Abstract
Background and Purpose
Brain microvascular disease leads to leukoaraiosis and lacunar infarcts, and contributes to risk of stroke and cognitive decline. Given a shared pathophysiology, retinal microvascular signs are expected to predict brain microvascular disease progression. We investigated if either leukoaraiosis volume progression measured continuously or combined with incident lacunar infarcts would better demonstrate expected associations with retinal disease than has previously been shown.
Methods
830 participants in the Atherosclerosis Risk in Communities study ages 55 and older and without previous stroke received an initial brain MRI, retinal photography, and 10 years later, a follow up MRI. We evaluated retinal vascular sign phenotypes as predictors of 1) leukoaraiosis volume increase and 2) a new score combining leukoaraiosis volume change and incident lacunar infarcts. Hypertension and diabetes were evaluated as confounders and effect modifiers.
Results
Individuals with any retinopathy (3.34 cm3; 95% CI 0.74–5.96) or with AV nicking (2.61cm3; 95% CI 0.80–4.42) each had greater progression of leukoaraiosis than those without these conditions. Any retinopathy (OR 3.18; 95% CI 1.71–5.89) or its components—microaneurysms (OR 3.06; 95% CI 1.33–7.07) and retinal hemorrhage (OR 3.02; 95% CI 1.27–7.20)—as well as AV nicking (OR 1.93; 95% CI 1.24–3.02) and focal arteriolar narrowing (OR 1.76; 95% CI 1.19–2.59), were associated with a higher quartile of a novel brain microvascular disease score combining leukoaraiosis progression with incident subclinical lacunes.
Conclusions
A novel scoring method revealed associations of retinal signs with leukoaraiosis progression and brain microvascular disease which have not been shown before.
Key Terms: retina, leukoaraiosis, lacune
Early detection of cerebral microvascular disease is critical for the prevention of further cerebrovascular injury. However, the earliest brain microvascular pathology precedes detectable changes on MRI. It is important, therefore, to identify predictors of cerebral small vessel disease occurring prior to the detection of brain abnormalities on MRI. As outlined in the STRIVE position paper, conventional MRI can show distinct forms of cerebral small vessel disease: most typically, symptomatic small subcortical infarcts, subclinical lacunes, white matter hyperintensities, and cerebral microbleeds.1 The microvascular bed of the retina mirrors the cerebral small vessels in embryologic origin, anatomic features, and physiologic properties2,3. Hypertensive and diabetic retinal signs are associated with incident stroke, independent of blood pressure and other risk factors4. Likewise, retinal signs are associated with5,6 and may predict subclinical brain microvascular pathology as an intermediary step towards clinical cerebrovascular disease.
Leukoaraiosis and silent lacunar infarcts are two variations of subclinical brain microvascular pathology that share pathological features and risk factors2. Both predict clinical stroke, dementia, and a worse prognosis after cerebral infarction7. Many studies seeking to elaborate risk factors for these phenomena separate these outcomes in analysis, as have previous Atherosclerosis Risk in Communities (ARIC) studies evaluating the relationship between retinal and brain microvascular disease. However, the two imaging findings are not always clearly distinguished, particularly when the leukoaraiosis, or white matter hyperintensity, is in areas typical of lacunar infarcts such as the basal ganglia and thalamus8. More importantly, because the two share similar pathophysiology, separate analysis of white matter hyperintensities and lacunar infarcts inappropriately limits a study’s power to detect statistically significant exposure-outcome relationships in brain microvascular disease.
In this study, we evaluated the extent of cerebral microvascular disease found in ARIC participants with and without retinal microvascular signs. We evaluated volumetric measurements of white matter hyperintensity progression (WMP) instead of a categorical rating of white matter disease. We combined incident lacunar infarcts with this measure of WMP, hypothesizing that retinal microvascular signs would be more likely to demonstrate associations with this combined measure than with separate cerebral microvascular measures.
