Abstract
OBJECTIVE
To screen a panel of targeted adenoviruses as vectors for endometriosis gene therapy.
STUDY DESIGN
Endometriotic cells were obtained from subjects with ovarian endometriomas. Liver tissues were taken from donors during hepatic transplantation surgery. Human endometriotic cells and liver tissues were transfected by targeted adenoviruses expressing luciferase reporter gene. Luciferase Activity mediated by each virus was expressed as a percentage of adenovirus serotype 5 activity. Two-tailed Student’s t test was used to compare the adenovirus data.
RESULTS
In endometriosic cells, the adenovirus-RGD (Ad5-RGD-luc), adenovirus under secretory leukocyte protease inhibitor promoter (Ad-SLPI-luc), and adenovirus under heparanse promoter (Ad-heparanase-luc) showed significantly higher activity as compared to the adenovirus serotype 5 (Ad5-CMV-luc). In liver tissues, adenovirus-survivin (Ad-survivin-luc) and Ad-heparanase-luc had significantly lower activity compared with Ad5-CMV-luc.
CONCLUSION
Ad-heparanase-luc showed “endometriosis on, liver off” phenotype and is promising vector for endometriosis gene therapy.
Keywords: adenovirus, targeting, endometriosis, gene therapy
Introduction
Endometriosis is a chronic and recurrent disease characterized by the presence of functional endometrial tissues (glands and stroma) outside the uterine cavity. It is estimated to affect 6-10 % of women in their reproductive years. Endometriosis-associated pain and infertility, in addition to cost and side effects of therapy, represent a substantial psychological and economical burden on the affected women.1
The use of endometriosis medical therapy is limited to no more than 6 months because long-term treatment is associated with serious hypoestrogenic side effects such as bone mineral density loss. In addition, medical therapy is not beneficial in treating endometriosis-associated infertility, and symptoms often recur after discontinuation of medical therapy or conservative surgery. Clearly, we are still in need of new treatment modalities for endometriosis that work at a molecular level rather than just the suppression of systemic ovarian estrogen production.2
Gene therapy is an emerging form of molecular medicine that has expanded in scope from malignant to benign diseases.3 Gene therapy involves the introduction of genetic material to the cell, whose subsequent products will address the therapeutic outcome. Endometriosis has been involved in gene therapy applications; Dabrosin et al (2002) eradicated endometriotic lesions in a murine model by inhibiting endometriosis-associated angiogenesis through the transient overexpression of angiostatin gene delivered to the peritoneal cavity of mice using the adenoviral vector.4 Recently, we successfully used the adenovirus to transfer the dominant negative estrogen receptor (DN-ER) gene into human endometriosis cells in vitro. This resulted in arrest of cellular proliferation, inhibition of cytokine production, and induction of apoptosis in these cells as a result of their deprivation of estrogen signaling.5
The development of effective and safe gene transfer vectors is integral to the success of gene therapy. Recombinant adenoviruses are promising gene therapy vectors because of their ease of propagation in the laboratory, limited pathogenecity in humans, and low mutagenesis potential. Moreover, adenoviruses can transfer genetic materials effectively in a wide spectrum of dividing and nondividing cells. This promiscuous tropism, however, represents a limitation because it leads to nonspecific gene transfer with sequestration of the virus and transgene into undesired tissues. Consequently, the fraction of the virus available for the intended tissue may be too limited to achieve a therapeutic effect. Additionally, toxicity to other organs my be increased.6
To achieve the desired effects of gene therapy, adenoviral vectors should be targeted as pathological tissue-specific and normal tissue sparing. Targeting strategies include transductional targeting, which depends on specific gene transfer by the vector to the target cells only. This is done through genetic modification of the adenovirus capsid proteins (fiber) to reroute its entry through receptors that are specifically expressed on pathological tissues. Transcriptional targeting allows for nonspecific gene transfer to a large number of cells, but the transgene is expressed only in the targeted tissues by driving its expression through a tissue-specific promoter that is active only in pathological tissue and exhibits minimal activity in normal tissues.7
In this study, we screened a panel of fiber-modified adenoviruses (transductionally targeted viruses) and adenoviruses in which transgene expression was driven by tissue-specific promoters (transcriptionally targeted viruses) to identify which would sustain a higher gene transfer and expression in human endometriosis cells, and would have lower activity in normal organs and tissues.
