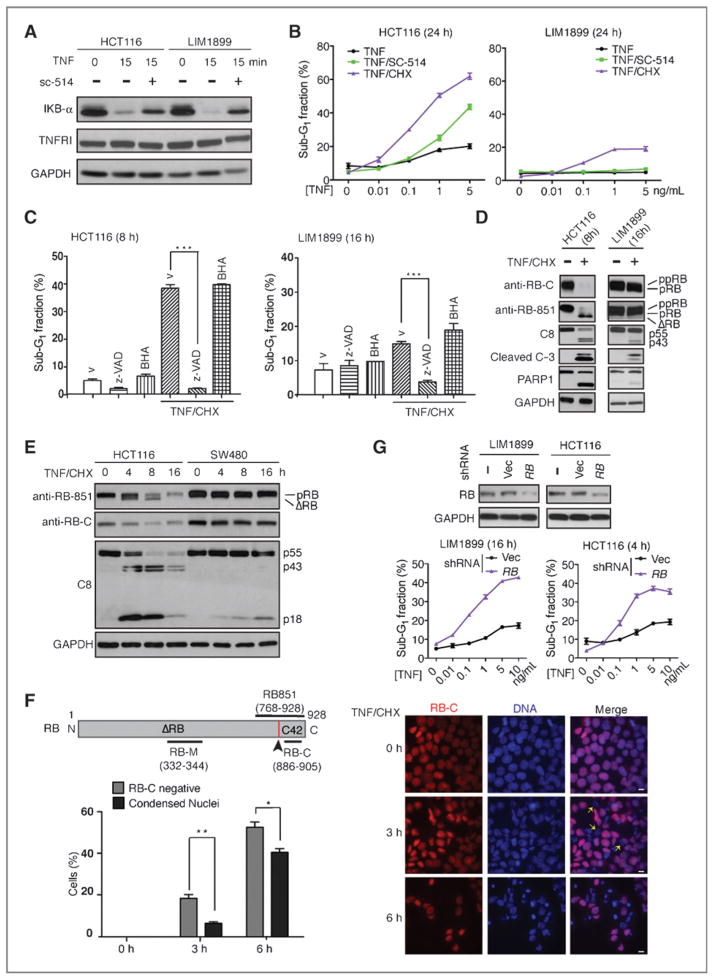
Both the HCT116 and the LIM1899 cells contain codon-45–mutated β-catenin (Fig. 3A; confirmed by resequencing; Supplementary Fig. S3A), except that HCT116 cells also contain a wild-type β-catenin allele (Supplementary Fig. S3A; ref. 28). Both cell lines express E-cadherin (Supplementary Fig. S3B), TNFR1, TRADD, RIPK1, TRAF2, cIAP, FADD, FLIP, and caspase-8 (Supplementary Fig. S3C). In both cell lines, the NF-κB signaling pathway is activated by TNF as shown by the degradation of IκB and the sensitivity to an inhibitor of IKKβ (sc-514, Fig. 4A). Treatment of HCT116 cells with TNF alone, TNF plus sc-514, or TNF plus cycloheximide (CHX) induced DNA fragmentation (Fig. 4B). However, LIM1899 cells were resistant to TNF or TNF/sc-514 and underwent DNA fragmentation only when treated with TNF/CHX but with a lower sensitivity than HCT116 cells (Fig. 4B). We therefore used the combined treatment of TNF/CHX to further compare the apoptotic response between HCT116 and LIM1899 cells.
Figure 4.
RB cleavage precedes chromatin condensation in apoptosis-sensitive HCT116 cells. A, TNF-induced IκBα degradation. Whole-cell lysates harvested at the indicated time following treatment with TNF (10 ng/mL) or the IKKβ inhibitor sc-514 (25 μmol/L) were probed with the indicated antibodies. B, TNF-induced DNA fragmentation was determined by FACS; mean and SDs from 3 independent experiments are shown. C, TNF induced caspase-dependent DNA fragmentation in colon cancer cells. Values shown are mean and SD from 3 independent experiments. ***, P < 0.001. D and E, TNF/CHX-induced protein cleavage in the indicated cells with indicated treatments. F, in situ detection of RB C-terminal cleavage with anti-RB-C and chromatin condensation in HCT116 cells. *, P < 0.05; **, P < 0.01. G, stable knockdown of RB (Western blots) sensitized colon cancer cells to TNF-induced apoptosis. CHX (2.5 μg/mL) was added to all TNF concentrations (0–10 ng/mL).
