Abstract
Objective
To examine the relationship between markers of vascular dysfunction and neurodevelopmental outcomes in perinatally HIV-infected (PHIV+) and perinatally HIV-exposed but uninfected (PHEU) youth.
Design
Cross-sectional design within a prospective, 15-site US-based cohort study.
Methods
Neurodevelopmental outcomes were evaluated in relation to nine selected vascular biomarkers in 342 youth (212 PHIV+, 130 PHEU). Serum levels were assessed for adiponectin, C-reactive protein (CRP), fibrinogen, interleukin-6 (IL-6), soluble vascular cell adhesion molecule-1 (sVCAM-1), E-selectin (sE-selectin), monocyte chemoattractant protein (sMCP-1), intercellular adhesion molecule-1 (sICAM-1), and P-selectin (sP-selectin). The Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) was administered at entry, yielding a Full-Scale IQ score, and four index scores. Factor analysis was conducted to reduce the biomarkers to fewer factors with related biological roles. Structural equation models (SEMs) were used to measure associations between resulting factors and WISC-IV scores.
Results
Mean participant age was 11.4 years, 54% were female, 70% black. The nine biomarkers were clustered into three factor groups: F1 (fibrinogen, CRP, and IL-6); F2 (sICAM-1 and sVCAM-1); and F3 (MCP-1, sP-selectin, and sE-selectin). Adiponectin showed little correlation with any factor. SEMs revealed significant negative association of F1 with WISC-IV processing speed score in the total cohort. This effect remained significant after adjusting for HIV status and other potential confounders. A similar association was observed when restricted to PHIV+ participants in both unadjusted and adjusted SEMs.
Conclusion
Aggregate measures of fibrinogen, CRP, and IL-6 may serve as a latent biomarker associated with relatively decreased processing speed in both PHIV+ and PHEU youth.
Keywords: HIV-affected children, inflammatory markers, neurodevelopmental outcomes, perinatal HIV infection
Background
Although the neurodevelopmental impact of perinatal HIV infection has been a concern since the first descriptions of pediatric HIV [1], questions remain regarding its physiological underpinnings. In the US-based Pediatric HIV/AIDS Cohort Study (PHACS), perinatally HIV-infected (PHIV+) youth without history of Center for Disease Control and Prevention (CDC) class C event and perinatally HIV-exposed but uninfected (PHEU) youth achieved mean Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) Full Scale IQ (FSIQ) scores in the low average range. Youth with prior class C event, particularly HIV encephalopathy, had even lower mean WISC-IV scores, suggesting significant impact of HIV disease severity. However, youth with a class C event, with or without encephalopathy, were at higher risk for processing speed deficits than their healthier PHIV+ or PHEU peers [2], suggesting that processing speed may be more susceptible to systemic effects of immune deficiency than other measured neurodevelopmental domains.
The relatively poor neurodevelopmental outcomes of both PHIV+ and PHEU youth suggest a possible role of psychosocial factors frequently associated with maternal HIV infection (e.g. poverty, nutrition, unstable housing) [3]. However, a physiological vulnerability for such outcomes may exist even before birth, as the developing fetus becomes exposed to an immune-dysregulated milieu in utero, possibly with long-term adverse impact on brain development, affecting neurodevelopmental outcomes for both PHIV+ and PHEU youth.
We have previously reported significant inverse correlations of blood levels of soluble P-selectin (sP-selectin) and fibrinogen with WISC-IV scores in 89 PHIV+ youth enrolled in the PHACS [4]. This finding suggested clinically relevant interactions between some of the microvascular cascades involving these biomarkers and the developing brains of PHIV+ youth. However, the study sample was enriched for dyslipidemia, which could have confounded the results given its reported association with both sP-selectin and fibrinogen in HIV+ children [5]. Further, it is not clear whether the observed correlations are specific for PHIV+ youth or extend to PHEU youth as well.
Here we present the results of analyses examining relationships between serum levels of the same nine candidate biomarkers and neurodevelopmental outcomes in a larger cohort of PHIV+ youth as well as a sample of PHEU youth. We hypothesized that serum levels of sP-selectin and fibrinogen would be inversely correlated with WISC-IV scores, in both PHIV+ and PHEU youth. On the basis of previous findings from PHACS [2], we also hypothesized that processing speed would be the most sensitive domain of the WISC-IV with respect to such associations.
Methods
Participants
The Adolescent Master Protocol (AMP) of the PHACS is a longitudinal, prospective cohort study that enrolled youth from March 2007 to November 2009 at 15 sites in the US including Puerto Rico. Children aged 7 to below 16 years and born to HIV+ mothers were eligible for enrollment into AMP. The present analysis included both PHIV+ and PHEU AMP participants who had vascular biomarkers evaluated from blood samples as part of an assessment of vascular dysfunction among those with and without hyperlipidemia [5], as well as evaluations of neurodevelopmental status at their entry visit.
The Institutional Review Boards at all clinical sites and the Harvard School of Public Health (Statistical and Data Management Center) approved the protocol, and informed consent from the parent(s) or guardian(s), and assent from the participants (when appropriate) were obtained.
