Abstract
Background
Rhinovirus and IgE act in concert to promote asthma exacerbations. While basophils are the principal cell type in the blood that is activated by IgE, their role in virus-induced asthma episodes remains elusive.
Objective
To monitor IgE responsiveness in circulating basophils of rhinovirus-infected atopic asthmatics during acute infection and convalescence.
Methods
The capacity for basophils to respond to IgE was assessed by testing the effects of allergen, or cross-linking anti-FcεRI and anti-IgE antibodies, on surface TSLP receptor in 24-hour PBMC cultures. Activation profiles of basophils from atopic asthmatics challenged intranasally with human rhinovirus 16 were monitored directly ex vivo or else in 24-hour cultures, at baseline (day 0), and then at days 4 and 21 post-challenge.
Results
Basophils in atopic asthmatics, but not in non-atopic controls, upregulated TSLP receptor upon IgE receptor ligation. The magnitude of this response was correlated with the proportion of serum total IgE that was allergen-specific (r=0.615, p<0.05). Following rhinovirus infection, all subjects developed nasal symptoms that peaked 3 to 5 days after viral challenge. Basophils displayed maximal IgE responsiveness three weeks post-challenge as judged by TSLP receptor levels in 24-hour cultures. No significant change in total IgE or specific IgE antibodies was detected during rhinovirus infection. By contrast, levels of IgE receptor-associated spleen tyrosine kinase, Syk, were increased on day 4 (p<0.05), and elevated levels were also detected three weeks post-challenge.
Conclusions and Clinical Relevance
Circulating basophils display increased IgE responsiveness three weeks after rhinovirus infection in atopic asthmatics. This observation, coupled with increased expression of Syk, implicates basophils in promoting, or else prolonging, rhinovirus-induced inflammation in atopic asthmatics.
Keywords: asthma, basophils, IgE, rhinovirus, Syk, TSLP receptor
Introduction
Infection with human rhinovirus (RV) is a major risk factor for asthma exacerbations among atopic patients [1]. In children older than 3 years of age, rhinovirus is the most common virus associated with asthma exacerbations [2, 3]. Several lines of evidence support the view that the interaction between virus and IgE contributes to this phenomenon. Asthma exacerbations among atopic children infected with RV display seasonal fluctuations that coincide with allergen exposure [2, 3]. Moreover, anti-IgE therapy markedly attenuates seasonal peaks in disease exacerbations [4]. In a recent study which explored the relationship between RV infection and specific IgE, the risk for active wheezing was highest among those children who tested positive for RV and had the highest IgE levels [5]. These observations suggest that rhinovirus infection and IgE interact to potentiate inflammatory responses in the lower airways.
Patients with asthma continue to wheeze long after cold symptoms resolve; however, the reasons for this remain unclear. Given that basophils are the principal cell type in the blood that is activated by IgE, we theorized that these cells contribute to the inflammatory sequelae that occur in atopic asthmatics following RV infection. Basophils have been implicated in potentiating airway inflammation among allergic subjects who received segmental allergen challenge following experimental infection with RV [6]. However, a role for these cells in RV-induced asthma exacerbations has largely been overlooked.
Here, we sought to interrogate the IgE responsiveness of circulating basophils in atopic asthmatics who received experimental intranasal challenge with RV-16. All subjects were challenged during peak allergen exposure season. The study was designed to sample circulating basophils both during acute infection, as well as several weeks after infection when cold symptoms had resolved. We aimed to exploit our previous work which established a link between Fc receptor triggering and surface TSLP receptor (TSLPR) in cells from atopic subjects. That work demonstrated the capacity for ligation of the high affinity IgG receptor, FcγRI, by allergen to induce TSLPR on dendritic cells (DCs) [7, 8]. TSLPR is expressed on activated human basophils [9]. Given that basophils express high levels of the high affinity IgE receptor, FcεRI, we first sought to establish whether IgE receptor triggering could modulate surface TSLPR in basophils of atopic subjects. By using TSLPR as a marker, we demonstrate that the IgE responsiveness of basophils is enhanced several weeks after RV infection, but not during acute infection. Our findings support the view that basophils contribute to chronic inflammatory processes in atopic asthmatics infected with RV.
