Summary
The freerunning period (τ) of the circadian pacemaker underlying the wheel-running activity rhythm of Mus musculus was found to be unaffected by the periods of environmental cycles (maternal and light/dark) under which the mice are raised. Mice born to mothers entrained to periods (T) of 28 or 20 h (ratio of light to dark of 14/10) and maintained on those cycle until beyond puberty showed only a temporary difference in freerunning period when placed into constant darkness. Such temporary ‘after-effects ‘ of entrainment were shown, as had been previously, to occur in animals exposed to non-24-h cycles as adults only.
After-effects on the ratio of activity to rest (α/ρ) were not even temporarily different in animals raised on T = 28 or T = 20.
Rearing on T = 28 or T = 20 did not affect the abilities of animals to entrain to these cycles later in life.
Measurements from young and old animals as well as remeasurement of the young animals later in their lives revealed several effects of age on the pacemaker: a) After-effects on freerunning period after T = 28 or T = 20 are not greater but last longer in older animals; b) Freerunning period is shorter in younger animals; and c) The ratio of activity to rest changes over time in constant darkness and is greater in young animals. Together these suggest that pacemaker ‘plasticity’ reflected in changes in τ and α/ρ over time in constant darkness decreases with age.
The length of gestation measured in ‘real’ time was the same in mice entrained to T = 28 or T = 20, demonstrating that gestation is not measured in circadian cycles.
Introduction
The innateness of circadian rhythmicity emerged as a principal question in the field of circadian rhythms as early as the field was recognizable as such. Early experiments with fruitflies (Pittendrigh 1954), lizards (Hoffmann 1959), chickens (Aschoff and Meyer-Lohmann 1954), mice (Aschoff 1960) and rats (Browman 1952) reared under constant lighting conditions, indicate that animals do not need to experience light/dark cycles during development in order to possess circadian rhythmicity as adults. More recently, this has been found to be the case for genetically blind rats (Richter 1971) and for a human, blind since birth (Miles et al. 1977).
Circadian rhythmicity is firmly established as an inherent property of biological organization. However, the question of the innateness of circadian rhythmicity and its underlying mechanisms encompasses more than simply the inherent presence or absence of rhythmicity. A circadian pacemaker such as that which underlies the wheel-running activity of nocturnal rodents possesses specific properties (e.g., its freerunning period) of presumed functional and adaptive significance relating the pacemaker’s interaction with the environment (Pittendrigh and Daan 1976b, c). Whether or not such properties are as inherent as the rhythmicity itself or are in any way shaped by the environment during development has yet to be determined. Aschoff (1960) found that the freerunning period of the mouse activity rhythm does not change over several generations in constant light. These animals were not, however, directly compared to animals raised otherwise. Hoffmann (1957) raised lizards on 18- or 36-h days and found no effect on freerunning period in the adult. It was not clear, however, if the animals entrained to these cycles, a condition which could well be a prerequisite for the cycles to have a developmental effect. There have been recent suggestions that raising rodents on non-24-h cycles may affect their freerunning period (Brown 1974) or ability to entrain (Lanman and Seidman 1977) later in life.
Entraining light cycles have well known effects (called after-effects) on freerunning periods of adult rodent activity rhythms measured in constant darkness following their entrainment (Pittendrigh 1960; Pittendrigh and Daan 1976a). For example, an entraining cycle with a period greater than 24 h will tend to lengthen the subsequent freerunning period relative to that following entrainment to a cycle with a period less than 24 h. Such after-effects can be very long lasting (as much as 100 days) but appear to be temporary (Pittendrigh and Daan 1976a). The extent to which non-24-h cycles can change the period of the pacemaker and the rate at which such changes decay in constant conditions, both measures of pacemaker ‘plasticity’, have only been studied in adult animals.
Work over the last decade has identified the suprachiasmatic nuclei of the hypothalamus as critical components in the neural mechanisms underlying circadian rhythmicity of rodents (for reviews, see Rusak and Zucker 1979; Moore 1978; Rusak 1979). The suprachiasmatic nuclei receive direct retinal input via the retinohypothalamic tract (Moore 1973). During development, fibers of this tract enter the suprachiasmatic nuclei between postnatal day three and seven in the rat (Stanfield and Cowan 1976; Mason et al. 1977; Lenn et al. 1977; Güldner 1978) and may, by morphological criteria, be functional in the second postnatal week (Lenn et al. 1977). Plasticity in the tract’s projection has been seen following unilateral enucleation in developing, but not in adult rats (Stanfield and Cowan 1976). Coincident with the development of retinal afferents is a diversification of synaptic types within the nuclei, followed by quantitative changes in synapses and neuropil that may continue up to five weeks of age (Lenn et al. 1977). Although the precise role of the suprachiasmatic nuclei in circadian rhythmicity is as yet unknown, its recognized importance and the above findings lend support to both the notion that pacemaker properties may depend on interactions among a population of neurons, and that this area important to the generation of circadian rhythms may be subject to environmental input during its development. The later possibility is supported by the recent findings of Fuchs and Moore (1980) that the suprachiasmatic nuclei are responsive to light as early as postnatal day 1 as measured by an increase in the uptake of 2-deoxy(l-14C) glucose.
We undertook to determine if there were differential effects on the circadian system of entrainment to non-24-h light/dark cycles (T = 28 and T = 20) during development. Mice were used in this study because of their wide range of entrainment. During the course of the study, which lasted more than a year, we also observed changes which occur in circadian rhythmicity over the life of the organism.
Methods
Female mice (Mus musculus, C57BL/st) obtained from Charles River Breeding Colony, Wilmington, Mass, were paired at 12.5 weeks of age with males (15 weeks of age) born in our laboratory to mice originally obtained from Charles River. These matings produced the experimental progeny of this investigation.
Before mating, the parents, initially maintained on L : D 14 : 10, were entrained to one of two different non-24-h light/dark cycles, L : D 16.3: 11.8 (period, T = 28.1 h, L/D = 1.38) or L : D 11.6 : 8.4 (T = 20.0 h, L/D=1.39). The probability of entrainment to these cycles was maximized by the gradual increase and decrease of cycles with initial periods of approximately 25 and 23 h over a period of four weeks (see Fig. 1b). Mothers were maintained on these cycles during mating, throughout gestation and lactation, and for approximately three weeks after the litters were weaned. The fathers were also on the cycles during this time, for a total of 14 weeks.
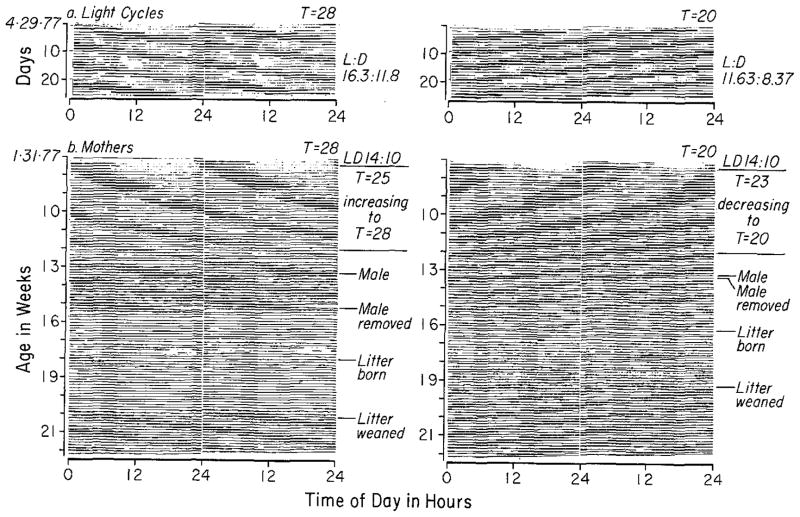
Fig. 1.
