Abstract
Background
Sexually transmitted infection (STI) diagnosis following diagnosis of acute HIV infection (AHI) indicates ongoing high-risk sexual behavior and possible risk of HIV transmission. We assessed predictors of STI acquisition and the effect of time since care entry on STI incidence in AHI patients in care and receiving consistent risk-reduction messaging.
Methods
Data on incident gonorrhea, chlamydia, trichomoniasis, primary/secondary syphilis, demographic, and clinical risk factors were abstracted from medical charts for patients diagnosed with AHI and engaged in care. Poisson regression models using generalized estimating equations were fit to estimate incidence rates (IR), incidence rate ratios (IRR), and robust 95% confidence intervals (CI).
Results
Among 185 AHI patients, 26 (14%) were diagnosed with ≥1 incident STI over 709.4 person-years; 46 STIs were diagnosed during follow-up (IR=6.8/100 person-years). The median time from HIV care entry to first STI diagnosis was 609 days (range=168–1681). Men who have sex with men (MSM) (p=0.03), a shorter time between presentation to medical care and AHI diagnosis (p=0.06), and STI diagnosis prior to AHI diagnosis (p=0.0003) were predictors of incident STI. STI IR >1 year after entering care was double that of patients in care ≤1 year (IRR=2.0 95% CI 0.8–4.9). HIV viral load was above the limits of detection within 1 month of 11 STI diagnoses in 6 patients (23.1%) (median=15,898 copies/mL, range=244–152,000 copies/mL).
Conclusions
Despite regular HIV care, STI incidence was high among this primarily young, MSM AHI cohort. Early antiretroviral initiation may decrease HIV transmission given ongoing risk behaviors despite risk-reduction messaging.
Keywords: acute HIV infection, sexually transmitted infection, incidence, antiretroviral therapy, HIV care
Introduction
Acute HIV infection (AHI) contributes disproportionately to HIV transmission,1–4 with elevated levels of HIV virus in blood and semen during AHI providing a plausible biological mechanism for efficient sexual transmission of disease.5,6 Elevated co-infection rates with sexually transmitted infections (STIs),7,8 increased numbers of partnerships,9,10 and ongoing high risk sexual behavior4,11,12 during AHI facilitate increase disease transmission. Prompt linkage and engagement in HIV clinical care, particularly following diagnosis of AHI, is paramount to reducing HIV transmission. Awareness of HIV diagnosis can alter behavior, whereby individuals limit their sexual encounters to partners whom they believe to be HIV-positive (serosorting) and decrease the number of sexual partners.11,13 Furthermore, initiation of effective antiretroviral therapy (ART) following linkage and engagement in care during AHI can reduce transmission given evidence of rapid viral suppression.14 However, some persons diagnosed with AHI maintain high risk sexual behaviors after diagnosis, including failure to use condoms.11,12 Accordingly, secondary prevention interventions during AHI remain critical given the increased infectiousness during this period and the temporal relation to behaviors leading to HIV acquisition.
By definition, individuals diagnosed with AHI become aware of their status very early following acquisition. The high risk of transmission during AHI and the inherent proximity to risk factors leading to HIV acquisition warrant evaluation of sexual behavior during and following diagnosis. An incident STI following HIV diagnosis can be used as a biomarker of high risk sexual behavior and possible risk of HIV transmission.7,15 In this study, we estimated STI incidence rates among a cohort of individuals diagnosed with AHI and receiving care between 1998 and 2012 in two clinics located in the Southeastern United States, to assess ongoing high risk sexual behavior. We also assessed predictors of STI acquisition while receiving care and the effect of time since entry into care on STI incidence.
Materials and Methods
Study Population and Design
We performed a retrospective cohort evaluation of STI incidence among all persons diagnosed with AHI between January 1, 1998 and March 1, 2012 linking to care at the University of North Carolina (UNC) or Duke University, and enrolling in an open-label treatment study within 30 days of AHI diagnosis and/or an AHI observational study as previously described.14,16 AHI was defined as a negative or indeterminate enzyme immunoassay or a negative HIV RNA test within 45 days of study enrollment plus reproducibly detectable HIV RNA by amplification methods. Patients enrolled in the observational cohort study typically maintain contact with HIV care providers at UNC or Duke University at least once every six months. During study visits, patients receive consistent prevention messaging, including targeted discussions with their provider and trained study coordinators about condom use, sexual and needle-sharing partners, and drug and alcohol use. Only patients with at least 12 weeks of follow-up through June 30, 2012 were included in this analysis.