Materials and Methods
Human Subjects
All participants were adults (ages 18–55) and included atopic subjects with physician-diagnosed asthma; atopic subjects who had physician-diagnosed atopic dermatitis with or without asthma (total IgE >150 IU/ml); and non-atopic healthy controls (total IgE <30 IU/ml and no specific IgE antibodies). All atopic subjects had high levels of specific IgE antibodies to dust mite (Table S1). Inclusion criteria for atopic asthmatics (ages 18–40) who received intranasal rhinovirus challenge included physician-diagnosed mild asthma; total IgE ≥200 IU/ml, no serum neutralizing antibody to RV-16, positive methacholine challenge test (≥20% decrease in FEV1 at a methacholine concentration of ≤8mg/ml), and positive skin prick tests to one or more aeroallergen. Asthma severity of rhinovirus challenged asthmatics fit the NHLBI guidelines for diagnosis and management of mild asthma with the exception of baseline FEV1 values which were permitted to be >70%, rather than > 80% predicted as per the guidelines.
All subjects received intermittent inhaled bronchodilators but no inhaled/oral corticosteroids, anti-leukotrienes or anti-histamines. Subjects were non-pregnant non-smokers without a history of allergen immunotherapy, nasal/sinus surgery, or chronic illness. Rhinovirus challenge studies were approved by the FDA and the NIAID safety committee (Clinical Trials.gov ID NCT02111772). All subjects were recruited through the UVA Allergic Diseases Clinic, or else by advertisement. All studies were approved by the University of Virginia Human Investigations Committee under protocols #13166, #12656 and #12673 and all study participants gave written informed consent.
Intranasal Challenge with Human Rhinovirus-16
Subjects were challenged as described previously [10]. Briefly, on day 0, subjects were challenged with 1 ml of inoculum containing 300 TCID of live RV-16 (0.5ml per nostril). Upper respiratory tract symptoms were scored on days 0 to 21 according to modified Jackson criteria [10].
Reagents
Allergens: Purified allergens (natural Der p 1, natural Der p 2, and recombinant H22-Fel d 1) with low endotoxin content (<25 IU/μg) were obtained from Indoor Biotechnologies, Inc. (Charlottesville, VA). Rhinovirus Preparation: FDA-approved RV-16 pool was kindly provided by Dr. Ronald Turner (University of Virginia). Flow Cytometry Antibodies: Fluorochrome-labeled monoclonal antibodies used for flow cytometry were: Lin3 cocktail (anti-CD3, -CD14, -CD19 and -CD20), Lin1 cocktail (anti-CD3, -CD14, -CD16, -CD19, -CD20 and -CD56), anti-CD123 (clone 6H6), and anti-HLA-DR (G46-6) purchased from BD Biosciences (San Jose, CA); anti-TSLPR (1B4), anti-CD1c (L161), anti-CD63 (H5C6) and anti-CD203c (NP4D6) (Biolegend, San Diego, CA); anti-CD11c (B-ly6) and anti-FcεRIα (CRA–1) (eBiosciences, San Diego, CA); and anti-Syk (4D10.1) (EMD Millipore, Billerica, MA). Compensation beads were purchased from BD Biosciences. Aqua viability dye was used to determine cell viability (Invitrogen, Carlsbad, CA). Other Reagents: Mouse anti-FcεRIα monoclonal antibody (clone AER-37) was purchased from Biolegend and rabbit anti-human IgE antibody was obtained from Bethyl Laboratories, Inc. (Montgomery, TX). BD FACS lysing solution for fresh whole blood staining was purchased from BD Biosciences.
Cells and Flow Cytometry
Cells were analyzed immediately using fresh whole blood specimens or after culture. For cultured cells, fresh PBMCs were isolated from venous blood and cultured for 24 hours in complete medium containing 10% autologous human serum in the presence or absence of allergen (25μg/ml for Der p1 and Der p 2 and 10μg/ml for H22-Fel d 1)[8]. Cells were stained for surface and intracellular markers, and then analyzed by flow cytometry using an LSRII Fortessa flow cytometer (BD Biosciences). Data analysis was performed using Flow Jo software version 9.5.2 (Tree Star Inc., Ashland, OR).
Microscopy of TSLPR+ Basophils and Dendritic Cells
Allergen-stimulated TSLPR+ basophils and myeloid DCs were sort-purified from 24-hour PBMC cultures using a Reflection Cell Sorter (iCyt, Champaign, IL) according to differential expression of HLA-DR and CD123 within the lineage-negative TSLPR+ gate. Cytospin preparations were obtained using a Thermo Shandon Cytospin 4 cytofuge (Harlow Scientific, Arlington, MA), and after Wright-Giemsa staining, cells were identified using a Nikon Eclipse E600 microscope (1000x magnification). Images were obtained using i2s 2008 software (Vashaw Scientific, Norcross, GA).
