Abstract
Background
Prior studies have reported adverse effects of either regional or near-roadway air pollution (NRAP) on lung function. However, there has been little study of the joint effects of these exposures.
Objectives
To assess the joint effects of NRAP and regional pollutants on childhood lung function in the Children’s Health Study.
Methods
Lung function was measured on 1,811 children from eight Southern Californian communities. NRAP exposure was assessed based on (1) residential distance to the nearest freeway or major road and (2) estimated near-roadway contributions to residential nitrogen dioxide (NO2), nitric oxide (NO), and total nitrogen oxides (NOx). Exposure to regional ozone (O3), NO2, particulate matter with aerodynamic diameter less than 10 μm (PM10) and 2.5 μm (PM2.5) was measured continuously at community monitors.
Results
A 17.9 ppb (two standard deviation) increase in near-roadway NOx was associated with deficits of 1.6% in FVC (p=0.005) and 1.1% in FEV1 (p=0.048). Effects were observed in all communities and were similar for NO2 and NO. Residential proximity to a freeway was associated with a reduction in FVC. Lung function deficits of 2–3% were associated with regional PM10 and PM2.5 (FVC and FEV1) and with O3 (FEV1), but not NO2, across the range of exposure between communities. Associations with regional pollution and NRAP were independent in models adjusted for each. Effects of NRAP were not modified by regional pollutant concentrations.
Conclusions
Results indicate that NRAP and regional air pollution have independent adverse effects on childhood lung function.
Keywords: traffic, lung function, air pollution, children, land-use regression
INTRODUCTION
Reduced lung function has been associated with subsequent increased risk of overall mortality, including coronary artery disease and respiratory disease in adults [1] and with asthma in children.[2] Therefore, identifying factors that reduce lung function but are modifiable could lead to interventions with large public benefits.
Regional air pollutants have been associated with reduced lung function in both adults and children.[3–4] Studies examining lung function in children exposed to local residential near-roadway air pollution (NRAP) have not found consistent associations,[5–11] although exposure metrics differed across studies. However, there has been little investigation of the joint effects of regional and NRAP exposures.
In this study, we assessed the joint effects of NRAP and regional exposures to ozone (O3), particulate matter with aerodynamic diameter of less than 10 μm and 2.5 μm (PM10 and PM2.5), and nitrogen dioxide (NO2) on childhood lung function in the Children’s Health Study (CHS). We examined associations with both traffic proximity measures and land-use regression modeled NRAP based on a prior dense air monitoring study of NOx conducted within CHS communities.
METHODS
Study Subjects
The CHS has enrolled over 11,000 children in a series of cohorts investigating the health effects of air pollution. The current analysis includes a cohort established in 2002–2003 when participants were 5–7 years of age.[12] During the 2007–2008 school year, lung function was measured on 1,811 cohort participants (82% of the active cohort) from eight communities, as described in detail in the Online Supplement.
Questionnaires
Questionnaires completed by parents or guardians at study enrollment provided information on participants’ health, socio-demographic and other exposures, which was updated yearly. A complete list of covariates is described in the Online Supplement.
Lung Function
Trained technicians measured lung function, weight, and height, and collected information about recent acute respiratory illness. Using pressure-transducer-based spirometers (Screenstar Spirometers, Morgan Scientific, Haverhill, MA), we identified the maximal forced expiratory volume during the first second (FEV1) and forced vital capacity (FVC) from a series of seven efforts from each child, as described previously.[13]
Air Pollution Exposure
NRAP exposures at each child’s residence and school were based on estimates of surrogates, including distance to freeways, highways, and large surface streets. Spatial land use regression models were developed based on an extensive monitoring campaign of nitrogen oxides (NOx) and nitrogen dioxide (NO2) and by subtraction nitrogen oxide (NO) at over 900 locations in CHS communities, as described previously.[14] Key predictors included distance to freeways and major roads, traffic volumes and their emissions-weighted dispersion estimates, with lesser contributions from population density and local variation in elevation. The resulting annual average predicted residential concentrations of near-roadway NO, NO2, and NOx, incrementally increased above regional background, was used in analyses, as described below.
The regional level of NO2, PM2.5, PM10, and O3 was computed as the mean of the six years of each pollutant measured continuously at a central monitoring location in each community from cohort recruitment (2002) to the recording of lung function tests (2007).
Additional details of NRAP and regional pollutant exposure assessment are provided in the Online Supplement.