Material and Methods
Human endometriosis cells
Human endometriotic cells were a kind gift from Drs. Serdar Bulun and Erkut Attar (Northwestern University, Chicago, Ill). These are primary cells (passage 3–4) established from ovarian endometrioma lesions as described previously.8 Cells were maintained at 37°C in 5% CO2/air in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (FBS).
Human liver tissue slices
Human liver samples were obtained from seronegative donor livers prior to transplantation into recipients (Department of Surgery, University of Alabama at Birmingham, Ala). All liver samples were flushed with University of Wisconsin solution (ViaSpan, Barr Laboratories, Inc, Pomona, NY) until sliced as described previously.9 Briefly, tissue cores of 8 mm were drilled and subsequently sliced using a Krumdieck slicer (Alabama R&D, Munfort, Ala), with a slice diameter of 4 mm and thickness of 150 μm. The cell number was estimated at 1 × 106 cells/slice, based on an approximate 10 cell slice thickness.10 Slices were washed and preincubated at 37°C for 1 hour in Williams’ medium E supplemented with D-glucose (25 mM) and gentamycin (50 μg/mL), saturated with 95% O2/5% CO2 before experiments were started.
All human cells and tissues were collected under a protocol approved by the Institutional Review Boards of the University of Texas Medical Branch, Galveston, Texas, and the University of Alabama at Birmingham, Birmingham, Alabama.
Adenoviruses
Seven recombinant adenoviruses were tested in both endometriosis cells and liver tissues. The names and description of the tested viruses are shown in Table I. All adenoviruses used were replication-defective viruses with a luciferase reporter gene in the E1 region under the transcriptional control of the corresponding promoters. The transductional activity and gene transfer efficiency of each virus were reflected by its luciferase transactivation level. All viruses were propagated for a large-scale production in 239 cells and purified by double CsCl density centrifugation as we have described previously.11
TABLE I.
Names, abbreviations, and structure of transductionally and transcriptionally targeted adenoviruses used in this study
| Targeting strategy |
Virus name | Abbreviation | Description | Reference |
|---|---|---|---|---|
| Wild type (nontargeted) |
Adenovirus serotype 5 |
Ad5-CMV- luc |
Encodes Ad-5 fiber with cytomegalovirus (CMV) promoter driving luciferase (luc) transgene expression |
21 |
| Transductionally targeted (fiber- modified viruses) |
Adenovirus- RGD |
Ad5-RGD- luc |
Integrin-binding peptide (arginine- glycine-aspartate) is attached to the adenovirus fiber HI loop to expand tropism to integrin expressing cells |
15 |
| Adenovirus sigma |
Ad-sigma-luc | Encode the wild type Ad5 fibers and the sigma-1 protein which is a reovirus attachment protein that can infect cells expressing sialic acid or junction adhesion molecule-1 (JAM-1) |
22 | |
| Adenovirus 5/3 |
Ad5/3-luc | Ad5 fiber knob has been replaced by that of Ad3 to redirect binding to the putative Ad3 receptors CD80, CD86, or CD46 |
23 | |
| Transcriptionally targeted (viruses with tissue- specific promoters) |
Adenovirus- survivin |
Ad-survivin- luc |
Transgene expression is derived by survivin promoter |
24 |
| Adenovirus- secretory leukocyte protease inhibitor |
Ad-SLPI-luc | Transgene expression is derived by SLPI promoter |
18 | |
| Adenovirus- heparanase |
Ad- heparanase luc |
Transgene expression is derived by heparanase promoter |
17 |
Ad, adenovirus; CMV, cytomegalovirus; luc, luciferase; RGD, integrin-binding peptide; SLPI, secretory leukocyte protease inhibitor.
Adenovirus transfections
Endometriotic cells were grown in 12-well plates to a density of 50,000 cells per well and fed regular medium for 24 hours. Cells were then incubated with the corresponding adenovirus, at 2 different multiplicities of infection (MOI) of 10 and 50 PFU/cell, in a special transfection medium containing 2% FBS with continuous gentle shaking for 5 hours. Cells were then rinsed with phosphate buffered saline (PBS), fresh medium was added, and cells were incubated at 37°C and 5% CO2.
Liver tissue slices were placed into 6-well plates (1 slice per well). All viral infections were performed in low serum transfection medium for 5 hours at a viral concentration of 500 vps/cell. Transfection medium was then replaced with regular medium, and tissue slices were incubated for up to 48 hours on a rocker set at 60 rpm to agitate slices to ensure adequate oxygenation and viability.