METHODS
Study Population
ARIC is a prospective cohort study designed to assess risk factors for cardiovascular disease and the natural history of atherosclerosis. ARIC has IRB approval and participants gave informed consent. Participants were middle-aged predominantly black and white men and women from four U.S. communities: Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD. 15,792 participants, aged 45–64, were enrolled at visit 1 (1987–89), with follow-up at visit 2 (1990–93), visit 3 (1993–95), and visit 4 (1996–99). Participants older than 55 at visit 3 were invited to undergo an initial brain MRI, and a subset had a follow-up MRI in 2004–6. Participants with prevalent stroke in 2004–6 were excluded from analysis.
Visit 3 MRI
As described elsewhere,8 1930 subjects drawn from two sites (Forsyth and Jackson) underwent MRI. The proton-density weighted images were graded for severity of leukoaraiosis on a scale of 0 to 9 developed for the Cardiovascular Health Study (CHS)9. Lacunar infarcts were defined as infarct-like lesions hyperintense to grey matter on T2 or hypointense on T1 with size >3mm but <20mm, in the caudate, lenticular nucleus, internal capsule, thalamus, brainstem, deep cerebellar white matter, centrum semiovale, or corona radiate.
Follow-up MRI
1134 participants returned for follow-up MRI. As reported elsewhere, those who returned were more likely to be black, with a comparable number of females10. CHS grades9 were assigned to scans as in visit 3. Scans also underwent a semi-automated volumetric analysis of leukoaraiosis using fluid-attenuated inversion recovery images and were standardized to an intracranial volume of 1500 cc10. Incident lacunes were present if seen on the follow-up but not visit MRI.
White Matter Hyperintensity Change
WMP has been more strongly associated with cognitive decline than baseline leukoaraiosis11,12. However, because leukoaraiosis volume from visit 3 MRIs was not directly measured volumetrically (thin-section images were not obtained on the baseline study), a direct volumetric calculation of WMP was not possible. Instead, we imputed visit 3 leukoaraiosis volumes using a previously published prediction quadratic equation (R2=.80)10 relating CHS score and leukoaraiosis at the follow-up MRI visit. WMP volume was calculated as follow-up MRI – visit 3 volumes. Progression of leukoaraiosis using the CHS score was defined as increase in score >2.
Brain Microvascular Disease Score
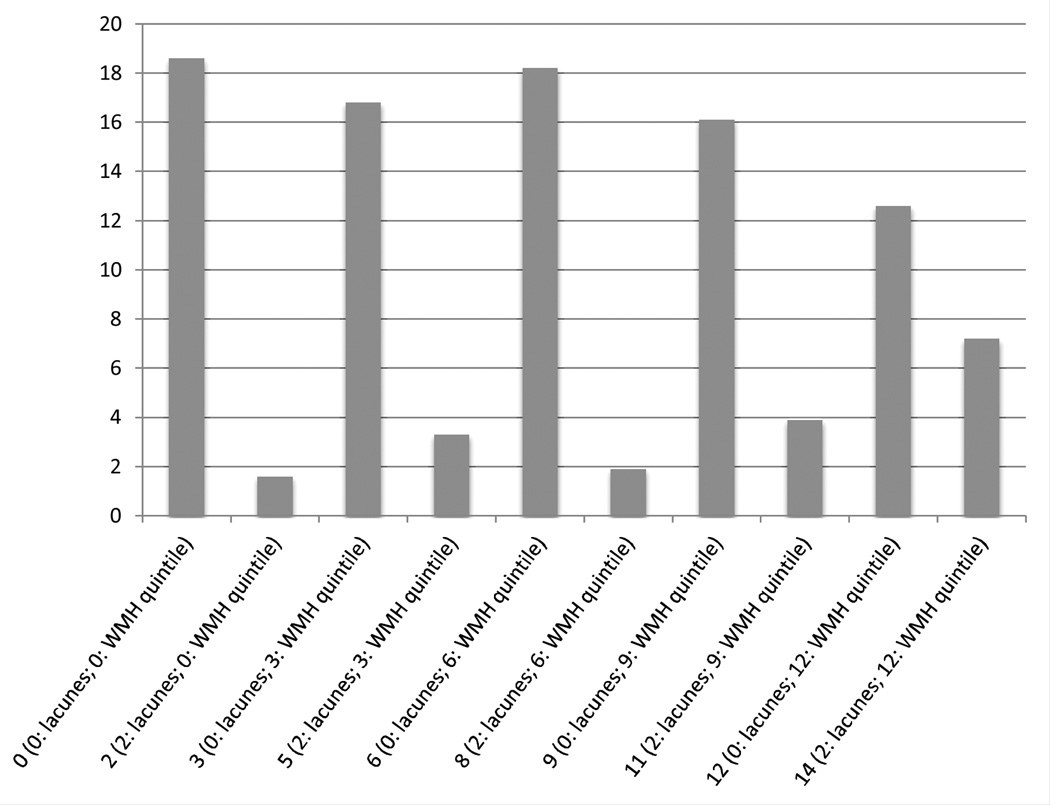
Lacunar infarcts and WMP were combined into one score. We used associations with cumulative systolic blood pressure (SBP) as a measure of validity, given the strong relationships previously observed in the literature between cumulative SBP and brain microvascular disease10. We created linear regression models, regressing SBP onto different combinations of lacunes and WMP, and using the likelihood ratio test, selected the model with the lowest Akaike Information Criteria (AIC). We used this model’s beta coefficients to derive our scoring system for cumulative brain microvascular disease (Figure 1), and also rounded these beta coefficients for ease of use.
