Abstract
Background
Cardiovascular disease is the leading cause of morbidity and mortality in patients on hemodialysis. To our knowledge, no studies have examined long-term outcomes of hemodialysis patients following coronary artery bypass grafting (CABG) in a predominately rural, low-income, and racially dichotomous population.
Methods
Long-term survival of hemodialysis patients undergoing non-emergent, isolated CABG was compared with non-hemodialysis patients. Survival probabilities were computed using the Kaplan-Meier product limit method and stratified by hemodialysis. Hazard ratios (HR) and 95% confidence intervals (95%CI) were computed using a Cox regression model.
Results
Hemodialysis patients (n=220) had shorter long-term survival than non-hemodialysis patients (median survival=3.3 versus 14 years, p<0.0001). The survival difference remained statistically significant after adjusting for clinically relevant variables (HR=5.2, 95%CI=4.4-6.2).
Conclusion
Hemodialysis patients had significantly shorter long-term survival compared with non-hemodialysis patients after CABG. Further research is needed to address the cost and policy implications of our findings, especially among priority populations.
Keywords: Hemodialysis, CABG, Survival
Introduction
The prevalence of chronic kidney disease (CKD) in the United States has steadily risen over the past 20 years due to the increasing number of obese individuals with diabetes and hypertension [1,2]. During this period, the prevalence of CKD stages 1-4 increased by 31%. Additionally, the number of individuals with end-stage renal disease (ESRD) requiring hemodialysis (HD) has increased from 209,000 to 472,000. Patientswith ESRD have a greater than 5-fold increased risk for all-cause mortality and a 3-fold increased risk for cardiovascular-related mortality [3].
Coronary artery bypass grafting (CABG) is the standard surgical approach for treatment of coronary artery disease (CAD). CKD patients undergoing CABG have worse short- and long-term outcomes postoperatively than the general population [4-11]. To our knowledge, no studies have examined long-term outcomes of HD patients following CABG in a predominately rural, low-income, and racially dichotomous population.
Materials and Methods
Patients
This was a retrospective cohort study of patients undergoing first-time, isolated CABG at the East Carolina Heart Institute between 1992 and 2011. Demographic data, comorbid conditions, CAD severity, and surgical data were collected at the time of surgery. Patients were stratified by preoperative HD status. Only black and white patients were included to minimize the potential for residual confounding (~1% other races). Racial identity was self-reported. Emergent cases were considered a clinically different population following surgery and were excluded in our analysis (n=420). The study was approved by the Institutional Review Board at the Brody School of Medicine, East Carolina University.
Definitions
Patients with CKD receiving dialysis treatment defined our HD population. Mortality was defined as any cause of death at any time after surgery. CAD was defined as at least 50% stenosis and confirmed by angiography before surgery.
Operative procedure
The left internal mammary artery was used for left anterior descending revascularization. Cardiopulmonary bypass or off-pump coronary artery bypass was selected depending upon patient presentation and surgeon preference. If cardiopulmonary bypass with cardiac standstill was achieved, cold-blood cardioplegia was used. Typically, distal anastomoses were performed first followed by proximal anastomoses. If off-pump coronary artery bypass was performed, left internal mammary artery to left anterior descending artery anastomosis was routinely performed first, followed by the remaining distal anastomoses. Proximal anastomoses of the saphenous vein conduits were sewn directly to the ascending aorta.
Setting
The East Carolina Heart Institute is a 120-bed cardiovascular hospital located in the center of eastern North Carolina, a rural region with a large black population. Cardiovascular disease is the number one cause of death in North Carolina with an unequal burden occurring in eastern North Carolina [12]. The institute is a population-based tertiary referral center. Nearly all patients treated at the East Carolina Heart Institute live and remain within a 150 mile radius of the medical center.
Data collection and follow-up
The primary sources of data extraction were the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery database and the electronic medical record at the Brody School of Medicine.
Cardiovascular surgery information at our facility has been reported to the STS since 1989. Data quality and cross-field validation are routinely performed by the Epidemiology and Outcomes Research Unit at the East Carolina Heart Institute. An electronic medical record was introduced at the Brody School of Medicine in 1997. Records from 1989 to 1997 were retrospectively scanned into the electronic medical record. Local and regional clinics were consolidated under a single electronic medical record in 2005 which allowed for efficient patient follow-up. The electronic medical record system applies multiple logic comparisons to reliably reduce mismatching of patient data across clinics and follow-up visits. The STS database is linked to the electronic medical record through a unique patient medical record number.