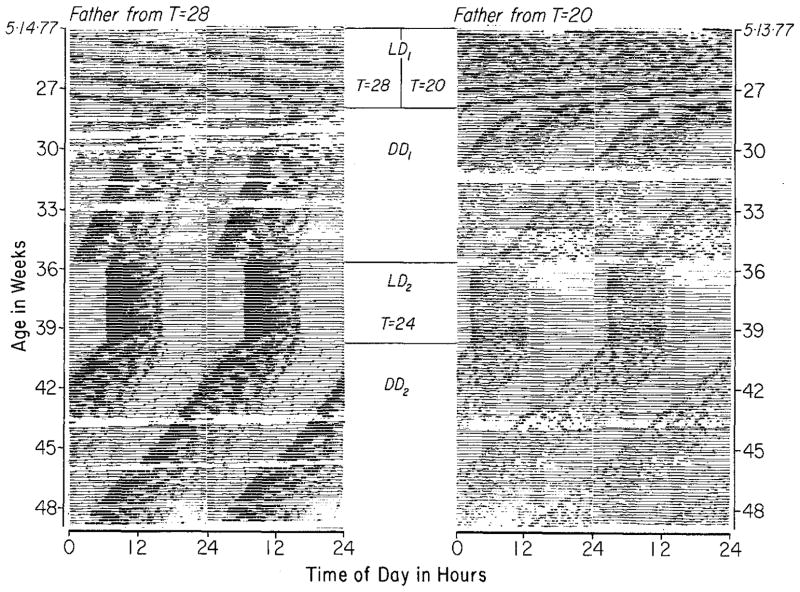
a Records of the non-24-h light/dark cycles to which mothers, fathers, and their progeny were exposed (LD1 of Figs 2 and 6). Records are double-plotted, dark bars indicate times when lights were off. b Activity records of two mothers, one entrained to T = 28 and one to T = 20. A male was with each of them during the days indicated, and all pups were taken from each mother at the time of weaning. Both records are double-plotted
Ten females (mothers) were kept in each light cycle and were each mated to a separate male. Males were left with the females until a vaginal plug was seen or until the females were clearly pregnant or clearly never going to be so. All males were removed by two weeks after their introduction. Mothers were individually housed (except during mating) in polypropylene cages (18×27× 20 cm) each with a running wheel (17 cm in diameter) and with food and water continuously available. Their wheel-running activity was recorded by an Esterline-Angus event recorder in order to confirm that they were each entrained to the non-24-h cycles. Each turn of a running-wheel resulted in two pen deflections on recording paper moving at 3/4″ per h. Each animal’s record was cut into 24-h segments and displayed as shown in Fig. 1b.
All animals, parental mice as well as their progeny, were kept in light-tight boxes (five running-wheel cages per box) with forced ventilation and a single 40 W cool white fluorescent bulb (2,000 lux at cage top). A single ‘Flexopulse’ clock (Gulf and Western Industries, Inc.) controlled each light cycle.
The litters were weaned, weighed, and grouped by litter when they were three weeks of age. At five weeks the sexes were separated in each litter. At approximately seven weeks of age, 20 progeny (15 males and 5 females) from each lighting condition (T = 20 and T = 28) were placed in running-wheel cages, still maintained on their respective cycles. Activity was recorded and displayed as previously described and as shown in Fig. 2. Progeny were obtained from six different litters in the case of T = 28 and five different litters for T = 20. At ten weeks of age the animals were transferred from the non-24- h cycles to constant darkness (DD).
Fig. 2.
Activity records of two mice raised on T = 28 and two mice raised on T = 20. Except for the changes in lighting conditions indicated and for food and water replenishment, the animals were left undisturbed for the duration of the records. Constant darkness indicated by DD; the T = 28, T = 20, and T = 24 cycles are described in the text. All records are double-plotted
The fathers, which had been on the experimental cycles for ten weeks, were also placed in running-wheel cages and maintained on the cycles for an additional four weeks before release into DD on the same day as were the experimental progeny. Subsequent treatment of the fathers as well as the progeny will be described where appropriate.
The measurements of freerunning period (τ) and the ratio of activity time to rest time (α/ρ), when the animals are in DD, were accomplished using eye-fit lines in a manner described by Davis and Menaker (1980). During entrainment to T = 28 and T = 20, α/ρ and the phase relationship between activity onset and lights off was determined for the progeny (see Table 2). Three consecutive α’s and three consecutive ρ’s were measured for each animal, and the mean α and mean ρ were used to determine the α/ρ for an individual. Phase relationship was estimated by two methods: 1) four consecutive onsets were picked, subtracted from the time of lights off, and averaged for each animal; and 2) onset for each animal on the day of the last lights off was determined by a projection back to that day by an eye-fit line through activity onsets of the following constant darkness. A negative phase relationship value indicates that activity begins after lights off. Similar phase relationship measurements were obtained for the fathers and the progeny during entrainment to T = 24 (Table 3). Where sample sizes are smaller than the number of animals that entered the experiment (i.e., 20 progeny from each cycle), animals with enough data missing to preclude a particular measurement have been excluded.
Table 2.
Activity/rest ratio (α/ρ) during entrainment to T = 28 and T = 20, and the phase relationship (Ψ) of activity onset to lights off (mean ± sem)
| α/ρ | Ψa | Ψb | |
|---|---|---|---|
| T = 28 | 1.842 ± 182* (n = 19) | 7.83 ± 0.406 (n =16) | 7.51 ± 0.624 (n = 16) |
| T = 20 | 2.507 ± 0.278 (n = 19) | −0.24 ± 0.085 (n = 19) | 1.11 ± 0.220 (n = 19) |
0.10 > P > 0.05 vs T = 20, Students t-test
Determined from onsets during entrainment
Determined from phase in constant darkness following entrainment
Table 3.
Phase relationship (Ψ) of activity onset to lights off on T = 24 (mean ± sem) and the direction of phase-shifts to entrain
| Animals raised on T = 28 and T = 20
|
Exposed to T = 28 and T = 20 as adults only
|
|||
|---|---|---|---|---|
| T = 28 | T = 20 | T = 28 | T = 20 | |
| Ψa | −0.13 ± 0.03 * (n = 15) | −0.26 ± 0.03 (n = 15) | −0.07 ± 0.09 (n = 9) | −0.15 ± 0.08 (n = 5) |
| Ψb | −0.25 ± 0.09 * (n = 15) | 0.17 ± 0.15 (n = 15) | 0.21 ± 0.20 (n = 9) | 0.03 ± 0.13 (n = 5) |
| Phase-shift (% advancing) | 50 (n = 16) | 53 (n = 17) | 29 (n = 7) | 50 (n = 6) |
P <0.05 vs T = 20, Student’s t-test
Determined from five onsets per animal during entrainment to T = 24(h)
Determined from phase in constant darkness following entrainment to T = 24 (h)
Figures 3, 4, 6, 7, 8 and 9 contain measurements of τ and α/ρ from mice in constant darkness following entrainment to light/dark cycles. A two factor, repeated measures analysis of variance (ANOVA) was performed on the data shown in Figs. 3, 4, 6 and 7 (previous LD cycle × time in constant darkness). A three factor, repeated measures ANOVA (LD cycle on which raised × LD cycle to which last entrained × time in constant darkness) was performed on the data shown in Fig. 8, and four factor, repeated measures ANOVA (entrainment to T = 28 × entrainment to T = 20×age×time in constant darkness) was performed on the data shown in Fig. 9. In some cases where interaction effects were found to be significant, groups of particular interest are compared using a Student’s t-test. If not given in the text, the findings of statistical analyses are included in the appropriate figure legends.
Fig. 3.