Procedures
Study assessments
At study entry, a complete physical exam was conducted with collection of body measurements and blood samples. Assessment of serum lipid levels for both PHIV+ and PHEU participants in the PHACS protocol was described in a previous related publication [5]. For PHIV+ participants, measurements of CD4+ T cells and HIV-1 RNA (viral load) were obtained along with lifetime history of antiretroviral therapy (ART) use, HIV disease severity as reflected by CDC disease class, nadir CD4%, and peak viral load. Maternal, primary caregiver (`caregiver'), and household characteristics were collected by caregiver interview.
Assessment of the biomarkers
Serum levels of nine biomarkers were assessed, including soluble monocyte chemoattractant protein/CCL-2 (referred to as sMCP-1), interleukin 6 (IL-6), C-reactive protein (CRP) (pro-inflammatory markers) [6–8], soluble vascular cell adhesion molecule-1/sCD106 (sVCAM-1), soluble E-selectin/sCD62E (sE-selectin), soluble intercellular adhesion molecule-1/sCD54 (sICAM-1) (endothelial activation markers) [8], fibrinogen (procoagulant state and inflammation marker) [8,9], sCD62P (sP-selectin) (procoagulant state and endothelial activation marker) [8–10], and adiponectin (modulator of endothelial inflammatory response and a metabolic marker) [8–10]. Biomarker assays were only conducted in those participants who were assessed to have adequate volumes of repository specimens, as described elsewhere [5]. The measurement methodology for each biomarker and intra-assay and inter-assay coefficients of variation have been previously described [4].
Assessment of neurodevelopmental outcomes
Children enrolled in AMP were administered the WISC-IV [11] at AMP study entry. Ten subtests of the WISC-IVare combined to yield four index scores: verbal comprehension, perceptual reasoning, working memory, and processing speed, and an overall composite score, the FSIQ. These are standardized scores based on the general US population (with mean = 100, SD = 15). Study psychometrists or psychologists administering the tests completed validity ratings. Only assessments which were considered valid were included in the analyses. Behavioral evaluations were also conducted for our study population, using the Behavior Assessment System for Children, Second Edition (BASC-2) [12], but the BASC-2 scores were not the focus of the present analysis.
Study participant characteristics
The following demographic covariates were considered in our analyses: participant age, sex, race, ethnicity, primary language, caregiver education, household income, relationship of participant and caregiver, hyperlipidemia status, BMI z-score, serum lipids [high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, and triglycerides], body composition measures assessed by dual energy X-ray absorptiometry (total body fat percentage, trunk fat percentage, trunk-to-extremity fat ratio), fasting glucose and insulin, and log homeostasis model assessment of insulin resistance. For models restricted to PHIV+ participants, we also considered receipt of HAART, protease inhibitors or non-nucleoside reverse transcriptase inhibitors, CDC HIV disease class, current CD4+ cell count, current and nadir CD4+ percentage (CD4%), log viral load, and log peak viral load.
Statistical methods
The cross-sectional analysis included youth participating in AMP who had a valid WISC-IV or BASC-2 and biomarker blood levels taken less than 6 months apart. Participants with CRP values above 10 mg/l were excluded from analyses because of high likelihood of an acute infection at the time blood was obtained. Biomarkers were log-transformed when necessary to reduce influence of outliers.
Linear regressions
We first fit separate sets of linear regression models for each biomarker with FSIQ as the outcome variable. We identified additional characteristics associated with FSIQ at significance level P less than 0.20, and fit-adjusted models including these characteristics as well as HIV status. We then used backwards selection models and retained covariates with P less than 0.15. This process was repeated restricted to PHIV+ participants, including HIV-specific clinical covariates. To explore whether HIV infection status modified the effect of the biomarker on the outcome, we fit regression models that included HIV infection status, the biomarker measurement, and an interaction term of these two covariates, adjusting for covariates selected previously.
Factor analyses and structural equation models
The results of the linear regressions indicated that some relationships between biomarkers and FSIQ previously reported as significant in PHIV+ youth [4] were not observed in this larger sample, but possible interactions with HIV status were noted. We therefore expanded our statistical approach to account for the fact that multiple biomarkers may reflect related signaling pathways. We conducted a factor analysis to reduce the nine biomarkers to a smaller number of latent factors. We then built SEMs to measure associations between the resulting factors and neurodevelopmental outcomes.
Factor analysis
Perinatally HIV-infected and PHEU participants were pooled for the analysis. Records with missing data for one or more biomarkers were omitted from the analysis. A z-score transformation (after log transformation, when appropriate) was used to standardize biomarker measurements. Factor analysis equations were solved using the principal components and the maximum-likelihood methods [13]. The squared multiple correlations were used as the initial estimate of communality for each variable. For each method, the factor loadings were calculated with no, varimax, and oblique varimax rotations. Scree plots and cumulative variance plots were generated to help select the appropriate number of factors. To check the stability of results, the dataset was divided into two random halves and for each method factor loadings were calculated for each half. For each factor, we identified those biomarkers that loaded with absolute value above 0.30 on the factor in the full dataset and (when convergent) the random halves of the dataset.
Structural equation models
The resulting factors were evaluated for association with the neurodevelopmental outcomes via SEMs from standardized path estimates. Each WISC-IV index score and the FSIQ were considered in a separate SEM model. A model with a latent variable representing neurodevelopmental status as the outcome (with four underlying index scores) was also fit. Each SEM was fit among all study participants and separately for each of the PHIV+ and PHEU cohorts. The models involving only PHEU youth did not converge and are not reported here. Model fit was assessed using the Comparative Fit Index (CFI) and root mean square error approximation (RMSEA). Adequate fit is generally described as having a CFI greater than 0.9 and RMSEA less than 0.05. SEMs were also fit adjusting for key covariates described above. As before, analyses with only PHIV+ youth included HIV-specific covariates. SEM analyses were performed in SAS 9.3 using Proc CALIS. All other analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA).