Statistical Methods
We fitted linear regression models (with fixed effects for each study community) to investigate associations of FVC and FEV1 with NRAP and a mixed model that included a random intercept for community to assess associations with regional pollutants and joint effects with NRAP. Each pulmonary function outcome was log transformed to satisfy the assumptions of the models. All models were adjusted for demographic and anthropomorphic characteristics (eg. height) and selected other potential confounders (eg. spirometry technician). In sensitivity analyses, other potential confounders and effect modifiers were examined using standard methods described in further detail in the Online Supplement.
The NRAP NOx (and NO and NO2) predicted residential exposures were deviated from a community-specific mean. Conceptually, this allowed examination of the effect of the complex NRAP mixture, for which the nitrogen oxides are only a surrogate, and to distinguish it from the regional NO2 effect, which was assessed based on the continuous measurements at the community monitor so as to be comparable to other regional pollutant assessments. This procedure was also necessary to make the NRAP NOx approximately orthogonal (uncorrelated) to cross-community regional exposures in the mixed models. Health effect estimates were scaled to the range of long-term average regional pollution across all communities and to two standard deviations in the predicted NRAP nitrogen oxides.
Based on our final model, we also computed estimated lung function representative of different combinations of high and low regional and NRAP environments. Low regional pollution was based on the minimum value of regional PM2.5 while low NRAP was defined as one standard deviation below the mean value for deviated NOx. Conversely, high regional pollution was based on the maximum value of regional PM2.5 and high NRAP was defined as one standard deviation above the mean value for deviated NOx. We expressed the predicted lung function in these different environments as percentages relative to those in the cleanest environment (low regional and low NRAP).
RESULTS
The average age at lung function measurement was 11.2 years (SD=0.6). A plurality of participants was White (40%) and a majority was of Hispanic ethnicity (57%, Table 1). Household income less than $30,000 and parental education less than high school were common, and secondhand tobacco smoke exposure was uncommon.
Table 1.
Characteristics of 1,811 CHS participants with lung function testing
| N (total=1811) | %† | |
|---|---|---|
| Male | 871 | 48.1 |
| Race | ||
| Asian | 86 | 4.8 |
| Black | 39 | 2.2 |
| Don’t Know | 239 | 13.2 |
| Mixed | 229 | 12.6 |
| Other | 486 | 26.8 |
| White | 732 | 40.4 |
| Hispanic ethnicity | ||
| Don’t Know | 92 | 5.1 |
| Hispanic | 1028 | 56.8 |
| Not Hispanic | 691 | 38.2 |
| SES | ||
| Household income | ||
| <$30,000 | 402 | 27.1 |
| $30,000 or more | 1084 | 73.0 |
| Parental education | ||
| Did not finish high school | 345 | 20.6 |
| High school diploma or some college | 854 | 51.0 |
| College diploma or greater | 477 | 28.5 |
| Health insurance covers child | 1508 | 89.3 |
| Home characteristics/Potential exposures | ||
| Gas stove | 1462 | 86.5 |
| Dog | 599 | 35.8 |
| Cat | 312 | 18.8 |
| Mold past 12 months | 172 | 10.5 |
| Secondhand smoke exposure | 67 | 3.8 |
| In-utero exposure to maternal smoking | 99 | 5.8 |
| Health conditions | ||
| Acute respiratory illness | 164 | 9.4 |
| Medical diagnosis of asthma | 334 | 19.5 |
Due to missing values, denominators (n) for each percentage may differ.
Overall, 27% of children lived within 500 m of a freeway, while 20%, 15% and 38% lived 500–1,000 m, 1,000–1,500 m, or >1,500 m from a freeway, respectively (Supplemental Material, Table S-1). There was 14% of children who lived within 75 m of a major road (mostly non-freeway), 17% between 75 and 150 m, 28% between 150 and 300 m, and 40% at least 300 m. The distributions of residential proximity to freeways and major roads varied substantially from community to community. Predicted residential near-roadway NOx, NO, and NO2 showed wide variation within most study communities (Figure 1). Correlations among regional pollutant levels ranged from 0.06 (between PM10 and NO2) to 0.80 (between PM10 and PM2.5; Supplemental Material, Table S-2). O3 had relatively strong positive correlations with PM2.5 and PM10. The correlation between predicted near-roadway NO, NO2 and NOx (within communities) exceeded 0.90 (Supplemental Material, Table S-3).
Figure 1.
Distribution of predicted local (A) NO, (B) NO2, and (C) NOx within each of the eight study communities based on a spatial land-use regression model.