Measurement of luciferase transactivation
Forty-eight hours posttransfection, endometriotic cells in each well were lysed with 150 μL/well of lysis buffer (Reporter Lysis Buffer; Promega, Madison, Wis) for 15 minutes. The wells were then scraped gently to collect the cell lysate. Twenty microliters of cell lysate from each well were mixed with 100 μL of luciferase assay reagent (Promega) and measured with a Berthold Lumat LB 9501 (Wildbad, Germany). Luciferase transactivation was normalized to total protein content of the cells.
The infected human liver tissue slices were placed in cell culture lysis buffer (Promega) and homogenized with an ultrasonicator (Fisher Scientific Model 100, Pittsburgh, Penn) at a setting of 15 watts for 10 seconds. Tissue homogenates were centrifuged to pellet the debris, and then the luciferase activities were measured using the Promega Luciferase Assay System and normalized to protein concentration in the tissue homogenates.
Statistical analysis
Relative luciferase transactivation for each modified adenovirus was expressed as a percentage of luciferase transactivation mediated by adenovirus serotype 5 (Ad5-CMV-luc, the wild type) in the particular tissue system. Two-tailed Student’s t test was used for comparison among different adenoviruses. Significance was reached when P < .05.
Results
A- Endometriosis cells (the target tissue)
Transductionally targeted adenoviruses
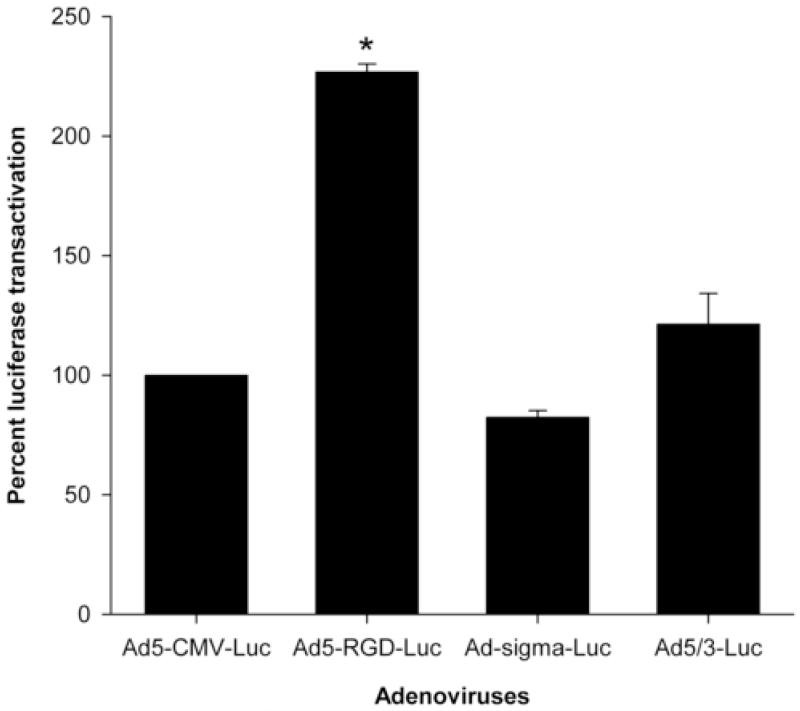
We compared the transductional activity of 3 fiber-modified adenoviruses (Ad5-RGD-luc, Ad-sigma-luc, and Ad5/3-luc) with the wild type adenovirus (Ad5-CMV-luc) in endometriosis cells at MOI of 10 PFU/cell. As seen in Figure 1, Ad5-RGD-luc significantly enhanced endometriotic cell transduction with a reporter gene expression 2.5-fold higher compared with Ad5-CMV-luc (P < .001). The same trend was seen at MOI of 50 PFU/cell (data not shown).
FIGURE 1.
Luciferase transactivation mediated by transductionally targeted adenoviruses at an MOI of 10 PFU/cell in endometriosis cells expressed as a percent of Ad5-CMV-luc activity.