The National Death Index was used to obtain death dates for patients lost to follow-up and also used to validate death information captured in our electronic medical record [13-15]. Linkage with the National Death Index was based on a multiple criteria, deterministic matching algorithm [15].
Statistical analysis
Categorical variables were reported as frequency and percentage while continuous variables were reported as mean ± standard deviation, median, and range. Variables not previously categorized were divided into quartiles prior to statistical analysis. Quartile categorization is advantageous because it limits the influence of outliers and allows for the assessment of trend across categories. Follow-up time was measured from the date of surgery to the date of death or last follow-up. Survival probabilities were computed using the Kaplan-Meier product limit method and stratified by HD status. The log-rank test was used to compare survival between HD patients and non-HD patients. Cox proportional hazard regression models were used to compute hazard ratios (HR) and 95% confidence intervals (95%CI) for long-term mortality. The initial multivariable models included variables that have been previously reported to be associated with cardiovascular-related mortality, regardless of their statistical significance in our dataset. These included age, sex, race, hypertension, CAD severity, congestive heart failure (CHF), and prior stroke. The post-hoc addition of other variables into the model was performed in a pairwise fashion. The test statistic of Grambsch and Therneau was used to check the proportional hazards assumption [16]. Statistical significance for categorical variables was tested using the chi-square (×2) test and the Deuchler-Wilcoxon procedure for continuous variables. PTrend was computed using a likelihood ratio test.
Few values were missing (<1%). However, when values were missing they were entered into the regression models as a separate category. A sensitivity analysis with missing values excluded also was performed to confirm that model beta coefficients did not substantively differ from the above results.
Statistical significance was defined as p<0.05. SAS Version 9.3 (Cary, NC) was used for all analyses.
Results
A total of 13,354 patients underwent non-emergent CABG between 1992 and 2011. The majority were male (71%), white (83%) and had multi-vessel CAD (93%). Less than half were smokers (24%), diabetic (35%), or had a prior myocardial infarction (MI) (40%). The mean age was 63±10 years. Hypertension was a common diagnosis (72%) while few patients had chronic obstructive pulmonary disease (7.4%) or prior stroke (7.8%). Approximately 19% had a history of prior percutaneous coronary intervention (PCI). The median follow-up for study participants was 8.1 years.