The means of FEV1 and FVC for males were 2,474 ml and 2,902 ml, respectively, and the corresponding means for females were 2,442 ml and 2,783 ml. Living within 500 m of a freeway was associated with a nearly 2 percent deficit in FVC (−1.96%; 95% CI: −3.41%, −0.49%; p=0.009) compared to those living at least 1,500 m from a freeway (Table 2). Mean FEV1 was also lower for children living within 500 m of a freeway but the association was not statistically significant. Although close proximity to a major road was negatively associated with each measure of lung function, these associations were not statistically significant.
Table 2.
Effects of measures of near-roadway air pollution on lung function level.
| FEV1†
|
FVC†
|
|||
|---|---|---|---|---|
| %Diff | 95% CI | %Diff | 95% CI | |
| Freeway | ||||
| >1,500 m | Ref | Ref | ||
| 1,000–1,500 m | 1.63 | (−0.05, 3.34) | 0.99 | (−0.65, 2.66) |
| 500–1,000 m | −0.50 | (−2.05, 1.07) | −1.01 | (−2.52, 0.53) |
| <500 m | −1.06 | (−2.55, 0.45) | −1.96 | (−3.41, −0.49)** |
| Trend (p-value) | 0.09 | 0.004 | ||
| Major Road | ||||
| >300 m | Ref | Ref | ||
| 150–300 m | −0.56 | (−1.90, 0.79) | −0.69 | (−2.00, 0.65) |
| 75–150 m | −0.50 | (−2.04, 1.06) | −0.82 | (−2.32, 0.72) |
| <75 m | −1.58 | (−3.21, 0.09) | −1.53 | (−3.14, 0.11) |
| Trend (p-value) | 0.09 | 0.06 | ||
| Predicted Near-roadway Pollution‡ | ||||
| NO2 | −1.00 | (−2.08, 0.09) | −1.40 | (−2.46, −0.33)* |
| NO | −1.19 | (−2.27, −0.09)* | −1.68 | (−2.74, −0.60)*** |
| NOx | −1.10 | (−2.19, −0.01)* | −1.56 | (−2.62, −0.49)*** |
All models include adjustments for log of height and its squared value, BMI and BMI2, sex, age, sex*age interaction, race, Hispanic ethnicity, respiratory illness at time of test, field technician, and study community.
Near-roadway residential pollutants were scaled to two standard deviations of their respective community-mean centered distributions (6.4 ppb for NO2, 12.3 ppb for NO, and 17.9 ppb for NOx).
p<0.05,
p<0.01,
p<0.005
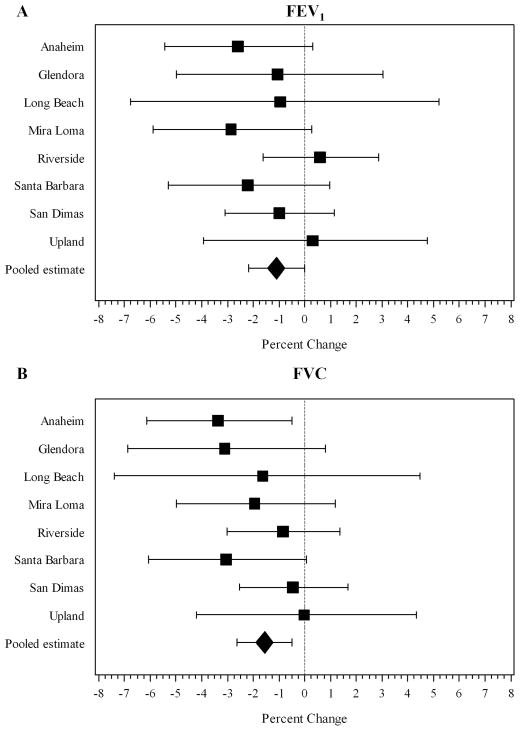
Near-roadway residential NOx, NO, and NO2 had statistically significant negative associations with both FVC and FEV1 (Table 2). For example, a two standard deviation increase in near-roadway NOx exposure (17.9 ppb) was associated with a 1.56% deficit in FVC (−2.62, −0.49; p=0.005), and a 1.10% deficit in FEV1 (−2.19, −0.01; p=0.048). Negative associations between near-roadway NOx and lung function were observed within six of the eight study communities for FEV1 (Figure 2A) and within all eight study communities for FVC (Figure 2B). There was not significant heterogeneity of near-roadway NOx effects across the eight communities for either FEV1 (p=0.61) or FVC (p=0.64).
Figure 2.
Associations of local NOx with (A) FEV1 and (B) FVC within each study community.