Results
Participant characteristics
Among 678 perinatally HIV-exposed (451 PHIV+ and 227 PHEU) AMP participants, 371 had vascular biomarkers measured. Of these, 342 participants (212 PHIV+ and 130 PHEU) had at least one valid WISC-IV within 6 months of their biomarker and CRP 10 mg/l or less and were included in the analysis. Demographic and clinical characteristics of the 342 participants included in the analysis (Table 1) were representative of the full cohort (n = 678)[5].
Table 1.
Demographic characteristics among 342 AMP participants and HIV-specific clinical characteristics among 212 HIV-infected AMP participants at the time when blood sample for biomarker measurement was obtained.
| Cohort |
||||
|---|---|---|---|---|
| Characteristics | PHIV+ (n = 212) | PHEU (n = 130) | Total (N = 342) | P-value* |
| Age (years), mean (SD) | 12.1 (2.51) | 10.3 (2.39) | 11.4 (2.60) | <0.001 |
| Men | 88 (42%) | 70 (54%) | 158 (46%) | 0.026 |
| Black race | 162 (76%) | 76 (58%) | 238 (70%) | <0.001 |
| Hispanic | 37 (17%) | 51 (39%) | 88 (26%) | <0.001 |
| English primary language | 203 (96%) | 112 (86%) | 315 (92%) | 0.001 |
| Caregiver: biological parent | 92 (43%) | 98 (75%) | 190 (56%) | <0.001 |
| Annual household income ≤ $20 000a | 96 (48%) | 84 (68%) | 180 (56%) | <0.001 |
| Caregiver education: high school or less | 127 (60%) | 91 (70%) | 218 (64%) | 0.059 |
| BMI, mean (SD) | 19.9 (4.54) | 20.7 (5.73) | 20.2 (5.03) | 0.73 |
| BMI z-score, mean (SD) | 0.31 (1.07) | 0.68 (1.30) | 0.45 (1.17) | 0.005 |
| CDC class C | 55 (26%) | |||
| Current antiretroviral use | ||||
| HAART | 185 (87%) | |||
| On PIs | 153 (72%) | |||
| On NNRTIs | 53 (25%) | |||
| HAART-based antiretroviral regimen grouping | ||||
| HAART with PI | 151 (71%) | |||
| HAART without PI | 34 (16%) | |||
| Non-HAART antiretroviral | 12 (6%) | |||
| Not on antiretroviral | 15 (7%) | |||
| Nadir CD4+ cell count, median (Q1, Q3) | 386 (220, 586) | |||
| Nadir CD4% | 20 (12, 26) | |||
| Most recent CD4+ cell count, median (Q1, Q3) | 713 (495, 920) | |||
| Most recent CD4% | ||||
| Median (Q1, Q3) | 33.0 (26.0, 38.5) | |||
| <15% | 11 (5%) | |||
| 15–25% | 34 (16%) | |||
| 25% or more | 167 (79%) | |||
| Log(10) viral load, median (Q1, Q3) | 2.60 (1.70, 3.32) | |||
AMP, Adolescent Master Protocol; CDC, Centers for Disease Control and Prevention; NNRTI, non-nucleoside reverse transcriptase inhibitor; PCG, primary caregiver; PHEU, perinatally HIV-exposed uninfected; PHIV+, perinatally HIV-infected; PI, protease inhibitor.
N = 324 (18 youth missing annual household income data).
P-values by chi-square test.
Biomarkers
All biomarkers had right-skewed distributions (and were thus log-transformed for statistical analysis) except sICAM-1 and sE-selectin. In unadjusted comparisons, PHIV+ participants had significantly higher levels of sMCP-1 (P = 0.014), sVCAM-1 (P <.001), sICAM-1 (P = 0.019), and fibrinogen (P = 0.003), and lower levels of sE-selectin (P = 0.008) than PHEU participants; the remaining four biomarkers did not differ by HIV status (Table 2).
Table 2.
Distributions of biomarker levels and WISC-IV scores for 342 AMP participants.