Patient characteristics were stratified by preoperative HD (Table 1). There were 220 (2%) HD patients. Patients with HD were more likely to be younger, black, hypertensive, and diabetic. They also presented more frequently with CHF, chronic obstructive pulmonary disease, peripheral arterial disease, prior MI, and prior stroke. A higher percentage of HD patients were receiving calcium channel blockers and angiotensin converting enzyme inhibitors or angiotensin receptor blocking agents prior to surgery (Table 2).
Table 1.
Patient characteristics and survival after CABG by hemodialysis (N=13,354).
| Characteristic | Hemodialysis | No Hemodialysis | Univariable HR (95%CI) |
||
|---|---|---|---|---|---|
|
| |||||
| n (%) | 5, 10, 15 Yr Survival (%) |
n (%) | 5, 10, 15 Yr Survival (%) |
||
|
| |||||
| Overall | 223 (2) | 36, 15, 12 | 13,131 (98) | 84, 65, 47 | 5.0 (4.3-5.9) |
|
| |||||
| Age (Years) | |||||
| Q1 (≤56) | 86 (39) | 50, 19, 14 | 3,753 (29) | 93, 82, 70 | 1.0 Referent |
| Q2 (>56-64) | 74 (33) | 37, 19, § | 3,420 (26) | 88, 70, 53 | 1.7 (1.6-1.9) |
| Q3 (>64-71) | 37 (17) | 20, 8, § | 3,279 (25) | 82, 58, 36 | 2.6 (2.4-2.8) |
| Q4 (>71) | 26 (12) | 13, 6, § | 2,679 (40)†† | 71, 41, 20 | 4.2 (3.9-4.6) |
| Mean ± SD, Median (Range) | 60±9.7, 60 (35-80) | 63±10, 64 (24-94)†† | Ptrend< 0.0001 | ||
|
| |||||
| Sex | |||||
| Male | 128 (57) | 43, 12, § | 9,296 (71) | 84, 66, 48 | 1.0 Referent |
| Female | 95 (43) | 28, 19, 19 | 3,835 (29)†† | 84, 63, 44 | 1.1 (1.05-1.2) |
|
| |||||
| Race | |||||
| White | 87 (39) | 28, 21, § | 10,943 (83) | 85, 65, 47 | 1.0 Referent |
| Black | 136 (61) | 42, 9, 9 | 2,188 (17)†† | 84, 62, 46 | 1.1 (1.06-1.2) |
|
| |||||
| BMI (kg/m2)* | |||||
| Obese (≥30) | 76 (34) | 47, 22, § | 5,353 (41) | 87, 68, 50 | 1.0 Referent |
| Overweight (25-29.9) | 85 (38) | 37, 11, § | 5,224 (40) | 85, 66, 48 | 1.1 (1.03-1.2) |
| Normal (18.5-24.9) | 57 (26) | 22, 15, 10 | 2,386 (18) | 79, 56, 38 | 1.5 (1.4-1.6) |
| Underweight (< 18.5) | 5 (2) | 40, 40, § | 95 (1)†† | 63, 44, 36 | 1.9 (1.5-2.5) |
| Mean ± SD, Median (Range) | 28±5.5, 27 (17-49) | 29±5.5, 28 (13-70)†† | Ptrend< 0.0001 | ||
|
| |||||
| Status | |||||
| Stable | 92 (41) | 49, 18, § | 5,364 (41) | 86, 69, 51 | 1.0 Referent |
| Urgent | 141 (59) | 28, 13, 13 | 7,767 (59) | 83, 62, 44 | 1.2 (1.16-1.3) |
|
| |||||
| CAD Severity | |||||
| 1 Vessel | 10 (4) | 66, 25, § | 879 (7) | 91, 78, 68 | 1.0 Referent |
| 2 Vessel | 66 (30) | 45, 20, § | 3,457 (26) | 87, 68, 51 | 1.6 (1.4-1.9) |
| 3 Vessel | 147 (66) | 31, 13, 13 | 8,795 (67) | 83, 62, 43 | 2.0 (1.7-2.3) Ptrend< 0.0001 |
|
| |||||
| Left Main Disease | |||||
| No | 176 (79) | 39, 15, § | 10,428 (79) | 85, 66, 48 | 1.0 Referent |
| Yes | 47 (21) | 28, 18, 18 | 2,703 (21) | 82, 61, 42 | 1.2 (1.1-1.3) |
|
| |||||
| Recent Smoker | |||||
| No | 184 (83) | 39, 17, 16 | 9,909 (75) | 84, 64, 46 | 1.0 Referent |
| Yes | 39 (17) | 23, 5, § | 3,222 (25)† | 85, 67, 50 | 0.89 (0.83-0.96) |
|
| |||||
| Hypertension | |||||
| No | 19 (9) | 22, 7, § | 3,666 (28) | 87, 70, 51 | 1.0 Referent |
| Yes | 204 (91) | 38, 16, 14 | 9,465 (72)†† | 84, 62, 45 | 1.3 (1.2-1.35) |
|
| |||||
| Diabetes | |||||
| No | 90 (40) | 35, 9, 6 | 8,593 (65) | 86, 69, 52 | 1.0 Referent |