Serum Antibody and Cytokine Assays
Serum total IgE and allergen-specific IgE antibodies were measured by ImmunoCAP assay (Phadia US, Portage, MI). Serum cytokines were measured by cytometric bead assay (EMD Millipore) using a Bio-Plex System (Bio-Rad, Hercules, CA).
Statistical Analysis
Linear mixed models with bonferroni correction were used to analyze within-group and between-group differences in cell percentages and mean fluorescent intensities for different conditions. Nasal symptoms were assessed by repeated measures one-way ANOVA. p values ≤0.05 were considered statistically significant.
Results
Allergen Induces TSLP Receptor on FcεRI+ Cells of Atopic Asthmatics
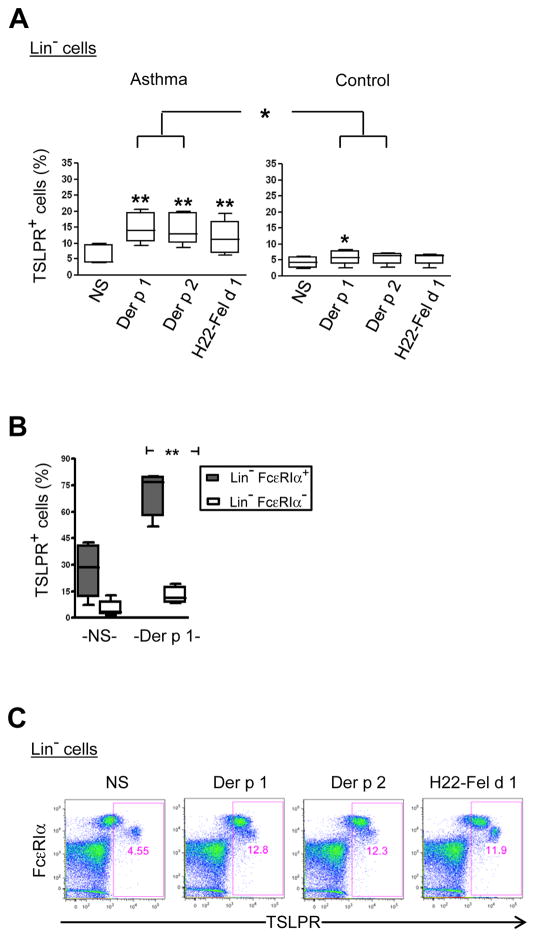
Our first objective was to establish a PBMC assay system for testing the IgE responsiveness of basophils, using TSLPR as a read out. This system allowed us to test basophil responses to allergen in vitro using a low blood volume, and to directly compare TSLPR expression on basophils with myeloid DCs within the same sample. First, we tested the ability for allergen to induce TSLPR on lineage-negative PBMCs (CD3negCD14negCD19negCD20neg) which are expected to contain both basophils and DCs (Fig. S1A). In cultures from atopic asthmatics, stimulation with major dust mite allergens induced a marked increase in the percentage of TSLPR+ lineage-negative cells as compared with non-stimulated cells (p<0.01). Moreover, the levels of TSLPR induced by dust mite allergens were comparable with those induced by targeting cat allergen to the high affinity IgG receptor, FcγRI, using H22-Fel d 1 (Fig. 1A) [8]. Levels of TSLPR expression induced in cells of atopic asthmatics were comparable to those observed in AD patients with markedly elevated total IgE (Fig. S1B and Table S1). While dust mite allergen induced TSLPR expression on lineage-negative cells from non-atopic controls, this effect was much lower than in atopic asthmatics (p<0.05). TSLPR was preferentially expressed on lineage-negative cells expressing the high affinity IgE receptor, FcεRI (Fig. 1B & C). These findings established the capacity for allergen to efficiently upregulate TSLPR on FcεRI+ cells from atopic asthmatics.
Figure 1. Allergen Induces TSLP Receptor on FcεRI+ Cells of Atopic Asthmatics.
PBMCs from atopic asthmatics and non-atopic controls were cultured for 24 hours and lineage-negative (Lin−) cells analyzed for TSLPR expression. (A) Percentage of TSLPR+ cells within the Lin− subset in asthmatics and controls (median and interquartile ranges; 5 subjects per group). NS, non-stimulated. *p<0.05 and **p<0.01. (B) Percentage of TSLPR+ cells within Lin−FcεRIα+ and Lin−FcεRIα− subsets in the presence or absence of allergen (n=5). (C) Effect of allergen on TSLPR expression on Lin− cells (representative data from 1 asthmatic subject).