TNF/CHX-induced DNA fragmentation was abolished by a pan-caspase inhibitor (zVADfmk) but not by an anti-oxidant (BHA, butylated hydroxyanisole; Fig. 4C), showing that TNF induced caspase-dependent apoptosis rather than necrosis in these colon cancer cells. Caspase activation, assessed by caspase-8 cleavage (which is required for the execution of apoptosis but not necrosis; ref. 9), caspase-3, and PARP1, occurred at a faster rate and to a greater extent in HCT116 than in LIM1899 cells (Fig. 4D). The cleavage of RB (loss of reactivity to anti-RB-C, and formation of ΔRB) was also more efficient in HCT116 than in LIM1899 cells (Fig. 4D). In another apoptosis-resistant colon cancer cell line, SW480, TNF/CHX also induced IκB degradation (Supplementary Fig. S3D) but not RB cleavage (Fig. 4E). We have previously shown that caspase-8 is required for TNF to induce RB cleavage, and the cleavage of RB is then required for TNF to induce apoptosis (21). We show here that RB cleavage, assessed by the loss of reactivity to anti-RB-C antibody (Fig. 4F schematic diagram), preceded TNF/CHX-induced nuclear condensation in HCT116 cells (Fig. 4F). Consistent with the conclusion that full-length RB suppresses apoptosis, RB knockdown with 3 different lentiviral shRNAs (Fig. 4G and Supplementary Fig. S4) sensitized both LIM1899 and HCT116 cells to TNF-induced apoptosis (Fig. 4G, Supplementary Fig. S4). Together, these results establish a correlation between nuclear β-catenin expression, inefficient RB cleavage, and reduced apoptotic response in colon cancer cells.
Enforced nuclear expression of β-catenin reduces TNF-induced apoptosis
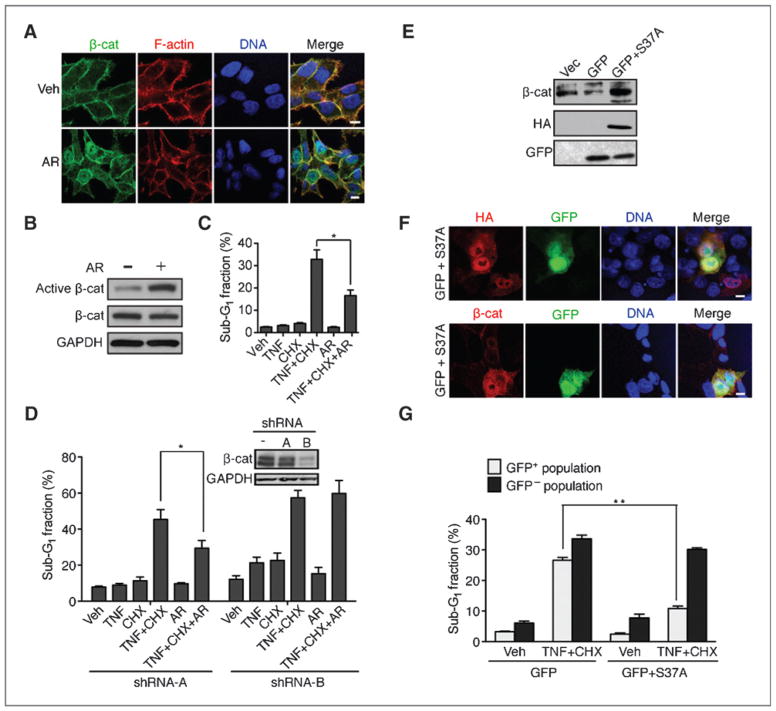
Because HCT116 cells contain a wild-type allele of β-catenin (ref. 28; Supplementary Fig. S3A), we treated them with a GSK3β inhibitor AR-A014418 (AR) to induce dephosphorylation and nuclear localization of the wild-type β-catenin (Fig. 5A and B). We found that TNF-induced DNA fragmentation (Fig. 5C) and caspase cleavage (Supplementary Fig. S5A) were reduced in AR-treated cells. To control for AR off-target effects, we stably knocked-down β-catenin and showed that the inhibitory effect of AR on TNF-induced DNA fragmentation was β-catenin–dependent because the apoptosis-inhibitory effect of AR was abolished by shRNA-B, which efficiently knocked-down β-catenin but not shRNA-A, which did not knockdown β-catenin (Fig. 5D). AR treatment did not alter the expression of TNFRI, FADD, TRADD, TRAF2, RIPK1, FLIP, XIAP, c-IAP, or survivin (Supplementary Fig. s5B). We also ectopically expressed an activated mutant S37A-β-catenin protein in HCT116 (Fig. 5E), found it localized to the nucleus (Fig. 5F), and caused a reduction in TNF/CHX-induced DNA fragmentation (Fig. 5G). Together, these results suggest that nuclear expression of β-catenin protein can suppress TNF-induced apoptosis in HCT116 cells.