| Yes | 133 (60) | 37, 20, § | 4,538 (35)†† | 81, 56, 36 | 1.6 (1.5-1.7) |
|
| |||||
| Congestive Heart Failure | |||||
| No | 125 (56) | 41, 17, 11 | 11,290 (86) | 86, 67, 49 | 1.0 Referent |
| Yes | 98 (44) | 30, 13, § | 1,841 (14)†† | 72, 43, 27 | 2.1 (2.0-2.3) |
|
| |||||
| COPD | |||||
| No | 197 (88) | 38, 16, 12 | 12,170 (93) | 85, 65, 47 | 1.0 Referent |
| Yes | 26 (12) | §, §, § | 961 (7)† | 74, 53, § | 1.8 (1.6-2.1) |
|
| |||||
| Peripheral Arterial Disease | |||||
| No | 156 (70) | 40, 18, 13 | 11,633 (89) | 86, 67, 49 | 1.0 Referent |
| Yes | 67 (30) | 27, 9, § | 1,498 (11)†† | 73, 45, 28 | 2.0 (1.8-2.6) |
|
| |||||
| Prior MI | |||||
| No | 120 (54) | 41, 19, 17 | 7,925 (60) | 86, 67, 48 | 1.0 Referent |
| Yes | 103 (46) | 30, 10, § | 5,206 (40)† | 82, 61, 44 | 1.2 (1.15-1.3) |
|
| |||||
| Prior Stroke | |||||
| No | 189 (85) | 40, 18, 14 | 12,130 (92) | 85, 66, 48 | 1.0 Referent |
| Yes | 34 (15) | 13, §, § | 1,001 (8)†† | 73, 45, 25 | 2.0 (1.8-2.2) |
|
| |||||
| Prior PCI | |||||
| No | 175 (78) | 37, 14, 13 | 10,647 (81) | 84, 64, 46 | 1.0 Referent |
| Yes | 48 (22) | 33, 27, § | 2,484 (19) | 87, 69, 51 | 0.85 (0.79-0.92) |
p < 0.05
p < 0.01
Last follow-up not reached
Missing category not shown;X2 (Categorical Variables);Deuchler-Wilcoxon Test (Continuous Variables)
BMI=body mass index; CAD=coronary artery disease; CI=confidence interval; COPD=chronic obstructive pulmonary disease; HR=hazard ratio;MI=myocardial infarction; PCI=percutaneous coronary intervention; Q1=quartile 1; Q2=quartile 2; Q3=quartile 3; Q4=quartile 4.
Table 2.
Preoperative Medications among CABG patients (N=13,354).
| Medication | Hemodialysis n (%) |
No Hemodialysis n (%) |
P-value |
|---|---|---|---|
| Aspirin | 136 (61) | 9,255 (70) | 0.0021 |
| Lipid Lowering Agents | 94 (42) | 5,337 (41) | 0.65 |
| Anticoagulants | 54 (24) | 4,303 (33) | 0.0069 |
| Antiplatelet Agents | 78 (35) | 6,848 (52) | < 0.0001 |
| β-Blockers | 133 (60) | 7,409 (56) | 0.34 |
| Calcium Channel Blockers | 97 (44) | 4,022 (31) | < 0.0001 |
| Diuretics | 38 (17) | 2,829 (22) | 0.10 |
| ACE Inhibitors/ARBs | 93 (42) | 4,030 (31) | 0.0004 |
| Digitalis | 17 (8) | 837 (6) | 0.45 |
| Nitrates | 35 (16) | 2,077 (16) | 0.96 |
| Inotropic Agents | 3 (1) | 127 (< 1) | 0.57 |
ACE=angiotensin converting enzyme; ARB=angiotensin receptor blocker; CABG=coronary artery bypass grafting.
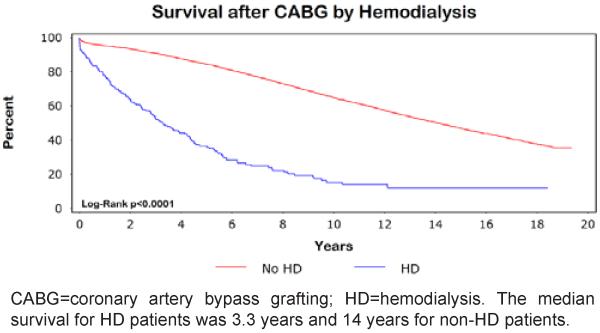
The Kaplan-Meier unadjusted survival curves were significantly different between HD and non-HD patients (Figure 1). The median survival for patients with and without HD was 3.3 years and 14 years, respectively.
Figure 1.
Kaplan-Meier survival after CABG by hemodialysis.
The unadjusted HR for HD was 5.0 (95%CI=4.3-5.9). The HR increased to 5.2 (95%CI=4.4-6.2) after adjusting for age, sex, race, hypertension, CAD severity, CHF, and prior stroke (Table 3). The multivariable results did not substantively change with the pairwise addition of other variables listed in Table 1.
Table 3.