Figure 1.
Rounded brain microvascular disease score distribution (derived directly from model beta coefficients). Having incident lacunes adds 2 points and each higher quintile of WMP beyond the lowest quintile adds 3 points. A person with lacunes and the 3rd quintile of WMP would get 2 points for lacunes and 3 points per higher WMP quintile (for a total of 6 points for WMP), for 8 total points.
Retinal Variables
Retinal photographs were taken at visit 3 of all participants, as described elsewhere5. Trained readers, masked to clinical history and risk factors, assessed photographs for the presence of microvascular abnormalities using standardized protocols. Any retinopathy was defined as the presence of any of the following: retinal microaneurysms, hemorrhages, soft exudates, hard exudates, macular edema, or optic disk swelling. Standardized definitions were also provided for retinal arteriovenous (AV) nicking, focal arteriolar narrowing, Central Retinal Arteriolar Equivalents (CRAE) and Central Retinal Venular Equivalents (CRVE). The quality control, grading and definition of these lesions were based on a standard protocol13. The reliability of retinal microvascular sign assessment is moderate to high in the ARIC study for most lesions, with repeated grading of photographs during earlier visits showing intragrader and intergrader weight kappas of 0.57 and 0.56 for AV nicking, 0.62 and 0.29 for focal arteriolar narrowing, and ranging from 0.81 to 1.00 for retinopathy lesions14. Intraindividual reliability coefficients were generally high15.
Covariates
Cardiovascular risk factors were ascertained at each ARIC visit through interview, physical exam, blood sample, and EKG. Diabetes was defined as fasting glucose ≥126mg/dl, nonfasting glucose ≥200mg/dl, or a self-reported history of physician-diagnosed diabetes or use of medications for diabetes. Three blood pressure measurements were taken with 5 minutes of rest between each measurement; the second and third measurements were averaged for the recorded measurement for that visit. Hypertension was defined as SBP ≥140, DBP ≥ 90 mmHg, or anti-hypertensive medication use within 2 weeks.. Cumulative SBP was calculated as time-weighted average of the 2nd and 3rd blood pressure reading from all 5 visits and can be interpreted as the mean daily SBP for the entire period. 10 mm Hg of SBP were added to the cumulative SBP of any participants who reported antihypertensive medication use at >50% of study visits10. Other covariates in the fully adjusted model included age, gender, race, study center, total cholesterol, HDL cholesterol, blood sugar, BMI, cigarette pack-years, and prevalent coronary heart disease. Other than cumulative SBP, visit 3 covariates were used in our analysis.