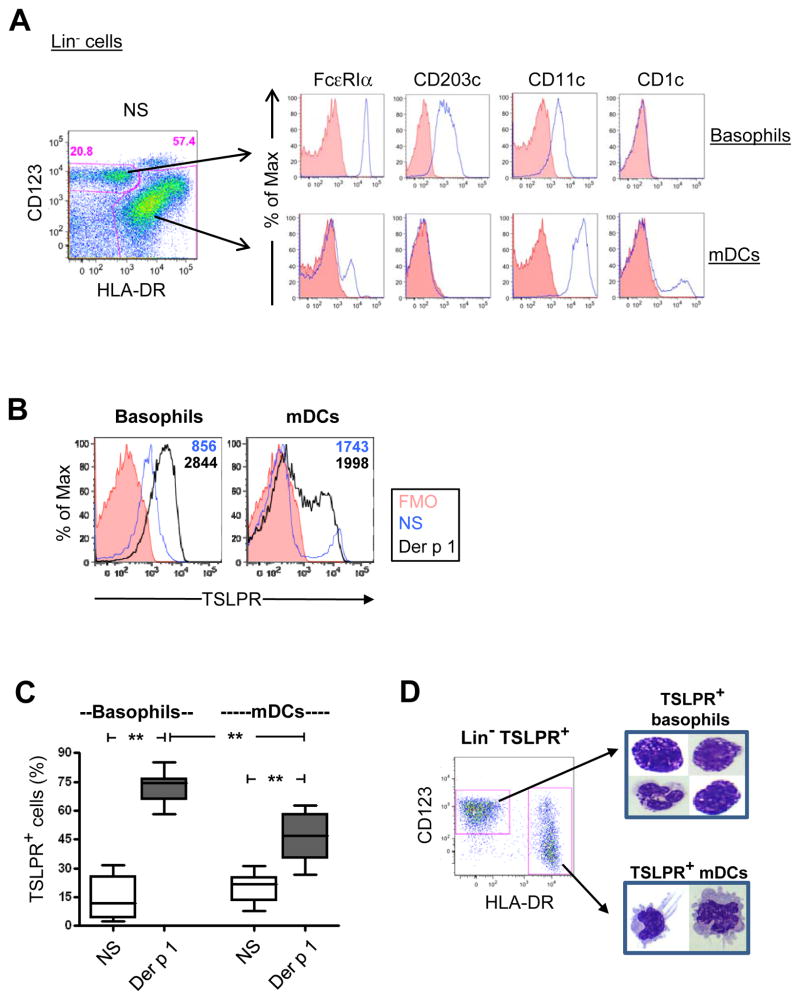
TSLP Receptor is Induced on Basophils
In atopic asthmatics, basophils were identified within the lineage-negative subset as FcεRIαhiHLA-DRnegCD123+ cells expressing the basophil activation marker CD203c. These cells were distinguishable from a major subset of HLA-DR+ myeloid DCs which expressed CD11c and CD1c, and lower levels of FcεRIα (Fig. 2A). Gating on basophils and myeloid DCs revealed different profiles of TSLPR expression on both cell types under non-stimulated conditions. Within DCs, a discrete population of TSLPR+ cells was observed, whereas TSLPR on basophils formed a continuum of low level expression (Fig. 2B). In the presence of allergen, TSLPR expression was increased, with higher levels of receptor expressed on basophils compared with DCs (p<0.01)(Figs. 2B & C). The identity of TSLPR+ basophils and DCs was confirmed by microscopy of flow-sorted TSLPR+ cells (Fig. 2D).
Figure 2. TSLP Receptor is Induced on Atopic Basophils.
(A) Phenotype of basophils and DCs within the Lin− subset (representative of 10 asthmatics). FMO, fluorescence-minus-one control. (B & C) Representative data (B) and summary data (C) for 13 atopic subjects showing the change in percentage of TSLPR+ basophils and DCs with allergen stimulation. (D) Microscopy of sort-purified TSLPR+ basophils and myeloid DCs using Wright-Giemsa staining (representative of 2 atopic subjects). Box plots represent median and interquartile ranges. **p<0.01.