Data Collection: Patient Information
Basic demographic and clinical information was collected on all persons enrolled in study, including age at diagnosis, gender, race (African-American [AA], other race), sexual risk group (female, heterosexual male, or men who have sex with men [MSM]), receipt of ART within 45 days of enrollment, CD4 cell count at enrollment, and viral load (VL) at enrollment. History of STI diagnosis prior to AHI diagnosis, including, Neisseria gonorrhoeae (GC), Chlamydia trachomatis (CT), Trichomonas vaginalis (TV), primary, secondary, or early latent syphilis, Condyloma (warts), herpes simplex virus, Hepatitis B, Pelvic Inflammatory Disease, and Nongonococcal Urethritis, was recorded. We also collected information on the time from first presentation to a health care facility with AHI-related illness to AHI diagnosis and the calendar year of AHI diagnosis.
Data Collection: STI Testing
STI testing and diagnosis dates for GC, CT, TV, and primary/secondary syphilis after enrolling in care were abstracted from medical and study charts for patients included in this analysis at UNC and Duke University. Diagnoses were based on clinical and laboratory data obtained as a part of routine HIV clinical care. Enrollment in the open-label treatment study included screening for GC, CT and syphilis. However, STI testing during follow-up was not part of study protocol for the treatment or observational studies. Screening varied among providers and was done as part of clinical care. Per study protocols, patients were also asked at each visit about intervening care and treatment, including STI screening, by outside providers. Verified STI testing results from these outside facilities were routinely requested and included in this analysis.
To limit the misclassification of prevalent STIs at the time of AHI diagnosis as incident STIs, we restricted STI testing to at least 28 days after study enrollment. An incident GC or CT diagnosis was defined as a positive cervical, urethral, rectal or urine test by culture or nucleic acid amplification. For TV, incident diagnoses included those with a clinical diagnosis supplemented by a positive wet mount or the presence of T. vaginalis on Papanicolaou smear or urine PCR. Incident primary/secondary syphilis were identified by a reactive non-treponemal test (RPR) confirmed by a treponemal test (MHA-TP). A clinician reviewed all data to confirm incident STI diagnosis. We included positive STI tests for which incidence could be verified (positive tests with a record of a prior negative test at or after AHI diagnosis or presence of STI-related symptoms at the time of the positive test). For syphilis only, positive tests in which the stage of disease could not be determined from RPR and/or MHA-TP results were excluded.
Statistical Analyses
We used χ2 and Wilcoxon rank-sum tests to compare demographic and clinical characteristics of patients with at least 1 incident STI to those who did not acquire a STI during follow-up. Patients were followed from date of enrollment on the treatment or observational study to the first of the following: administrative censoring at the last date of STI ascertainment (June 30, 2012) or loss to follow-up (defined as 8 months without a clinic or study visit from the last date of any clinical activity). Because a person could have more than 1 STI, Poisson regression models, using generalized estimating equations and an exchangeable correlation matrix, were fit to estimate incidence rates (IR), incidence rate ratios (IRR), and robust 95% confidence intervals (CI) for all STIs and by age, race, gender, sexual risk group, calendar year of diagnosis, and history of a STI diagnosis prior to AHI diagnosis. We repeated this analysis to assess differences in screening rates.
We also estimated the effect of time since entering care on STI incidence. Time since HIV care entry was categorized as ≤1, 2, 3, 4, and ≥5 years, based on minimizing heterogeneity within categories, preserving power to provide robust estimates, and allowing a longitudinal representation. Based on a review of the literature and a construction of a directed acyclic graph, we adjusted our model for sexual risk group and a history of STI diagnosis prior to AHI diagnosis. All statistical analyses were conducted in SAS version 9.3 (Cary, NC). The study was approved by the UNC and Duke University Institutional Review Boards. All participants provided written informed consent.