Statistical Analysis
Stata version 12.0 for Macintosh was used. WMP volume was analyzed continuously. Retinal vascular signs were analyzed in separate models as binary variables except for Central Retinal Arteriolar Equivalents (CRAE) and Central Retinal Venular Equivalents (CRVE), which are continuous measures (in microns) of retinal vessel caliber. Using linear regression, we tested the association between retinal variables and continuous WMP, and used ordinal logistic regression of brain microvascular disease progression score on the retinal variables. Based on small numbers in some score categories, we combined scores based on quartile distribution. Multinomial logistic regression and ordinal logistic regression were used to compare combined groups of scores (quartiles) with a reference group of a score from 0–3. We used ordinal logistic regression models for the total score distribution but proportional odds assumptions were violated for some retinal variables so do not present those results.
Interactions between diabetes and hypertension, each, were evaluated with presence of retinal disease, by introducing an interaction term. We also stratified models by diabetes and hypertension status.
RESULTS
Participant Characteristics
A total of 830 participants in our study had interpretable baseline and follow-up MRIs, gradable retinal photography, and were without prevalent stroke at visit 3. Of these, 157 participants had lacunes on follow-up MRI, 147 of which were incident. The median WMP over 10 years was 2.5 cm3. The number of participants with each retinal sign was as follows: AV nicking (n=108), focal arteriolar narrowing (n=121), and any retinopathy (n=50) or its components retinal hemorrhage (n=24) and microaneurysms (n=28). Other signs of retinopathy were seen less frequently. Four participants had retinopathy, AV nicking, and focal arteriolar narrowing. Participants with two MRI’s (versus only one) did not differ with respect to age, sex, or race, but had less hypertension and diabetes, and more retinopathy.
Participants with retinopathy were more likely to be male, black, hypertensive, and with diabetes than participants without retinopathy (table 1). Individuals with focal arteriolar narrowing were older than those without (62.4 vs 61.2 y, p=0.005), and more likely to have hypertension (52.9% vs 40.1%, p=0.008) than persons without focal arteriolar narrowing. Sex, race, and frequency of diabetes were not significantly different between the groups with and without focal narrowing.
Table 1.
Baseline characteristics of participants.
| Any Retinopathy (n=50) |
No Retinopathy (n=722) |
p-value | AV Nicking (n=108) |
No AV nicking (n=709) |
p-value | |
|---|---|---|---|---|---|---|
| Age, y | 60.9 (3.9) | 61.5 (4.4) | 0.39 | 62.2 (4.0) | 61.3 (4.4) | 0.04 |
| Men (%) | 48.0 | 39.4 | 0.23 | 44.4 | 39.8 | 0.36 |
| Black Race (%) | 64.0 | 45.7 | 0.01 | 57.4 | 46.5 | 0.04 |
| Study Center Jackson, MS (%) |
60.0 | 40.4 | 0.007 | 53.7 | 41.2 | 0.01 |
| Hypertension (%) | 64.0 | 41.0 | 0.001 | 50.9 | 40.7 | 0.05 |
| Cumulative SBP, mm Hg |
130.9 (18.3) | 123.1 (16.5) | 0.002 | 127.3 (16.3) | 123.2 (16.6) | 0.02 |
| Diabetes (%) | 36.0 | 11.4 | <0.001 | 13.3 | 13.1 | 0.96 |
| Blood Glucose, mg/dl |
142.9 (75.3) | 106.4 (31.5) | 0.001 | 106.7 (24.5) | 109.1 (38.3) | 0.39 |
| Ever Smoked (%) | 54.0 | 53.3 | 0.92 | 57.1 | 53.0 | 0.43 |
| Pack Years | 7.9 (16.7) | 13.7 (25.2) | 0.03 | 13.1 (23.1) | 13.3 (24.7) | 0.95 |
| Total Cholesterol, mg/dl |
212.8 (37.0) | 209.1 (37.2) | 0.50 | 205.2 (37.2) | 209.4 (37.3) | 0.28s |
| HDL Cholesterol, mg/dl |
51.5 (17.8) | 55.9 (18.9) | 0.11 | 56.1 (18.0) | 55.5 (18.8) | 0.76 |
| BMI | 28.1 (4.2) | 27.1 (4.7) | 0.14 | 27.6 (4.3) | 27.1 (4.7) | 0.25 |
| CHD (%) | 4.2 | 3.5 | 0.82 | 3.7 | 3.3 | 0.82 |
Data are mean (SD) for continuous data and % for categorical data.