Multivariable proportional hazards model.
| Characteristic | Adjusted HR (95%CI) |
|---|---|
|
| |
| Hemodialysis | |
| No | 1.0 Referent |
| Yes | 5.2 (4.4-6.2) |
|
| |
| Age (Years) | |
| Q1 (≤56) | 1.0 Referent |
| Q2 (>56-64) | 1.7 (1.5-1.8) |
| Q3 (>64-71) | 2.5 (2.3-2.8) |
| Q4 (>71) | 4.0 (3.7-4.4) PTrend< 0.0001 |
|
| |
| Sex | |
| Male | 1.0 Referent |
| Female | 0.92 (0.86-0.97) |
|
| |
| Race | |
| White | 1.0 Referent |
| Black | 1.0 (0.97-1.1) |
|
| |
| CAD Severity | |
| 1 Vessel | 1.0 Referent |
| 2 Vessel | 1.4 (1.2-1.6) |
| 3 Vessel | 1.6 (1.4-1.9) PTrend< 0.0001 |
|
| |
| Hypertension | |
| No | 1.0 Referent |
| Yes | 1.1 (1.05-1.2) |
|
| |
| Congestive Heart Failure | |
| No | 1.0 Referent |
| Yes | 1.8 (1.6-1.9) |
|
| |
| Prior Stroke | |
| No | 1.0 Referent |
| Yes | 1.6 (1.5-1.8) |
CAD=coronary artery disease; CI=confidence interval; HR=hazard ratio; Q1=quartile 1; Q2=quartile 2; Q3=quartile 3; Q4=quartile4.
Discussion
HD patients undergoing CABG generally have unfavorable outcomes in the perioperative, in-hospital setting compared with non-HD patients [4-6,11]. However, little is known about long-term survival after CABG in this population [6,11]. The current study represents a tertiary referral heart institute’s examination of long-term (20 years) survival of HD patients after CABG among a rural, low-income, and racially dichotomous population. Furthermore, it builds upon previous studies of shorter duration showing that HD is associated with decreased survival after CABG [5,6,11].
Our study is unique because of its priority target population which has historically experienced discrimination with respect to cardiac surgery [17]. Twenty-eight (97%) of the 29 counties in eastern North Carolina fall below the national per capita income of $27,915, with half reporting a value less than $20,000 [18]. Similarly, 90% of the counties have a higher percentage of blacks than the national value of 13.1% [18].
A recent 10-year study examining the influence of renal dysfunction on long-term survival after CABG reported an adjusted HR=11.6 (95%CI=9.62-13.9) for HD patients compared with HR=5.2 (95%CI=2.5-4.1) in the current analysis [6]. Differences likely were due to the inclusion of emergent cases and a higher percentage of previous MI (63.4% versus 46%) and prior stroke (22.1% versus 15%) in their HD population. Similarly, a 5-year regional cohort study of 15,574 consecutive patients undergoing CABG in northern New England (Maine, New Hampshire, Vermont, and Massachusetts) reported an increased risk of mortality (HR=3.2, 95%CI=2.5-4.1) [11]. In contrast, our HD patients had an increased prevalence of diabetes (60% versus 42.3%), which may account for the observed difference. Although race was not reported in this study, presumably their black population was lower than our study based on Census data [18].
The decreased survival observed among HD patients possibly is explained by differences in preoperative comorbid conditions. HD patients also have a greater prevalence of cardiovascular disease (CVD) risk factors leading to CAD [3]. Furthermore, HD patients have been shown to have more complex and diffuse CAD [19]. This reflects a population with advanced atherosclerotic disease at the time of surgery.
The type of revascularization procedure has been shown in some studies to influence the survival of ESRD and HD patients [20-22]. However, the comparative disadvantage of PCI versus CABG has been small (e.g., HR < 1.2). Our results (HR=5.2) highlight the detrimental impact of HD on survival after CABG regardless of revascularization method.
Strengths and limitations
Our study is strengthened by its large sample size and long-term follow-up. Furthermore, we were able to accurately determine time of death using a combination of the National Death Index and our comprehensive electronic medical record. Furthermore, this study is generalizable to other low-income, rural, and racially diverse populations, a group that has not been extensively studied in previous reports examining survival among HD patients after CABG.
Similar to any natural history study in which the comparison groups were not randomly assigned, there may be residual confounding in our models even though we adjusted for a large number of covariates. Additionally, we acknowledge that other unmeasured factors could have influenced our results.
HD duration was not collected in our data set and could have influenced our findings [23-25]. Mortality may have been unrelated to HD status. Cause of death is not recorded in the National Death Index and death certificate information was not collected in this study. Death information was limited by a 2-year lag between data collection and data availability in the National Death Index [15].