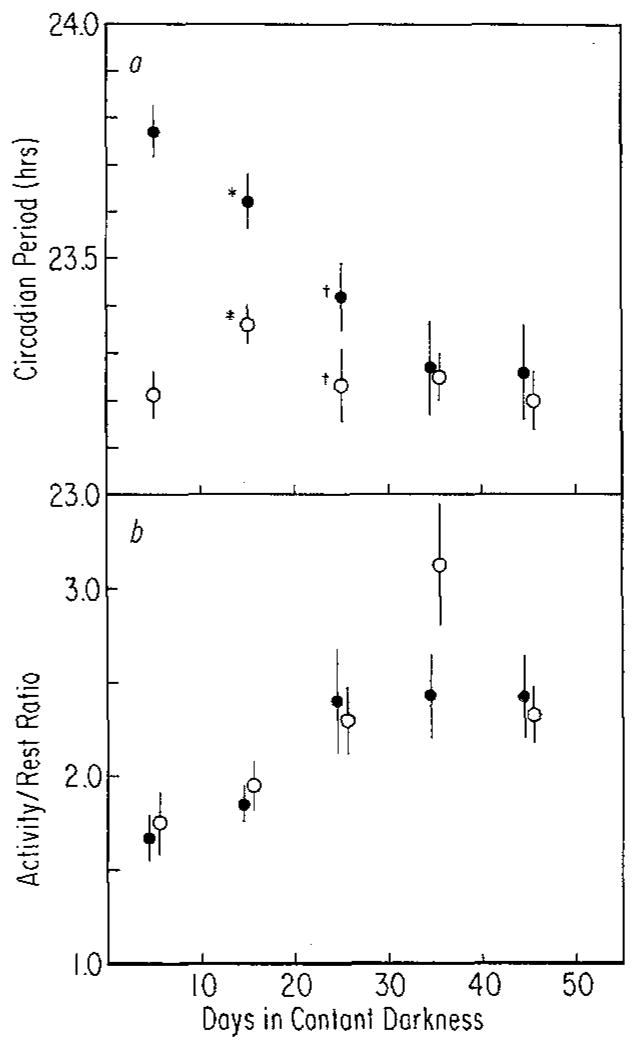
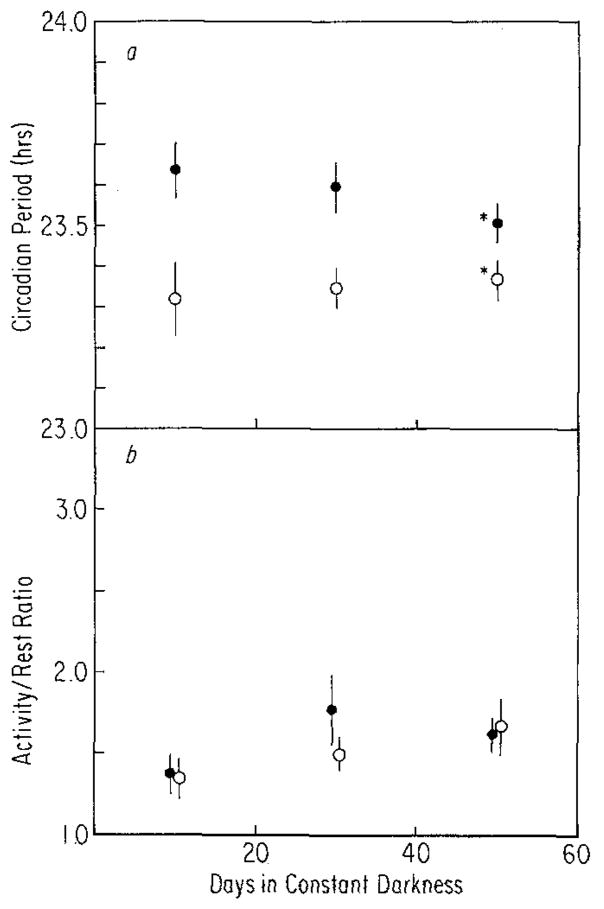
a After-effects on period (τ) measured over 10-day intervals for 50 days in constant darkness in mice raised on T = 28 (●, n = 17) or T = 20 (○, n = 16). Vertical lines: standard errors of the means (SEM). Both main effects (T and time in constant darkness) and their interaction were significant (P <0.01) by ANOVA (*P <0.01, †0.01 > P >0.05, t-test), b After-effects on activity/rest ratio (α/ρ) measured over 10-day intervals for 50 days in constant darkness in mice raised on T = 28 (●, n = 16) or T = 20 (○, n = 16). Vertical lines: SEM. Only the main effect of time in constant darkness was significant (P <0.001)
Fig. 4.
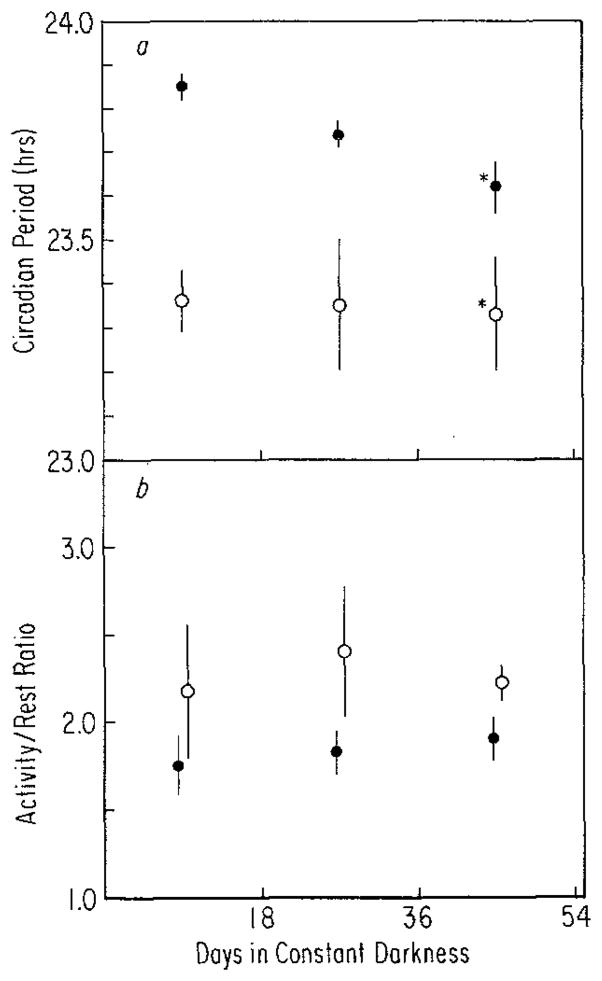
a After-effects on period (τ) measured over 18-day intervals for 54 days in constant darkness in mice (the fathers) exposed to T = 28 (●, n = 8) or T = 20 (○, n = 5) as adults. The large measurement intervals (compared to Fig. 3) were used because several records were too sloppy immediately following release into constant darkness to obtain reliable period estimates with smaller intervals. Vertical lines: SEM. The main effects of T (P <0.001) and time in constant darkness (P <0.025) were significant by ANOVA (*0.01 > P >0.05, t-test). b After-effects on activity/rest ratio (α/ρ) measured over 18-day intervals for 54 days in constant darkness in mice (the fathers) exposed to T = 28 (●, n = 8) or T = 20 (○, n = 5) as adults. Vertical lines: SEM. In neither group does α/ρ change with time. Neither main effects or their interaction were significant by ANOVA
Fig. 6.
a After-effects on period (τ) measured over 20-day intervals for 80 days in constant darkness following entrainment to T = 24 in mice raised on T = 28 (●, n = 17) or T = 20 (○, n = 17). Vertical lines SEM. Both main effects (T and time in constant darkness) and their interaction were significant (P <0.05) by ANOVA (*0.10 > P > 0.05, t-test). b After-effects on activity/rest ratio (α/ρ) measured over 20-day intervals for 80 days in constant darkness following entrainment to T = 24 in animals raised on T = 28 (●, n = 17) or T = 20 (○, n = 17). Vertical lines: SEM. The main effect of T was not significant (P >0.05) although the effects of time in constant darkness and its interaction with T were significant (P <0.01) by ANOVA (*P <0.05, t-test)
Fig. 7.
a After-effects on period (τ) measured over 20-day intervals for 60 days in constant darkness following entrainment to T = 24 in mice (the fathers) exposed to T = 28 (●, n = 9) or T = 20 (○, n = 5) as adults. Vertical lines: SEM. Main effect of T and its interaction with the main effect of time in constant darkness were significant (P <0.05) by ANOVA (*0.01 > P >0.05, t-test), b After effect on activity/rest ratio (α/ρ) measured over 20-day intervals for 60 days in constant darkness following entrainment to T = 24 in mice (the fathers) exposed to T = 28 (●, n = 7) or T = 20 (○, n = 4) as adults. Vertical lines: SEM
Fig. 8.