SBP indicates systolic blood pressure, HDL, high-density lipoprotein; LDL, low-density lipoprotein; BMI, body mass index; CHD, Coronary Heart Disease.
WMP and Retinal Signs
Table 2 shows the results of each linear regression between WMP (defined both as a volumetric change, in cm3, and as a categorical change (an increase of at least two categories using the CHS scale), and the separate retinal variables. As a continuous variable, in separate adjusted models, continuous WMP was higher in the presence of AV nicking or any retinopathy. In contrast, when WMP was treated as an ordinal variable using the CHS rating scale, WMP was significantly associated only with AV nicking, in unadjusted models.
Table 2.
Regression models evaluating distinct signs of retinal microvascular disease, each, with MRI small vessel disease.
| Incident Lacunes (OR**) | WMP category (OR**) | WMP volume (Beta***) | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| Any retinopathy |
2.16 (1.14,4.09) |
2.31 (1.10,4.86) |
1.46 (0.77,2.77) |
1.39 (0.65,2.95) |
3.42 (1.07,5.78) |
3.47 (1.00,5.93) |
| AV Nicking |
1.94 (1.21,3.11) |
1.75 (1.04,1.14) |
1.73 (1.10,2.70) |
1.48 (0.88,2.48) |
3.57 (1.93,5.21) |
2.58 (0.24,0.51) |
| Focal Arteriolar Narrowing |
2.00 (1.28,3.14) |
1.60 (0.98,2.62) |
0.98 (0.61,1.57) |
0.87 (0.51,1.48) |
2.46 (0.89,4.03) |
1.45 (−0.13,3.03) |
| CRAE | 0.99 (0.96,1.01) |
0.99 (0.96,1.02) |
1.006 (0.98,1.03) |
1.00 (0.97,1.03) |
0.02 (−0.09,0.12) |
0.03 (−0.09,0.15) |
| CRVE | 1.00 (0.97,1.02) |
1.00 (0.97,1.04) |
1.006 (0.98,1.03) |
1.02 (0.98,1.05) |
0.02 (−0.09,0.13) |
0.05 (−0.07,0.18) |
| Microaneurysms | 1.55 (0.65,3.73) |
1.61 (0.58,4.47) |
0.99 (0.40,2.49) |
0.81 (0.27,2.43) |
2.55 (−0.56,5.65) |
2.16 (−1.18,5.51) |
| Retinal Hemorrhage |
2.53 (1.05,6.08) |
2.46 (0.87,6.93) |
2.22 (0.95,5.15) |
1.93 (0.71,5.28) |
3.33 (0.03,6.63) |
3.27 (−0.14,6.69) |
Adjusted for age, gender, race, study center, total and HDL cholesterol, BMI, pack-years, cumulative SBP, blood glucose, and presence of CHD.
Results for lacunes (incident) and categorical progression of WMP are odds ratios (OR) with 95% CI’s, since these were derived from logistic regressions.
Volume change in WMP results are beta coefficients from linear regressions (with 95% CI).