Patients in this study were recruited over a relatively long period (20 years), over which practice methods and clinical care may have changed considerably. However, results were consistent throughout the study after stratifying by 3 time periods, indicating the robustness of the data to temporal changes. Also, the status of several variables in our analysis may have changed over time. We did not adjust for these variables in a time-dependent manner due to their potential to be in the causal pathway. Similarly, postoperative complications were not included in our analysis because of their time-dependent status. While our Cox proportional hazard model diverged from the proportional hazards assumption, there were no interactions over time and results remained clinically interpretable in terms of the average relative hazard over the observation period.
We considered missing values to be a distinct category and they were entered into the regression models as a separate category rather than being imputed. Imputation methods require data to be “missing at random” which is difficult to verify given the sparseness and unknown distribution of the missing values [26,27]. However, we cannot rule out misclassification bias due to grouping missing values into a distinct category. Our use of quartile boundaries, while desirable for minimizing the influence of outliers, may have yielded overly broad categories and the potential for residual confounding. However, the substitution of continuous variables in our models did not materially alter results. Additionally, multivariable Cox regression models, rather than propensity score matching, were used to control for confounding because of potential “non-collapsibility bias” inherent to logistic regression-based propensity scores [28].
Implications
The results of this study have some intriguing implications for healthcare reform and policy aimed at preventing ESRD and the need for HD, especially among patients with concomitant CAD and those living in rural, low-income, and racially diverse areas. The United States vastly outspends virtually all other developed countries on healthcare yet continues to rank near the bottom for life expectancy [29]. A key strategy for containing healthcare expenditures will be identifying diseases and treatment scenarios that account for excessive charges and implementing targeted measures to effectively reduce costs. The median survival of HD patients in our study receiving CABG was significantly shorter than those not receiving HD (3.3 versus 14 years), suggesting a greater force of mortality among HD patients. Approximately 33% of deaths in the United States are attributable to CVD and mortality is especially high among the subset of patients on HD who receive CABG, as illustrated in our results [30].The estimated direct cost of CVD in the United States at the beginning of the study period was ~$209 billion, with the in-hospital cost for CABG averaging over $20,000 per surgery [31]. Although a cost-effectiveness analysis was beyond the scope of the current study, future efforts should be directed at assessing the cost of life-course wellness programs in light of quality years of life and the benefit, or lack thereof, for HD patients receiving CABG.
Our data further suggests the need for debate at the legislative level regarding the reimbursement for CABG among HD patients. Options range from the complete denial of benefits to increasing payments to cover the extra intra- and post-operative costs incurred by HD patients undergoing CABG. Additional study and data analysis will be required to gauge the impact and cost-effectiveness of incentives or other health promotion strategies to minimize patient acuity at the time of surgery. Policy discussions in this area are complex. Optimally, discourse will encompass the careful integration of individual and public health perspectives prior to implementing sweeping changes with unintended consequences.
Lifestyle modification and pharmaceutical intervention following the diagnosis of ESRD and CAD typically do not reverse the damage accumulated over the lifetime of these patients [32]. Healthcare costs generally are highest in the year before death, accounting for up to 31% of total Medicare expenditures [32]. Having a favorable cardiovascular risk profile earlier in life has been associated with lower costs at the end of life [32]. Reducing costs for end-of-life care is an important goal of healthcare reform and will have direct policy implications for HD patients receiving CABG. Targeted measures will need to focus on primary prevention during youth to promote an enduring healthful lifestyle (improved diet, routine exercise, and abstinence of tobacco use).
Reducing and removing barriers to health through lifestyle interventions, and linking health policy innovations with practice changes, poses tremendous challenges as we move forward to reform healthcare in the United States. A disproportionate burden of illness continues to be borne by the poor and racial and ethnic minorities. This is implicitly seen in the study at hand in which approximately 61% of HD patients undergoing CABG were black, compared with 17% in non-HD patients, and the majority of white HD patients undergoing CABG were low income. In many regions of the country, similar to eastern North Carolina, the financial well-being of the healthcare system is characterized by unsustainable costs, an ageing population, increasing economic disparities, and an insatiable hunger for healthcare services.