Results
Between January 1, 1998 and March 1, 2012, 185 AHI diagnoses enrolled in a treatment or longitudinal study at UNC or Duke University. The majority of participants were AA (57%, N=106) and male (88%, N=162). Among the men, 82% (N=135) self-identified as MSM. The median age at diagnosis was 26 years (IQR 22–36). At enrollment, the median baseline CD4 cell count was 489 cells/mm3 (IQR 343–674) and the median VL was 457,895 copies/mL (IQR 78,115–2,030,000). Patients were followed for a median of 3.3 years (IQR 1.8–5.5) and contributed 709.4 person-years of observation.
A total of 591 laboratory tests for STIs in 113 patients were performed during follow-up, (116 GC, 116 CT, 3 TV, and 356 primary/secondary syphilis tests). The median time to the first STI test was 216 days (IQR 112–520). Seventy-two (39%) patients had no recorded history of STI testing during follow-up. Statistically significant differential screening rates were observed among patients with a history of a STI diagnosis (IRR=2.3, 95% CI 1.5–3.6), ≤25 years (IRR=0.4, 95% CI 0.3–0.7), and who self-identified as MSM (IRR=2.0, 95% CI 1.1–3.5).
Twenty-six (14%) patients were diagnosed with at least 1 STI during follow-up and 9 (35%) of who were diagnosed with >1 STI (range 2–7). Nearly 80% of patients diagnosed with an incident STI had previously been diagnosed with a STI and one-third had a bacterial STI diagnosis in the 8 weeks preceding their AHI diagnosis. Patients diagnosed with at least 1 STI were more likely to be MSM, experience a shorter time period between first presentation to a healthcare facility and AHI diagnosis, and have a history of STI diagnoses than patients without an incident STI (Table 1). A total of 3 incident STIs were diagnosed in 2 individuals who did not identify as MSM. Patients diagnosed with an incident STI were followed longer than those not diagnosed with an incident STI (Table 1). When considering only patients with a record of STI testing, the follow-up time for patients without an incident STI diagnosis increased (median 3.5 years (IQR 2.4–5.8)) and the difference between groups became statistically non-significant (p=0.3). The median time to first incident STI was 609 days (range 168–1681) after enrollment.
Table 1.
Patient demographic and clinical characteristics stratified by acquisition of an incident sexually transmitted infection (STI)
| ≥ 1 STI | No STI | ||||
|---|---|---|---|---|---|
| N | % | N | % | p | |
| TOTAL | 26 | 14.1% | 159 | 85.9% | |
| Sexual Risk Group | |||||
| Female | 1 | 3.8% | 22 | 13.8% | 0.03 |
| Heterosexual Male | 1 | 3.8% | 26 | 16.4% | |
| MSM | 24 | 92.3% | 111 | 69.8% | |
| Dichotomized Race/Ethnicity | |||||
| African-American | 17 | 65.4% | 89 | 56.0% | 0.4 |
| Non-African American | 9 | 34.6% | 70 | 44.0% | |
| History of STI Diagnosis | |||||
| Yes | 20 | 79.9% | 62 | 39.0% | 0.0003 |
| No | 6 | 23.1% | 97 | 61.0% | |
| On Antiretroviral Therapy within 45 Days of HIV Diagnosis | |||||
| Yes | 19 | 73.1% | 122 | 76.7% | 0.7 |
| No | 7 | 26.9% | 37 | 23.3% | |
| Site | |||||
| Duke | 6 | 23.1% | 73 | 45.9% | 0.003 |
| UNC | 20 | 76.9% | 86 | 54.1% | |
| Calendar Year Diagnosed with HIV | |||||
| 1998–2002 | 1 | 3.8% | 19 | 11.9% | 0.4 |
| 2003–2005 | 5 | 19.2% | 35 | 22.0% | |
| 2006–2008 | 11 | 42.3% | 51 | 32.1% | |
| 2009–2012 | 9 | 34.6% | 54 | 34.0% | |
| Age (years) | |||||
| Median [Interquartile Range (IQR)] | 24 | (21–32) | 26 | (22–36) | 0.3 |
| Time Presentation to Diagnosis (days) | |||||
| Median (IQR) | 0 | (0–2) | 1 | (0–7) | 0.06 |
| Initial viral load (VL) (copies/mL) | |||||
| Median (IQR) | 723,365 | (92,581–1,846,596) | 439,884 | (73,296–2,251,415) | 0.5 |
| Initial CD4 (cells/mL) | |||||
| Median (IQR) | 413 | (311–629) | 492 | (354–686) | 0.2 |
| Follow-up Time (years) | |||||
| Median (IQR) | 4.1 | (3.3–5.7) | 2.9 | (1.5–5.5) | 0.03 |
During follow-up, 46 STIs were diagnosed, including 21 cases of primary/secondary syphilis, 15 GC, 9 CT, and 1 TV. Five additional positive STI results were excluded because timing of STI acquisition could not be determined (3 syphilis and 2 GC diagnoses). This resulted in an overall incidence rate of 6.8 diagnoses/100 person-years (95% CI 4.2–11.0) (Table 2). The highest STI-specific incidence rate was identified for syphilis (2.9 diagnoses/100 person-years).