Transcriptionally targeted adenoviruses
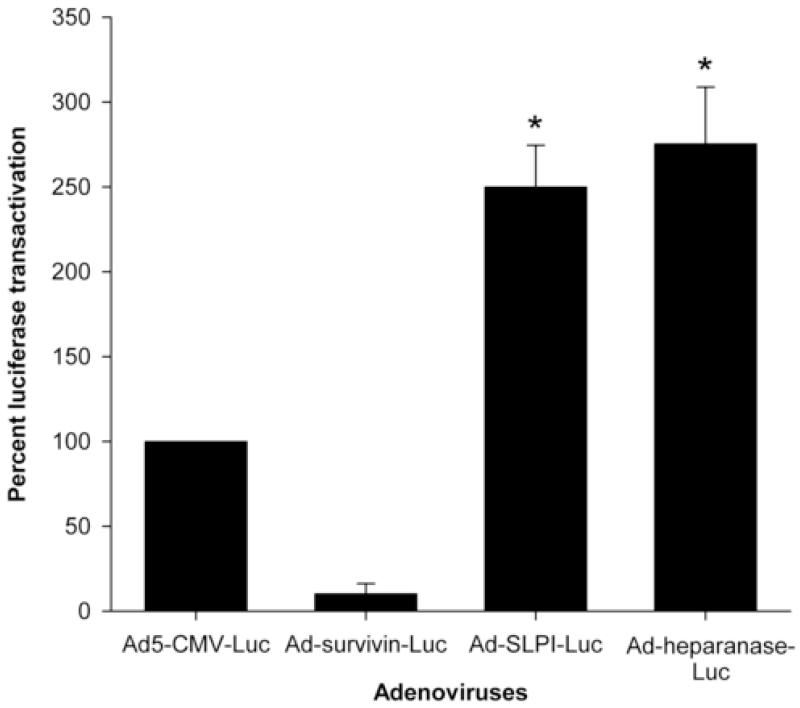
We also compared the transcriptional activity mediated by 3 adenoviruses with tissue-specific promoters (Ad-survivin-luc, Ad-SLPI-luc, and Ad-heparanase-luc) to Ad5-CMV-luc (the wild type adenovirus under the ubiquitously activated CMV promoter) at MOI of 10 PFU/cell in endometriosis cells. As observed in figure 2, the Ad-SLPI-luc–and Ad-heparanase-luc–mediated luciferase activation was 2.5- and 3-fold higher as compared with Ad5-CMV-luc (P = .008 and P = .012, respectively). A similar trend was seen at MOI of 50 PFU/cell (data not shown).
FIGURE 2.
Luciferase transactivation mediated by transcriptionally targeted adenoviruses at an MOI of 10 PFU/cell in endometriosis cells expressed as a percent of Ad5-CMV-luc activity.
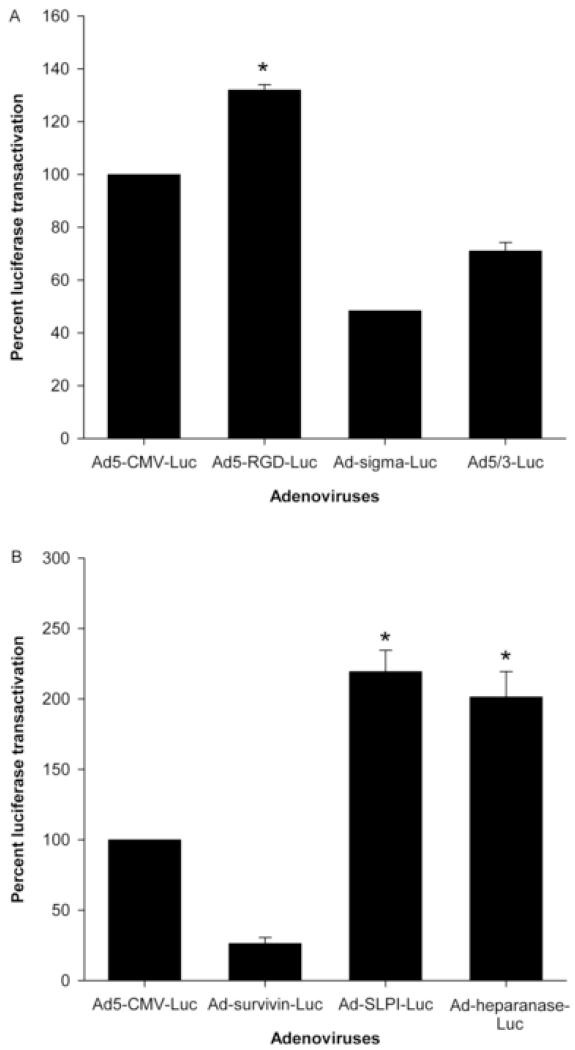
Modified adenoviruses at 10 PFU/cell versus Ad5-CMV-luc at MOI of 50 PFU/cell
As we have previously shown, using Ad5 expressing beta galactosidase (LacZ) as a marker gene, near optimal transduction of endometriosis cells (>80% of the cells) was achieved at MOI of 50 PFU/cell.12 Here, we wanted to compared transductionally and transcriptionally targeted adenoviruses at a low MOI (10 PFU/cell) to Ad5-CMV-luc at an MOI that is known to achieve the best transduction of endometriosis cells (50 PFU/cell). As shown in Figure 3 A&B, Ad5-RGD-luc, Ad-SLPI-luc, and Ad-heparanase-luc at an MOI of 10 PFU/cell mediated a significantly higher reporter gene expression than Ad5-CMV-luc at an MOI of 50 PFU/cell (P < .001, P < .03, and P < .04, respectively) (Figure 3A, B).