| Cohort |
||||
|---|---|---|---|---|
| PHIV+ | PHEU | Total | P-value | |
| Vascular biomarker level | ||||
| Number with samplesa | 212 | 130 | 342 | |
| Median (Q1, Q3) | ||||
| C-reactive protein (mg/l) | 0.40 (0.20, 0.95) | 0.60 (0.20, 2.00) | 0.40 (0.20, 1.20) | 0.095 |
| Interleukin-6 (pg/ml) | 1.00 (0.70, 1.50) | 1.10 (0.70, 1.70) | 1.00 (0.70, 1.60) | 0.48 |
| Soluble monocyte chemotactic protein-1 (pg/ml) | 103 (80.3, 135) | 90.9 (74.0, 119) | 97.9 (76.7, 129) | 0.014 |
| Soluble intercellular adhesion molecule-1 (ng/ml) | 284 (174, 341) | 245 (164, 320) | 265 (169, 331) | 0.019 |
| Soluble vascular cell adhesion molecule-1 (ng/ml) | 784 (667, 975) | 689 (570, 816) | 749 (624, 917) | <0.001 |
| Fibrinogen (mg/dl) | 365 (323, 407) | 337 (292, 396) | 359 (311, 402) | 0.003 |
| Soluble P-selectin (ng/ml) | 32.1 (23.7, 45.9) | 35.5 (26.3, 48.8) | 33.3 (24.4, 46.5) | 0.21 |
| Soluble E-selectin (ng/ml) | 44.6 (32.5, 61.6) | 51.1 (36.2, 70.7) | 46.6 (34.0, 64.4) | 0.008 |
| Adiponectin (g/ml) | 9.41 (6.98, 12.21) | 9.19 (7.68, 11.56) | 9.30 (7.43, 11.99) | 0.89 |
| WISC-IV scores | ||||
| Number with valid testsb | 201 | 127 | 328 | |
| Mean (SD) | ||||
| Full scale IQ score | 84.8 (15.6) | 84.7 (15.7) | 84.8 (15.6) | 0.66 |
| Verbal comprehension | 86.8 (15.7) | 85.1 (16.2) | 86.2 (15.9) | 0.28 |
| Perceptual reasoning | 90.1 (15.0) | 89.7 (15.0) | 89.9 (15.0) | 0.48 |
| Working memory | 87.9 (16.1) | 86.7 (14.3) | 87.4 (15.4) | 0.40 |
| Processing speed | 86.6 (15.1) | 89.7 (13.4) | 87.8 (14.5) | 0.099 |
AMP, Adolescent Master Protocol; IQR, interquartile range; PHEU, perinatally HIV-exposed uninfected; PHIV+, perinatally HIV-infected; WISC-IV, Wechsler Intelligence Scales for Children, fourth edition. P-values by Wilcoxon test.
One additional PHEU participant missing soluble P-selectin, and one PHEU and one PHIV+ patient each missing interleukin-6 soluble intercellular adhesion molecule-1, and soluble vascular adhesion molecule-1 measurements.
One additional PHIV+ participant missing full scale IQ score.
Neurodevelopmental outcomes
The four WISC-IV index scores and FSIQ were approximately normally distributed. The mean scores were in the low average–average range (86–90) and did not differ significantly by HIV status (Table 2).
Relationships between biomarkers and neurodevelopmental outcomes
Individually, none of the nine candidate biomarkers showed significant relationships with FSIQ in linear regression models, both overall and in the models restricted to PHIV+ participants alone. The relationships remained nonsignificant after adjusting for caregiver education and household income and forcing BMI z-score into the model for adiponectin (P > 0.05). In adjusted models that contained PHIV+ and PHEU participants, PHIV+ status was not associated with FSIQ (P-value >0.40 for all biomarkers).
There was a significant interaction effect between log (adiponectin) and HIV infection status on FSIQ (P = 0.004). A unit increase in log10 (adiponectin), adjusted for caregiver education and BMI z-score, was associated with a 2.07 unit increase in FSIQ in PHIV+ participants (P = 0.05), but was associated with a 3.97 unit decrease in PHEU participants (P = 0.04).
Factor analysis
Among 342 participants with biomarker measurements, three were missing at least one biomarker, leaving 339 participants available for the factor analysis. Kaiser's measure of sampling adequacy was calculated as K = 0.63, above the acceptable threshold of 0.5 or 0.6 [14]. Scree plots suggested models with three or four factors.
Results of the factor analyses are shown in Table 3. The four-factor principal component analysis and three-factor maximum likelihood analysis gave consistent results, suggesting one factor (F1) for fibrinogen, CRP, and IL-6; one (F2) for sICAM-1 and sVCAM-1; and one (F3) for sMCP-1, P-selectin, and sE-Selectin. The four factor model identified using principal component analysis had a high loading factor only for adiponectin (0.90), and low loading factors (<0.40) for all other biomarkers. These models explained 65 and 38% of the biomarker variance, respectively. The suggested three factors and adiponectin were used as the predictors of neurodevelopmental outcomes in the SEMs.
Table 3.
Factor analysis results (factor loadings, communalities, and variance explained for 342 AMP participants).
| Maximum likelihood fit with 3 factors |
||||
|---|---|---|---|---|
| Biomarker | F1 | F2 | F3 | Communality |
| log(CRP) | 0.94 | −0.05 | −0.09 | 0.89 |
| log(IL-6) | 0.54 | 0.26 | 0.02 | 0.36 |
| log(fibrinogen) | 0.54 | −0.02 | 0.11 | 0.30 |
| slCAM-1 | 0.16 | 0.45 | 0.13 | 0.25 |
| log(sVCAM-1) | −0.02 | 0.82 | −0.11 | 0.69 |
| sE-selectin | 0.09 | 0.16 | 0.40 | 0.19 |
| log(sMCP-1) | −0.12 | 0.24 | 0.32 | 0.17 |
| log(sP-selectin) | −0.02 | −0.05 | 0.69 | 0.48 |
| log(adiponectin) | −0.12 | 0.10 | −0.19 | 0.06 |
| Cumulative proportion of variance explained | 0.17 | 0.28 | 0.38 | 0.38 |
CRP, C-reactive protein; IL-6, interleukin 6; sE-selectin, soluble E-selectin; sICAM-1, soluble intracellular adhesion molecule; sMCP-1, soluble monocyte chemoattractant protein; sP-selectin, soluble P-selectin; sVCAM-1, soluble vascular cell adhesion molecule. F1–F3: factor loadings for factors 1–3, respectively. Communality is defined as the sum of the squares of the factor loadings for a variable. All factor loadings rotated using an oblique varimax rotation. Variables that have loading factor above 0.30 or below −0.30 on the combined cohort are in bold face (models did not converge on separate halves of the dataset).