We also tested the association of retinal signs with WMP across strata defined by the presence or absence of hypertension and diabetes (Supplemental table I, II). With smaller numbers in each stratum, the associations were attenuated and fewer were statistically significant. In linear regression models for each separate retinal sign, however, the direction of the associations was similar across strata with no significant interactions between most retinal signs and hypertension or diabetes. The exception was for AV nicking, where a significant (multivariable p-interaction=0.003) interaction was found for the association of AV nicking and diabetes, with WMP.
Retinal Associations with Cumulative Microvascular Disease Score
Among the different combinations of lacunar infarcts and WMP tested for strength of association with cumulative SBP, dividing WMP into quintiles and treating lacunes as present or absent yielded the lowest AIC (Table 3); we therefore used the score resulting from this regression equation as the endpoint for evaluating the associations of small vessel disease with retinal disease. Table 4 shows the associations between retinal variables and the grouped new cumulative brain microvascular disease scores using multinomial and ordinal logistic regression. Results using the rounded scoring system from Figure 1 were identical to results using the exact score. AV nicking, focal arteriolar narrowing, and any retinopathy or its individual components microaneurysms and retinal hemorrhage were all significantly associated with composite brain microvascular disease. Associations with CRAE and CRVE remained nonsignificant.
Table 3.
Distinct models comparing WMP (cc’s) and incident lacunes (present vs absent). “WMP*Lacunes” is an interaction term. Cumulative SBP is regressed onto each set of variables in separate models. Akaike Information Criteria (AIC) scores derived from likelihood ratio tests comparing model to a null model with no independent variables. Quintiles of WMP + Lacunes selected for lowest AIC.
| Model | AIC |
|---|---|
| WMP | 6557 |
| Lacunes | 6657 |
| WMP + Lacunes | 6447 |
| WMP + Lacunes + WMP*Lacunes | 6442 |
| Quintiles of WMP + Lacunes | 6429 |
| Quartiles of WMP + Lacunes | 6431 |
| Sextiles of WMP + Lacunes | 6431 |
Table 4.
Multinomial and ordinal logistic regression results for each retinal disease phenotype, comparing higher scores on the cumulative brain microvascular disease with the lowest possible scores. Adjusted as in Table 2. Odds ratios (95% CI) are displayed. A score of 0–3 is the reference group.
| Multinomial logistic regression | Unadjusted ordinal logistic regression |
Adjusted Ordinal logistic regression |
||||
|---|---|---|---|---|---|---|
| Retinal finding | Score 0–3 | Score 5–6 | Score 8–9 | Score 11–14 | OR per higher category |
OR per higher score category |
| Any retinopathy | 1.0 | 0.86 (0.25–2.97) | 3.57 (1.26–10.13) | 4.21 (1.54–11.45) | 2.78 (1.63–4.73) | 3.18 (1.71–5.89) |
| AV nicking | 1.0 | 0.57 (0.25–1.29) | 1.04 (0.50–2.17) | 2.31 (1.26–4.26) | 2.65 (1.78–3.94) | 1.93 (1.24–3.02) |
| Focal Arteriolar Narrowing |
1.0 | 1.71 (0.91–3.21) | 2.45 (1.29–4.65) | 2.08 (1.12–3.86) | 1.92 (1.35–2.73) | 1.76 (1.19–2.59) |
| CRAE/10 | 1.0 | 1.00 (0.96–1.04) | 0.97 (0.93–1.01) | 0.98 (0.94–1.02) | 0.99 (0.97–1.01) | 0.98 (0.96–1.01) |
| CRVE/10 | 1.0 | 0.99 (0.95–1.04) | 1.01 (0.97–1.05) | 1.02 (0.98–1.06) | 1.00 (0.98–1.02) | 1.01 (0.99–1.03) |
| Microaneurysms | 1.0 | 0.34 (0.05–2.19) | 3.24 (0.78–13.38) | 3.39 (0.89–12.88) | 2.59 (1.29–5.21) | 3.06 (1.33–7.07) |
| Retinal Hemorrhage | 1.0 | 0.92 (0.18–4.74) | 2.55 (0.58–11.21) | 4.20 (1.07–16.52) | 2.85 (1.34–6.06) | 3.02 (1.27–7.20) |
DISCUSSION
In our study, we aimed to improve the characterization of brain microvascular disease as an outcome so that we would be more likely to detect associations with signs of retinal disease. We predicted that by (1) converting WMP into a continuous measurement rather than an ordinal score and (2) combining WMP and lacunar infarcts into a cumulative brain microvascular disease score, more retinal signs would be significantly associated with brain microvascular disease in a manner meeting a priori expectations. Indeed, more retinal signs were associated with brain microvascular disease using these methods than has been shown in previous studies. WMP alone was associated with AV nicking and with any retinopathy. The cumulative score was associated with AV nicking, focal arteriolar narrowing, and any retinopathy as well as its components.
