Abstract
Since its discovery, peroxynitrite has been known as a potent oxidant in biological systems, and a rapidly growing body of literature has characterized its biochemistry and role in the pathophysiology of various conditions. Either directly or by inducing free radical pathways, peroxynitrite damages vital biomolecules such as DNA, proteins including enzymes with important functions, and lipids. It also initiates diverse reactions leading eventually to disrupted cell signaling, cell death, and apoptosis. The potential role and contribution of this deleterious species has been the subject of investigation in several important diseases, including but not limited to, cancer, neurodegeneration, stroke, inflammatory conditions, cardiovascular problems, and diabetes mellitus. Diabetes, obesity, insulin resistance, and diabetes-related complications represent a major health problem at epidemic levels. Therefore, tremendous efforts have been put into investigation of the molecular basics of peroxynitrite-related mechanisms in diabetes. Studies constantly seek new therapeutical approaches in order to eliminate or decrease the level of peroxynitrite, or to interfere with its downstream mechanisms. This review is intended to emphasize the latest findings about peroxynitrite and diabetes, and, in addition, to discuss recent and novel advances that are likely to contribute to a better understanding of peroxynitrite-mediated damage in this disease.
Keywords: Diabetes, diabetic complications, insulin resistance, lipid peroxidation, nitrotyrosine, peroxynitrite
INTRODUCTION
The possible role of peroxynitrite and the correlation of peroxynitrite-mediated damage with pathophysiological mechanisms in various disease models have been extensively studied in the past decade. Several disease conditions are associated or accompanied with proinflammation or low-grade inflammatory processes to such an extent that both superoxide and NO production are elevated, making the chance of peroxynitrite generation extremely high. The physiological and pathological implications of peroxynitrite are numerous and are the focus of several well established research groups and investigators. Diseases that are major health problems such as neurodegenerative disorders, cardiovascular diseases, infectious problems and inflammation, as well as diabetes and its related conditions and complications (for reviews see [1-8]), are studied to address potential peroxynitrite-mediated mechanisms and the damaging effects of peroxynitrite in protein oxidation/nitration, in hopes of finding novel therapeutic approaches to mitigate peroxynitrite-related oxidative stress processes.
The past few years have provided a large number of studies and excellent, wide-ranging reviews on the contribution of peroxynitrite to cell signaling and major pathophysiological conditions and diseases [4, 7, 9-10]. In this recent review, we would like to focus on the major lines of evidence and findings in diabetes mellitus and its complications. We emphasize the importance of complex peroxynitrite biochemistry in light of the latest novel findings in diabetes, including type 1 models, complications, type 2 models, metabolic syndrome, and insulin resistance studies. Our intention is also to highlight some of the new postulated mechanisms that may link lipid peroxidation and peroxynitrite-mediated damage and can explain the frequent co-occurrence of these events.
This review was written in response to the growing interest in the relationship of oxidative stress and peroxynitrite to diabetes outside of the community of free radical biochemists. As such, we attempt to bridge the gaps between the highly mechanistic, largely in vitro investigation of the kinetic probabilities of different radical reactions occurring, and the primarily in vivo analysis of the correlation between loosely defined biomarkers of oxidative stress and diabetes progression. Several investigators have already begun to integrate these two approaches, applying novel techniques to rigorously identify specific free radicals and their biological compartmentalization and role in vivo in diabetes models – the pool of these contains a few hundred papers at least. The literature presented here reviews the biochemistry of free radicals in relation to diabetes, specifically focusing on peroxynitrite and its possible products, as well as recent work using specific and novel approaches. We feel these papers represent important steps in advancing to a much more mechanistic and nuanced analysis of the role of such specific oxidative metabolites like peroxynitrite in diabetes.
THE CHEMISTRY OF PEROXYNITRITE – ONE- AND TWO-ELECTRON OXIDATION MECHANISMS IN BIOLOGY
The production of peroxynitrite, a potent oxidant and its role in in vivo endothelial damage was proposed in 1990 [11]. Since then, several studies and investigators have provided solid evidence to support the possibility of the in vivo formation of this molecule, once a controversial subject. In addition, they have unraveled its role in a diversity of pathological conditions.
A simultaneous flux of nitric oxide and superoxide anion overproduction in a given system with close proximity to one another leads to the formation of peroxynitrite in a diffusion controlled fashion [12]. Superoxide anion and nitric oxide are not necessarily toxic but normally exist in a fine balance with physiological levels participating in several signaling events [13-14]. If overproduced in a pathophysiological case, in a diseased organ or tissue, efficient defense mechanisms such as superoxide dismutases (SOD) are available to neutralize superoxide. NO is able to diffuse rapidly, eventually being converted to nitrate [15]. Problems can occur when the formation of these species happens within very close proximity, a few molecules of distance apart, and NO production is increased several folds, diffusing to different places; it is difficult to kinetically outcompete the reaction between su-peroxide and NO in vivo. Various studies indicate slightly different rate constants, but they are commonly reported to be in the range of 109, which is essentially a diffusion controlled condition [12, 16]. This phenomenon rendered the focus of biological research moving from hydroxyl radical and the possibility of Fenton reaction towards peroxynitrite mediated biochemical pathways and described NO as a Janus-faced molecule and peroxynitrite as a biological oxidant [11, 17]. Though free radical production from peroxynitrite was initially controversial, currently peroxynitrite biochemistry is well described and widely accepted by many investigators and research groups. The molecule is considered to be a strong oxidant and can react with biomolecules as direct targets or in the absence of those, through its various ways of decomposition will likely mediate biologically relevant free radical reactions. These modes of action, and the factors that influence which pathways are most likely to occur in different in vivo situations, are detailed below.
At neutral pH, peroxynitrite decomposes by a homolytic cleavage giving rise to hydroxyl radicals (•OH) and nitrogen dioxide (•NO2) in about 30 % and nitrate in about 70 % [18-21]. These species can then further initiate various free radical reactions (both oxidation and nitration). They can damage biomembranes, DNA, start lipid peroxidation processes, and modify proteins [22-26]. Protein modification often occurs either by oxidation or nitration of tyrosines, cysteins (thiols), or tryptophanes. These residues can be key functional parts in active sites of enzymes, receptors, and other proteins. The decomposition is considered very slow at neutral pHs but has much more relevance at low acidic pHs, which can occur, for example, in inflamed tissues, ischemia, or in phagosomes.
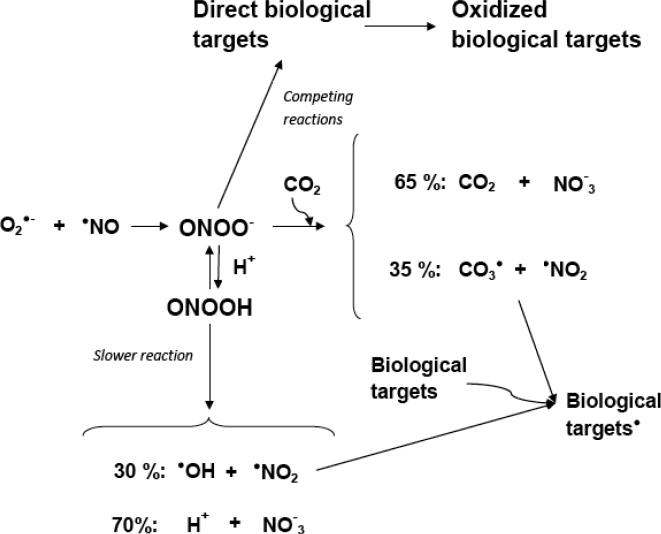
At neutral pH, the reaction of peroxynitrite with thiols and heme proteins, as well as with CO2, becomes significantly more important than spontaneous decomposition because both the concentrations of these biomolecules and their rapid reactions will favor this route [27-28]. The result is a greatly reduced half-life of peroxynitrite (in the millisecond range) and the two electron-oxidation route of the targets. Functionally important parts of enzymes such as thiols or methionine can be oxidized, which can lead to loss of function. Peroxynitrite also reacts with the in vivo often ubiquitous carbon dioxide; this reaction gives ~35 % carbonate radical anion (CO3•−) and •NO2, and ~65 % nitrate through a rapidly decomposing intermediate product [29-33]. CO2 will efficiently compete with other biological targets for peroxynitrite; therefore, most of the reactions will occur from carbonate radical anion and nitrogen dioxide, resulting in one electron oxidation mechanisms [34]. Fig. (1) shows the summary of the above mentioned biological reactions of peroxynitrite.