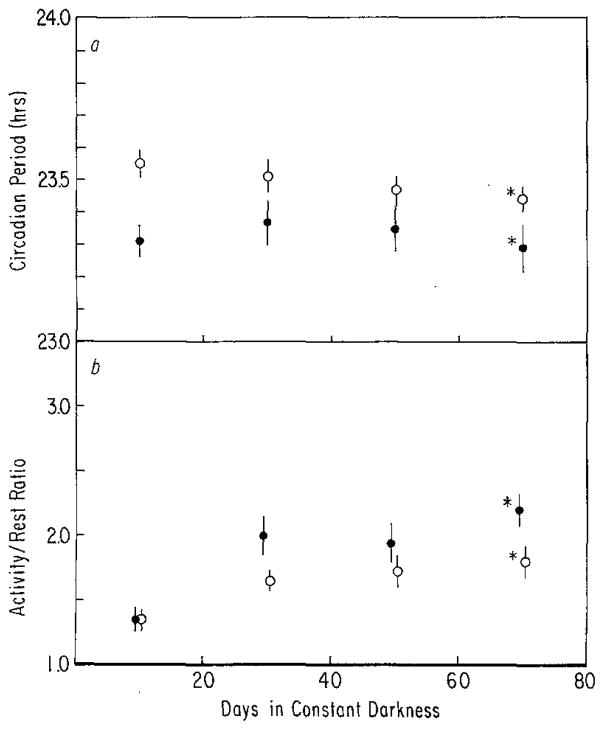
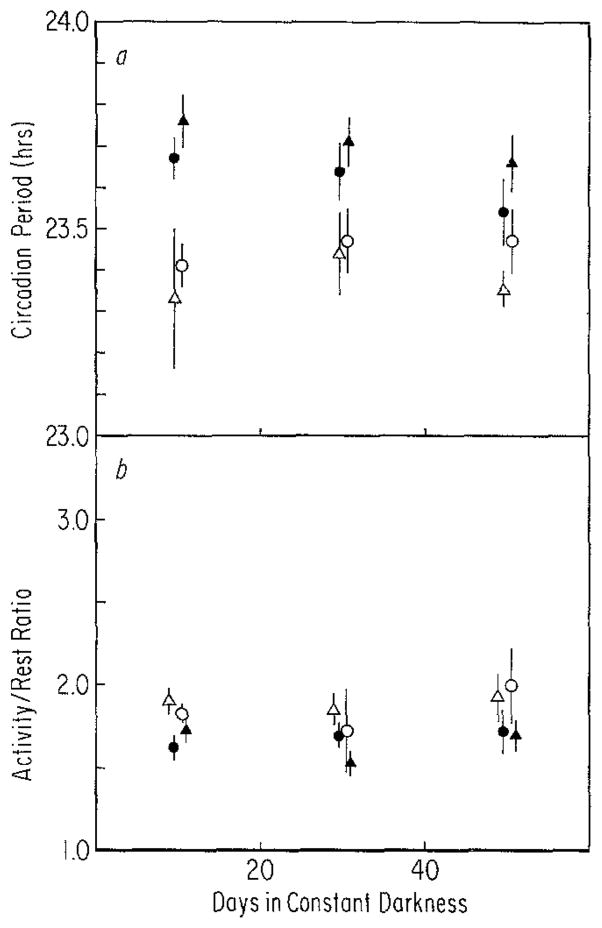
a After-effects on period (τ) measured over 20-day intervals for 60 days in constant darkness following entrainment to T =28 (closed symbols) or T = 20 (open symbols). Triangles (▲, n = 9 and △, n = 6) represent animals raised on T = 28. Circles (●, n = 9 and ○, n = 2) represent animals raised on T = 20. Vertical lines: SEM. ANOVA indicates that the animals raised on different cycles are not different from each other following entrainment to either T = 28 or T = 20 at any time, i.e., the main effect of rearing condition was not significant (P>0.10). Main effects of T and time in constant darkness as well as their interaction were significant (P< 0.01). b After-effects on activity/rest (α/ρ) measured over 20-day intervals for 60 days in constant darkness following entrainment to T = 28 or T = 20. Symbols and sample size are the same as in Fig. 8a. Irrespective of rearing conditions, mean α/ρ of animals from T = 20 was greater than that of animals from T = 28, i.e., only the main effect of T is significant (P<0.05)
Fig. 9.
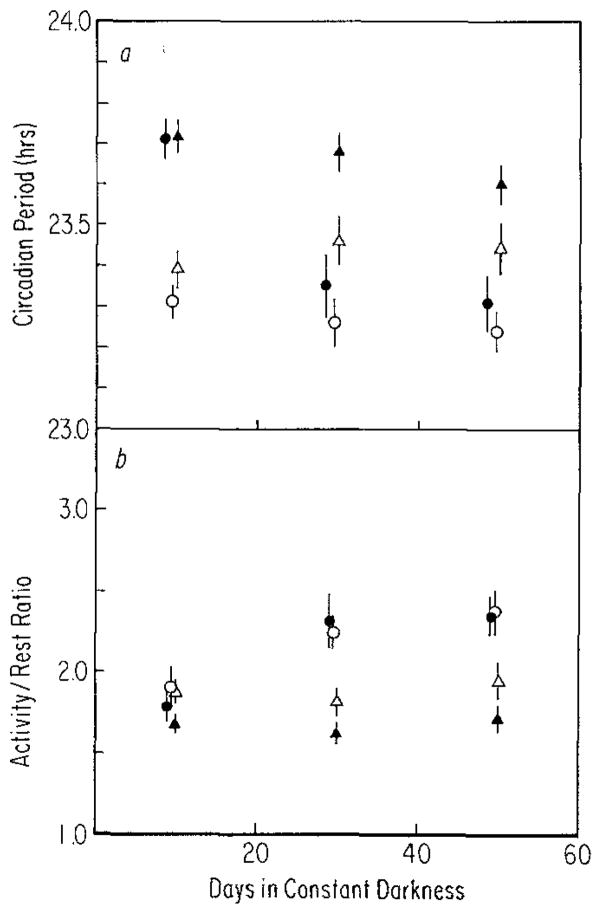
a After-effects on period (τ) following entrainment to T = 28 (closed symbols) or T = 20 (open symbols) in mice 10 weeks of age (●, n = 17 and ○, n = 16) or 41 weeks of age (▲, n = 18 and △, n = 8). Measurements were made over either 18-day (circles) or 20-day (triangles) intervals. Vertical lines: SEM. ANOVA performed on these data utilized slightly smaller sample sizes than indicated in order to include only those animals for which measurements were available both when they were young and older. The main effect of age (triangles versus circles) is significant (P< 0.005). b After-effects on activity/rest ratio (α/ρ) following entrainment to T = 28 (closed symbols) or T = 20 (open symbols) in mice 10 weeks of age (●, n = 18 and ○, n = 16) or 41 weeks of age (▲, n = 18 and △, n = 8). Vertical lines: SEM. The main effect of age (circles versus triangles) is significant (P< 0.001)
Results
Maternal Entrainment
The non-24-h light/dark cycles to which the mother mice were entrained (T = 20 and T = 28) and under which their progeny were reared are shown in Fig. 1a. Figure 1b contains the records of two mothers, one from each cycle, and indicates their treatment from before mating to beyond weaning. Seven out of ten females became pregnant under T = 28 (one litter was lost during lactation) and five out of ten under T = 20. In all cases mothers were entrained both before the birth of their litters and after weaning.