FIGURE 3.
Luciferase transactivation mediated by transductionally (A) and transcriptionally (B) targeted adenoviruses in endometriosis cells at an MOI of 10 PFU/cell versus Ad5-CMV-luc at an MOI of 50 PFU/cell (optimal dose).
B- Liver tissues (the nontarget tissues)
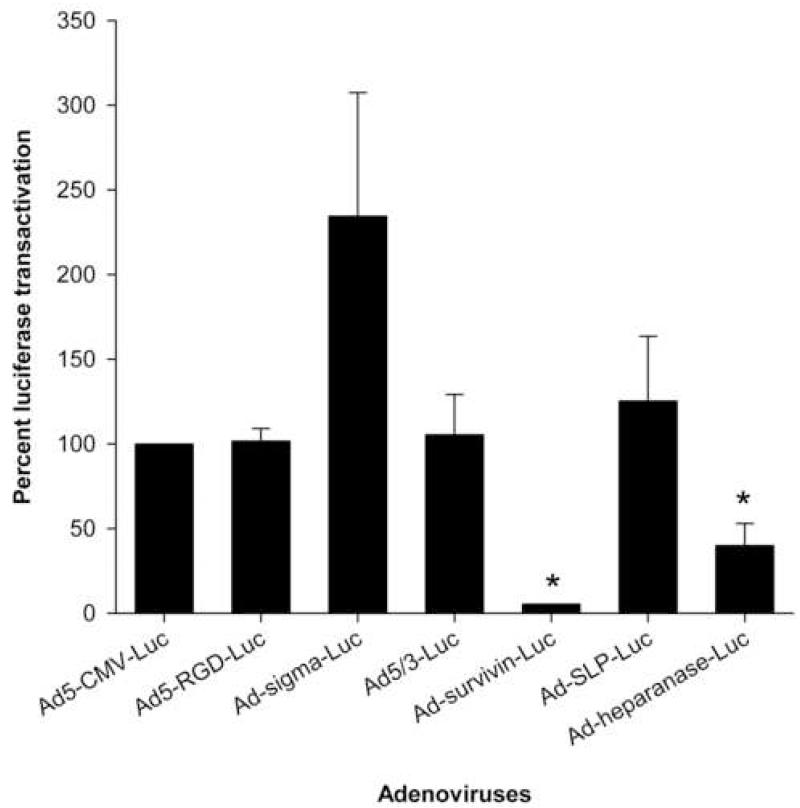
One of the major limitations of systemically administered adenovirus vectors for gene therapy is the uptake of a major part of the virus by the liver tissues. To overcome this problem and limit hepatotoxicity associated with adenovirus administration, the modified viruses should exhibit minimal gene transfer and expression activity in the liver. We tested the gene transfer and expression levels mediated by the modified adenoviruses in the liver tissues. Only Ad-survivinluc and Ad-heparanase-luc mediated significantly lower luciferase transactivation in liver tissues as compared with Ad5-CMV-luc (P = .004, P =.01, respectively) (Figure 4).
FIGURE 4.
Luciferase transactivation mediated by modified adenoviruses in human liver tissue slices expressed as percent of Ad5-CMV-luc activity.
Comment
Adenoviral infection is initiated by the recognition of the primary cell surface receptor, the coxsackie-adenovirus receptor (CAR), by the C-terminal part of an adenovirus fiber protein termed knob. CAR dependency of the adenovirus represents a problem in gene therapy, as this receptor is widely distributed over many cells and tissues in the body.13 This means that nontargeted CAR-expressing tissues will also be transfected by the administered adenovirus. This is true with regard to the liver, in particular, which sequesters the majority of systemically administered adenovirus particles.7
To maximize therapeutic effects and minimize toxicity of endometriosis gene therapy, specific adenoviral targeting strategies need to be implemented. Transductional targeting employs genetically engineered adenoviral particles with specific modifications in the adenovirus fiber, including attachment of targeting peptides, serotype knob switching, or fiber replacement. These modifications should reroute the adenovirus cell entry through CAR-independent pathways.14 In this study, we tested 3 transductionally targeted adenoviruses in the context of endometriosis gene therapy: Ad5/3-luc, Ad-5RGD-luc, and Ad-sigma-luc. We showed that Ad5-RGD-luc-mediated reporter gene activity in endometriosis cells is significantly higher than unmodified (wild type) Ad5-CMV-luc at 10 and 50 PFU/cell.