Structural equation models
In models with FSIQ, verbal comprehension, perceptual reasoning, working memory, and latent neurodevelopmental variable, there were no significant associations between any of the factors or adiponectin and these outcomes in any of the models, adjusted or unadjusted (Table 4).
Table 4.
Summary of structural equation models among 342 AMP participants included in the analysis.
| Standardized path coefficient for each neurodevelopmental outcome |
||||||
|---|---|---|---|---|---|---|
| Paths in model | FSIQ | PS | WM | VC | PR | Latent ND variable |
| Overall model (including PHIV+ and PHEU) | ||||||
| F1 → outcome | −0.10 | −0.19* | −0.12 | 0.03 | −0.08 | −0.10 |
| F2 → outcome | −0.07 | −0.01 | −0.01 | −0.12 | −0.11 | −0.09 |
| F3 → outcome | −0.04 | 0.03 | −0.15 | 0.02 | 0.01 | −0.04 |
| log(adiponectin) → outcome | 0.02 | 0.01 | −0.04 | 0.04 | 0.04 | 0.02 |
| PHIV+ model | ||||||
| F1 → outcome | −0.11 | −0.21* | −0.13 | −0.02 | −0.04 | −0.12 |
| F2 → outcome | −0.11 | −0.07 | −0.07 | −0.11 | −0.13 | −0.13 |
| F3 → outcome | 0.01 | −0.02 | −0.04 | 0.01 | 0.07 | 0.02 |
| log(adiponectin) → outcome | 0.11 | 0.09 | 0.08 | 0.09 | 0.12 | 0.12 |
| Model description and fit Overall model | ||||||
| Overall model | ||||||
| N | 307 | 308 | 325 | 308 | 308 | 308 |
| RMSEA | 0.088 | 0.093 | 0.086 | 0.088 | 0.088 | 0.072 |
| CFI | 0.781 | 0.765 | 0.787 | 0.770 | 0.777 | 0.859 |
| PHIV+ model | ||||||
| N | 198 | 187 | 199 | 199 | 199 | 199 |
| RMSEA | 0.065 | 0.071 | 0.066 | 0.066 | 0.065 | 0.063 |
| CFI | 0.842 | 0.804 | 0.828 | 0.840 | 0.831 | 0.872 |
CFI, Comparative Fit Index; FSIQ, full scale IQ; ND, neurodevelopmental; PHEU, perinatally HIV-exposed uninfected; PHIV+, perinatally HIV-infected; PR, perceptual reasoning; PS, processing speed; RMSEA, root mean square error approximation; VC, verbal comprehension; WM, working memory. F1 has loading factors to C-reactive protein, interleukin-6, and fibrinogen; F2 has loading factors to soluble vascular cell adhesion molecule-1 and soluble intercellular adhesion molecule-1; F3 has loading factors to soluble monocyte chemoattractant protein, soluble E-selectin, and soluble P-selectin. Overall models control for: FSIQ, PR, and the latent variable: HIV infection status, caregiver income, caregiver education, and BMI z-score; WM: HIV infection status, non-English exposure, and BMI z-score; PS: HIV infection status, caregiver income, and BMI z-score; VC: HIV infection status, non-English exposure, caregiver income, and caregiver education. PHIV+ only models control for: FSIQ, PR, VC, and the latent variable: peak viral load, nadir CD4%, non-English exposure, and caregiver education; WM: peak viral load, nadir CD4%, non-English exposure, and BMI z-score; PS: peak viral load, nadir CD4%, non-English exposure, BMI z-score, caregiver education, and caregiver income.
P < 0.05.
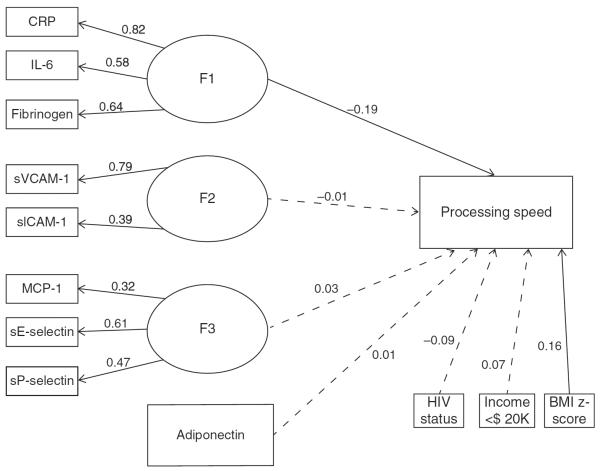
In models for the processing speed outcome in the total cohort, F1 (reflecting CRP, IL-6, and fibrinogen) was negatively associated with processing speed in both an unadjusted model and a model adjusting for HIV status, caregiver income, and BMI z-score (adjusted estimate = −0.19, P = 0.014) (Fig. 1). This finding persisted when restricted to PHIV+ participants even after adjusting for peak viral load, nadir CD4%, non-English exposure, BMI z-score, caregiver education, and household income (adjusted estimate = −0.21, P = 0.032). There were no other significant associations.