Fig. (1).
Possible peroxynitrite reaction paths in a biological environment. NO and superoxide anion reacts in a diffusion controlled fashion to form peroxynitrite. The decomposition of peroxynitrous acid is considered slow at pH=7.4, to produce hydroxyl radicals. Peroxynitrite reacts with various biotargets via the two electron-oxidation mechanism. Carbon dioxide competes with this reaction, and may divert reactions to the one electron oxidation route where most of the biotargets will be oxidized by nitrogen dioxide and the carbonate radical anion. The scheme does not take compartmentalization, biomembranes, or non-homogenous milieu into account. Based on Augusto et al., Free Radic Biol Med 32:841-859, 2002.
The numerous studies of peroxynitrite-mediated oxidation of biotargets in vitro and in vivo provided a very good background to support the theory of how free radical production from this toxic oxidant can play a role in various conditions. While carbonate radical anion and •NO2 will mediate most of these reactions in a neutral biological milieu, spontaneous decomposition and hydroxyl radical will also contribute at low pHs and in certain pathological conditions or hydrophobic environments [34]. Conversely, while hydroxyl radical reacts mostly at a diffusion limited rate oxidizing amino acids or DNA bases, carbonate radical is considerably more selective. Tyrosine in a hydrophilic environment, for example, is more accessible to this charged species, and carbonate radical anion reacts about a hundred times faster with Tyr than nitrogen dioxide [29]. Carbonate radical anion also increases the efficiency of •NO2 for protein tyrosine nitration [33, 35-36]. The in vivo consequence can be a very efficient protein tyrosine nitration due to the combination of these radicals.
Compartmentalization, biomembranes, and the heterogenous nature of cells further complicate the reactions of peroxynitrite-derived species in biology. Uncharged species such as the peroxynitrous acid or nitrogen dioxide can diffuse through membranes, while charged ones such as the carbonate radical anion or peroxynitrite anion are unable to do so [37-40]. It has also been demonstrated that peroxynitrite can travel through anion channels in erythrocytes [41-42], reaching various parts and components of the cell. Therefore, the diversity of the cellular environment including membranes, protein structures and domains, and phospholipids makes peroxynitrite chemistry and biochemistry rather complex. Due to different permeability and diffusibility of the different peroxynitrite-derived radicals, the intra- or extracellular production site of these species will strongly determine and influence the outcome and the possible targets in a given biological system. A very thorough demonstration of these possibilities was shown for the first time in Augusto et al.'s studies [40]. The group demonstrated how peroxynitrous acid and nitrogen dioxide penetrated the cell membrane in a macrophage cell line model and oxidized intracellular protein tyrosine and thiol residues. The addition of bicarbonate/carbon dioxide resulted in an inhibition of the formation of these residues, due to the quick reaction of peroxynitrite with carbon dioxide. Moreover, they also discussed how the results reflect on physiological conditions in a biological environment and how different radicals play a role in biotarget oxidation when peroxynitrite is produced extra- vs. intracellularly. Another very thorough investigation shows kinetic predictions and gives important considerations for reactive nitrogen species chemistry in biology [43]. Various studies (demonstrating both in vitro and in vivo) have since emphasized the important role of hydrophobic and hydro-philic compartments, lipid membranes, and organelles, such as mitochondria in the diverse chemical biology of peroxynitrite and its derived species [37, 39-40, 44-45]. Yet, in clinical medicine and medicinal chemistry, these considerations, the variability and the limitation of the reactions mediated directly by peroxynitrite or peroxynitrite-derived species are still often overlooked.
DIABETES, DIABETIC PATHOGENESIS AND PEROXYNITRITE
It is well known that the number of patients with this chronic disease is on the rise – and by 2030 is expected to double, reaching approximately 300 million according to the World Health Organization [46]. Around 90 % are type 2 diabetic, usually with long years of metabolic syndrome combined with cardiovascular risk factors, insulin resistance and obesity. Metabolic syndrome-related conditions and complications such as arteriosclerosis, associated cardiac problems like stroke and infarction, and cardiomyopathy are among the leading causes of death for these patients in the United States and worldwide [46]. The microvascular complications such as nephropathy and retinopathy lead to renal failure, cataract formation, and seriously impaired vision [47-48]. Peripheral neuropathy is another member of late complications and can be associated with juvenile or type 2 diabetes, leading to impaired sensation, numbness, and often painful conditions. The potential role of peroxynitrite has been investigated by a large number of groups supporting the involvement of this molecule in type 1 and type 2 diabetic models - STZ-mice, NOD mice, ob/ob and high fat diet mice - as well as in diabetic complications including cardiovascular problems, neuropathy, retinopathy, and nephropathy [5, 9, 49-52].
A body of work focuses on the diabetes pathogenesis itself, where the emphasis is on β-cell death in type 1 diabetes, as this type of diabetes is characterized by the total loss of β-cells in the pancreas, mainly due to autoimmune processes. A myriad of factors have been proposed to participate in the triggering mechanism including cytokines, free radicals, and peroxynitrite itself.
In an in vitro model system, when Langerhans islet cells from rodents or human patients are exposed to peroxynitrite, insulin secretion is inhibited, with concomitant DNA damage and eventually apoptosis [53]. Similar results were obtained in studies using nitric oxide donors or various ROS generating environments [54-56]. Because of DNA damage, the activation of the enzyme poly-ADP-ribose polymerase (PARP) as a downstream mechanism is also under the scope of intense investigation. PARP is a protein involved in a number of cellular processes related mainly to DNA repair and programmed cell death. One important function of PARP is assisting in the repair of single-strand breaks, on the other hand, upon DNA cleavage by enzymes involved in cell death (such as caspases), PARP can deplete the ATP pool of a cell in an attempt to repair the damaged DNA. ATP depletion in a cell leads to lysis and cell death [57-59].
In in vivo animal models, iNOS often mediates a possibly at least partially peroxynitrite-driven mechanism. Although first discovered in macrophages, the enzyme is over-expressed in various tissues, including the pancreas, [60] and knocking out iNOS protects against chemically induced diabetes [61]. Genetic disruption or pharmacological inhibition of iNOS are also shown to be protective in different models including the non-obese diabetic (NOD) mice [62]. Furthermore, apart from the above mentioned in vitro models, the islets from a NOD mice pancreas show significant nitrotyrosine staining compared to healthy controls [62], suggesting a correlation between this biomarker and the consequent protein oxidation and damage in β cell death. The application of peroxynitrite scavengers as well as PARP inhibitors also reduces nitrotyrosine damage in the Langerhans, further confirming this correlation [62-64]. A few years ago, a unifying mechanism was proposed in relation to diabetes and diabetic complications where, probably through the decomposition of peroxynitrite, excess superoxide production from the mitochondria (due to hyperglycemia) induces single strand breaks, and therefore, PARP activation. A key enzyme in the glycolytic pathway, GAPDH is modified by PARP, which leads to the impairment of various biochemical pathways involved in oxidative stress, such as the polyol pathway and aldose reductase activation [65].