Ad5-RGD-luc is an expanded tropism adenovirus that contains a targeting peptide (Arg-Gly-Asp) attached to the HI loop of its fiber to redirect its entry through integrin receptors rather than the ubiquitously expressed CAR. Previous reports have shown significantly increased transductional efficiency mediated by this virus as compared with unmodified Ad5 in integrin expressing ovarian cancers and squamous cell carcinomas of the head and neck.15 Endometriosis tissues were found to express various types of integrins that were implicated in the adhesion of endometriotic cells to the peritoneal surfaces.16 This conceivably explains our finding of enhanced transductional efficiency mediated by this integrin targeting virus in endometriosis cells as compared with wild type Ad5-CMV-luc.
An additional level of targeting specificity can be gained by restricting the transgene expression to the target tissue. This strategy of transcriptional targeting is based upon the use of promoters that display preferential activity in pathological tissues. An ideal tissue-specific promoter for transcriptional targeting exhibits a selective “pathological tissue on” phenotype. To mitigate hepatotoxicity, candidate promoters additionally should exhibit a “liver off” phenotype.17 We evaluated 3 tissue-specific promoters for their activity in endometriosis cells: survivin, secretory leukocyte protease inhibitor (SLPI), and heparanase promoters. According to our results, Ad-SLPI-luc and Ad-heparanase-luc showed significantly higher reporter gene activity than Ad5-CMV-luc in endometriosis cells at MOIs of 10 and 50 PFU/cell.
SLPI was identified as a potent inhibitor of leukocyte serine proteases, and it plays a role in the protection of mucosal surfaces against injury associated with inflammation. The SLPI gene promoter retains its fidelity in the adenoviral vectors and was used as a tissue-specific promoter in ovarian cancer because it is activated in ovarian cancer cell lines and in primary cells, while it has low expression levels in normal organs, such as the liver.18 In endometriosis, SLPI mRNA was detected in ovarian endometrioma, peritoneal endometriosis, and deep rectovaginal endometriosis, but not in normal ovarian tissue or eutopic endometrium from control women.19 Other studies have shown that SLPI concentration in the peritoneal fluid of endometriosis patients was significantly higher than that of controls. The SLPI to elastase, a proteolytic enzyme involved in endometriosis pathogenesis, is higher in women with endometriosis. This difference may be perceived as a result of either increased macrophage numbers in the peritoneal environment of endometriosis patients or the selective expression of protease inhibitors in response to endometriosis-associated inflammation.20 This data suggested that the SLPI promoter is active in endometriosis tissue and can work as a tissue-specific promoter for targeted endometriosis gene therapy. Our results support this concept where Ad-SLPI-luc significantly increased transgene expression levels over Ad-5-luc in endometriosis cells at both 10 and 50 PFU/cell.
Heparanase is a heparan sulfate-specific glucuronidase that plays an important role in tumor cell metastasis due to its capability of cleaving heparan sulfate and degrading extracellular matrix. Heparanase has been implicated in many important physiological and pathological processes including tumor cell metastasis, angiogenesis, and leukocyte migration. Heparanase promoter is an attractive candidate for a tissue-specific promoter. It retains its fidelity in an adenovirus context and has been used as a tissue-specific promoter driving gene expression with high activity in breast cancer cells but shows low activity in normal breast cells and in the murine liver.17 Endometriosis has some characteristics that are similar to malignant tumors, such as the ability to invade and spread. Cells can obtain this characteristic by decomposing extracellular matrix through the production of enzymes, such as matrix metalloproteinases and heparanase. Moreover, heparanase, through cleaving extracellular matrix proteins, can release heparan sulfate-bound cytokines and growth factors, such as basic fibroblast growth factor, which allow proliferation and angiogenesis of endometriosis cells. Endometriosis tissues were found to express heparanase at mRNA and protein levels, and the expression level correlated with the clinical stage of endometriosis. Also, the expression level of heparanase, both the transcript and protein, was found to be significantly higher in ectopic endometrium and eutopic endometrium of endometriosis patients than eutopic control endometium. Furthermore, immunohitochemical analysis showed positive heparanase immunostaining in epithelial and stromal cells of ectopic endometrium and eutopic endometrium of endometriosis patients, whereas only epithelial cells stained positively for heparanase in control endometrium.21 The higher level and different pattern of expression of heparanase in endometriosis tissue than normal endometrium might represent a dysregulation of its gene induced by the action of pro inflammatory cytokines and prostaglandins in the peritoneal environment of endometriosis patients. Based on this data, we tested the heparanase promoter as a potential tissue-specific promoter in endometriosis cells. Reporter gene expression levels mediated by Ad-heparanase-luc in endometriosis cells were significantly higher than unmodified Ad5-CMV-luc.