In all mothers, wheel-running activity declined during lactation to such a low level that for ten of the eleven mothers it could not be determined with absolute certainty that they either were or were not entrained. Some of the mothers, however, (e.g., the right side of Fig. 1b) gave some evidence of being entrained throughout lactation, and all mothers were clearly entrained immediately following the return of activity at weaning. The loss of activity during lactation could be attributed to nest-building beneath the running-wheel in some but not all cases. Activity returned just prior to or at the time of weaning, either abruptly (e.g., the left side of Fig. 1b) or gradually (e.g., the right side of Fig. 1b).
During mating, the females were examined daily for vaginal plugs, thus allowing, together with litter dates, the calculation of gestation time. Table 1 summarizes gestation length, litter size, and average pup weight, none of which were significantly different for mothers under T = 28 or T = 20. The similarity of gestation length, as measured in 24-h days, confirms the finding of Lanman and Seidman (1977) who kept mice on T = 21, that the length of gestation is not measured in circadian cycles, but rather is an absolute amount of developmental time.
Table 1.
Comparison of litters raised on T = 28 and T = 20
| Gestation (days) | Litter size at weaning | Average pup weight at weaning (g) | |
|---|---|---|---|
| T = 28 | |||
| Mean | 19.33 | 6.83 | 9.24 |
| sem | 0.615 | 0.792 | 0.444 |
| n | 6 | 6 | 6 |
| T = 20 | |||
| Mean | 19.33 | 7.20 | 9.95 |
| sem | 0.333 | 0.837 | 0.567 |
| n | 3 | 5 | 5 |
After-Effects of Entrainment
Animals raised on T = 28 or T = 20.
Of the progeny conceived and reared on T = 28 and T = 20, only one (from T = 20) of the 40 animals tested did not entrain to the non-24-h cycle. This one animal clearly showed a freerunning period longer than 24 h in the presence of the T = 20 light/dark cycle.
Figure 2 contains representative records of two animals from each of the two rearing conditions. After three weeks. in running-wheels on the non-24-h cycles (LD1 of Fig. 2) the animals were released into constant darkness (DD1). Freerunning period and changes in it for 50 days following release into DD1 are compared for animals from the two rearing conditions in Fig. 3a. For the first 20 days in constant darkness, the mean freerunning periods are clearly different. Somewhere between 20 and 30 days, however, this difference disappears and the two groups, despite their different rearing conditions, become indistinguishable. Hence the after-effects of entrainment are not permanent or even long lasting despite entrainment throughout development.
Figure 3b shows α/ρ measurements for these same animals. The two groups are indistinguishable at all but one measurement interval, including the first two intervals when freerunning period is different. The ratio of activity to rest increased with time in all animals but appeared to do so more rapidly in the T = 20 group between 20 and 40 days after release into constant darkness. By 50 days, however, the groups were again similar.
The initial similarity in α/ρ of the T = 28 and T = 20 groups may be related to the equality of light/dark ratios of the two cycles. Although complicated by possible ‘masking’ effects of the light or dark on activity, α/ρ of the two groups while entrained are only marginally different as shown in Table 2, with that of the T = 20 animals being larger. The similarity of these ratios suggests that there is no absolute required maximum or minimum amount of activity or rest in these mice but rather it is the ratio of these per cycle that is important. Despite the high light intensity used (2,000 lux), animals entrained to T = 28, as a consequence of their phase relationship to the cycle, spent a significant portion of their active time in the light (approximately seven and a half h). The animals entrained to T = 20 began activity at approximately lights off (Table 2) but generally continued to be active several hours after lights on.
The increase in α/ρ and the decrease in τ with time in constant dark in the T = 28 animals (Fig. 3a and b) results in a negative correlation between these properties. A similar correlation is not obvious in the T = 20 animals. In the T = 20 animals, τ first increased and then decreased so that the mean values of the first and last intervals were similar (Fig. 3 a). The mechanism by which τ could first change in one direction and then in another is presently unknown. The lengthening of τ may reflect the decay of after-reflects due to T = 20, while the shortening of τ may reflect a process which occurs in constant dark, irrespective of previous entrainment conditions. This later process combined with the decay of after-effects due to T = 28 could result in the rapid shortening of τ in the T = 28 animals. If a change in α/ρ is correlated only with the shortening of τ that occurs independently of the previous light/dark cycle, then an increase in α/ρ would be expected and in fact can be seen (Fig. 3) in the T = 20 as well as the T = 28 animals.
After-Effects in Adults
Figure 4 a compares the after-effects on freerunning period of T = 28 and T = 20 in adult males (the fathers) that were entrained to the cycles between 14 and 28 weeks of age. Figure 5 shows the records of two of these males, one from each cycle. In contrast to the animals raised on T = 28 and T = 20, freerunning periods of adults from these conditions were still different after approximately 36 days in constant darkness. Pittendrigh and Daan (1976a) found that after-effects in adult mice due to entrainment to cycles with the same periods as used here, lasted for up to 100 days in constant darkness. This apparent difference in the persistence of after-effects between the younger animals raised on the cycles (approximately 25 days) and animals entrained to them as adults (either ours or those of Pittendrigh and Daan (1976a)) is contrary to the original expectations of this investigation, i.e., that development on the cycles would result in, if not permanent, at least more dramatic after-effects.
Fig. 5.
Activity records of two adult mice (fathers), one initially entrained to T = 28 and the other to T = 20. Except for the indicated changes in lighting conditions and regular replenishment of food and water, the animals were undisturbed for the duration of the records. Both records are double plotted
Any discussion of the question of the persistence of after-effects must be qualified by a general weakness in the design of these and similar experiments. When the animals are placed on non-24-h light/dark cycles with periods near the limits to which they can entrain, some animals are likely not to entrain. This was true of one of the progeny and two of the adults on T = 20. Pittendrigh and Daan (1976a) also mention that some of their animals did not entrain to T = 20 and T = 28. Such animals are naturally not included in the freerunning period measurements following subsequent release into constant darkness. Whatever the reason may be for such animals not entraining to the experimental cycles, the consequence is that the remaining animals have been selected, as well as affected, by entrainment. Those animals which did not entrain to T = 20 may have failed to do so because their freerunning periods were longer than average and consequently less likely to adjust (i.e., to be phase-shifted by the light/dark cycle a sufficient amount each day) to the short period. The remaining T = 20 animals would then represent a group selected for short freerunning period. If a similar but opposite selection occurred with the T = 28 animals, then a permanent difference between the groups would have been established.
To what extent an animal’s inability to entrain depends on its freerunning period as it would subsequently be expressed in constant darkness cannot be determined. It is possible, however, to measure freerunning period of a group of animals prior to entrainment and observe any correlation with an inability to entrain. In an experiment examining the effect of rearing conditions on entrainment, a group of animals were placed on T = 20. Several of them did not entrain, allowing a comparison of their average freerunning period just prior to placement on the light cycle with that of those that did entrain. Such a comparison reveals a significant difference, with those animals that did not entrain having a longer average freerunning period (23.224 ± 0.044 (n = 11) vs 23.686 ± 0.083 (n = 3), P<0.01, Student’s t-test). This therefore, supports the idea that the non-24-h cycles may change the mean freerunning periods both by after-effects and spuriously as a result of inadvertent selection by the investigator. However, in the experiments reported here only a few animals did not entrain, so that the effects of the non-24-h cycles on period are likely to reflect primarily after-effects induced by these cycles.