Table 2.
Crude Incidence Rates (IR) and Robust 95% Confidence Intervals (CI)
| Events | Incidence Rate* |
Robust 95% CI |
|
|---|---|---|---|
| All Incident STIs | 46 | 6.8 | (4.2–11.0) |
| By Disease | |||
| Chlamydia | 9 | 1.3 | (0.6–2.8) |
| Syphilis | 21 | 2.9 | (1.8–4.8) |
| Gonorrhea | 15 | 2.2 | (1.0–4.7) |
| Trichomonias | 1 | 0.1 | (0.0–0.9) |
Rates and robust 95% CI were calculated using an exchangeable correlation matrix. Rates are based on 709.2 person-years of follow-up and are presented per 100 person-years
STI IRs varied by demographic and clinical categories (Table 3). Patients with a history of STI diagnoses were more likely to be diagnosed with an incident STI during follow-up than those with no history (IRR 3.0 95% CI 1.0–9.6). IRs were also highest among AA (IRR 1.9, 95% CI 0.7–5.6), while rates were lower among older (>25 years) patients (IRR 0.2 95% CI 0.1–0.6). IRs increased monotonically by calendar year of AHI diagnosis, ranging from 1.7 (95% CI 0.2–11.6) if diagnosed between 1998–2002 to 10.4 (95% CI 4.6–23.3) if diagnosed between 2009–2012, despite constant STI testing across diagnosis-year cohorts. During follow-up, the proportion of all AHI infections that were among persons aged ≤25 years increased from approximately 45% in 1998–2002 to 62% in 2009–2012 (p=0.05). The STI IR within this younger population was larger than that in the cohort as a whole at every time point, except 2003–2005 (Table 3).
Table 3.
Sexually transmitted infection (STI) incidence rates (IR), incidence rate ratios (IRR), and robust 95% confidence intervals (CI) stratified by patient demographic and clinical characteristics