Adjustment for potential confounding variables resulted in only small changes to the estimated effects of near-roadway residential NOx on FEV1 and FVC (Table 3). For example, across models that included various additional adjustments, the near-roadway NOx-related deficits ranged from −0.96% to −1.12% (main model: −1.10%) for FEV1, and from −1.40% to −1.60% (main model: −1.56%) for FVC. In an analysis restricted to children without asthma, the effect of near-roadway NOx was similar to that in the entire study population (1.19% decline in FEV1 and 1.51% decline in FVC). The difference in effects between children with and without asthma was not statistically significant. There was also no significant heterogeneity in near-roadway NOx effects on lung function in girls compared to boys. Although we have observed associations of lung function with exposure at schools of participants in this study in conjunction with psychosocial stress,[15] we observed no main effects of exposure in schools in this analysis (results not shown).
Table 3.
Sensitivity analysis for lung function effects of near-roadway residential NOx.
| FEV1†
|
FVC†
|
|
|---|---|---|
| % diff (95% CI) | % diff (95% CI) | |
| Main model | −1.10 (−2.19, −0.01) | −1.56 (−2.62, −0.49) |
| Additional covariates | ||
| Main model + family income | −1.04 (−2.13, 0.07) | −1.51 (−2.58, −0.43) |
| Main model + parental level of education | −0.96 (−2.05, 0.14) | −1.44 (−2.51, −0.36) |
| Main model + diagnosis of asthma by medical doctor | −1.06 (−2.14, 0.03) | −1.55 (−2.61, −0.47) |
| Main model + dogs in home | −0.97 (−2.06, 0.13) | −1.40 (−2.47, −0.33) |
| Main model + cats in home | −1.09 (−2.18, 0.00) | −1.55 (−2.61, −0.47) |
| Main model + exposure to gas stove | −1.10 (−2.18, −0.01) | −1.56 (−2.62, −0.49) |
| Main model + in-utero exposure to maternal smoking | −1.09 (−2.17, 0.01) | −1.60 (−2.66, −0.53) |
| Main model + exposure to tobacco smoke at home | −1.12 (−2.20, −0.02) | −1.57 (−2.63, −0.50) |
| Main model + exposure to mold | −1.12 (−2.21, −0.03) | −1.57 (−2.64, −0.50) |
| Main model + insurance coverage | −1.10 (−2.18, −0.01) | −1.55 (−2.61, −0.48) |
| Subgroup analysis | ||
| Non-asthmatics | −1.19 (−2.41, 0.05) | −1.51 (−2.72, −0.29) |
| Asthmatics | −0.65 (−3.35, 2.14) | −1.20 (−3.91, 1.58) |
| Boys | −0.96 (−2.48, 0.58) | −1.13 (−2.60, 0.36) |
| Girls | −1.10 (−2.65, 0.48) | −1.81 (−3.34, −0.25) |
See Table 2 for adjustment variables and scaling factor for pollutant effects.
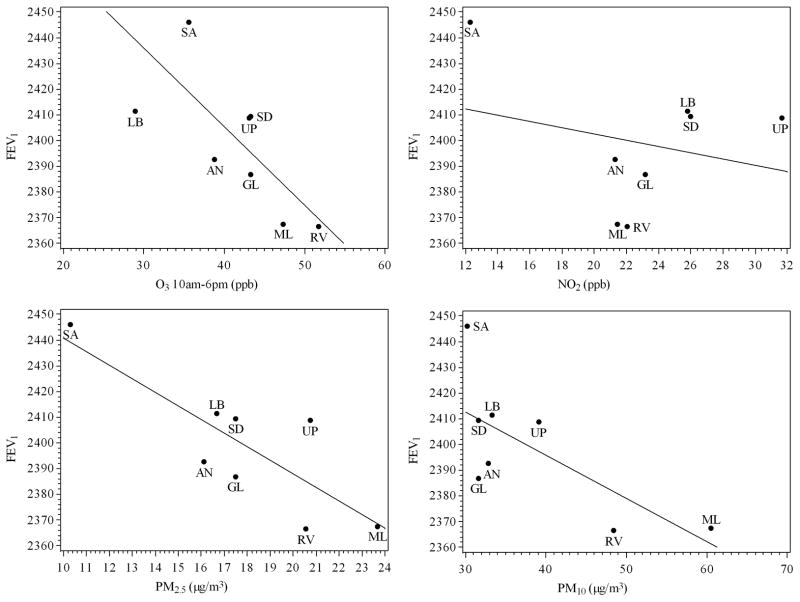
Deficits in FEV1 of approximately 3% were observed across the range of community O3 and PM2.5 levels (p=0.006 for O3 and 0.001 for PM2.5, Table 4 and Figure 3). A greater than 2% deficit was observed across the range of PM10 exposure. Deficits in FVC of over 2% were also observed across the range of both PM2.5 and PM10 (Table 4 and Figure 4); however, a single community (Mira Loma) appears to have driven the association between FVC and PM10.