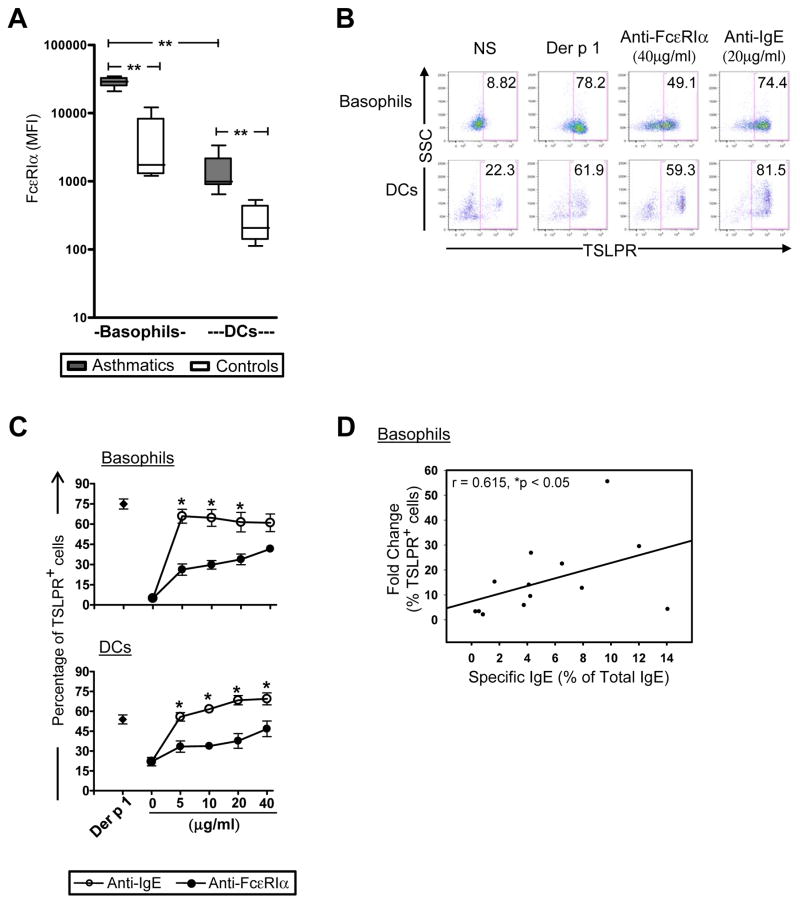
TSLP Receptor is Induced in an IgE-Dependent Manner
We confirmed that basophils expressed higher levels of IgE receptor as compared with DCs (p<0.01), and that levels of IgE receptor were higher on basophils and DCs in asthmatics versus non-atopic controls (p<0.01)(Fig. 3A)[11, 12]. Cross-linking IgE receptor using anti-IgE antibody induced TSLPR in both basophils and DCs at levels comparable to those induced by allergen, though anti-FcεRIα was less efficient at inducing TSLPR (p<0.01)(Figs. 3B & C). It has been reported that the efficiency of IgE receptor cross-linking on basophils by allergen is increased when a higher proportion of IgE in the system is allergen-specific [13]. In keeping with this, the fold change in TSLPR expression induced by dust mite allergen on basophils was positively correlated with the proportion of serum IgE that was dust mite-specific (r=0.615, p<0.05) (Fig. 3D). This relationship was not observed in DCs (data not shown). Taken together, these findings support the view that the propensity for basophils to upregulate TSLPR in response to allergen occurs in an IgE-dependent fashion.
Figure 3. TSLPR is Induced in an IgE-Dependent Manner.
(A) Levels of FcεRIα expression on basophils and DCs in non-stimulated 24-hour PBMC cultures from asthmatics and non-atopic controls (median and interquartile ranges, n=13 and 5 respectively). **p<0.01. (B & C) Representative data (B) and dose-response curves (C, n=6 asthmatics) for anti-FcεRIα and anti-IgE antibodies in 24-hour PBMC cultures. Values represent the mean ± SEM. *p<0.01. (D) Correlation between the proportion of allergen-specific IgE antibodies (anti-D. pteronyssinus IgE antibodies as a percentage of total IgE) and fold change in TSLPR expression on basophils (n=13 asthmatics).