The pattern of freerunning period changes that were observed in animals raised on T = 20 (a lengthening followed by shortening, Fig. 3 a) was not seen in the T = 20 adults. Either these same changes did not occur in the adults or they could not be seen because of the larger measurement intervals used (see legend to Fig. 4). In the T = 28 adults, freerunning period shortened less rapidly than in those raised on T = 28 and may have continued to shorten if they had been left for a longer time in constant darkness. Also in contrast to the progeny there is an absence in the adults of any change in α/ρ with time.
After-Effects of T = 24
The unexpected rapid decay of after-effects in animals raised on T = 28 and T = 20, relative to that in adults, raised two hypotheses concerning the way in which these animals might differ from those exposed to the non-24-h cycles as adults only. One is that the younger animals are different because of their age only, and for unknown reasons changes in τ and α/ρ that occur over time in constant darkness occur more rapidly in them. The other is that animals raised on T = 20 and T = 28 have in some way been changed by the experience. The latter hypothesis predicts a permanent change in the way the animals raised on the cycles will respond to them as well as to other cycles. In order to test this, we measured the response (i.e., after-effects) of the two groups to the same intermediate cycle of T = 24 (LD2 of Figs. 2 and 5).
All of the animals, both progeny and adult, entrained to T = 24 (LD, 14:10). Although there were no obvious or striking differences in the phase relationships of the T = 20 and T = 28 animals, either progeny or adults, there were, as Table 3 indicates, small but nevertheless significant differences in the time of activity onset relative to that of lights off between animals raised on T = 28 and T = 20. The adults showed no such difference. Also shown in Table 3 are the directions of phase-shifts taken by animals in each group during entrainment to T = 24. The cycle was imposed on each group in such a way so as to avoid a predominance of a particular direction of phase-shift in any one group. Such phase-shifts can themselves produce long-lasting after-effects (Pittendrigh and Daan 1976a).
The phase relationship differences (ψ) shown in Table 3 suggest that light had a longer lasting inhibitory effect on activity in the T = 20 animals, resulting in a greater phase lag in activity onset during entrainment. As indicated by the projected onset times, the pacemaker in the T = 20 animals actually had a phase slightly advanced relative to that of the T = 28 animals. Based on entrainment theory and the phase response curve for mice (Pittendrigh and Daan 1976a, b) the difference based on projected onsets suggests that the T = 20 animals were receiving greater delays while entrained to T = 24, or the delay portion of their phase response curve has a smaller amplitude than does that of the T = 28 animals.
The after-effects due to T = 24 in the animals raised on T = 20 or T = 28 (measured during DD2 of Fig. 2) are shown in Fig. 6. Although mean freerunning periods of these two groups were indistinguishable at the end of DD1 (see Fig. 3a), they were surprisingly different immediately following release into constant darkness from T = 24 (Fig. 6a). Comparison of the first measurement interval of Fig. 6a with the last interval of Fig. 3a indicates that entrainment to T = 24 had a substantial lengthening effect on the T = 20 animals but not on the T = 28 animals. This effect on the T = 20 group may be a temporary after-effect because the two groups become less different with time.
This apparent differential effect of T = 24 on the T = 20 and T = 28 progeny was not seen in the adults. The T = 20 and T = 28 adults retained the difference in freerunning period that was seen prior to T = 24 (Fig. 7 a). Because the adults continue to converge throughout DD2 (Fig. 7 a), it is likely that the difference seen immediately following T = 24 is in fact a long-lasting after-effect which was not abolished by entrainment to T = 24. This suggests that not only was the response of the adults to T = 24 different from that of the progeny, but that the after-effect due to T = 20 and T = 28 were much longer-lasting in the adults.
The after-effects to T = 24 on α/ρ were similar in the progeny and adults. In both cases there were no consistent differences between the T = 20 and T = 28 groups. In both young and adult animals α/ρ increased significantly with time in constant dark (Figs. 6b and 7b).
Effect of Rearing Condition on Entrainment to T = 20 and T = 28
The evidence that animals raised on T = 20 and T = 28 interpret a T = 24 cycle differently (i.e., the difference in entrained phase relationship and in after-effects) suggest that these groups might also interpret other cycles differently. We therefore tested to see if the rearing conditions affected the ability of animals to entrain to the extreme periods of T = 28 and T = 20.
At the end of DD2 each progeny group was divided in half and placed into either T = 28 or T = 20 for eight weeks (LD3 of Fig. 2). There was no difference between the progeny groups in their abilities to entrain although both entrained less easily to T = 20 (Table 4).
Table 4.
Effect of rearing condition on entrainment to T = 28 and T= 20
| T = 28 | T = 20 | |||
|---|---|---|---|---|
| Reared on | Reared on | |||
| T = 28 or | T = 20 | T=28 or | T = 20 | |
| Number entrained | 9 | 9 | 7 | 5 |
| Number not entrained | 0 | 0 | 1 | 3 |
The after-effects due to T = 20 and T = 28 are shown for animals raised on either T = 20 or T = 28 in Fig. 8. Although the animals raised on T = 20 appeared to converge more rapidly than the T = 28 animals, no such significant interaction was indicated by statistical analysis (i.e., rearing condition × T-cycle × time in DD, P>0.50), indicating that rearing on T = 20 or T = 28 does not affect the after-effects induced by these cycles later in the life of the animal. If the after-effects are compared without regard to rearing conditions, they persist throughout the second and possibly the third measurement interval (i.e., for overall comparison of T-cycles and for T-cycles × time in constant darkness, P<0.01, and comparison of T-cycles in final interval by t-test gives 0.10 > P> 0.05) suggesting that after-effects persist longer in older animals (although see below) and that the rapid decay of after-effects initially seen in these animals (Fig. 3a) was related to their age rather than to rearing on the non-24-h cycles, i.e., they now show after-effects similar to those seen in adult animals (Fig. 5a).
The only finding which emerges from the α/ρ data of these animals is a small difference between the combined T = 20 and combined T = 28 animals (Fig. 8b).
Effects of Age on After-Effects and Freerunning Period
The rapid decay of after-effects in mice raised on T = 20 and T = 28 was surprising, based on previous studies of after-effects in mice (Pittendrigh and Daan 1976a), and in comparison to the decay of after-effects in our own adult controls. Two hypotheses were proposed to account for this result; that young animals in general show less persistence of after-effects (i.e., more rapid changes in τ and α/ρ in constant darkness) and, alternatively, that exposure to the non-24-h cycles during development alters the way in which animals will respond to such cycles. The results described in the preceding section show that after-effects following T = 28 and T = 20 were not influenced by the rearing conditions, but indicate that the decay of after-effects initially seen in these animals raised on experimental light cycles appears to be due to an aging phenomenon rather than to a permanent developmental effect of the rearing conditions.
An age difference in after-effects is itself an intriguing possibility. Figure 9a compares the after-effects of T = 20 and T = 28 on freerunning period in young and old mice. The rapid decay of after-effects is clear in the young mice, while the after-effects in older mice appear to persist much longer. The statistical analysis of these data indicate, however, that the older T = 20 and T = 28 animals may have been different, by chance prior to their exposure to T = 20 and T = 28 as adults. Consequently a strong statement about the persistence of after-effects cannot be made.