Table 4.
Effect of averaged regional pollutants on lung function level.
| Regional Pollutant | % Diff† | 95% CI | |
|---|---|---|---|
| FEV1 | O3 (10am-6pm) | −3.10 | (−5.24, −0.91)** |
| PM2.5 | −2.94 | (−4.65, −1.20)*** | |
| PM10 | −2.19 | (−3.98, −0.37)* | |
| NO2 | −1.19 | (−4.14, 1.85) | |
| FVC | O3 (10am-6pm) | −0.31 | (−3.11, 2.57) |
| PM2.5 | −2.25 | (−3.94, −0.52)* | |
| PM10 | −2.05 | (−3.54, −0.54)** | |
| NO2 | −0.79 | (−3.52, 2.02) |
See footnote to Table 2 for adjustment variables (community adjustment not included). Each pollutant was scaled to the range of the 24-hour average over the study period from 2002 until 2007 with the exception of O3, which was scaled to the 8-hour average from 10am to 6pm (22.7 ppb for O3 10-6, 13.3 μg/m3 for PM2.5, 30.3 μg/m3 for PM10, 19.4 μg/m3 for NO2).
p<0.05,
p<0.01,
p<0.005
Figure 3.
Adjusted average FEV1 versus 2002–2007 community-average pollutant levels.
Average FEV1 values are referenced to a white, non-hispanic female of average height and BMI and without a respiratory infection on the day pulmonary function was examined.
Figure 4.
Adjusted average FVC versus 2002–2007 community-average pollutant levels.
Average FVC values are referenced to a white, non-hispanic female of average height and BMI and without a respiratory infection on the day pulmonary function was examined.
In models assessing the joint effects of regional and NRAP, there was little change in the strength of the regional pollutant associations with either FVC or FEV1, after adjusting for near-roadway NOx (Table 5). For FEV1, there was little change in the unadjusted association of near-roadway NOx (1.10% deficit in Table 2) after adjusting for regional pollutants effects (1.04% to 1.14% deficits in Table 5). For FVC, the unadjusted association with near-roadway NOx (1.56% deficit in Table 2) was somewhat attenuated after adjusting for regional pollutants (1.40% to 1.49% deficits in Table 5), although the associations remained significant. Similar patterns of lung function deficits in two-pollutant models were observed for near-roadway NO and NO2 (results not shown). The patterns of effects of freeway proximity associations were also similar in models including a regional pollutant and in models unadjusted for regional pollution (results not shown). We examined the possibility that background pollutant exposures might up-regulate pulmonary response to near-roadway pollutants resulting in larger lung function deficits in communities with high regional pollutants. However, none of the regional pollutants significantly modified the association between near-roadway residential NOx and each of the lung function endpoints (results not shown).
Table 5.
Joint analysis of regional air pollution and near-roadway NOx on lung function.
| Regional Pollutant | Effect of Regional Pollutant†
|
Effect of Near-roadway NOx†
|
|||
|---|---|---|---|---|---|
| % Diff | 95% CI | % Diff | 95% CI | ||
| FEV1 | O3 (10am-6pm) | −3.24 | (−5.32, −1.11)*** | −1.04 | (−2.11, 0.05) |
| PM2.5 | −3.00 | (−4.76, −1.21)*** | −1.07 | (−2.14, 0.01) | |
| PM10 | −2.24 | (−4.04, −0.41)* | −1.14 | (−2.22, −0.06)* | |
| NO2 | −1.22 | (−4.23, 1.88) | −1.07 | (−2.15, 0.02) | |
| FVC | O3 (10am-6pm) | −0.34 | (−3.21, 2.63) | −1.47 | (−2.53, −0.41)** |
| PM2.5 | −2.35 | (−4.09, −0.57)** | −1.40 | (−2.46, −0.34)** | |
| PM10 | −2.17 | (−3.68, −0.63)** | −1.49 | (−2.54, −0.43)** | |
| NO2 | −0.78 | (−3.62, 2.15) | −1.46 | (−2.52, −0.39)** | |
DISCUSSION
These results indicate that exposure to near-roadway air pollution adversely affects childhood lung function. Strengths of the study were the ability to demonstrate consistent effects of NRAP using both roadway proximity and validated predicted NOx markers for the NRAP mixture in communities with differing regional air quality, roadway networks, and geographical characteristics. The study design offered an unusual opportunity to demonstrate that associations of lung function with NRAP pollutant variation were independent of associations also observed with regional air pollution.