The intersection of genes and lifestyle, in the context of predicting who ultimately benefits from CABG, represents a dynamic opportunity to tailor future treatment options at the individual level for HD patients. Certainly, some patients will fare better than others and potentially knowing (probabilistically) in advance which HD patients are not good candidates for CABG will be a significant step forward in successfully managing their healthcare and minimizing unnecessary and costly surgery.
A cost-benefit approach to treatment allocation structured on evidence-based research and outcome measures ultimately is the path of the future if healthcare reform is to be successful. Monies will need to be allocated to those interventions and treatments that achieve the greatest health impact. This will translate into making difficult but necessary health policy decisions, especially for HD patients in need of CABG. Other strategies may include the use of financial incentives directed toward physicians to improve patient compliance (pay-for-outcomes models). Adopting the use of ethical consultations for patients with critical care needs in which care is not beneficial or disproportionate to cost, must be carefully weighed against our moral obligations as a civilized nation [29].
The findings of this study also have important implications for patient risk stratification. To be effective, risk stratification models must reflect the underlying community from whom it was derived and will be applied [33]. The current study has identified several factors important for predicting long-term survival in this population (age, CAD, CHF, and stroke) and sets the foundation for future risk stratification models. Countering beliefs of therapeutic nihilism, risk stratification models may have potential worth for targeted subsets of lower risk patients who might benefit from cardiac surgery and subsequent rehabilitation. In a study of outpatient claims, HD patients who received cardiac rehabilitation after CABG had a 35% reduced risk for all-cause mortality [34]. The importance of the latter in the context of healthcare reform is further highlighted by the fact that cardiac rehabilitation is a covered expense under Medicare for HD patients who have undergone CABG.
Conclusions
In our rural, low-income, and racially dichotomous population, HD patients undergoing CABG had significantly shorter long-term survival compared with non-HD patients. This provides useful outcome information for surgeons, policy makers, and patients. Further research is needed to evaluate the cost-effectiveness of CABG in HD patients and the utility of risk stratification models.
Acknowledgements
We would like to thank Katherine Jones, PhD (Center for Health Systems Research and Development at East Carolina University) for editorial help and research assistance. Also, special thanks to Lloyd Novick, MD (Department of Public Health, Brody School of Medicine) for technical advice and encouragement in the writing of this manuscript. Dr. Efird is supported in part by NIH Grant 5R34DE022272-02.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Charytan DM, Kuntz RE. Risks of coronary artery bypass surgery in dialysis-dependent patients--analysis of the 2001 National Inpatient Sample. Nephrol Dial Transplant. 2007;22:1665–1671. doi: 10.1093/ndt/gfl835. [DOI] [PubMed] [Google Scholar]
- 5.Liu JY, Birkmeyer NJ, Sanders JH, Morton JR, Henriques HF, et al. Northern New England Cardiovascular Disease Study Group Risks of morbidity and mortality in dialysis patients undergoing coronary artery bypass surgery. Circulation. 2000;102:2973–2977. doi: 10.1161/01.cir.102.24.2973. [DOI] [PubMed] [Google Scholar]
- 6.Boulton BJ, Kilgo P, Guyton RA, Puskas JD, Lattouf OM, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg. 2011;92:595–601. doi: 10.1016/j.athoracsur.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Zheng Z, Li Y, Yuan X, Hou J, et al. Impact of renal dysfunction on long-term survival after isolated coronary artery bypass surgery. Ann Thorac Surg. 2009;87:1079–1084. doi: 10.1016/j.athoracsur.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 8.Holzmann MJ, Hammar N, Ahnve S, Nordqvist T, Pehrsson K, et al. Renal insufficiency and long-term mortality and incidence of myocardial infarction in patients undergoing coronary artery bypass grafting. Eur Heart J. 2007;28:865–871. doi: 10.1093/eurheartj/ehl508. [DOI] [PubMed] [Google Scholar]