| N | Person- Years |
IR* | Robust 95% CI |
IRR | Robust 95% CI |
|
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤25 years | 34 | 284.6 | 11.8 | (6.6–21.3) | REF | |
| >25 years | 12 | 424.8 | 2.8 | (1.5–5.1) | 0.2 | (0.1–0.6) |
| Dichotomized Race/Ethnicity | ||||||
| Non-Black | 17 | 386.7 | 2.6 | (5.1–15.7) | REF | |
| Black | 29 | 322.8 | 4.7 | (1.9–11.6) | 1.9 | (0.7–5.6) |
| Sexual Risk Group | ||||||
| Female | 2 | 82.6 | 1.9 | (0.3–11.8) | REF | |
| Heterosexual Male | 1 | 133.1 | 0.7 | (0.1–4.6) | 0.4 | (0.0–5.3) |
| MSM | 43 | 493.8 | 8.9 | (5.4–14.6) | 4.8 | (0.7–32.5) |
| History of STI Diagnosis | ||||||
| No | 14 | 420.3 | 3.7 | (1.3–10.4) | REF | |
| Yes | 32 | 289.4 | 11.2 | (6.6–18.8) | 3.0 | (1.0–9.6) |
| On Antiretroviral Therapy within 45 Days of HIV Diagnosis | ||||||
| No | 16 | 127.2 | 11.0 | (4.2–29.0) | REF | |
| Yes | 30 | 582.3 | 5.7 | (3.3–9.7) | 0.5 | (0.2–5.6) |
| Calendar Year Diagnosed with HIV | ||||||
| 1998–2002 | 2 | 148.6 | 1.7 | (0.2–11.6) | 0.6 | (0.1–4.9) |
| 2003–2005 | 6 | 197.3 | 2.8 | (1.2–6.5) | REF | |
| 2006–2008 | 24 | 240.8 | 9.4 | (4.6–19.2) | 3.3 | (1.1–9.9) |
| 2009–2012 | 14 | 122.7 | 10.4 | (4.6–23.3) | 3.7 | (1.1–11.7) |
| Calendar Year Diagnosed with HIV (≤25 years only) | ||||||
| 1998–2002 | 2 | 62.6 | 3.9 | (0.6–25.8) | 2.9 | (0.2–40.7) |
| 2003–2005 | 1 | 62.8 | 1.3 | (0.2–8.5) | REF | |
| 2006–2008 | 19 | 87.6 | 19.3 | (5.6–43.2) | 14.4 | (1.9–107.1) |
| 2009–2012 | 12 | 71.7 | 15.2 | (6.1–37.6) | 11.3 | (1.5–87.9) |
| Time Since Entry into Care by Year | ||||||
| ≤1 year | 7 | 177.0 | 3.9 | (1.6–9.7) | REF | |