Fig. 1. Structural equation models evaluating relationship between biomarkers factors and processing speed among 342 perinatally HIV-infected or perinatally HIV-exposed but uninfected children included in the analysis.
Model for the processing speed outcome in the total cohort adjusted for HIV status, caregiver income, and BMI z-score. Ovals represent latent, unobserved variables. Rectangles represent observed variables. Nonsignificant paths are marked with dashed lines.
Discussion
We examined relationships between serum levels of nine candidate biomarkers and neurodevelopmental outcomes in PHIV+ and PHEU youth from the PHACS/AMP cohort. The mean WISC-IV scores were in the low average–average range for the four index scores and did not differ significantly by HIV status. The SEMs suggested a significant negative relationship between the combined fibrinogen, CRP, and IL-6 factor and processing speed in the total cohort. This effect remained significant when examined among PHIV+ participants alone and after adjusting for HIV viral load and other relevant clinical and socio-demographic variables, suggesting a mechanism independent from HIV disease severity to which processing speed might be particularly sensitive.
In a typically developing brain, processing speed progressively increases throughout childhood and adolescence [15], mirroring the ongoing process of white matter myelination which continues into late adolescence [16]. Data from neuroimaging studies suggest that white matter integrity and resulting efficiency in neuronal signal conduction determines the speed of information processing [17]. Thus, one way to interpret data from the present analysis is that combined levels of IL-6, CRP, and fibrinogen may be a marker of altered white matter myelination in the brains of PHIV+ youth. In particular, the presence of fibrinogen in the central nervous system (CNS) after blood–brain barrier (BBB) disruption induces rapid and sustained microglial activation with axonal and myelin damage [18], which, along with changes in CRP and IL-6, may provide enough of an ensemble pro-inflammatory signal to ultimately affect processing speed. This hypothesis could be tested in a longitudinal study combining neuroimaging and neurophysiological studies, neurodevelopmental testing, and blood biomarkers.
IL-6 is produced by multiple cell types, including endothelial, glia, T and B cells. Among its many roles, IL-6 induces B-cell proliferation and differentiation and IgG production, as well as T-cell growth and cytotoxic T-cell differentiation and macrophage differentiation [19,20]. Importantly, latent HIV-1 infection in the CNS has been associated with elevated BCL11B, an epigenetic chromatin modifier associated with deregulation of IL-6 [21]. The relationship observed in the present analysis suggests that IL-6 might be involved in mechanisms of chronic CNS injury in PHIV+ youth, possibly by facilitating trafficking of activated T cells and macrophages across the BBB and perpetuating chronic microglia activation or even via direct neurotoxic effect [22]. In adult populations of HIV+ patients, IL-6 is weakly negatively associated with impairments in memory and learning [23], but remains elevated in the cerebrospinal fluid (CSF) of treated patients, regardless of cognitive status [24], suggesting persistent neuroinflammation despite suppressive ART. The absence of independent effect of IL-6 in linear regressions, and presence of significant effect of combined IL-6, CRP, and fibrinogen levels in the present study may suggest that a certain degree of systemic pro-inflammatory or pro-coagulant activity or both is needed to promote IL-6 activity associated with CNS injury in PHIV+ youth.
Although the model for PHEU participants did not converge, the persistence of significant relationship between `factor F1' and processing speed in the total cohort after adjusting for HIV status leaves open the possibility that such relationship exists in PHEU youth, but perhaps to a lesser degree and, given the absence of HIV infection, via a different mechanism. Evidence suggests presence of abnormalities of fetal immune maturation associated with in-utero exposure to HIV which may persist into childhood in a subtle form even in the absence of perinatal HIV transmission, including lower naïve CD4+ cell counts and thymic output in PHEU infants [25]; presence of HIV-specific CD4+ and CD8+ T cells in PHEU children as old as 7 years [26]; increased IgG, IgM, and IgA levels at 24 months of age [27]; and altered cell-mediated or antibody responses to certain vaccines (Bacillus Calmette-Guérin, pertussis, pneumococcus, and tetanus toxoid) [28]. In-utero exposure to maternal infections and immune activation during critical times of development has been associated with increased risk of autism [29], schizophrenia [30], and bipolar disorder [31]. Bilbo and Schwarz [32] hypothesized that, following fetal exposure to maternal infection, a subset of glia cells enter permanently activated or primed state, resulting in overproduction of cytokines in response to subsequent immune challenges, with lifelong consequences for brain function. Similar mechanisms might plausibly be at play in PHEU youth. Finally, children born into and living in poverty (who are disproportionately vulnerable to perinatal HIV exposure in the US) may be at risk for chronically altered immune responses due to other nonspecific factors associated with socio-economic disadvantage. Meta-analysis of 32 studies reported strong inverse associations of CRP levels with measures of socioeconomic status and significantly higher CRP levels among members of nonwhite racial/ethnic groups relative to whites [33]. In the present analysis, the PHEU youth were more likely to come from families from the lowest income bracket than their PHIV+ counterparts.