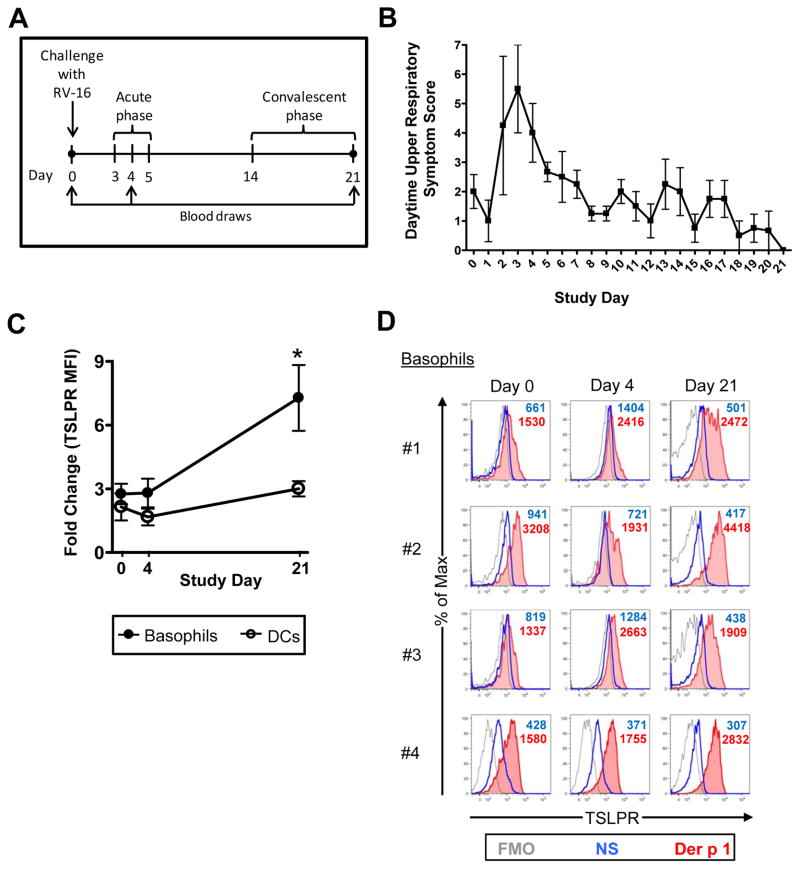
Rhinovirus Infection Augments IgE Responsiveness of Basophils Three Weeks after Experimental Challenge
Having established that surface TSLPR is upregulated in an IgE-dependent fashion, we used TSLPR as a marker to monitor the IgE responsiveness of circulating basophils during rhinovirus infection. Atopic asthmatics (geometric mean total IgE = 421.3 IU/ml [95% CI 245.6, 722.7]) were challenged intranasally with RV-16. Expression of TSLPR was analyzed on basophils stimulated with Der p 1 immediately before challenge, 4 days post-challenge during peak cold symptoms, and during convalescence (day 21) (Figs. 4A & B). As expected, stimulation with allergen induced TSLPR expression on basophils at all time points; however, this effect was significantly greater 21 days post-challenge (mean fold change in MFI = 7.23 ± 3.1 on day 21 versus 2.76 ±0.96 and 2.8 ± 1.35 on days 0 and 4 respectively, p<0.01) (Figs. 4C & D). This phenomenon was not observed in DCs which showed similar responsiveness to allergen at each time point (Fig. 4C). Similar results were obtained using anti-IgE and anti-FcεRI antibodies (Fig. S2A).
Figure 4. Rhinovirus Exposure Augments IgE Responsiveness in Circulating Basophils.
(A) Schematic illustrating rhinovirus challenge study design. (B) Upper respiratory symptom scores (mean ± SEM) based on sore throat, sneezing, runny nose, nasal congestion, and eye irritation. (C & D) Allergen-induced TSLPR expression at days 0, 4 and 21 in 24-hour PBMC cultures. Values in (C) represent the mean ± SEM. *p<0.01 for day 21 versus days 0 and 4. Experiments were performed in 4 asthmatics.