DIABETIC COMPLICATIONS AND CONSIDERATIONS ABOUT PEROXYNITRITE AND NITROTYROSINE FORMATION
Regardless of oral treatments or insulin injection, almost half of the patients with either type of diabetes develop late complications, such as retinopathy, nephropathy, peripheral neuropathy, or various forms of vascular and cardiac problems. These complications can lead to more severe conditions including ulcers, renal failure, cataract, and blindness, as well as endothelial dysfunction, atherosclerosis, and myocardial injury [66-70]. The possible contribution from peroxynitrite and its derived species has been intensely investigated by a number of groups studying various complications.
In diabetic neuropathy, one of the first studies where the possible role of peroxynitrite-derived species was discussed showed that preventing superoxide formation in epineurial arterioles of the sciatic nerve restored endothelium-dependent vasodilation [71]. Later on, a body of studies focused on potent peroxynitrite decomposition catalysts to improve neuronal function and counteract peripheral neuropathy in the disease, using the streptozotocin-diabetic model, the NOD mice, as well as the leptin deficient ob/ob mice [72-77]. Furthermore, focusing on human patients, nitrosylated proteins have been suggested as a new biomarker for peroxynitrite related stress in diabetic subjects with macroangiopathy [78]. As diabetes is associated generally with chronic low-grade inflammation, the potential role of iNOS has been investigated in neuropathy as well. iNOS knockout mice treated with streptozotocin showed improved function in multiple manifestations of peripheral neuropathy, including normal nerve conduction velocities and less severe small-fibre sensory neuropathy [79]. In relation to peroxynitrite-mediated downstream mechanisms, several lines of evidence prove the role of PARP activation in diabetic neuropathy [52, 80-81]. These studies include a range of PARP inhibitors and knockout mice experiments. The possibility for new therapeutic strategies to ameliorate oxidative stress and peroxynitrite-related damage in neuropathy was covered and discussed in comprehensive overviews [5, 9, 59].
Studies on diabetic nephropathy first reported increased levels of nitrotyrosine in the kidney of human patients [82]. One of our previous studies suggested the involvement of nitric oxide and peroxynitrite in pathological changes of various tissues, including the diabetic kidney [83]. Similarly to neuropathy, DNA damage and the activation of PARP in peroxynitrite-mediated cytotoxicity were studied in the pathogenesis of nephropathy as well [84-85]. Peroxynitrite also seems to play a role in glomerular lesions in the kidney of diabetic rats [86], possibly through the JAK/STAT signaling pathway [87]. As the peroxynitrite-induced damage – PARP activation – metabolic changes pathway probably works both ways, the inhibition of aldose reductase by the compound fidarestat ameliorated oxidative/nitrosative stress and PARP activation in tissues that are involved in complications [88], such as the diabetic kidney as well as in human mesangial cells in a high glucose environment [89].
In diabetic retinopathy, the role of PARP activation has also been proposed, together with the involvement of NADPH oxidase, iNOS, and peroxynitrite [90]. In a streptozotocin-induced long term model, nitrotyrosine formation was abundant in the microvessels of the retina, and more alarming, this nitrotyrosine accumulation was not normalized after 6 months of glycemic control [91]. This failure to normalize protein oxidation/nitration in the retina, and the accumulation of these oxidized protein aggregates, may be at least partially responsible for the resistance of diabetic retinopathy. In in vitro studies, indications for peroxynitrite-mediated oxidative changes were also found where retinal endothelial cells were exposed to high glucose [92]. Furthermore, at the site of pathological changes in the retina, in the retinal microvasculature, peroxynitrite has been shown to contribute to permeability changes. Also, lipid peroxidation and peroxynitrite were linked to retinal ischemia/reperfusion injury [93-94]. In vivo studies compared various rat and mice models of diabetes in regard of how early diabetes-related changes occur in the retina [95]. iNOS inhibitors such as aminoguanidine or peroxynitrite scavengers, like the compound FP15, and furthermore, PARP inhibitors also successfully corrected some major alterations in this complication [96-98].
As peroxynitrite is able to interfere with several bio-molecules in the endothelium, vascular smooth muscle, and in the cardiac tissues; its contribution has also been postulated in cardiovascular complications of diabetes [99-103]. The various cardiac problems - cardiomyopathy, hypertension, infarction, and endothelial dysfunction - related to long term diabetes are still among the major complication problems. Observations in human aortic endothelial cells confirmed that high glucose via peroxynitrite can cause tyrosine nitration, which in this case lead to important functional changes, inactivating enzymes such as prostacyclin synthase, in association with apoptosis [104]. Others confirmed as well that peroxynitrite is a major trigger leading to cardiomyocyte apoptosis in vitro and in vivo [105]. In high glucose-perfused hearts, iNOS upregulation was observed together with increasing NO and superoxide production. These conditions favor peroxynitrite formation, indicated by elevated nitrotyrosine levels, and furthermore, apoptosis [99]. Moreover, evidence was found for increased nitrotyrosine and a correlation between the degree of pathological change and nitrotyrosine levels in human patients, in heart biopsies [101]. Peroxynitrite was shown to be able to oxidize the zinc-thiolate complex of eNOS, which can lead to an important vicious cycle, as the enzyme gets uncoupled, producing both super-oxide and NO simultaneously, resulting in more peroxynitrite formation [106]. The same research group showed direct S-glutathiolation of p21 ras by peroxynitrite in endothelial cells, which event mediates its direct activation, as well as endothelial insulin resistance caused by oxidized LDL [107-108]. As DNA strand breaks and peroxynitrite are usually linked, regardless of the site or tissue, PARP activation was intensively studied in cardiovascular and endothelial dys-function and heart failure as well [109-114]. Streptozotocin-diabetic rats showed increased peroxynitrite production in the aorta, cardiac hypertrophy, protein carbonylation or nitrotyrosine formation [115-116]. Similarly to studies in other complications, aminoguanidine or other NOS inhibitors, peroxynitrite scavengers or PARP inhibitors were shown to be effectively attenuating the pathological changes in cardiac studies as well [64, 115-119].
In the light of all these studies, it is important to note that careful consideration should be given regarding whether 3-nitrotyrosine indicates functional alteration on enzymes and a mechanistic contribution to the pathological condition or if it is only a biomarker of the oxidative stress process in the disease [120]. Although nitrotyrosine is not exclusively formed from the reaction with peroxynitrite, it indicates the involvement of Tyr• and protein oxidation, and is still probably the most valid marker to suspect at least a contribution from peroxynitrite-modulated mechanisms. It is a post-translational modification of protein tyrosine residues, which can lead to loss [121-122] or gain of function [123-125] or even no change in function of the protein [126]. In general, the nitrotyrosine formation yield in biology is relatively low, site specific, and affecting only a few Tyr residues of a given protein, which raises the question of relevancy in certain cases. Protein methionine and cystein residues can also be oxidized, contributing to loss of function. Hence, the influence of protein tyrosine nitration on enzyme function may be a finely modulated mechanism in diabetes as well. It should always be evaluated whether nitration, nitrosylation, oxidation or other peroxynitrite-derived species dependent mechanisms play a role in the disease progression at molecular levels or only serve as markers of oxidative stress. Nevertheless, the accumulating data suggest a strong correlation between 3-nitrotyrosine formation and important pathophysiological consequences in diabetes.
NOVEL FINDINGS ABOUT PEROXYNITRITE IN DIABETES, INSULIN RESISTANCE AND RELATED CONDITIONS
The past decade has seen the continuation of research on the currently recognized and established pathways, as well as novel investigations that further indicate and confirm the correlation of peroxynitrite-mediated damage in various diabetes related conditions.