Because endometriosis is typically confined within the peritoneal cavity, in a typical clinical scenario, one can envision freely administering the adenovirus vector into the peritoneal cavity as an ultrasound-guided office procedure or as an augmentation step during laparoscopy. This would allow more direct delivery of gene therapy vector to endometriosis tissues. Conversely, other unintended intraabdominal organs may also be exposed to the adenovirus and its carried transgene, which may lead to increased toxicity to these organs. Of particular concern is the liver, which is already known for its high transfectability to the adenovirus. We tested the gene transfer and expression levels of the transductionally and transcriptionally targeted adenoviruses in liver tissues using the liver tissue slice model. We found that only Ad-survivin-luc and Adheparanase-luc have significantly lower activity in liver tissue as compared with Ad5-CMV-luc.
We used the liver as representative of normal non targeted organs because it is known to sequester most of the administered adenovirus into the hepatocytes and the Kupffer cells.6 Other organs that might be involved during intaperitoneal instillation of the virus include the spleen, kidneys, intestine and bladder. A logical future step would be to study these targeted viruses in a animal model of endometriosis to detect the ones that can achieve best gene transfer into endometriosis tissues. It would then be possible to study the effect on different types of endometriosis (eg, ovarian, peritoneal) and to collect the liver in addition to other organs to detect potential adenovirus related toxicity in these organ systems. This in vivo work would be an essential step towards the ultimate goal of applying this technology in a clinical trial on humans.
Selective targeting of the adenovirus vectors provides a number of advantages. First, cell type-specific targeting will result in transgene expression only in the cell type of choice (endometriosis cells), which prevents toxicity to other organs (eg, the ovaries or liver). Second, the inflammatory and immune responses against the vector are reduced. This is thought to arise from the uptake of the virus and transgene into antigen-presenting cells, and from the virus binding to native receptors, promoting the stimulation of cytokine secretion. Third, the dose of the virus used can be potentially reduced, since transfection specificity is increased by targeting.22 This would reduce toxicity and immunological reaction against the viral vector even further. The dose reduction with targeted viruses has been shown in our study where Ad5-RGD-luc, Ad-SLPI-luc, and Ad-heparanase-luc mediated significantly higher reporter gene expression in endometriosis cells at an MOI of 10 PFU/cell as compared with untargeted wild type Ad5-CMV-luc at MOIs of 50 PFU/cell (Figure 3).
In conclusion, Ad5-RGD-luc, Ad-SLPI-luc, and Ad-heparanse-luc showed high activity in endometriosis cells. Among those candidate viruses, only Ad-heparanse-luc showed relatively lower activity in the liver tissues. Thus, Ad-heparanse-luc exhibits an “endometriosis on, liver off” phenotype and, consequently, this modified adenovirus represents a promising vector for endometriosis gene therapy in the future.
ACKNOWLEDGEMENTS
This work was supported by the following grants: DOD W81XWH-05-1-0035 and NIH R01CA083821 (D.C.); G12 RR03032 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (A.A.); and a grant from the Egyptian Mission Department (E.R.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at Society of Gynecologic Investigations 54th annual scientific meeting, Reno, Nevada, March 2007.