NRAP is a complex mixture of particles and reactive gases with oxidant and pro-inflammatory properties that could plausibly cause the observed lung function deficits.[16–17] Oxides of nitrogen were selected to develop prediction models for likely near-roadway variation of the mixture because they are inexpensive to measure with the spatial density needed to develop valid models. NO2 also has known oxidant and immune-modulatory properties and could contribute to the near-roadway lung functions effects,[18] although in our analysis it was not possible to distinguish NRAP NO2 effects from other components of the mixture. The association of regional PM2.5 and PM10 with both FEV1 and FVC, and no effect of regional NO2, suggests that there were independent effects of transported or secondary regional particulate matter and of the NRAP mixture (rather than NO2). In addition, previous reports from the CHS (and other studies) showing associations of NRAP, but not regional pollutants, with prevalent and incident asthma [12 19–20] also are consistent with separate and independent effects of these diverse pollutant mixtures.
It is also possible that more complex combinations of regional and NRAP account for the observed associations, as toxicological and experimental studies indicate that interaction with other pollutants may enhance the effects of particle exposure.[21–22] Although the study design allowed us to examine the heterogeneity of NRAP health effects across multiple communities, we found little evidence for interaction between regional pollutants and NRAP. Rather, the adverse effects were relatively consistent in all eight study communities, although there was limited precision to each estimate because of limited community-specific sample size.
We have previously observed associations of regional PM [23] and traffic proximity [7] with growth of FVC, but accompanied by larger effects in FEV1 in an older cohort of CHS participants. Other studies of traffic and lung function in elementary school and adolescent children have also found larger associations with flow rates than with FVC.[8–9 24] However, the current results are consistent with an observed effect of regional pollutants on FVC in a cross sectional analysis of prior CHS cohorts.[13] Additional follow up of this cohort is ongoing and may help elucidate these relationships.
Some previous studies that have looked at associations between residential traffic related pollution and lung function were performed in multiple geographical regions,[5 7–8 10–11] but many of these studies used only roadway proximity or traffic count/density metrics rather than validated exposure models. Other studies that have used land-use regression to estimate the relationship between NRAP and childhood lung function were performed in relatively limited geographical regions.[6 9] Results have not been consistent across studies.
These inconsistencies in the strength of association between near-roadway residential traffic exposure and respiratory health across several prior studies[5–11] may result in part from the use of different types of NRAP measures, with differing degrees of uncertainty as proxies for pollution exposure. A strength of this study was the use of quantitative residential NOx exposure assignments derived from a spatial land-use regression model calibrated to measurements at well characterized locations in study communities.[14] Additionally, the association between lung function and predicted NOx was consistent with the inverse relationship between residential distance to a freeway and lung function, which was also observed in an earlier CHS cohort,[7] as concentrations of NRAP decrease with increasing distance from a freeway.[25] Comparable, high quality, exposure assessment across studies would facilitate qualitative comparisons or pooled analyses and might lead to more consistent epidemiologic findings.
The adverse associations of lung function with O3, PM2.5, and PM10 are consistent with other studies.[3] In earlier CHS cohorts we reported associations of lung function with PM2.5 and PM10, as well as NO2, but not with O3.[7 13] However, O3 and PM were correlated across communities of the current cohort, and it was therefore not possible to distinguish effects of each.
This study replicates the general design and general age range of a cross-sectional report from a previous CHS cohort [19] but expands the scope of that earlier work by examining both between and within-community pollutant effects. The amount of between-community regional variation in the present study is less than that found in previous CHS studies due to our focus on more-urban communities with larger gradients in NRAP. However, a nearly two-fold difference in the six-year averaged regional pollution concentrations (Figures 3 and 4) exists between the highest and lowest polluted communities, which allowed us to identify between and within-community effects. We have been collecting additional lung function data and will examine longitudinal pollutant effects separately.
We considered the possibility that bias explained our results. Participants and non-participants from the cohort were generally similar across a broad range of demographic, social and housing characteristics (Supplemental Material, Table S-4). The only significant difference was for boys, who were more likely than girls to be non-participants. However, adjusting for sex and for other characteristics had little impact on the NRAP effect estimate (Table 3). Furthermore, the effect of NOx on lung function in analyses restricted to girls was generally similar to the effect among all participants. Although selection bias and residual confounding by other factors cannot be excluded as an explanation for our results, these analyses provide little reason to believe that this occurred.