Figure 5.
Nuclear accumulation of β-catenin reduces TNF-induced apoptosis in HCT116 cells. A, GSK3β inhibitor AR-A014418 (AR; 20 μmol/L) induced nuclear accumulation of β-catenin in HCT116 cells. Scale bars, 10 μm. B, AR treatment induced β-catenin dephosphorylation. Whole-cell lysates were probed with the indicated antibodies. C, AR treatment reduced TNF-induced apoptosis. Values shown are mean and SD from 3 independent experiments. *, P < 0.05. D, inhibitory effect of AR on TNF-induced apoptosis was dependent on β-catenin. HCT116 cells stably transduced with the indicated shRNA (see Western blots for β-catenin knockdown efficiency) were treated with the indicated reagents and the sub-G1 fractions measured by FACS at 8 hours. Values shown are mean and SD from 3 independent experiments. *, P < 0.05. E and F, expression and nuclear localization of HA-S37-β-catenin (S37A) in HCT116 cells cotransfected with GFP. Scale bars, 10 μm. G, ectopic expression of nuclear β-catenin reduced TNF-induced apoptosis. Transfected cells were sorted into GFP-positive or negative fractions and then treated as indicated, and sub-G1 fractions were determined. Values shown are mean and SD from 3 independent experiments. **, P < 0.01.
β-catenin knockdown enhances TNF-induced apoptosis in colon cancer cells expressing nuclear β-catenin
It is generally accepted that colon cancer cells are dependent on β-catenin for proliferation and survival (11). However, we found that the knockdown of β-catenin had different effects on the survival of HCT116, SW480, LIM1215, and LIM1899 cells. We could stably propagate HCT116 cells in which β-catenin was knocked-down with lentiviral shRNA (Fig. 5D), consistent with a previous report showing that the proliferation and survival of HCT116 cells are independent of β-catenin (28). Similarly, we could stably propagate β-catenin knockdown SW480 cells (Fig. 6B). However, with LIM1215 and LIM1899, β-catenin–positive cells overtook the knockdown cells within 2 to 3 passages postinfection with the shRNA lentivirus. The dependency of LIM1899 and LIM1215 cells on β-catenin for survival was further shown by the induction of DNA fragmentation (Fig. 6A, LIM1899) or caspase activation (Fig. 6E, LIM1215) when β-catenin was transiently knocked-down with siRNA.
Figure 6.