Unlike our preliminary analysis [4], and contrary to our hypothesis, this analysis did not indicate a significant relationship between sP-selectin and neurodevelopmental outcomes in either PHIV+ or PHEU youth, whereas fibrinogen was only significantly associated with processing speed outcomes in combination with CRP and IL-6. In the multivariate linear regressions, serum adiponectin levels were significantly associated with a higher FSIQ among PHIV+ youth and a lower FSIQ in PHEU youth, but this significance was lost in the SEMs. Such inconsistencies illustrate the limitations of single-marker-oriented approach aimed at understanding physiological mechanism(s) that contribute to less optimal neurodevelopmental outcomes among PHIV+ and PHEU youth; rather, cause is almost certainly multifactorial. To address this issue, we expanded our analyses to include factor analysis of the nine biomarkers of interest and then used the resulting factors to build SEMs. Fibrinogen, CRP, and IL-6 grouped as one factor ('F1'); the two adhesion molecules (sICAM-1 and sVCAM-1), which are both considered markers of endothelial activation, formed another ('F2'); whereas sP-selectin (endothelial activation and procoagulant state marker), sE-selectin (endothelial activation marker), and sMCP-1 (inflammation marker) constituted the third factor ('F3'). The nonassociation of adiponectin with any of the factors coincides with its somewhat unique status as adipocyte-specific secretory protein which promotes insulin sensitivity [34], as well as a role as modulator of endothelial inflammatory response [10]. In the SEMs, factors F2, F3, and adiponectin predicted no neurodevelopmental outcomes, perhaps suggesting that abnormalities of endothelial activation, metabolism and cell adhesion, which are known to occur in PHIV+ youth [35] are not as salient to CNS damage in PHIV+ youth as are chronic immune activation and residual inflammation which has been demonstrated even after 12 years of well controlled viremia in HIV+ adults [36]. The lack of significant association between blood sMCP-1 levels (either alone or as part of `factor F3') with lower WISC-IV scores is consistent with previously reported experimental data in Simian immunodeficiency virus-infected macaques: blood levels of sMCP-1 did not predict CNS dysfunction, whereas CSF-to-plasma sMCP-1 ratios above 1 predicted moderate-to-severe encephalopathy, suggesting that brain parenchyma was the main source of increased sMCP-1 production [37].
The study has limitations. Due to the cross-sectional design, the temporality and causal direction of the observed associations cannot be inferred. PHEU youth from the AMP cohort provided a control for the effect of HIV infection, but not for the effect of in-utero exposure to HIV. Future studies should include a comparison cohort of generally healthy, demographically comparable youth without history of perinatal HIV exposure. Next, although processing speed index score provides a valuable measure of neurodevelopmental functioning, it is based on only two subtests of the WISC-IV. Whereas this limitation warrants careful interpretation of our finding, one potential clinical implication is that, if this finding is validated by future studies, processing speed could serve as a cost-effective screen for identifying those PHIV+ youth who need more thorough neurocognitive follow-up and appropriate accommodations as indicated. Finally, having chosen to focus on WISC-IV scores only, we did not evaluate the relationship between biomarker factors and behavioral outcomes. We intend to address this limitation in future longitudinal analyses which will be informed by the factor loadings presented here and include the BASC-2 scores as additional outcomes.
In conclusion, we observed a significant inverse correlation between serum levels of three combined pro-inflammatory markers (IL-6, CRP, and fibrinogen) and processing speed (measured by WISC-IV) in a subset of youth from the PHACS cohort. This finding suggests that residual inflammation may be associated with decreased processing speed in PHIV+ youth despite well controlled HIV disease. Decreased processing speed and aggregate increase in levels of fibrinogen, CRP, and IL-6 might have value in identifying PHIV+ youth, and, possibly, PHEU youth, who need more intensive neurocognitive follow-up. Longitudinal studies are indicated to elucidate the mechanisms of this association and assess potential clinical utility in predicting CNS impairment.
Acknowledgements
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS.
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP in 2012, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, MahboobullahBaig, Anna Cintron; Children's Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Doyle Patton, Deyana Leon; Children's Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Ann & Robert H. Lurie Children's Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; St. Christopher's Hospital for Children: Janet Chen, Latreca Ivey, Maria Garcia Bulkley, Mitzie Grant; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Medea Jones, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
S.K. thanks Dr Pim Brouwers, PhD, for his critical feedback and review of the manuscript.
Sources of funding: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute of Allergy and Infectious Diseases, the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, National Institute of Neurological Disorders and Stroke, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (U01 HD052102-04) (Principal Investigator: George R. Seage, III; Project Director: Julie Alperen) and the Tulane University School of Medicine (U01 HD052104-01) (Principal Investigator: Russell B. Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc. (PI: Mercy Swatson).
Footnotes
Author contributions: S.K. participated in developing the hypothesis, study design, analysis, and interpretation of study results, and was the primary writer of the manuscript; R.G., B.Z., and E.L. conducted statistical analyses and participated in the writing and revision of the manuscript; S.N. was involved in study design, analysis, and interpretation of study results, and manuscript writing; T.L.M. participated in development of research concept of vascular inflammation in HIV and editing the manuscript; R.H. was involved in developing the original idea, data analysis plan, data interpretation, manuscript review, and finalization; H.A.G. helped with interpretation of data in terms of translating the preclinical model to explain the data in terms of the neuropathogenesis of HIV-1; K.M.M. was involved in study design, analysis, and interpretation of study results, and manuscript writing; B.K. was involved in study design, data interpretation, and manuscript writing; A.J.M.'s laboratory provided all of the biomarker testing, and he aided in the writing and revision of the manuscript; P.L.W. participated in the study design, statistical analysis, and aiding in the writing and revision of the manuscript.