A previous ARIC study evaluated retinal microvascular pathology in association with silent lacunar infarcts and WMP as separate outcomes8. WMP (defined as an increase in the CHS grade of 2 or more points between visit 3 and the follow-up MRI) was only associated with AV nicking. Lacunar infarcts were associated with AV nicking, microaneurysm, retinal hemorrhage, and retinopathy, but not with focal arteriolar narrowing. These findings can be explained by a lower number of WMP events and lacunes coupled with the infrequency of retinal changes, limiting the power to detect associations with WMP. By using a quadratic model to impute white-matter volume from the CHS scores,10 this created a measure of WMP with a greater ability to discriminate small changes in disease burden, allowing us to detect the additional association between any retinopathy and WMP. Our cumulative brain microvascular disease score also allows us to identify associations between brain microvascular disease and retinal changes not previously identified, by combining lacunar infarcts and WMP.
Other groups have proposed combining lacunar infarcts and white matter disease into one measure. In the Women’s Health Initiative16, Haan et al. summed volumes of lacunar infarcts and white matter disease across different brain regions, combining both types of brain microvascular disease. Our method of combining lacune and WMP information involved exploring the relative strength of associations of different combinations with SBP. Although our definition of lacune does not exactly match that recently recommended by the STRIVE group1(we used a maximum lacunar size of 20 mm, vs 15 mm in STRIVE), we have used it consistently throughout the study, allowing for assessment of incident lacunes over time.
We believe our combined index represents a measure of brain microvascular disease reflecting the shared pathophysiology between these two MRI-detected signs. This score is therefore more likely to detect true risk factors for brain microvascular disease than when the risk factors are evaluated with either lacunar infarcts or white matter disease alone. Moreover, if this score is a more sensitive measure of microvascular disease as an exposure, it might have greater value as a risk factor for cognitive decline, incident stroke, and stroke prognosis. If retinal microvascular changes precede progression of cerebral microvascular disease, this might indicate a point at which aggressive preventive therapies (e.g. aimed at control of hypertension or diabetes) might be implemented which could even lead to reduction in brain microvascular disease, and possibly in turn, in mild cognitive impairment and dementia. Future studies might evaluate the utility of retinal screening as an indicator of a need for more aggressive vascular risk factor control.
Our score includes the MRI components (lacunes, WMP) that are most strongly driven by microvascular disease. We did not include measures of cerebral atrophy or enlarged perivascular spaces, although these may also represent a form of cerebral small vessel disease.17,18 The study of retinal microvascular disease in association with cerebral atrophy might allow for a better understanding of the vascular contribution to cerebral atrophy, but atrophy is not pathognomonic for microvascular disease, and, although it might be at least partially due to small vessel disease, it is probably a multifactorial process. We were not able to evaluate progression of microbleeds, which is another likely sign of brain microvascular disease, as evaluation for these had not been included in the MRI scans presented here.