REFERENCES
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Ferrero S, Abbamonte LH, Anserini P, Remorgida V, Ragni N. Future perspectives in the medical treatment of endometriosis. Obstet Gynecol Surv. 2005;60:817–26. doi: 10.1097/01.ogx.0000189153.87365.dc. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: Adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–31. doi: 10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Dabrosin C, Gyorffy S, Margetts P, Ross C, Gauldie J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am J Pathol. 2002;161:909–18. doi: 10.1016/S0002-9440(10)64251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Othman E, Salama SA, Ismail N, Al-Hendy A. Gene therapy of endometriosis: adenovirus mediated expression of dominant negative estrogen receptor induces apoptosis in human endometriotic cells. Fertil Steril. 2007;88:462–71. doi: 10.1016/j.fertnstert.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Rein DT, Breidenbach M, Curiel DT. Current developments in adenovirus-based cancer gene therapy. Future Oncol. 2006;2:137–43. doi: 10.2217/14796694.2.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004;4:1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- 8.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–06. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 9.Stoff-Khalili MA, Stoff A, Rivera AA, Banerjee NS, Everts M, Young S, et al. Preclinical evaluation of transcriptional targeting strategies for carcinoma of the breast in a tissue slice model system. Breast Cancer Res. 2005;7:R1141–R1152. doi: 10.1186/bcr1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby TO, Rivera A, Rein D, Wang M, Ulasov I, Breidenbach M, et al. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res. 2004;10:8697–703. doi: 10.1158/1078-0432.CCR-04-1166. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hendy A, Magliocco AM, Al Tweigeri T, Braileanu G, Crellin N, Li H, et al. Ovarian cancer gene therapy: repeated treatment with thymidine kinase in an adenovirus vector and ganciclovir improves survival in a novel immunocompetent murine model. Am J Obstet Gynecol. 2000;182:553–59. doi: 10.1067/mob.2000.104837. [DOI] [PubMed] [Google Scholar]
- 12.Othman E, Curiel D, Al-Hendy A. Towards gene therapy of endometiriosis: targeting adenovirus to human endometriotic cells using tissue-specific promoters and fiber-modified virus. Reprod Sci. 2007;14:70A. [Google Scholar]
- 13.Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005;57:869–82. doi: 10.1016/j.addr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Everts M, Curiel DT. Transductional targeting of adenoviral cancer gene therapy. Curr Gene Ther. 2004;4:337–46. doi: 10.2174/1566523043346372. [DOI] [PubMed] [Google Scholar]
- 15.Dehari H, Ito Y, Nakamura T, Kobune M, Sasaki K, Yonekura N, et al. Enhanced antitumor effect of RGD fiber-modified adenovirus for gene therapy of oral cancer. Cancer Gene Ther. 2003;10:75–85. doi: 10.1038/sj.cgt.7700529. [DOI] [PubMed] [Google Scholar]
- 16.Puy LA, Pang C, Librach CL. Immunohistochemical analysis of alphavbeta5 and alphavbeta6 integrins in the endometrium and endometriosis. Int J Gynecol Pathol. 2002;21:167–77. doi: 10.1097/00004347-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Breidenbach M, Rein DT, Schondorf T, Khan KN, Herrmann I, Schmidt T, et al. A new targeting approach for breast cancer gene therapy using the heparanase promoter. Cancer Lett. 2006;240:114–22. doi: 10.1016/j.canlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Barker SD, Coolidge CJ, Kanerva A, Hakkarainen T, Yamamoto M, Liu B, et al. The secretory leukoprotease inhibitor (SLPI) promoter for ovarian cancer gene therapy. J Gene Med. 2003;5:300–10. doi: 10.1002/jgm.341. [DOI] [PubMed] [Google Scholar]
- 19.Suzumori N, Sato M, Yoneda T, Ozaki Y, Takagi H, Suzumori K. Expression of secretory leukocyte protease inhibitor in women with endometriosis. Fertil Steril. 1999;72:857–67. doi: 10.1016/s0015-0282(99)00381-7. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Agarwal A, Sekhon L, Krajcir N, Cocuzza M, Falcone T. Serum and peritoneal abnormalities in endometriosis: potential use as diagnostic markers. Minerva Ginecol. 2006;58:527–51. [PubMed] [Google Scholar]
- 21.Jingting C, Yangde Z, Yi Z, Mengxiong L, Rong Y, Yu Z, et al. Expression of heparanase and angiopoietin-2 in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol. doi: 10.1016/j.ejogrb.2006.09.018. In press. [DOI] [PubMed] [Google Scholar]
- 22.Schagen FH, Wensveen FM, Carette JE, Dermody TS, Gerritsen WR, van Beusechem VW. Genetic targeting of adenovirus vectors using a reovirus sigma1-based attachment protein. Mol Ther. 2006;13:997–1005. doi: 10.1016/j.ymthe.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–80. [PubMed] [Google Scholar]
- 24.Van Houdt WJ, Haviv YS, Lu B, Wang M, Rivera AA, Ulasov IV, et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg. 2006;104:583–92. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]