- 9.Chonchol MB, Aboyans V, Lacroix P, Smits G, Berl T, et al. Long-term outcomes after coronary artery bypass grafting: preoperative kidney function is prognostic. J Thorac Cardiovasc Surg. 2007;134:683–689. doi: 10.1016/j.jtcvs.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 10.van de Wal RM, van Brussel BL, Voors AA, Smilde TD, Kelder JC, et al. Mild preoperative renal dysfunction as a predictor of long-term clinical outcome after coronary bypass surgery. J Thorac Cardiovasc Surg. 2005;129:330–335. doi: 10.1016/j.jtcvs.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Dacey LJ, Liu JY, Braxton JH, Weintraub RM, DeSimone J, et al. Long-term survival of dialysis patients after coronary bypass grafting. Ann Thorac Surg. 2002;74:458–462. doi: 10.1016/s0003-4975(02)03768-2. [DOI] [PubMed] [Google Scholar]
- 12.Morris PJ. Heart Disease and Stroke in North Carolina NC Med J. 2012;73:448–449. [PubMed] [Google Scholar]
- 13.Welke KF, Ferguson TB, Jr, Coombs LP, Dokholyan RS, Murray CJ, et al. Validity of the Society of Thoracic Surgeons National Adult Cardiac Surgery Database. Ann Thorac Surg. 2004;77:1137–1139. doi: 10.1016/j.athoracsur.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology. 2001;12:259–261. doi: 10.1097/00001648-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Morales DL, McClellan AJ, Jacobs JP. Empowering a database with national long-term data about mortality: the use of national death registries. Cardiol Young. 2008;18(Suppl 2):188–195. doi: 10.1017/S1047951108002916. [DOI] [PubMed] [Google Scholar]
- 16.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 17.Hannan EL, van Ryn M, Burke J, Stone D, Kumar D, et al. Access to coronary artery bypass surgery by race/ethnicity and gender among patients who are appropriate for surgery. Med Care. 1999;37:68–77. doi: 10.1097/00005650-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Bureau USC . US Census Bureau State & County Quick Facts. 2010. [Google Scholar]
- 19.Gruberg L, Rai P, Mintz GS, Canos D, Pinnow E, et al. Impact of renal function on coronary plaque morphology and morphometry in patients with chronic renal insufficiency as determined by intravascular ultrasound volumetric analysis. Am J Cardiol. 2005;96:892–896. doi: 10.1016/j.amjcard.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 20.Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation. 2002;106:2207–2211. doi: 10.1161/01.cir.0000035248.71165.eb. [DOI] [PubMed] [Google Scholar]
- 21.Herzog CA, Ma JZ, Collins AJ. Long-term outcome of dialysis patients in the United States with coronary revascularization procedures. Kidney Int. 1999;56:324–332. doi: 10.1046/j.1523-1755.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–2049. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI. Revisiting survival differences by race and ethnicity among hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol. 2006;17:2910–2918. doi: 10.1681/ASN.2005101078. [DOI] [PubMed] [Google Scholar]
- 24.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55:100–112. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen WF, Jr., Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. JAMA. 1998;280:1764–1768. doi: 10.1001/jama.280.20.1764. [DOI] [PubMed] [Google Scholar]
- 26.Heitjan DF. Incomplete data: what you don’t know might hurt you. Cancer Epidemiol Biomarkers Prev. 2011;20:1567–1570. doi: 10.1158/1055-9965.EPI-11-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 28.Efird JT, Lea S, Toland A, Phillips CJ. Informational odds ratio: a useful measure of epidemiologic association in environment exposure studies. Environ Health Insights. 2012;6:17–25. doi: 10.4137/EHI.S9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz MH. Decreasing hospital costs while maintaining quality: can it be done? Arch Intern Med. 2010;170:317–318. doi: 10.1001/archinternmed.2009.519. [DOI] [PubMed] [Google Scholar]
- 30.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg MJ, Filion KB, Azoulay A, Brox AC, Haider S, et al. Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165:1506–1513. doi: 10.1001/archinte.165.13.1506. [DOI] [PubMed] [Google Scholar]
- 32.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, et al. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Arch Intern Med. 2005;165:1028–1034. doi: 10.1001/archinte.165.9.1028. [DOI] [PubMed] [Google Scholar]
- 33.Berger AK, Herzog CA. CABG in CKD: untangling the letters of risk. Nephrol Dial Transplant. 2010;25:3477–3479. doi: 10.1093/ndt/gfq536. [DOI] [PubMed] [Google Scholar]
- 34.Kutner NG, Zhang R, Huang Y, Herzog CA. Cardiac rehabilitation and survival of dialysis patients after coronary bypass. J Am Soc Nephrol. 2006;17:1175–1180. doi: 10.1681/ASN.2005101027. [DOI] [PubMed] [Google Scholar]