The evaluation of PARP inhibition as a downstream mechanism stayed in the focus of research [127]. In recent studies, PARP inhibition was found to counteract cataract formation and early retinal changes [128], on the other hand, the aldose reductase inhibitor fidarestat suppressed ischemia/reperfusion induced changes in the retina of rats [129]. Inhibition of PARP was also efficient to abrogate multiple manifestations of type 1 diabetic early nephropathy [130] where rats were streptozotocin-diabetic for ~ 3 months, as well as in a longer term model to successfully ameliorate kidney disease [131], where rats were diabetic for about 6 months. PARP gene deficiency or inhibition successfully ameliorated neuropathic pain [132]. Novel research papers and reviews discuss the role of the peroxynitrite-PARP pathway in human diseases, in diabetic vascular complications, and in experimental neuropathy, and moreover, show a novel side of this mechanism in gestational diabetes and diabetic pregnancies [133-135].
The various diabetic model studies have been expanded to focus on type 2 diabetes and metabolic syndrome-like situations, including high-fat diet fed mice, leptin deficient ob/ob mice, or type 2 diabetic rats. The relationship between nitrosative stress and peripheral neuropathy has been evaluated in the ob/ob model [77, 136] and in high-fat diet induced obesity [137]. The pathogenesis and the potential treatments in neuropathy have been recently reviewed [138-139]. New results support the role of superoxide, nitric oxide, peroxynitrite, and PARP in diabetic retinopathy as well [140]. In relation to diabetes, early intervention of protein tyrosine nitration helps to prevent neovascularization in ischemic retinopathy [141].
Beside the inducible form of nitric oxide synthase (iNOS), studies extended to the other constitutive isoforms of the enzyme (eNOS/nNOS) to investigate their potential role to the peroxynitrite pathway in diabetes related patho-physiological changes. nNOS gene deficiency prevented diabetes-induced peroxynitrite injury, as diabetic nNOS −/− mice had near normal nitrotyrosine and PARP levels in dorsal root ganglia neurons but not in peripheral nerve, which is indicative of different roles of nNOS-derived NO in peroxynitrite formation in these two tissue-sites of peripheral diabetic neuropathy [142]. The nNOS knockout mice also developed motor and sensory nerve conduction velocity deficits as well as thermal hypoalgesia, which indicates that nNOS is required for maintaining the normal peripheral nerve function and small sensory nerve fibre innervation. In a db/db mouse model, nNOS expression was reduced in the sciatic where the compound rosuvastatin reversed the reduction of nNOS expression by mechanisms that involved phosphoinositide 3-kinase and phosphorylation of Akt [143]. In the same study, co-treatment of diabetic mice with rosuvastatin and a selective nNOS inhibitor blunted beneficial effects of rosuvastatin on conduction velocity deficits, thermal sensitivity, and nerve vascularity, which also suggests an important role for nNOS-derived NO in peripheral nerve function.
Interestingly, a study that recently examined renal biopsies from patients showed that in human diabetic nephropathy there is an overexpression of eNOS – the endothelial form of nitric oxide synthase in the diseased tissue [144].
Others are focusing on the role of iNOS as a player mediating chronic low-grade inflammation in diabetes and, furthermore, contributing to insulin resistance through peroxynitrite as a possible intermediate. Even after the diabetes has developed, a transition metal-independent hydroxyl radical generation was observed with direct in vivo and EPR approaches in a streptozotocin-induced rat model. The specific iNOS inhibitior 1400W reduced lipid radical and hydroxyl radical production, indicating peroxynitrite and its decomposition as initiators of protein- and lipid damage [145]. It is possible that a lower pH in a chronic inflammatory condition favors the generation of hydroxyl radicals, which in turn starts lipid peroxidation. These lipid radicals then initiate tyrosyl radical formation and, hence, increase the possibility of nitration in a hydrophobic environment.
As it has been demonstrated that targeted disruption of iNOS protects against obesity-related insulin resistance [146-147], more studies are currently investigating the pathways with potential peroxynitrite involvement in relation to insulin signaling and insulin resistance, an important condition accompanying metabolic syndrome and type 2 diabetes. These novel studies showed that peroxynitrite may also contribute to insulin resistance. Exogenous peroxynitrite from SIN-1 as a donor, for example, in the muscle resulted in increased nitration of insulin receptor or IRS-1 and Akt [148]. The stimulation of peroxynitrite catalysis improved insulin sensitivity in a high fat diet-fed mice model [149]. Furthermore, in a lipid infusion model, tyrosine nitration of insulin signaling proteins was observed [150]. Although in vitro studies have shown before that peroxynitrite can induce tyrosine nitration of IRS-1 [151], this study shows for the first time that protein tyrosine nitration regulates hepatic insulin signaling and disturbs glucose metabolism in vivo. Other investigators have previously also observed iNOS induction in diet-induced obesity and in ob/ob mice models, in association with enhanced S-nitrosation of IRS-1 and its reduced protein level in muscle [152]. Pharmacological or genetic blockade of iNOS prevented the reduction in the IRS-1 protein level in these two models of insulin resistance. Similar mechanisms were suggested in other inflammation and obesity related models [153]. The same aforementioned considerations apply in the case of insulin resistance and phosphorylation studies as well – at the mechanistic level, very interestingly, various studies showed inactivation and decreased tyrosine phosphorylation [154], while others demonstrated enhanced Tyr phosphorylation of proteins after nitration [155-156]. These investigations point out a finely modulated regulatory mechanism balancing tyrosine phosphorylation and nitration.
It is noteworthy that in relation to all these studies, novel in vitro works are focusing on the possible mechanisms of how protein tyrosine nitration, dimerization, and hydroxylation happens in hydrophobic compartments such as lipoproteins and cell membranes. The studies clarify the kinetic possibility, the role and relevance of peroxynitrite and peroxynitrite-derived radicals in these reactions, as well as provide a possible link between lipid peroxidation and protein oxidation/nitration [157-158]. When protein tyrosine nitration and/or dimerization caused by biological oxidants happen, it usually requires the formation of a Tyr• as an intermediate. In the case of an in vivo system, where proteins are often associated with hydrophobic compartments, membranes for example, the participation of unsaturated fatty acids is quite possible, as they often serve as targets for an initial free radical attack. In the latest study, it has been demonstrated that lipid-derived radicals mediate the one-electron oxidation of tyrosine to Tyr•, which can afterward react with another Tyr•, or with nitrogen dioxide to yield 3,3'-dityrosine or 3-nitrotyrosine [158]. This data indicates that lipid peroxide radicals (LOO•) can mediate tyrosine oxidation processes in hydrophobic biocompartments and provide a new mechanistic insight to understand protein oxidation and nitration in lipoproteins and biomembranes. The findings provide a new biochemical background to test the relationship of peroxynitrite and nitration, as well as lipid peroxidation in pathological conditions like obesity, metabolic syndrome, diabetes, and insulin resistance (a summary scheme is shown on Fig. 1). In these various models of chronic metabolic disturbances, or the excess lipid accumulation models like the infusion, the elevated levels of free fatty acids, toxic lipid metabolites, and oxidation/nitration of proteins are often observed together, in time and location. These processes may harm several proteins and contribute to the development of oxidative imbalance, while worsening the disease conditions such as poor glucose metabolism or insulin resistance. Therefore an optional new therapeutic way may be to optimally block both lipid peroxidation and protein nitration processes by pharmacological inhibition or possible scavenging and neutralization of the species.