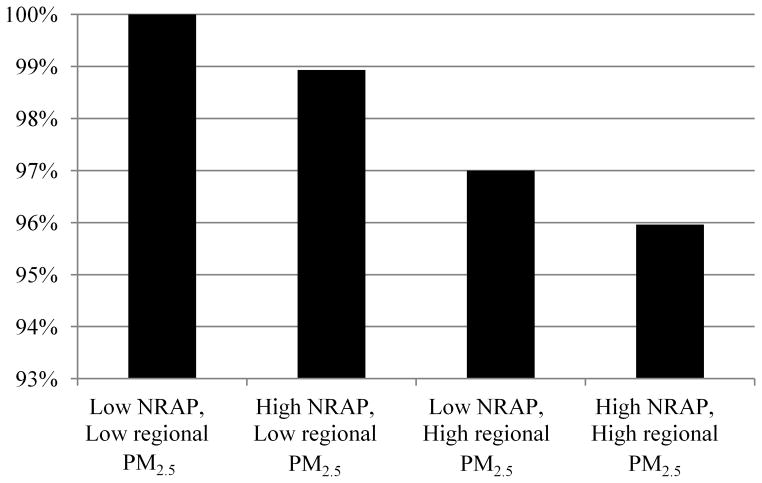
There are potentially large public health implications of these findings because NRAP exposure due to proximity of homes and other locations where children spend time is common [26–27] and lung function in childhood tracks into adult life.[28–30] Furthermore, the strong association between exposure and lung function in non-asthmatic children suggests that traffic-related pollution did not affect only a sensitive subgroup but rather has a potential impact on all children. Although direct comparison of the magnitude of effects of regional and near-roadway pollution is difficult, the deficits associated with near-roadway NOx across a (two-standard deviation) range of within-community variation encompassing most children in our study communities was only modestly less than the effects of regional pollutants across the range of community-average exposure. Compared with a child living in a low NRAP environment in a low regional PM2.5 community, the results suggest that a child living in a high NRAP environment in a community with high PM in Southern California would experience a greater than 4% decrease in FEV1 (Figure 5) For comparison with another common exposure, maternal secondhand smoking of 1 pack/day has been shown to be associated with a 0.4% deficit in childhood level of FEV1.[31] Prevention of these large pollutant effects poses a challenge to the current air pollution regulatory framework, which historically has set standards using risk calculations that consider effects of regional air quality but not near-roadway traffic-related variation in exposure.
Figure 5.
Joint effect of regional PM2.5 and NRAP on FEV1.
Percentages in different exposure environments are relative to a low regional PM2.5 and low NRAP environment as described in the Statistical Methods section.
Supplementary Material
What is the key question?
Do residential near-roadway and regional air pollution cause reduced lung function?
What is the bottom line?
This study found that increased near-roadway and regional air pollutants were independently associated with lower FEV1 and FVC.
Why read on?
A design including multiple communities and predicted near-roadway residential air pollution exposure from well-validated models allowed this study to demonstrate associations of lung function deficits with regional ozone and particulate matter that were independent of associations with indicators of the near-roadway pollutant mixture in multiple communities.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health (grant #s 5P30ES007048, 5P01ES009581, 5P01ES011627, P01ES022845, 5R01 ES016535, 5R03ES014046, 1P50 CA180905, 5R01HL061768, 5R01HL076647, 5R01HL087680, and 1RC2HL101651), the Environmental Protection Agency (grant #s RD83544101, R826708, RD831861, and R831845), and the Hastings Foundation.
Footnotes
COMPETING INTERESTS
Frederick Lurmann is employed by Sonoma Technology, Inc (Petaluma, CA). Rob McConnell has received research support from an air quality violations settlement agreement between the South Coast Air Quality Management District, a California state regulatory agency, and BP. The other authors declare they have no actual or potential competing interests.