Publisher's Disclaimer: S.K. contributed to this article in his personal capacity. The views expressed are his own and do not necessarily represent the views of the National Institutes of Health or United States Government.
Conflicts of interest There are no conflicts of interest.
References
- 1.Le Doaré K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:e1326–e1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- 2.Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31:592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donenberg GR, Pao M. Youths and HIV/AIDS: psychiatry's role in a changingepidemic. J Am Acad Child Adolesc Psychiatry. 2005;44:728–747. doi: 10.1097/01.chi.0000166381.68392.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapetanovic S, Leister E, Nichols S, Miller T, Tassiopoulos K, Hazra R, et al. Relationship between markers of vascular dysfunction and neurodevelopmental outcomes in perinatally HIV-infected youth. AIDS. 2010;24:1481–1491. doi: 10.1097/QAD.0b013e32833a241b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller TL, Borkowsky W, DiMeglio LA, Dooley L, Geffner ME, Hazra R, et al. Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Med. 2012;13:264–275. doi: 10.1111/j.1468-1293.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballantyne CM, Nambi V. Markers of inflammation and their clinical significance. Atheroscler Suppl. 2005;6:21–29. doi: 10.1016/j.atherosclerosissup.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Papanicolaou DA, Vgontzas AN. Interleukin-6: the endocrine cytokine. J Clin Endocrinol Metab. 2000;85:1331–1333. doi: 10.1210/jcem.85.3.6582. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 9.Woollard KJ, Kling D, Kulkarni S, Dart AM, Jackson S, Chin-Dusting J. Raised plasma soluble P-selectin in peripheral arterial occlusive disease enhances leukocyte adhesion. Circ Res. 2006;98:149–156. doi: 10.1161/01.RES.0000199295.14073.69. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler D. Wechsler Intelligence Scale for children. 4th ed Harcourt Assessment, Inc; San Antonio, Texas: 2003. [Google Scholar]
- 12.Reynolds CR, Kamphaus RW. Behavior assessment system for children. 2nd ed American Guidance Service; Circle Pine, MN: 2004. [Google Scholar]
- 13.Johnson RA, Wichern DW. Applied multivariate statistical analysis. 5th ed Prentice Hall; 2002. pp. 481–537. [Google Scholar]
- 14.Kaiser HF. An index of factorial simplicity. Psychometrika. 1974;39:31–36. [Google Scholar]
- 15.Fry AF, Hale S. Processing speed, working memory and fluid intelligence: evidence for a developmental cascade. Psychol Sci. 1996;7:237–241. [Google Scholar]
- 16.Pfefferbaum A, Mathalon DH, Sullivan EV, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SL, Glass JO, Li Y, Ogg R, Qaddoumi I, Armstrong GT, et al. White matter integrity is associated with cognitive processing in patients treated for a posterior fossa brain tumor. Neuro Oncol. 2012;14:1185–1193. doi: 10.1093/neuonc/nos154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto T. IL-6: from laboratory to bedside. Clin Rev Allergy Immunol. 2005;28:177–186. doi: 10.1385/CRIAI:28:3:177. [DOI] [PubMed] [Google Scholar]
- 21.Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen SD, Jeppesen DL, Kolte L, Clark DR, Sørensen TU, Dreves AM, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood. 2001;98:398–404. doi: 10.1182/blood.v98.2.398. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen KK, Pedersen M, Gaardbo JC, Ronit A, Hartling HJ, Bruunsgaard H, et al. Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long term treatment with combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63:272–279. doi: 10.1097/QAI.0b013e318289bced. [DOI] [PubMed] [Google Scholar]
- 24.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–3871. [PubMed] [Google Scholar]
- 26.Bunders M, Pembrey L, Kuijpers T, Newell ML. Evidence of impact of maternal HIV infection on immunoglobulin levels in HIV-exposed uninfected children. AIDS Res Hum Retroviruses. 2010;26:967–975. doi: 10.1089/aid.2009.0241. [DOI] [PubMed] [Google Scholar]
- 27.Ensoli F, Fiorelli V, Muratori DS, De Cristofaro M, Vincenzi L, Topino S, et al. Immune-derived cytokines in the nervous system: epigenetic instructive signals or neuropathogenic mediators? Crit Rev Immunol. 1999;19:97–116. Review. [PubMed] [Google Scholar]
- 28.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–340. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 29.Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- 30.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. J Am Med Assoc Psychiatry. 2013;8:1–8. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- 32.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.vanWijk JP, Cabezas MC. Hypertriglyceridemia, metabolic syndrome, and cardiovascular disease in HIV-infected patients: effects of antiretroviral therapy and adipose tissue distribution. Int J Vasc Med. 2012;2012:201027. doi: 10.1155/2012/201027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller TL, Somarriba G, Orav EJ, Mendez AJ, Neri D, Schaefer N, et al. Biomarkers of vascular dysfunction in children infected with human immunodeficiency virus-1. J Acquir Immune Defic Syndr. 2010;55:182–188. doi: 10.1097/QAI.0b013e3181e222c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rönsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR. Persistent inflammation and endothelial activation in HIV-1 infected patients after 12 years of antiretroviral therapy. PLoS One. 2013;8:e65182. doi: 10.1371/journal.pone.0065182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, et al. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]