SUMMARY
Past years showed an increasing number of studies and the investigation of peroxynitrite in diabetes and its related conditions in further and deeper details. The major approaches, mechanisms, and possible prevention was discussed in this review to a certain extent, focusing on the major lines of evidence of how peroxynitrite, this powerful biological oxidant, contributes to many aspects of diabetes, metabolic syndrome, and insulin resistance, and how the markers and footprints related to peroxynitrite and its derived species seem to correlate with the progression of all complications. Careful evaluation and interpretation of the data should always be carried out to emphasize whether the relationship is mechanistic or serves as a correlative biomarker. These studies continuously contribute to a better understanding of the biochemical mechanisms underlying these conditions, and also provide possible therapeutic options (through inhibition, scavenging, or downstream effectors related to peroxynitrite) to at least partially ameliorate the disease progression. They are highlighting the importance and the multi-faceted nature of peroxynitrite, and that the actions of this molecule, including the modulation of lipid peroxidation and protein nitration, are in fine tuned balance. Further understanding the precise chemistry and the biochemical pathways, with present and future medicinal chemistry studies in such a common disease as diabetes and in the midst of an increasing obesity crisis, are extremely important and will certainly lead to novel paradigms, and therefore, new treatment designs in the battle against this disease and all its related, long-term affecting conditions.
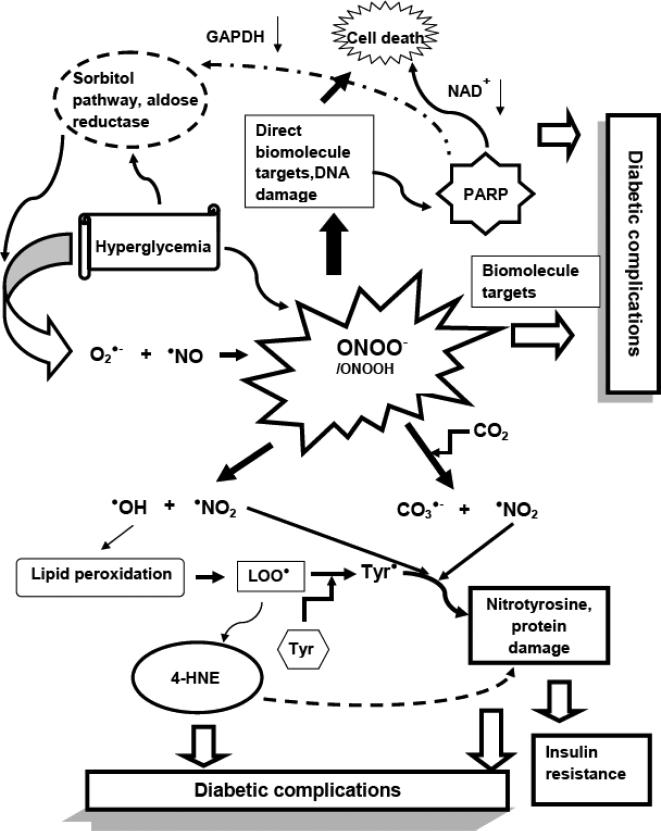
Fig. (2).
A short summary of peroxynitrite-mediated reactions and their possible relation to diabetic complications. Superoxide anion and nitric oxide reacts in a diffusion controlled fashion to form the potent oxidant peroxynitrite. The sources of superoxide in hyperglycemia can be several, including the activation of the polyol pathway and the enzyme aldose reductase as a major mechanism; mitochondrial oxidative stress, NADPH oxidases, uncoupled NOS and several others. NO levels can be elevated due to chronic low grade inflammation. Peroxynitrite affects biomolecules either directly, or by its homolytic cleavage or through its reaction with the ubiquitous CO2. When DNA is targeted, causing single strand breaks, it activates PARP. PARP activation leads to loss of NAD+ and ATP and eventually necrosis and cell death, contributing to diabetic pathogenesis or complications. From the various reactions of peroxynitrite, radicals can initiate lipid peroxidation, leading to the accumulation of lipid peroxide radicals (LOO•) and the toxic end product 4-hydroxynonenal (4-HNE). 4-hydroxynonenal has the ability to covalently bind to proteins and enzymes with vital role, interfering with their function. According to novel findings, lipid peroxide radicals may mediate the oxidation of tyrosine to Tyr radicals (Tyr•) in hydrophobic environments. The tyrosyl radical then can react with •NO2 to form nitrotyrosine, contributing to protein oxidation in tissue sites of diabetic complications.
ACKNOWLEDGEMENTS
The research work of the author is supported by the K99/R00 Grant DK086315 from the National Institute of Diabetes Digestive and Kidney Disease, NIH, and the Pennington Foundation. I would like to thank Dr. Marcelo Bonini for all the helpful discussions about peroxynitrite and its various biochemical aspects in diabetes, Dr. Shawna Wicks for her advice and input, and Mrs. Katie Bailey for careful proofreading of the manuscript.
ABBREVIATIONS
- 1400W
N-(3-(Aminomethyl)benzyl)acetamidine
- 4-HNE
4 - hydroxynonenal
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- IRS-1
insulin receptor substrate - 1
- NOD mice
non-obese diabetic mice
- NOS
nitric oxide synthase
- PARP
poly-ADP-ribose polymerase
- ROS
reactive oxygen species
- SIN-1
molsidomine
- SOD
superoxide dismutase
- STZ
streptozotocin
REFERENCES
- 1.Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lubos E, Handy DE, Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2008;13:5323–5344. doi: 10.2741/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada S, Bolanos JP. Nitric oxide cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 4.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacher P, Szabo C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6:136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dys-function in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 7.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 8.Hasko G, Mabley JG, Nemeth ZH, Pacher P, Deitch EA, Szabo C. Poly(ADP-ribose) polymerase is a regulator of chemokine production: relevance for the pathogenesis of shock and inflammation. Mol Med. 2002;8:283–289. [PMC free article] [PubMed] [Google Scholar]
- 9.Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci. 2009;14:4809–4814. doi: 10.2741/3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 13.Ignarro LJ. Nitric oxide: a unique endogenous signaling molecule in vascular biology. Biosci Rep. 1999;19:51–71. doi: 10.1023/a:1020150124721. [DOI] [PubMed] [Google Scholar]
- 14.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Joshi MS, Ferguson TB, Jr., Han TH, Hyduke DR, Liao JC, Rassaf T, Bryan N, Feelisch M, Lancaster JR., Jr. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci U S A. 2002;99:10341–10346. doi: 10.1073/pnas.152149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauser T, Koppenol WH. The rate constant of the reaction of superoxide with nitrogen monoxide: Approaching the diffusion limit. Journal of Physical Chemistry A. 2002;106:4084–4086. [Google Scholar]
- 17.Hogg N, Darley-Usmar VM, Wilson MT, Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992;281:419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatti RM, Alvarez B, Vasquez-Vivar J, Radi R, Augusto O. Formation of spin trap adducts during the decomposition of peroxynitrite. Arch Biochem Biophys. 1998;349:36–46. doi: 10.1006/abbi.1997.0451. [DOI] [PubMed] [Google Scholar]
- 19.Lehnig M. Radical mechanisms of the decomposition of peroxynitrite and the peroxynitrite-CO(2) adduct and of reactions with L-tyrosine and related compounds as studied by (15)N chemically induced dynamic nuclear polarization. Arch Biochem Biophys. 1999;368:303–318. doi: 10.1006/abbi.1999.1268. [DOI] [PubMed] [Google Scholar]
- 20.Merenyi G, Lind J, Goldstein S, Czapski G. Peroxynitrous acid homolyzes into *OH and *NO2 radicals. Chem Res Toxicol. 1998;11:712–713. doi: 10.1021/tx980043h. [DOI] [PubMed] [Google Scholar]
- 21.Radi R. Peroxynitrite reactions and diffusion in biology. Chem Res Toxicol. 1998;11:720–721. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- 22.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 23.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JS. Ischaemic injury mediator. Nature. 1990;345:27–28. doi: 10.1038/345027b0. [DOI] [PubMed] [Google Scholar]
- 25.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 26.Darley-Usmar VM, Hogg N, O'Leary VJ, Wilson MT, Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 27.Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 28.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 29.Augusto O, Bonini MG, Amanso AM, Linares E, Santos CC, De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med. 2002;32:841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 30.Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 31.Lymar SV, Hurst JK. Carbon dioxide: physiological catalyst for peroxynitrite-mediated cellular damage or cellular protectant? Chem Res Toxicol. 1996;9:845–850. doi: 10.1021/tx960046z. [DOI] [PubMed] [Google Scholar]