References
- 1.Knuiman MW, James AL, Divitini ML, et al. Lung function, respiratory symptoms, and mortality: results from the Busselton Health Study. Ann Epidemiol. 1999;9:297–306. doi: 10.1016/s1047-2797(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 2.Islam T, Gauderman WJ, Berhane K, et al. Relationship between air pollution, lung function and asthma in adolescents. Thorax. 2007;62:957–63. doi: 10.1136/thx.2007.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotschi T, Heinrich J, Sunyer J, et al. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008;19:690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- 4.Downs SH, Schindler C, Liu LJ, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357:2338–47. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- 5.Pujades-Rodriguez M, Lewis S, McKeever T, et al. Effect of living close to a main road on asthma, allergy, lung function and chronic obstructive pulmonary disease. Occup Environ Med. 2009;66:679–84. doi: 10.1136/oem.2008.043885. [DOI] [PubMed] [Google Scholar]
- 6.Dales R, Wheeler A, Mahmud M, et al. The influence of living near roadways on spirometry and exhaled nitric oxide in elementary schoolchildren. Environ Health Perspect. 2008;116:1423–7. doi: 10.1289/ehp.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauderman WJ, Vora H, McConnell R, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–7. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 8.Brunekreef B, Janssen NA, de Hartog J, et al. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. 1997;8:298–303. doi: 10.1097/00001648-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Rosenlund M, Forastiere F, Porta D, et al. Traffic-related air pollution in relation to respiratory symptoms, allergic sensitisation and lung function in schoolchildren. Thorax. 2009;64:573–80. doi: 10.1136/thx.2007.094953. [DOI] [PubMed] [Google Scholar]
- 10.Gauvin S, Amro S, Zmirou D, et al. Road traffic, NO2 exposure and respiratory function among children (VESTA study) International Journal of Vehicle Design. 2001;27:251–61. [Google Scholar]
- 11.Janssen NA, Brunekreef B, van Vliet P, et al. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect. 2003;111:1512–8. doi: 10.1289/ehp.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–72. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters JM, Avol E, Gauderman WJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159:768–75. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 14.Franklin M, Vora H, Avol E, et al. Predictors of intra-community variation in air quality. J Expo Sci Environ Epidemiol. 2012;22:135–47. doi: 10.1038/jes.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam T, Urman R, Gauderman WJ, et al. Parental stress increases the detrimental effect of traffic exposure on children’s lung function. Am J Respir Crit Care Med. 2011;184:822–7. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aust AE, Ball JC, Hu AA, et al. Particle characteristics responsible for effects on human lung epithelial cells. Res Rep Health Eff Inst. 2002:1–65. discussion 67–76. [PubMed] [Google Scholar]
- 17.Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–60. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EPA. Integrated Science Assessment for Oxides of Nitrogen – Health Criteria (Final Report) U.S. Environmental Protection Agency; 2008. [accessed 26 Mar 2013]. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=194645. [Google Scholar]
- 19.Peters JM, Avol E, Navidi W, et al. A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159:760–7. doi: 10.1164/ajrccm.159.3.9804143. [DOI] [PubMed] [Google Scholar]
- 20.HEI. HEI Special Report. Health Effects Institute; 2010. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. [Google Scholar]
- 21.Mauderly JL, Samet JM. Is there evidence for synergy among air pollutants in causing health effects? Environ Health Perspect. 2009;117:1–6. doi: 10.1289/ehp.11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YC, Rappold AG, Graff DW, et al. Synergistic effects of exposure to concentrated ambient fine pollution particles and nitrogen dioxide in humans. Inhal Toxicol. 2012;24:790–7. doi: 10.3109/08958378.2012.718809. [DOI] [PubMed] [Google Scholar]
- 23.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 24.Oftedal B, Brunekreef B, Nystad W, et al. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19:129–37. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- 25.Zhu YF, Hinds WC, Kim S, et al. Concentration and size distribution of ultrafine particles near a major highway. Journal of the Air & Waste Management Association. 2002;52:1032–42. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 26.Appatova AS, Ryan PH, LeMasters GK, et al. Proximal exposure of public schools and students to major roadways: a nationwide US - survey. Journal of Environmental Planning and Management. 2008;51:631–46. [Google Scholar]
- 27.Perez L, Lurmann F, Wilson J, et al. Near-roadway pollution and childhood asthma: implications for developing “win-win” compact urban development and clean vehicle strategies. Environ Health Perspect. 2012;120:1619–26. doi: 10.1289/ehp.1104785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hibbert ME, Hudson IL, Lanigan A, et al. Tracking of lung function in healthy children and adolescents. Pediatr Pulmonol. 1990;8:172–7. doi: 10.1002/ppul.1950080308. [DOI] [PubMed] [Google Scholar]
- 29.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 30.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Wypij D, Gold DR, et al. A longitudinal study of the effects of parental smoking on pulmonary function in children 6–18 years. Am J Respir Crit Care Med. 1994;149:1420–5. doi: 10.1164/ajrccm.149.6.8004293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.