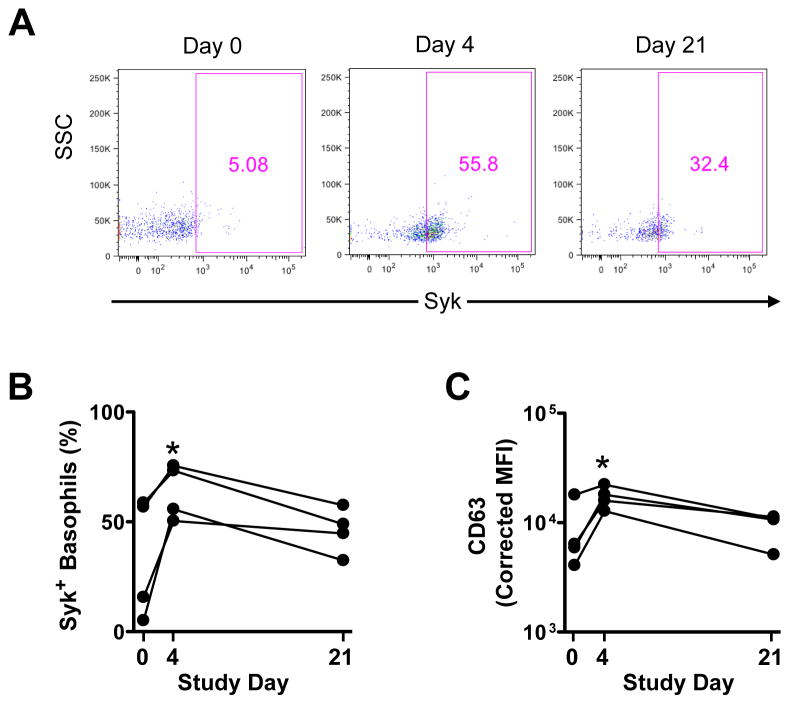
Enhanced IgE responsiveness in basophils might be explained by an increase in components of the IgE receptor signaling pathway during RV infection. Neither total IgE nor specific IgE antibody levels were significantly altered in the serum of RV-challenged asthmatics. Moreover, no change in expression of FcεRIα on basophils was detected at any time point (data not shown). Subsequent experiments in RV-challenged subjects revealed that expression of the FcεRI-associated protein tyrosine kinase, Syk, was increased in all subjects tested on day 4 (p<0.05). Moreover, levels remained elevated at day 21 in those subjects who had low Syk levels at baseline (Fig. 5A & B). Expression of the basophil activation marker, CD63, also peaked at day 4 (p<0.05), while a similar, though less marked, trend was observed for CD203c (Fig. 5C & Fig. S2B). To further probe how rhinovirus might condition enhanced IgE responsiveness of basophils in vivo, cytokines were measured in the serum of RV-challenged subjects. Levels of TNF-α, but not IL-3 or other pro-inflammatory cytokines, were significantly increased at day 4 (p=0.028)(Fig. S2C). Together, these findings demonstrated the ability for rhinovirus infection to augment the IgE/TSLPR axis in basophils of atopic asthmatics.
Figure 5. Expression of Syk and CD63 is Enhanced During RV Infection.
Phenotype of basophils in atopic asthmatics analyzed directly ex vivo on days 0, 4 and 21 post-challenge using whole blood specimens. (A) Representative data showing change in Syk expression. (B) Percentage of total basophils expressing Syk. *p<0.05. (C) Levels of CD63 expression. *p<0.05. Experiments were performed in 4 asthmatics.
Discussion
Using an in vivo challenge model, we have demonstrated that exposure to rhinovirus preferentially augments IgE responsiveness of circulating basophils in atopic asthmatics. We have taken advantage of a novel assay to test IgE responsiveness, which exploits the link between IgE and TSLP receptor. Amplification of the IgE/TSLPR axis in basophils was observed three weeks after inoculation of the virus, at a time when nasal symptoms had resolved. This finding points to a possible role of basophils in the “late” response to rhinovirus. Increased IgE responsiveness in circulating basophils would be expected to promote, or else prolong, inflammatory processes following rhinovirus infection in atopic asthmatics. As such, our findings integrate key concepts arising from other RV challenge studies. Early work in allergic rhinitics demonstrated enhanced histamine release and eosinophil recruitment in the lungs during the acute phase of RV infection, as well as one month after infection, following segmental antigen challenge [6]. In a more recent study, rhinovirus-stimulated cells harvested from the lungs of infected asthmatics displayed enhanced production of Th2 cytokines as compared with cells from healthy infected individuals [14]. Basophils have also been implicated as initiators of chronic inflammatory processes [15]; however, further work is needed to explore this concept.