- 32.Radi R, Cosgrove TP, Beckman JS, Freeman BA. Peroxynitrite-induced luminol chemiluminescence. Biochem J. 1993;290:51–57. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lymar SV, Jiang Q, Hurst JK. Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry. 1996;35:7855–7861. doi: 10.1021/bi960331h. [DOI] [PubMed] [Google Scholar]
- 34.Bonini MG, Augusto O. Carbon dioxide stimulates the production of thiyl, sulfinyl, and disulfide radical anion from thiol oxidation by peroxynitrite. J Biol Chem. 2001;276:9749–9754. doi: 10.1074/jbc.M008456200. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein S, Czapski G, Lind J, Merenyi G. Tyrosine nitration by simultaneous generation of (.)NO and O-(2) under physiological conditions. How the radicals do the job. J Biol Chem. 2000;275:3031–3036. doi: 10.1074/jbc.275.5.3031. [DOI] [PubMed] [Google Scholar]
- 36.Santos CX, Bonini MG, Augusto O. Role of the carbonate radical anion in tyrosine nitration and hydroxylation by peroxynitrite. Arch Biochem Biophys. 2000;377:146–152. doi: 10.1006/abbi.2000.1751. [DOI] [PubMed] [Google Scholar]
- 37.Marla SS, Lee J, Groves JT. Peroxynitrite rapidly permeates phospholipid membranes. Proc Natl Acad Sci U S A. 1997;94:14243–14248. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero N, Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite in the presence of carbon dioxide. Arch Biochem Biophys. 1999;368:23–30. doi: 10.1006/abbi.1999.1272. [DOI] [PubMed] [Google Scholar]
- 39.Khairutdinov RF, Coddington JW, Hurst JK. Permeation of phospholipid membranes by peroxynitrite. Biochemistry. 2000;39:14238–14249. doi: 10.1021/bi001270x. [DOI] [PubMed] [Google Scholar]
- 40.Lopes de Menezes S, Augusto O. EPR detection of glutathionyl and protein-tyrosyl radicals during the interaction of peroxynitrite with macrophages (J774). J Biol Chem. 2001;276:39879–39884. doi: 10.1074/jbc.M104012200. [DOI] [PubMed] [Google Scholar]
- 41.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci U S A. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macfadyen AJ, Reiter C, Zhuang Y, Beckman JS. A novel superoxide dismutase-based trap for peroxynitrite used to detect entry of peroxynitrite into erythrocyte ghosts. Chem Res Toxicol. 1999;12:223–229. doi: 10.1021/tx980253u. [DOI] [PubMed] [Google Scholar]
- 43.Lancaster JR., Jr. Nitroxidative, nitrosative, and nitrative stress: kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol. 2006;19:1160–1174. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Joseph J, Feix J, Hogg N, Kalyanaraman B. Nitration and oxidation of a hydrophobic tyrosine probe by peroxynitrite in membranes: comparison with nitration and oxidation of tyrosine by peroxynitrite in aqueous solution. Biochemistry. 2001;40:7675–7686. doi: 10.1021/bi002958c. [DOI] [PubMed] [Google Scholar]
- 45.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 46.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 47.Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- 48.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopa thy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Mabley JG, Soriano FG. Role of nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic vascular dys-function. Curr Vasc Pharmacol. 2005;3:247–252. doi: 10.2174/1570161054368571. [DOI] [PubMed] [Google Scholar]
- 51.Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hadjivassiliou V, Green MH, James RF, Swift SM, Clayton HA, Green IC. Insulin secretion, DNA damage, and apoptosis in human and rat islets of Langerhans following exposure to nitric oxide, peroxynitrite, and cytokines. Nitric Oxide. 1998;2:429–441. doi: 10.1006/niox.1998.0203. [DOI] [PubMed] [Google Scholar]
- 54.Cunningham JM, Mabley JG, Delaney CA, Green IC. The effect of nitric oxide donors on insulin secretion, cyclic GMP and cyclic AMP in rat islets of Langerhans and the insulin-secreting cell lines HIT-T15 and RINm5F. Mol Cell Endocrinol. 1994;102:23–29. doi: 10.1016/0303-7207(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 55.Eizirik DL, Delaney CA, Green MH, Cunningham JM, Thorpe JR, Pipeleers DG, Hellerstrom C, Green IC. Nitric oxide donors decrease the function and survival of human pancreatic islets. Mol Cell Endocrinol. 1996;118:71–83. doi: 10.1016/0303-7207(96)03768-9. [DOI] [PubMed] [Google Scholar]
- 56.Fehsel K, Jalowy A, Qi S, Burkart V, Hartmann B, Kolb H. Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes. 1993;42:496–500. doi: 10.2337/diab.42.3.496. [DOI] [PubMed] [Google Scholar]
- 57.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radons J, Heller B, Burkle A, Hartmann B, Rodriguez ML, Kroncke KD, Burkart V, Kolb H. Nitric oxide toxicity in islet cells involves poly(ADP-ribose) polymerase activation and concomitant NAD+ depletion. Biochem Biophys Res Commun. 1994;199:1270–1277. doi: 10.1006/bbrc.1994.1368. [DOI] [PubMed] [Google Scholar]
- 59.Szabo C. Roles of poly(ADP-ribose) polymerase activation in the pathogenesis of diabetes mellitus and its complications. Pharmacol Res. 2005;52:60–71. doi: 10.1016/j.phrs.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC. Inducible nitric oxide synthase (iNOS) in pancreatic islets of nonobese diabetic mice: identification of iNOS- expressing cells and relationships to cytokines expressed in the islets. Endocrinology. 1996;137:2093–2099. doi: 10.1210/endo.137.5.8612552. [DOI] [PubMed] [Google Scholar]
- 61.Flodstrom M, Tyrberg B, Eizirik DL, Sandler S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes. 1999;48:706–713. doi: 10.2337/diabetes.48.4.706. [DOI] [PubMed] [Google Scholar]
- 62.Suarez-Pinzon WL, Szabo C, Rabinovitch A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet beta-cells. Diabetes. 1997;46:907–911. doi: 10.2337/diab.46.5.907. [DOI] [PubMed] [Google Scholar]
- 63.Suarez-Pinzon WL, Mabley JG, Strynadka K, Power RF, Szabo C, Rabinovitch A. An inhibitor of inducible nitric oxide synthase and scavenger of peroxynitrite prevents diabetes development in NOD mice. J Autoimmun. 2001;16:449–455. doi: 10.1006/jaut.2001.0507. [DOI] [PubMed] [Google Scholar]
- 64.Szabo C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, Van Duzer JH, Williams W, Salzman AL, Groves JT. Part I: pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol Med. 2002;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- 65.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 67.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 68.Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev. 2001;22:36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 69.Da Ros R, Assaloni R, Ceriello A. Antioxidant therapy in diabetic complications: what is new? Curr Vasc Pharmacol. 2004;2:335–341. doi: 10.2174/1570161043385538. [DOI] [PubMed] [Google Scholar]
- 70.Obrosova IG. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid Redox Signal. 2005;7:1543–1552. doi: 10.1089/ars.2005.7.1543. [DOI] [PubMed] [Google Scholar]
- 71.Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res. 2003;37:33–40. doi: 10.1080/1071576021000028442. [DOI] [PubMed] [Google Scholar]
- 72.Drel VR, Pacher P, Vareniuk I, Pavlov I, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur J Pharmacol. 2007;569:48–58. doi: 10.1016/j.ejphar.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drel VR, Pacher P, Vareniuk I, Pavlov IA, Ilnytska O, Lyzogubov VV, Bell SR, Groves JT, Obrosova IG. Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetramesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes. Int J Mol Med. 2007;20:783–792. [PMC free article] [PubMed] [Google Scholar]