Effects of β-catenin knockdown on basal and TNF-induced apoptosis in colon cancer cells. A, LIM1899 but not HCT116 cells were sensitized to basal and TNF-induced apoptosis upon transient knockdown of β-catenin. Values shown are mean and SD from 3 independent experiments. B, stable knockdown of β-catenin enhanced TNF/CHX-induced DNA fragmentation in SW480 cells. Values shown are mean and SD from 3 independent experiments. C, stable knockdown of β-catenin enhanced caspase activation in LIM1899 cells. D, transient knockdown of β-catenin sensitized LIM1899 but not HCT116 cells to TNF/CHX-induced protein cleavage. C and D, TNF/CHX treatment was for 8 hours at 48 hours posttransfection. E, transient knockdown of β-catenin sensitized LIM1215 cells to caspase activation at 48 hours posttransfection. F, expression of a panel of proteins at 48 hours after the knockdown of β-catenin in the indicated cells.
We then examined the effect of transient or stable β-catenin knockdown on TNF/CHX-induced apoptotic response in LIM1899, HCT116, and SW480 cells. In HCT116 cells, β-catenin knockdown did not affect the DNA fragmentation (Fig. 6A) or the caspase cleavage (Fig. 6D) response to TNF/CHX. In contrast, in LIM1899 cells, β-catenin knockdown enhanced the DNA fragmentation (Fig. 6A), caspase activation (Fig. 6C), and cleavage (Fig. 6D) response to TNF/CHX. In SW480 cells, the stable knockdown of β-catenin also enhanced the DNA fragmentation response to TNF/CHX relative to parental cells or populations infected with vector or irrelevant shRNA (Fig. 6B). The knockdown of β-catenin did not alter the levels of TNFR1, RIPK1, TRADD, FLIP, and XIAP in HCT116, LIM1899, or SW480 cells (Fig. 6F). A reduction in the levels TRAF2, caspase-8, FADD, and IκB were detected in β-catenin knockdown LIM1899 cells but because caspase activity was actually enhanced, this reduction was not pertinent to the survival function of nuclear β-catenin. These results suggest that the suppression of TNF-induced apoptosis by nuclear β-catenin did not involve previously established resistance mechanisms, for example, the upregulation of FLIP, the downregulation of XIAP, or altered expression of other known components of the extrinsic apoptosis pathway.
β-catenin–dependent RB protein expression
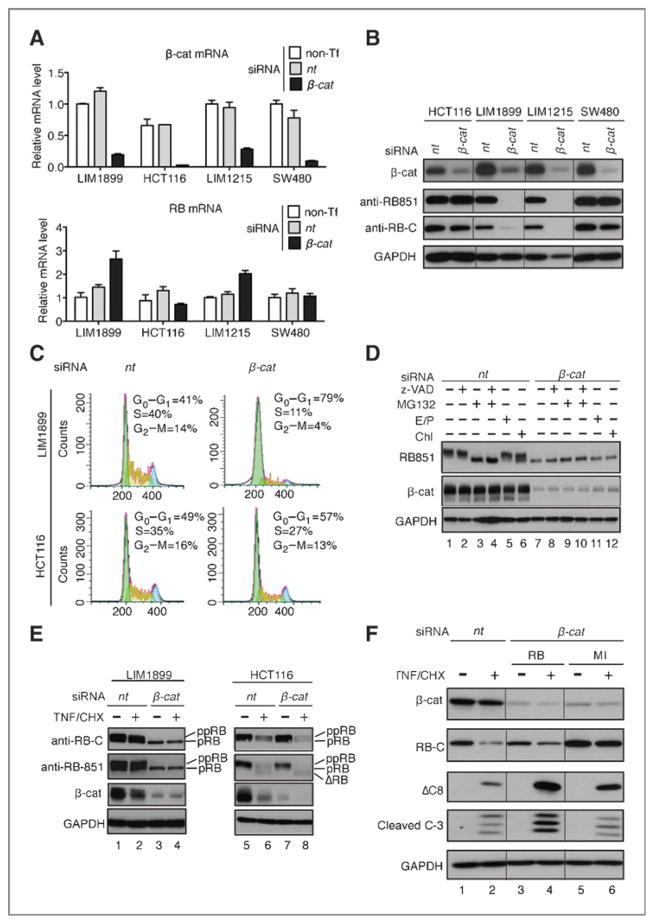
Because nuclear expression of β-catenin or the MI mutation of RB is associated with resistance to TNF-induced apoptosis in the mouse colonic epithelial cells and because TNF-induced RB cleavage is reduced in colon cancer cells expressing nuclear β-catenin, we examined whether nuclear β-catenin might affect the cleavage or the stability of RB protein. The knockdown of β-catenin did not reduce the levels of RB mRNAs in the 4 colon cancer cell lines examined (Fig. 7A). However, β-catenin knockdown had different effects on the RB protein levels, with significant reductions in the phosphorylation and the overall levels of RB observed with LIM1899 and LIM1215 cells (Fig. 7B). As the knockdown of β-catenin caused a higher level of G1 increase in LIM1899 than in HCT116 cells (Fig. 7C), the reduction in RB phosphorylation could be the result of G1 arrest. The RB protein level but not its phosphorylation could be partially restored by treatment with a proteasome inhibitor (Fig. 7D), suggesting that the knockdown of β-catenin also destabilized RB in LIM1899 cells. When treated with TNF/CHX, RB and β-catenin were both cleaved and degraded in HCT116 cells (Fig. 7E, lane 6). In β-catenin knockdown LIM1899 cells, the already lower levels of RB were not further reduced by TNF/CHX treatment (Fig. 7E lanes 3, 4). Because the knockdown of RB could sensitize LIM1899 cells to TNF-induced apoptosis (Fig. 4F), the reduction in RB protein was likely to account for the enhanced apoptotic response to TNF upon β-catenin knockdown in LIM1899 cells. Together, these results show that nuclear expression of β-catenin is required for the stable expression of RB protein, which is required to suppress the apoptotic response to TNF in colon cancer cells.
Figure 7.
Ectopic expression of RB-MI, but not RB, reduced caspase-8 cleavage in β-catenin knockdown cells. A, effect of β-catenin knockdown (top histograms) on RB mRNA levels (bottom histograms). B, effect of β-catenin knockdown on RB protein levels. C, effect of β-catenin knockdown on the cell cycle. D, effect of inhibitors z-VADfmk (pan-caspase, 20 μmol/L), MG132 (proteasome, 20 μmol/L), E/P (E64D and pepstatin, lysosome, 10 μg/mL each), and Chl (chloroquine, lysosome, 100 μmol/L) on RB phosphorylation and protein levels in LIM1899 cells posttransfection with nontarget (nt) or β-catenin (β-cat) siRNA. Drugs were added at 48 hours posttransfection for 6 hours. E, effect of transient β-catenin knockdown on TNF/CHX-induced RB cleavage and degradation in the indicated cells. F, ectopic expression of RB-MI, but not RB-wt, suppressed TNF/CHX-induced caspase-cleavage. Cells transfected with the indicated siRNA and the RB expression plasmids were treated with TNF/CHX, and the levels of cleaved caspase-8 and caspase-3 were determined from whole-cell lysates.
In the apoptosis-resistant LIM1899 cells, knockdown of β-catenin enhanced TNF/CHX-induced caspase-8 cleavage (Fig. 6D, lane 4), which is required for caspase-8 to induce apoptosis (9). If stabilization of RB is required for the suppression of caspase-8 cleavage, the ectopic expression of RB-MI (cleavage-resistant RB) would inhibit TNF/CHX-induced caspase cleavage more efficiently than RB-wt (cleavage-sensitive RB) in β-catenin–deficient LIM1899 cells. As shown in Fig. 7F, cotransfection of RB with the β-catenin siRNA transiently restored RB levels to that of the endogenous RB (in the nt-siRNA–transfected cells); however, TNF/CHX-induced caspase cleavage remained higher than that of the nt-siRNA–transfected LIM1899 cells (Fig. 7F, compare lane 4 to 2). In contrast, ectopic expression of RB-MI reduced caspase cleavage in these β-catenin–deficient cells (Fig. 7F, lane 6). Together, these results suggest that sustaining the expression of full-length RB is a mechanism by which nuclear β-catenin suppresses TNF-induced apoptosis in colon cancer cells.