Questions remain regarding the mechanism of enhanced IgE responsiveness detected several weeks after rhinovirus infection. Our findings support the view that this reflects intrinsic changes to the basophil that act downstream of IgE receptor. Enhanced IgE responsiveness was not linked to increased expression of circulating IgE antibodies or increased expression of FcεRI on the basophil surface. By contrast, enhanced expression of Syk detected both at 4 days and 3 weeks post-challenge, suggests that increased expression of signaling components contributes to altered IgE responsiveness. Consistent with this view, levels of Syk expression have been correlated with IgE-mediated histamine releasability in human basophils [16–18]. Given the complexity of IgE receptor signaling events, further work is necessary to elucidate how Syk, and other molecules such as Lyn and the phosphatase SHIP1, which are known to act early during IgE-mediated signaling, contribute to enhanced basophil responsiveness in RV infection. It should also be acknowledged that the propensity to augment TSLPR following RV infection may not be solely IgE-dependent. For example, basophils express a variety of toll-like receptors and stimulation with toll-like receptor ligands can enhance surface TSLPR (data not shown). Thus, RV infection might, in theory, promote enhanced activation of basophils through convergence of multiple pathways.
It remains to be addressed whether circulating basophils present 3 weeks post-challenge represent the same population of cells present in the blood during the acute phase of infection. It is generally accepted that the average lifespan of basophils is only 3–5 days; however, there is a paucity of data on their longevity in disease. Circulating basophils present 3 weeks post-challenge might constitute a new population of cells that is primed to enhance IgE responsiveness in the bone marrow post-infection. Alternatively, they may represent recirculating basophils that were primed in the lung to prolong their lifespan. Our ability to detect elevated levels of TNF-α in the serum of RV-challenged subjects during the acute phase of infection supports the view that pro-inflammatory cytokines may be involved in RV-induced basophil priming. Our findings provide a basis for further investigating these important aspects of basophil biology in the context of allergic disease.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the following grants from NIH: NIAID R01 AI052196 and NIAMS R01 AR059058 (to J. Woodfolk), and R01 AI020565 (T. Platts-Mills).
The authors thank: Joanne Lannigan, MS and Michael Solga, MS (University of Virginia) for assistance with flow cytometry; and Holliday Carper, MS and Deborah Murphy, RN (University of Virginia) for assistance with blood draws. We also thank Steven Ziegler, PhD of Benaroya Research Institute for helpful discussions.
Abbreviations
- DC
dendritic cell
- RV-16
human rhinovirus-16
- TSLPR
thymic stromal lymphopoietin receptor
Footnotes
Conflicts of Interest
J.A. Woodfolk receives research funds from NIH/NIAID and NIH/NIAMS. T.A. Platts-Mills and P.W. Heymann receive research funds from NIH/NIAID. All other authors declared no conflicts of interest.
References
- 1.Duff AL, Pomeranz ES, Gelber LE, Price GW, Farris H, Hayden FG, Platts-Mills TA, Heymann PW. Risk factors for acute wheezing in infants and children: viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics. 1993;92:535–40. [PubMed] [Google Scholar]
- 2.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, Hayden FG, Hatley TK, Chamberlain R. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–90. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 4.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, Murphy DD, Odio S, James HR, Patrie JT, Hunt W, O’Rourke AK, Davis MD, Steinke JW, Lu X, Kennedy J, Heymann PW. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–505. e5. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–8. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulse KE, Reefer AJ, Engelhard VH, Satinover SM, Patrie JT, Chapman MD, Woodfolk JA. Targeting Fel d 1 to FcgammaRI induces a novel variation of the T(H)2 response in subjects with cat allergy. J Allergy Clin Immunol. 2008;121:756–62. e4. doi: 10.1016/j.jaci.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Hulse KE, Reefer AJ, Engelhard VH, Patrie JT, Ziegler SF, Chapman MD, Woodfolk JA. Targeting allergen to FcgammaRI reveals a novel T(H)2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J Allergy Clin Immunol. 2010;125:247–56. e1–8. doi: 10.1016/j.jaci.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–33. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TA, Hayden FG, Gwaltney JM, Jr, Hatley TK, Owens AM, Heymann PW. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–16. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 11.Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112:1132–8. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 13.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, Watanabe N, Karasuyama H. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–20. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein LM, MacGlashan DW., Jr The concept of basophil releasability. J Allergy Clin Immunol. 1986;77:291–4. doi: 10.1016/s0091-6749(86)80106-3. [DOI] [PubMed] [Google Scholar]
- 17.MacGlashan DW., Jr Relationship between spleen tyrosine kinase and phosphatidylinositol 5′ phosphatase expression and secretion from human basophils in the general population. J Allergy Clin Immunol. 2007;119:626–33. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 18.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Syk deficiency in nonreleaser basophils. J Allergy Clin Immunol. 1999;104:279–84. doi: 10.1016/s0091-6749(99)70367-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.