Although we controlled for the major cardiovascular risk factors in our regression models, unmeasured confounders may still exist. In addition, the sample of participants who were included and underwent two MRI’s had less retinopathy and less comorbidity than those with only one MRI- however, this might dilute the true findings, so our estimates would be conservative. Measurements of our neuroimaging endpoints could also be a limitation. By imputing volumes at the visit 3 MRI, there is a possibility of measurement error or erroneous predicted associations. Moreover, we were only able to define lacunes as present or absent, and, disallowed anyone with any lacune at visit 3 from having incident lacunes at the follow-up MRI, although additional lacunes could in fact be incident. Lastly, we validated our cumulative brain microvascular disease score (via strength of association with cumulative SBP) using the same dataset that we then used to test associations between our score and retinal disease. Ideally, we would validate this in a separate sample in the future. It is possible that by finding a score that is highly associated with SBP, we are only representing one type of small vessel disease. Future evaluation of composite scores might evaluate them in regards to other vascular risk factors as well.
SUMMARY
These data are consistent with previous reports that retinal microvascular signs predict white matter disease and lacunar infarcts. In treating WMP as a continuous measure and combining it with lacunar infarcts, we see associations between brain microvascular disease and retinal signs that, though expected based on common pathophysiology, were not seen before. The cumulative brain microvascular disease score that we developed could be a useful research tool in further studies seeking to elaborate on the risk factors and outcomes associated with lacunar infarcts and white matter disease.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding: The Atherosclerosis Risk in Communities Study is a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Brain MRI examinations were funded by R01-HL70825.
Footnotes
Disclosures: None.
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 3.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005;206:319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker ML, Hand PJ, Wang JJ, Wong TY. Retinal signs and stroke: Revisiting the link between eye and brain. Stroke. 2007;39:1371–1379. doi: 10.1161/STROKEAHA.107.496091. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 6.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, et al. Retinal microvascular abnormalities and mri-defined subclinical cerebral infarction: The atherosclerosis risk in communities study. Stroke. 2006;37:82–86. doi: 10.1161/01.STR.0000195134.04355.e5. [DOI] [PubMed] [Google Scholar]
- 7.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurology. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 8.Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: A prospective study. Brain. 2010;133:1987–1993. doi: 10.1093/brain/awq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, et al. Magnetic resonance abnormalities cardiovascular disease in older adults The cardiovascular health study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, et al. Blood pressure and white matter disease progression in a biethnic cohort: Atherosclerosis risk in communities (aric) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith EE. Leukoaraiosis and stroke. Stroke. 2010;41:S139–143. doi: 10.1161/STROKEAHA.110.596056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silbert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: White matter hyperintensity progression matters. Neurology. 2009;73:120–125. doi: 10.1212/WNL.0b013e3181ad53fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/ sclerosis in the atherosclerosis risk in communities study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Sharrett AR, Klein BE, Chambless LE, Cooper LS, Hubbard LD, et al. Are retinal arteriolar abnormalities related to atherosclerosis? The atherosclerosis risk in communities study. Arterioscler. Thromb. Vasc. Biol. 2000;20:1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 15.Couper DJ, Klein R, Hubbard LD, Wong TY, Sorlie PD, Cooper LS, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics” the atherosclerosis risk in communities study. Am. J. Ophthalmol. 2002;133:78–88. doi: 10.1016/s0002-9394(01)01315-0. [DOI] [PubMed] [Google Scholar]
- 16.Haan M, Espeland MA, Klein BE, Casanova R, Gaussoin SA, Jackson RD, et al. Cognitive function and retinal and ischemic brain changes: The women’s health initiative. Neurology. 2012;78:942–949. doi: 10.1212/WNL.0b013e31824d9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grau-Olivares M, Arboix A, Junque C, Arenaza-Urquijo EM, Rovira M, Bartres-Faz D. Progressive gray matter atrophy in lacunar patients with vascular mild cognitive impairment. Cerebrovasc. Dis. 2010;30:157–166. doi: 10.1159/000316059. [DOI] [PubMed] [Google Scholar]
- 18.Duering M, Righart R, Csanadi E, Jouvent E, Herve D, Chabriat H, et al. Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology. 2012;79:2025–2028. doi: 10.1212/WNL.0b013e3182749f39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.