Discussion
Previous studies of the Rb-MI mice (19–21) have shown that caspase cleavage of RB is required for TNF to induce apoptosis in intestinal epithelial cells. TNF-induced gene expression is not altered in Rb-MI cells (21). The RB cleavage products (ΔRB and the C-terminal 42 amino acid peptide) are unstable in apoptotic cells and ectopic expression of either or both fragments did not stimulate apoptosis in LIM1899 cells (J. Han, unpublished). These findings suggest that preservation of the full-length RB is required to inhibit TNF-induced epithelial apoptosis. We show here that C-terminal cleavage of RB occurred in the apoptosis-sensitive HCT116 cells before nuclear condensation. Recent studies have shown that RB can regulate the centromeric localization of condesin to ensure the fidelity of chromosomal segregation during mitosis (30). Perhaps RB could function as a barrier for premature chromatin condensation and has to be destabilized for apoptosis to proceed.
Recent studies have found that intestinal-specific knockout of caspase-8 causes spontaneous colitis, severe sensitivity to DSS, and premature death (ref. 8; L. Eckmann, unpublished). This is consistent with the fact that caspase-8 is required to inhibit TNF-induced necrosis (6, 7). The Rb-MI mice do not suffer from spontaneous colitis, instead, are protected from DSS-induced ulceration (Fig. 1J). Thus, the antinecrosis function of caspase-8 must be intact in the Rb-MI mice. Given that RB cleavage is specifically required for the activation of TNF-induced apoptosis in intestinal epithelial cells, it is not surprising to find that caspase-8–positive colon cancer cells suppress TNF-induced apoptosis by stabilizing the full-length RB protein.
The mechanism by which nuclear β-catenin regulates the expression of RB protein is presently unknown. Our results indicate that the nuclear expression, besides the mutational activation, of β-catenin is involved. We have observed the coimmunoprecipitation of RB and β-catenin in LIM1899, SW480, and HCT116 cells and found that this association was not altered by TNF treatment (Supplementary Fig. S6). We have also shown that the RB protein immunoprecipitated with β-catenin or with E2F1 can be cleaved by caspase-8 or caspase-3 in vitro (Supplementary Fig. S6), suggesting that β-catenin by itself does not protect RB from caspase cleavage. Elucidation of the mechanism underlying nuclear β-catenin–dependent RB stabilization will await further investigation.
The TCGA data have shown a consistent upregulation of RB1 expression in human CRC samples (12). A previous report has suggested that wild type RB expression is required to restrain the inhibitory activity of E2F1 on β-catenin in colon cancer cells (31). Our finding provides an additional explanation for the upregulation of RB expression in colon cancer cells, as it is also required to inhibit TNF-induced apoptosis. The TCGA data have shown that the Wnt pathway is activated by genetic and epigenetic alterations in more than 90% of human colorectal cancer (12); however, nuclear β-catenin expression has been detected in only 47% of 742 sporadic human colon cancer tissue samples (16), consistent with the fact that activation of the canonical Wnt pathway is necessary but not sufficient for the nuclear accumulation of β-catenin (13). Our results suggest that the expression of nuclear β-catenin is associated with cancer cell resistance to TNF-induced apoptosis and therefore may be used as a biomarker to identify tumors that are dependent on β-catenin to suppress the apoptotic activation of caspase-8.
Supplementary Material
Acknowledgments
The authors thank Dr. Tony Burgess at the Ludwig Cancer Research Institute in Melbourne, Australia, for the generous gifts of the LIM1899 and LIM1215 cells, which were previously described in reference 24.