- 74.Nangle MR, Cotter MA, Cameron NE. Effects of the peroxynitrite decomposition catalyst, FeTMPyP, on function of corpus cavernosum from diabetic mice. Eur J Pharmacol. 2004;502:143–148. doi: 10.1016/j.ejphar.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 75.Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, Yorek MA. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 2007;293:E1645–1655. doi: 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- 76.Obrosova IG, Mabley JG, Zsengeller Z, Charniauskaya T, Abatan OI, Groves JT, Szabo C. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005;19:401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- 77.Vareniuk I, Pavlov IA, Drel VR, Lyzogubov VV, Ilnytska O, Bell SR, Tibrewala J, Groves JT, Obrosova IG. Nitrosative stress and peripheral diabetic neuropathy in leptin-deficient (ob/ob) mice. Exp Neurol. 2007;205:425–436. doi: 10.1016/j.expneurol.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 78.Julius U, Drel VR, Grassler J, Obrosova IG. Nitrosylated proteins in monocytes as a new marker of oxidative-nitrosative stress in diabetic subjects with macroangiopathy. Exp Clin Endocrinol Diabetes. 2009;117:72–77. doi: 10.1055/s-2008-1078710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vareniuk I, Pavlov IA, Obrosova IG. Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia. 2008;51:2126–2133. doi: 10.1007/s00125-008-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mash-talir N, Pacher P, Yorek MA, Obrosova IG. Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes. 2006;55:1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 82.Thuraisingham RC, Nott CA, Dodd SM, Yaqoob MM. Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int. 2000;57:1968–1972. doi: 10.1046/j.1523-1755.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 83.Stadler K, Jenei V, von Bolcshazy G, Somogyi A, Jakus J. Increased nitric oxide levels as an early sign of premature aging in diabetes. Free Radic Biol Med. 2003;35:1240–1251. doi: 10.1016/s0891-5849(03)00499-4. [DOI] [PubMed] [Google Scholar]
- 84.Kiss L, Szabo C. The pathogenesis of diabetic complications: the role of DNA injury and poly(ADP-ribose) polymerase activation in peroxynitrite-mediated cytotoxicity. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):29–37. doi: 10.1590/s0074-02762005000900007. [DOI] [PubMed] [Google Scholar]
- 85.Minchenko AG, Stevens MJ, White L, Abatan OI, Komjati K, Pacher P, Szabo C, Obrosova IG. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 86.Xiao H, Li Y, Qi J, Wang H, Liu K. Peroxynitrite plays a key role in glomerular lesions in diabetic rats. J Nephrol. 2009;22:800–808. [PubMed] [Google Scholar]
- 87.Wang H, Li Y, Liu H, Liu S, Liu Q, Wang XM, Shi Y, Duan H. Peroxynitrite mediates glomerular lesion of diabetic rat via JAK/STAT signaling pathway. J Endocrinol Invest. 2009;32:844–851. doi: 10.1007/BF03345756. [DOI] [PubMed] [Google Scholar]
- 88.Obrosova IG, Pacher P, Szabo C, Zsengeller Z, Hirooka H, Stevens MJ, Yorek MA. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes. 2005;54:234–242. doi: 10.2337/diabetes.54.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drel VR, Pacher P, Stevens MJ, Obrosova IG. Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic Biol Med. 2006;40:1454–1465. doi: 10.1016/j.freeradbiomed.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ellis EA, Guberski DL, Hutson B, Grant MB. Time course of NADH oxidase, inducible nitric oxide synthase and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat. Nitric Oxide. 2002;6:295–304. doi: 10.1006/niox.2001.0419. [DOI] [PubMed] [Google Scholar]
- 91.Kowluru RA, Kanwar M, Kennedy A. Metabolic memory phenomenon and accumulation of peroxynitrite in retinal capillaries. Exp Diabetes Res. 2007;2007:21976. doi: 10.1155/2007/21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- 93.Marumo T, Noll T, Schini-Kerth VB, Harley EA, Duhault J, Piper HM, Busse R. Significance of nitric oxide and peroxynitrite in permeability changes of the retinal microvascular endothelial cell monolayer induced by vascular endothelial growth factor. J Vasc Res. 1999;36:510–515. doi: 10.1159/000025694. [DOI] [PubMed] [Google Scholar]
- 94.Shibuki H, Katai N, Yodoi J, Uchida K, Yoshimura N. Lipid peroxidation and peroxynitrite in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2000;41:3607–3614. [PubMed] [Google Scholar]
- 95.Obrosova IG, Drel VR, Kumagai AK, Szabo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 2006;49:2525–2533. doi: 10.1007/s00125-006-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 97.Sugawara R, Hikichi T, Kitaya N, Mori F, Nagaoka T, Yoshida A, Szabo C. Peroxynitrite decomposition catalyst, FP15, and poly(ADP-ribose) polymerase inhibitor, PJ34, inhibit leukocyte entrapment in the retinal microcirculation of diabetic rats. Curr Eye Res. 2004;29:11–16. doi: 10.1080/02713680490513146. [DOI] [PubMed] [Google Scholar]
- 98.Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, Stevens MJ, Szabo C. Poly(ADP-ribose) polymerase inhibitors counteract diabetes- and hypoxia-induced retinal vascular endothelial growth factor overexpression. Int J Mol Med. 2004;14:55–64. [PubMed] [Google Scholar]
- 99.Ceriello A, Quagliaro L, D'Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D. Acute hyper-glycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51:1076–1082. doi: 10.2337/diabetes.51.4.1076. [DOI] [PubMed] [Google Scholar]
- 100.Cohen RA. Role of nitric oxide in diabetic complications. Am J Ther. 2005;12:499–502. doi: 10.1097/01.mjt.0000178776.77267.19. [DOI] [PubMed] [Google Scholar]
- 101.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 102.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 104.Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- 105.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med. 2006;41:886–895. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 108.Clavreul N, Bachschmid MM, Hou X, Shi C, Idrizovic A, Ido Y, Pimentel D, Cohen RA. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 109.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 111.Szabo C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADPRibose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- 112.Pacher P, Liaudet L, Mabley J, Komjati K, Szabo C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- 113.Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002;9:659–664. [PubMed] [Google Scholar]
- 114.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7:1568–1580. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001;50:1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 116.Stadler K, Jenei V, Somogyi A, Jakus J. Beneficial effects of aminoguanidine on the cardiovascular system of diabetic rats. Diabetes Metab Res Rev. 2005;21:189–196. doi: 10.1002/dmrr.501. [DOI] [PubMed] [Google Scholar]
- 117.Esberg LB, Ren J. Role of nitric oxide, tetrahydrobiopterin and peroxynitrite in glucose toxicity-associated contractile dysfunction in ventricular myocytes. Diabetologia. 2003;46:1419–1427. doi: 10.1007/s00125-003-1183-8. [DOI] [PubMed] [Google Scholar]