An obvious difference between young and old animals in Fig. 9a which may be related to the apparent difference in after-effects is the clear difference in freerunning period of the two groups. Although there is no age difference immediately following release into constant darkness (the first measurement interval of Fig. 9a), the younger animals (especially those from T = 28) show a shorter freerunning period than do the older animals (after 20 to 40 days in constant darkness). This age difference is significant if compared across all other treatments (see legend to Fig. 9) and is also significant if compared in those few animals that individually were treated identically when they were young and when they were older (Table 5).
Table 5.
Comparison of freerunning period (τ) and activity/rest ratio (α/ρ) in mice at 16 and 49 weeks of age
|
τ (h)
|
α/ρ
|
|||||
|---|---|---|---|---|---|---|
| 16 | 49 | Δτ | 16 | 49 | Δα/ρ | |
| Previously entrained to T = 28 | 23.56 | 23.52 | −0.04 | 2.174 | 1.704 | −0.470 |
| 23.09 | 23.81 | 0.72 | 1.760 | 1.433 | −0.327 | |
| 23.47 | 23.82 | 0.35 | 1.630 | 1.281 | −0.349 | |
| 23.69 | 23.77 | 0.08 | 1.880 | 1.571 | −0.309 | |
| 22.95 | 23.50 | 0.55 | 2.227 | 1.517 | −0.710 | |
| 23.39 | 23.32 | −0.07 | 4.143 | 1.593 | −2.550 | |
| 23.61 | 23.54 | −0.07 | 1.483 | 2.217 | 0.236 | |
| 23.08 | 23.20 | 0.12 | 3.786 | 2.550 | −1.236 | |
|
| ||||||
| Previously entrained to T = 20 | 23.23 | 23.38 | 0.15 | 1.692 | 1.769 | 0.077 |
| 22.69 | 23.32 | 0.63 | 2.650 | 2.227 | −0.423 | |
|
| ||||||
| Mean | Δτ | 0.24 | Mean | Δα/ρ | −0.556 | |
| sem | 0.09 | sem | 0.273 | |||
| P <0.05* | 0.10> P >0.05* | |||||
Paired t-test
Table 5 gives α/ρ measurements for individuals and, together with the old and young group differences shown in Fig. 9b, indicates a decrease in α/ρ with age. Figure 9b shows that α/ρ does not change with time in constant darkness in the older animals as it does in the young ones.
Discussion
Pacemaker Development
The freerunning period of the circadian pacemaker that underlies locomotor activity/rest rhythmicity of Mus musculus is not influenced by the period of the environmental cycle under which the animal is raised. In earlier studies with mammals raised under constant conditions (Aschoff 1960; Richter 1971) the contribution of the mother to the development of rhythmicity in the progeny has been a confounding issue. We have eliminated this problem by using abnormal periodicities to which the mothers could entrain, thereby confronting the developing animal with both non-24-h light/dark information and non-24-h maternal rhythmicity. These cycles apparently did not have a developmental effect on the pacemaker. Either, (1) the pacemaker does not have a period of development, (2) the developing pacemaker was not exposed to the non-24-h cycles, or (3) pacemaker development is ‘preprogrammed’ and insensitive to environmental periodicity.
The first of these possibilities requires that the pacemaker mechanism is intracellular and consequently its development consist only of replication with each cellular division. The cellular or multicellular nature of mammalian circadian pacemakers is currently unkown. However, because the complexity of pacemaker properties in rodents strongly suggests that the mechanism involves the interaction of at least two circadian oscillators (Pittendrigh and Daan 1976c), it is likely that the mechanism requires some degree of intercellular interaction and therefore a period of development, however brief.
Given the existence of such a developmental period, the second possibility requires that in our experimental situation the pacemaker must not have been exposed to the abnormal, non-24-h cycles as it developed. Although this possibility cannot be eliminated, maternal rhythmicity can prenatally influence the developing fetus in rats. Rat fetuses have been found to show a daily rhythm in growth which is synchronized either to the mother or to the light/dark cycle to which she is exposed (Barr 1973). Furthermore, rhythms measured in rat pups shortly after birth appear to be synchronized prenatally to the mother (Deguchi 1975; Fuchs and Moore 1980). Even so, it is possible, but unlikely, that in our experiments some aspect of circadian maternal rhythmicity was not entrained to the non-24-h cycles as was wheel-running activity. Although entrainment of the wheel-running activity rhythm was confirmed in all mothers, it has been found in primates (but not rodents) that different rhythms can have different limits of entrainment (Aschoff et al. 1967; Sulzman et al. 1978).
Postnatally, the mice in our experiment were unquestionably exposed to the non-24-h light/dark cycles. Although the age at which the pacemaker is first sensitive to light has not been determined, Fuchs and Moore (1980) have found that the suprachiasmatic nuclei of rats are responsive to light (measured as increased uptake of 2-deoxy-D-glucose) on postnatal day one. Deguchi (1975) observed an apparent difference between the periods of pineal gland rhythms in LL vs. DD treated 12-day-old rats, indicating a sensitivity of the pacemaker before that age to either light, maternal rhythmicity, or both. Although these findings indicate sensitivity of the neonatal pacemaker to environmental rhythmicity, it is not known whether entrainment to non-24-h cycles occurs.
Given the assumption that the circadian pacemaker underlying wheel-running activity does have a period of development and can be entrained to maternal or light/dark rhythmicity during its development, our findings indicate that the mechanism reflected in the mature freerunning period develops in an inflexible, genetically predetermined manner even though it is being driven at abnormal periodicities during its entire developmental history.
The different developmental histories of the experimental progeny did not affect their freerunning periods, their ability to entrain to periods of 20 or 28 h as adults, or the after-effects induced by these cycles. The small difference between the two developmental groups in their entrained phase relationships to T=24 and the different after-effects induced by this entrainment stand as the only possible effects of development under the non-24-h cycles.
Because after-effects following T = 20 and T=28 appear to be extremely long-lasting in the adult controls (even in the face of entrainment to T=24) the difference between progeny groups following T = 24 may not reflect a special developmental effect of entrainment to non-24-h cycles. In general, the existence of very long-lasting after-effects in adults makes it impractical to use the duration of after-effects as a measure of developmental effects of environmental rhythmicity. It appears that the adult circadian pacemaker is affected by entrainment as much as, or more than, that of the developing animal. As Pittendrigh and Daan (1976c) have contended, plasticity of the pacemaker, as reflected in after-effects, is likely to be a genetically determined feature of the pacemaker as are other properties such as period. Plasticity is usefully viewed not as an inconsequential property of the pacemaker, but rather as a funtional and adaptive feature of its design. The adaptive significance of pacemaker plasticity may be related to seasonal changes in day length and the maintenance of an adaptive phase relationship to the environment as such changes occur (Pittendrigh and Daan 1976c; Daan and Aschoff 1975). That the period of the pacemaker and the range of its plasticity are under complete genetic control is, if not expected, at least reasonable in view of the astronomical regularities to which these properties are adapted.
It remains to be determined if postnatal synaptogenesis in the suprachiasmatic nuclei (Lenn et al. 1977) can be altered by environmental rhythmicity or by any other means, and if so, what the consequences for circadian rhythmicity would be. Unpublished observations (Davis and Menaker) indicate that depriving the suprachiasmatic nuclei of retinal afferents immediately after birth does not affect the period of the pacemaker underlying hamster wheel-running activity.
Effects of Age on the Pacemaker
Several differences between the circadian systems of young and old animals were observed in our data. Firstly and most notable, was a difference in the persistence of after-effects on period following entrainment to T =28 and T = 20. The difference in period between young mice from these two cycles (Fig. 3a) disappeared more rapidly than did the difference between their fathers (Figs. 4a and 7a), between these same animals when they were older (Fig. 8a), or between adult mice exposed to similar cycles in a previously published study (Pittendrigh and Daan 1976a). Secondly, the freerunning period of young animals was found to be shorter than that of older animals (Fig. 9a and Table 5). This difference in period is apparent only after several weeks in constant darkness; i.e., young and old animals are not different immediately following transfer from the light/dark cycles to constant darkness (Fig. 9a).