Grant Support
The study was supported by RO1CA058320 to J.Y.J. Wang, P30DK80506 to L. Eckmann, and the National Council for Scientific and Technological Development, Brazil to R.C. Soletti, and H.L. Borges.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Research Online (http://mcr.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: J. Han, R.C. Soletti, A. Sadarangani, L. Eckmann, H.L. Borges, J.Y.J. Wang
Development of methodology: J. Han, R.C. Soletti, P. Sridevi, J.Y.J. Wang
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J. Han, R.C. Soletti, A. Sadarangani, P. Sridevi, M.E. Ramirez, J.Y.J. Wang
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J. Han, R.C. Soletti, A. Sadarangani, P. Sridevi, L. Eckmann, J.Y.J. Wang, J.Y.J. Wang
Writing, review, and/or revision of the manuscript: R.C. Soletti, P. Sridevi, L. Eckmann, J.Y.J. Wang
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Sadarangani
Study supervision: A. Sadarangani, H.L. Borges, J.Y.J. Wang
References
- 1.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–23. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 2.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–5. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 4.Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 5.Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS. RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell. 2011;44:9–16. doi: 10.1016/j.molcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–42. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12:757–63. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–9. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181:2522–32. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- 10.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–26. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Network TT. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman A, Nusse R. Location, location, location: FoxM1 mediates beta-catenin nuclear translocation and promotes glioma tumorigenesis. Cancer Cell. 2011;20:415–6. doi: 10.1016/j.ccr.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–54. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–42. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452–62. doi: 10.1158/1078-0432.CCR-10-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan X, Martin SJ, Green DR, Wang JY. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J Biol Chem. 1997;272:9613–6. doi: 10.1074/jbc.272.15.9613. [DOI] [PubMed] [Google Scholar]
- 18.Tan X, Wang JY. The caspase-RB connection in cell death. Trends Cell Biol. 1998;8:116–20. doi: 10.1016/s0962-8924(97)01208-7. [DOI] [PubMed] [Google Scholar]
- 19.Borges HL, Bird J, Wasson K, Cardiff RD, Varki N, Eckmann L, et al. Tumor promotion by caspase-resistant retinoblastoma protein. Proc Natl Acad Sci U S A. 2005;102:15587–92. doi: 10.1073/pnas.0503925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chau BN, Borges HL, Chen TT, Masselli A, Hunton IC, Wang JY. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat Cell Biol. 2002;4:757–65. doi: 10.1038/ncb853. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Masselli A, Frisch SM, Hunton IC, Jiang Y, Wang JY. Blockade of tumor necrosis factor-induced Bid cleavage by caspase-resistant Rb. J Biol Chem. 2007;282:29401–13. doi: 10.1074/jbc.M702261200. [DOI] [PubMed] [Google Scholar]
- 22.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–20. [PubMed] [Google Scholar]
- 24.Zhang HH, Walker F, Kiflemariam S, Whitehead RH, Williams D, Phillips WA, et al. Selective inhibition of proliferation in colorectal carcinoma cell lines expressing mutant APC or activated B-Raf. Int J Cancer. 2009;125:297–307. doi: 10.1002/ijc.24289. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi M, Fukuda K, Sugimura T, Wakabayashi K. Beta-catenin is frequently mutated and demonstrates altered cellular location in azoxymethane-induced rat colon tumors. Cancer Res. 1998;58:42–6. [PubMed] [Google Scholar]
- 27.Kaiser S, Park YK, Franklin JL, Halberg RB, Yu M, Jessen WJ, et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekine S, Shibata T, Sakamoto M, Hirohashi S. Target disruption of the mutant beta-catenin gene in colon cancer cell line HCT116: preservation of its malignant phenotype. Oncogene. 2002;21:5906–11. doi: 10.1038/sj.onc.1205756. [DOI] [PubMed] [Google Scholar]
- 29.Bottomly D, Kyler SL, McWeeney SK, Yochum GS. Identification of {beta}-catenin binding regions in colon cancer cells using ChIP-Seq. Nucleic Acids Res. 2010;38:5735–45. doi: 10.1093/nar/gkq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manning AL, Dyson NJ. RB: mitotic implications of a tumour suppressor. Nat Rev Cancer. 2012;12:220–6. doi: 10.1038/nrc3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, et al. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature. 2008;455:552–6. doi: 10.1038/nature07310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.