- 118.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, Batkai S, Kollai M, Szabo C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- 120.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration--functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 121.Quijano C, Hernandez-Saavedra D, Castro L, McCord JM, Freeman BA, Radi R. Reaction of peroxynitrite with Mnsuperoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J Biol Chem. 2001;276:11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- 122.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hodara R, Norris EH, Giasson BI, Mishizen-Eberz AJ, Lynch DR, Lee VM, Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 124.Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J Biol Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 125.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 126.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, 3rd, Finkel B, Lanken PN, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;278:L961–967. doi: 10.1152/ajplung.2000.278.5.L961. [DOI] [PubMed] [Google Scholar]
- 127.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drel VR, Xu W, Zhang J, Kador PF, Ali TK, Shin J, Julius U, Slusher B, El-Remessy AB, Obrosova IG. Poly(ADP-ribose)polymerase inhibition counteracts cataract formation and early retinal changes in streptozotocin-diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:1778–1790. doi: 10.1167/iovs.08-2191. [DOI] [PubMed] [Google Scholar]
- 129.Agardh CD, Agardh E, Obrosova IG, Smith ML. The aldose reductase inhibitor fidarestat suppresses ischemia-reperfusion-induced inflammatory response in rat retina. Pharmacology. 2009;84:257–263. doi: 10.1159/000241733. [DOI] [PubMed] [Google Scholar]
- 130.Drel VR, Xu W, Zhang J, Pavlov IA, Shevalye H, Slusher B, Obrosova IG. Poly(Adenosine 5′-diphosphate-ribose) polym-erase inhibition counteracts multiple manifestations of experimental type 1 diabetic nephropathy. Endocrinology. 2009;150:5273–5283. doi: 10.1210/en.2009-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shevalye H, Stavniichuk R, Xu W, Zhang J, Lupachyk S, Maksimchyk Y, Drel VR, Floyd EZ, Slusher B, Obrosova IG. Poly(ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of kidney disease in long-term streptozotocin-diabetic rat model. Biochem Pharmacol. 2010;79:1007–1014. doi: 10.1016/j.bcp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Obrosova IG, Xu W, Lyzogubov VV, Ilnytska O, Mashtalir N, Vareniuk I, Pavlov IA, Zhang J, Slusher B, Drel VR. PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Radic Biol Med. 2008;44:972–981. doi: 10.1016/j.freeradbiomed.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horvath EM, Magenheim R, Kugler E, Vacz G, Szigethy A, Levardi F, Kollai M, Szabo C, Lacza Z. Nitrative stress and poly(ADP-ribose) polymerase activation in healthy and gestational diabetic pregnancies. Diabetologia. 2009;52:1935–1943. doi: 10.1007/s00125-009-1435-3. [DOI] [PubMed] [Google Scholar]
- 134.Negi G, Kumar A, Sharma SS. Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010;391:102–106. doi: 10.1016/j.bbrc.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 135.Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 137.Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mash-talir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 138.Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arora M, Kumar A, Kaundal RK, Sharma SS. Amelioration of neurological and biochemical deficits by peroxynitrite decomposition catalysts in experimental diabetic neuropathy. Eur J Pharmacol. 2008;596:77–83. doi: 10.1016/j.ejphar.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 140.Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci. 2009;14:3974–3987. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 141.Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al- Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther. 2010;332:125–134. doi: 10.1124/jpet.109.157941. [DOI] [PubMed] [Google Scholar]
- 142.Vareniuk I, Pacher P, Pavlov IA, Drel VR, Obrosova IG. Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. Int J Mol Med. 2009;23:571–580. doi: 10.3892/ijmm_00000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ii M, Nishimura H, Kusano KF, Qin G, Yoon YS, Wecker A, Asahara T, Losordo DW. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation. 2005;112:93–102. doi: 10.1161/CIRCULATIONAHA.104.511964. [DOI] [PubMed] [Google Scholar]
- 144.Hohenstein B, Hugo CP, Hausknecht B, Boehmer KP, Riess RH, Schmieder RE. Analysis of NO-synthase expression and clinical risk factors in human diabetic nephropathy. Nephrol Dial Transplant. 2008;23:1346–1354. doi: 10.1093/ndt/gfm797. [DOI] [PubMed] [Google Scholar]
- 145.Stadler K, Bonini MG, Dallas S, Jiang J, Radi R, Mason RP, Kadiiska MB. Involvement of inducible nitric oxide synthase in hydroxyl radical-mediated lipid peroxidation in streptozotocin-induced diabetes. Free Radic Biol Med. 2008;45:866–874. doi: 10.1016/j.freeradbiomed.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 147.Dallaire P, Bellmann K, Laplante M, Gelinas S, Centeno-Baez C, Penfornis P, Peyot ML, Latour MG, Lamontagne J, Trujillo ME, Scherer PE, Prentki M, Deshaies Y, Marette A. Obese mice lacking inducible nitric oxide synthase are sensitized to the metabolic actions of peroxisome proliferator-activated receptor-gamma agonism. Diabetes. 2008;57:1999–2011. doi: 10.2337/db08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou J, Huang K. Peroxynitrite mediates muscle insulin resistance in mice via nitration of IRbeta/IRS-1 and Akt. Toxicol Appl Pharmacol. 2009;241:101–110. doi: 10.1016/j.taap.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 149.Duplain H, Sartori C, Dessen P, Jayet PY, Schwab M, Bloch J, Nicod P, Scherrer U. Stimulation of peroxynitrite catalysis improves insulin sensitivity in high fat diet-fed mice. J Physiol. 2008;586:4011–4016. doi: 10.1113/jphysiol.2008.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Charbonneau A, Marette A. iNOS induction underlies lipid-induced hepatic insulin resistance in mice: Potential role of tyrosine nitration of insulin signaling proteins. Diabetes. 2010;59:861–871. doi: 10.2337/db09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nomiyama T, Igarashi Y, Taka H, Mineki R, Uchida T, Ogihara T, Choi JB, Uchino H, Tanaka Y, Maegawa H, Kashiwagi A, Murayama K, Kawamori R, Watada H. Reduction of insulin-stimulated glucose uptake by peroxynitrite is concurrent with tyrosine nitration of insulin receptor substrate-1. Biochem Biophys Res Commun. 2004;320:639–647. doi: 10.1016/j.bbrc.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 152.Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 153.Carvalho-Filho MA, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. Targeted disruption of iNOS prevents LPS-induced S-nitrosation of IRbeta/IRS-1 and Akt and insulin resistance in muscle of mice. Am J Physiol Endocrinol Metab. 2006;291:E476–482. doi: 10.1152/ajpendo.00422.2005. [DOI] [PubMed] [Google Scholar]
- 154.Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–66. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- 155.Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota AM. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- 156.MacMillan-Crow LA, Greendorfer JS, Vickers SM, Thompson JA. Tyrosine nitration of c-SRC tyrosine kinase in human pancreatic ductal adenocarcinoma. Arch Biochem Biophys. 2000;377:350–356. doi: 10.1006/abbi.2000.1799. [DOI] [PubMed] [Google Scholar]
- 157.Bartesaghi S, Peluffo G, Zhang H, Joseph J, Kalyanaraman B, Radi R. Tyrosine nitration, dimerization, and hydroxylation by peroxynitrite in membranes as studied by the hydrophobic probe N-t-BOC-l-tyrosine tert-butyl ester. Methods Enzymol. 2008;441:217–236. doi: 10.1016/S0076-6879(08)01212-3. [DOI] [PubMed] [Google Scholar]
- 158.Bartesaghi S, Wenzel J, Trujillo M, Lopez M, Joseph J, Kalyanaraman B, Radi R. Lipid Peroxyl Radicals Mediate Tyro-sine Dimerization and Nitration in Membranes. Chem Res Toxicol. 2010;23:821–835. doi: 10.1021/tx900446r. [DOI] [PMC free article] [PubMed] [Google Scholar]