Thirdly, a difference in the ratio of activity time to rest time (α/ρ) between young and old animals also emerges as α/ρ changes over time in constant darkness (Fig. 9b). In young animals, α/ρ increases with time (Fig. 3b) but in the fathers of these animals (Fig. 4b) or in these same animals when they are older (Fig. 9b), α/ρ increases very little if at all. Taken together, the age differences in pacemaker properties suggest that pacemaker ‘plasticity’ is greater in young mice; changes occur more often (e.g., α/ρ) or more rapidly (e.g., decay of after-effects on period) in young than in old mice.
In contrast to our finding that freerunning period lengthens with age, Pittendrigh and Daan (1974) found in three species of rodents (two species of deer-mice and hamsters) that freerunning period shortens with age. Consistent with this, they later showed that freerunning period is shorter in old than in young Mus musculus (Pittendrigh and Daan 1976a). Although unlikely, it is possible that in Mus musculus the freerunning periods of individuals lengthen with age, as we found, but that the average period of very old mice is shorter than that of young mice as a result of the selective deaths of animals with the longest periods. The age differences that we have reported as well as the possible discrepancies between our results and the findings of others point to a need for further work on the effects of aging on the circadian organization.
Abbreviations
- LD
light/dark
- DD
constant darkness
- T
period of the entraining cycle
- τ
period of the pacemaker
- α/ρ
ratio of activity time to rest time
- ANOVA
analysis of variance
- SEM
standard error of the mean
Footnotes
Research supported by NIH Grant 1 RO1 HD13162-01 to M. Menaker
References
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harbor Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Meyer-Lohman J. Angeborene 24-Stunden-Perio-dik beim Küken. Pflügers Arch. 1954;260:170–176. [PubMed] [Google Scholar]
- Aschoff J, Gerecke U, Wever R. Desynchronization of human circadian rhythms. Jpn J Physiol. 1967;17:450–457. doi: 10.2170/jjphysiol.17.450. [DOI] [PubMed] [Google Scholar]
- Barr M. Prenatal growth of Wistar rats: circadian periodicity of fetal growth late in gestation. Teratology. 1973;7:283–287. doi: 10.1002/tera.1420070309. [DOI] [PubMed] [Google Scholar]
- Browman LG. Artificial sixteen-hour day activity rhythms in the white rat. Am J Physiol. 1952;168:694–697. doi: 10.1152/ajplegacy.1952.168.3.694. [DOI] [PubMed] [Google Scholar]
- Brown FM. 27-hour day effects on reproduction and circadian activity period in rats. In: Scheving LE, Halberg F, Pauley JE, editors. Chronobiology. Igoku Shoin; Tokyo: 1974. pp. 466–471. [Google Scholar]
- Daan S, Aschoff J. Circadian rhythms of locomotor activity in captive birds and mammals: their variations with season and latitude. Oecologia. 1975;18:269–316. doi: 10.1007/BF00345851. [DOI] [PubMed] [Google Scholar]
- Davis FC, Menaker M. Hamsters through time’s window: temporal structure of hamster locomotor rhythmicity. Am J Physiol. 1980;239:R149–R155. doi: 10.1152/ajpregu.1980.239.1.R149. [DOI] [PubMed] [Google Scholar]
- Deguchi T. Ontogenesis of a biological clock for serotonin: acetylcoenzyme A N-acetyltransferase in pineal gland of rat. Proc Natl Acad Sci USA. 1975;72:2814–2828. doi: 10.1073/pnas.72.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs JL, Moore RY. Development of circadian rhythmicity and light responsiveness in the rat suprachiasmatic nucleus: a study using the 2-deoxy[1–14C]glucose method. Proc Natl Acad Sci USA. 1980;77:1204–1208. doi: 10.1073/pnas.77.2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldner FH. Synapses of optic nerve afferents in the rat suprachiasmatic nucleus. I. Identification, qualitative description, development and distribution. Cell Tissue Res. 1978;194:17–35. doi: 10.1007/BF00209231. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. Angeborene Tagesperiodik bei Eidechsen. Naturwissenschaften. 1957;44:359–360. [Google Scholar]
- Hoffmann K. Die Aktivitätsperiodik von im 18- und 36- Stundentag erbrüteten Eidechsen. Z Vergl Physiol. 1959;42:422–432. [Google Scholar]
- Lanman TJ, Seidman L. Length of gestation in mice under a 21-hour day. Biol Reprod. 1977;17:224–227. doi: 10.1095/biolreprod17.2.224. [DOI] [PubMed] [Google Scholar]
- Lenn NJ, Bruce B, Moore RY. Postnatal development of the suprachiasmatic hypothalamic nucleus of the rat. Cell Tissue Res. 1977;178:463–475. doi: 10.1007/BF00219568. [DOI] [PubMed] [Google Scholar]
- Mason CA, Sparrow N, Lincoln DW. Structural features of the retinohypothalamic projection in the rat during normal development. Brain Res. 1977;132:141–148. doi: 10.1016/0006-8993(77)90711-9. [DOI] [PubMed] [Google Scholar]
- Menaker M, Takahashi JS, Eskin A. The physiology of circadian pacemakers. Annu Rev Physiol. 1978;40:501–526. doi: 10.1146/annurev.ph.40.030178.002441. [DOI] [PubMed] [Google Scholar]
- Miles LEM, Raynal DM, Wilson MA. Blind man living in normal society has circadian rhythms of 24.9 hours. Science. 1977;198:421–423. doi: 10.1126/science.910139. [DOI] [PubMed] [Google Scholar]
- Moore RY. Retinohypothalamic projection in mammals: a comparative study. Brain Res. 1973;49:403–409. doi: 10.1016/0006-8993(73)90431-9. [DOI] [PubMed] [Google Scholar]
- Moore RY. Central neural control of circadian rhythms. In: Ganong WF, Martini L, editors. Frontiers in neuroendocrinology. Vol. 5. Raven Press; New York: 1978. p. 185. [Google Scholar]
- Pittendrigh CS. On temperature independence in the clock system controlling emergence time in Drosophila. Proc Natl Acad Sci USA. 1954;40:1018–1029. doi: 10.1073/pnas.40.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. Circadian oscillations in rodents: a systematic increase in their frequency with age. Science. 1974;186:548–550. doi: 10.1126/science.186.4163.548. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J Comp Physiol. 1976a;106:223–252. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: the clocklike properties of the pacemaker. J Comp Physiol. 1976b;106:291–331. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol. 1976c;106:333–355. [Google Scholar]
- Richter CP. Inborn nature of the rat’s 24-hour clock. J Comp Physiol Psychol. 1971;75:1–4. doi: 10.1037/h0030681. [DOI] [PubMed] [Google Scholar]
- Rusak B. Neural mechanisms for entrainment and generation of mammalian circadian rhythms. Fed Proc. 1979;38:2589–2595. [PubMed] [Google Scholar]
- Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Stanfield B, Cowan WM. Evidence for a change in the retino-hypothalamic projection in the rat following early removal of one eye. Brain Res. 1976;104:129–136. doi: 10.1016/0006-8993(76)90652-1. [DOI] [PubMed] [Google Scholar]
- Sulzman FM, Fuller CA, Hiles LG, Moore-Ede MC. Circadian rhythm dissociation in an environment with conflicting temporal information. Am J Physiol. 1978;235:R175–R180. doi: 10.1152/ajpregu.1978.235.3.R175. [DOI] [PubMed] [Google Scholar]