| 2 years | 15 | 145.9 | 10.0 | (5.3–18.8) | 2.5 | (1.0–6.4) |
| 3 years | 13 | 118.3 | 10.5 | (5.5–20.3) | 2.7 | (0.9–7.7) |
| 4 years | 8 | 87.3 | 8.7 | (3.6–20.8) | 2.2 | (0.7–7.4) |
| ≥5 years | 3 | 181.0 | 2.4 | (0.8–7.3) | 0.6 | (0.2–2.3) |
| Dichotomized Time Since Entry into Care | ||||||
| ≤1 year | 7 | 177.0 | 3.9 | (1.6–9.7) | REF | |
| >1 year | 39 | 532.4 | 7.9 | (4.7–13.2) | 2.0 | (0.8–4.9) |
Rates and robust 95% CI were calculated using an exchangeable correlation matrix. Rates are presented per 100 person-years
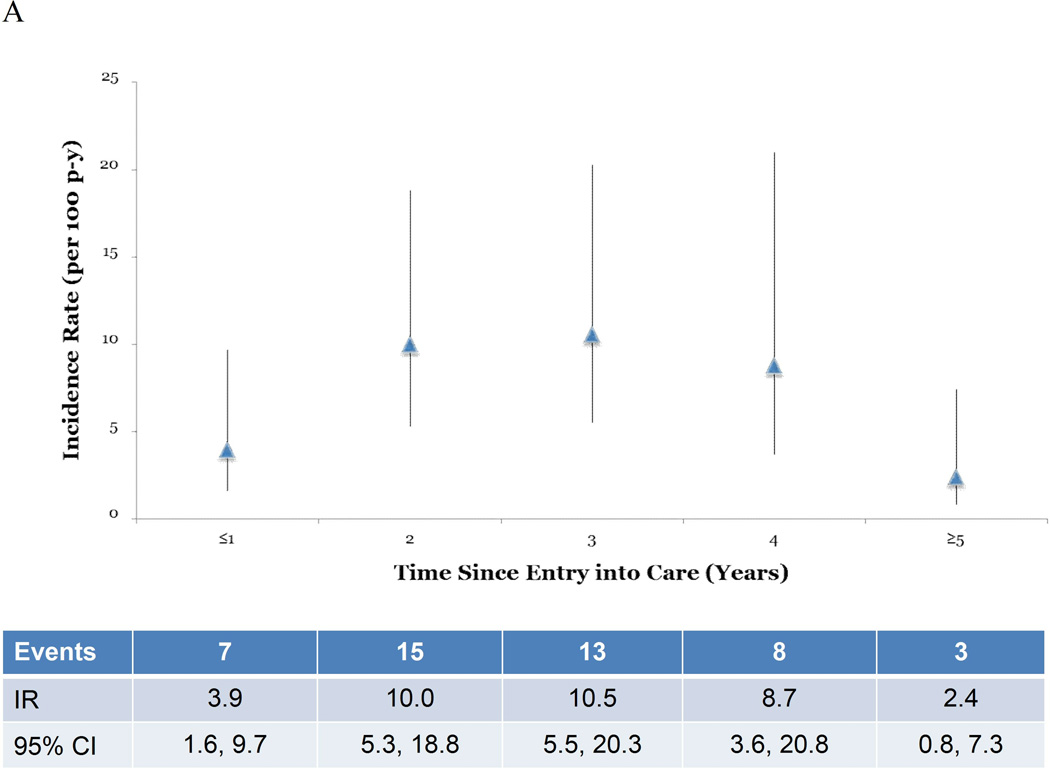
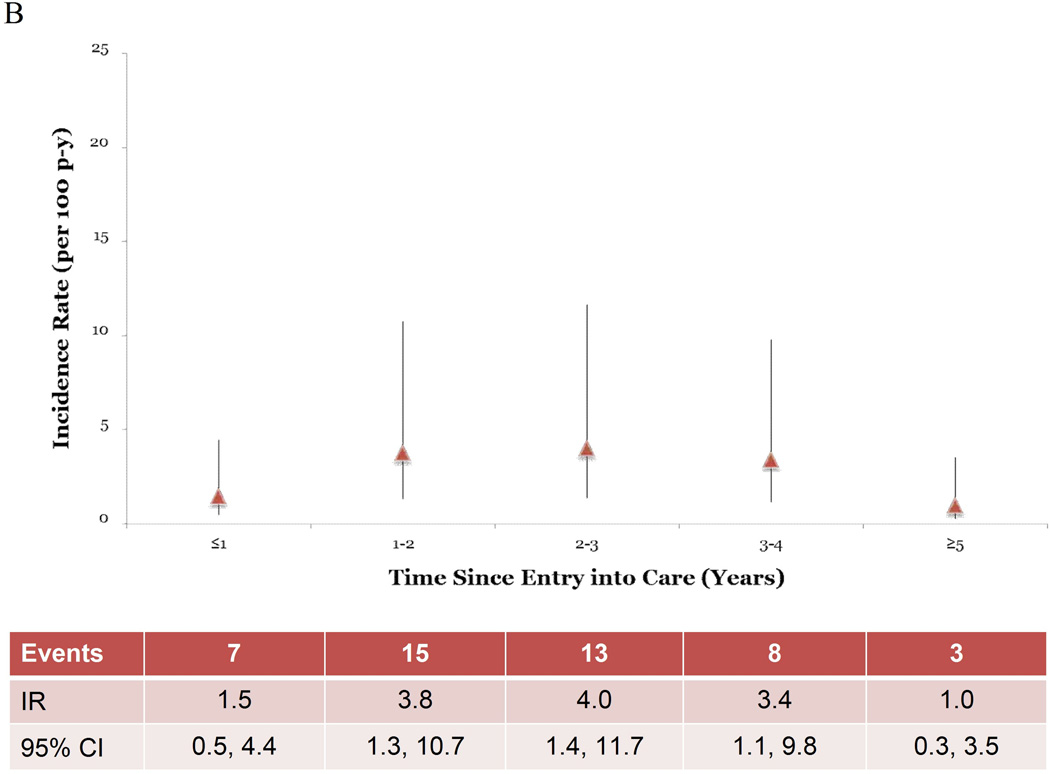
STI IRs increased as the time following entry into care increased. For all STIs combined, the crude IR increased through 3 years after enrollment, at which point rates began declining (Figure 1A). This same trend was observed in point estimates in multivariable analysis controlling for history of STI diagnosis and sexual risk group, although the point estimates were attenuated (Figure 1B). When the time since entering care was dichotomized at 1 year after AHI diagnosis, the STI IR >1 year after entering care was double that of patients in care ≤1 year (IRR=2.0 95% CI 0.8–4.9) (Table 3). This comparison in both the unadjusted rates (p=0.1) and the rates adjusted for history of STI diagnosis and sexual risk group (p=0.1) were not statistically significant.
Figure 1.
Incidence rates (IR) and robust 95% confidence intervals (CI) by time since entry into care. Figure 1A shows unadjusted IR and robust 95% CI over the study period. Figure 1B shows the IR and robust 95% CI adjusted for sexual risk group and history of a STI diagnosis.
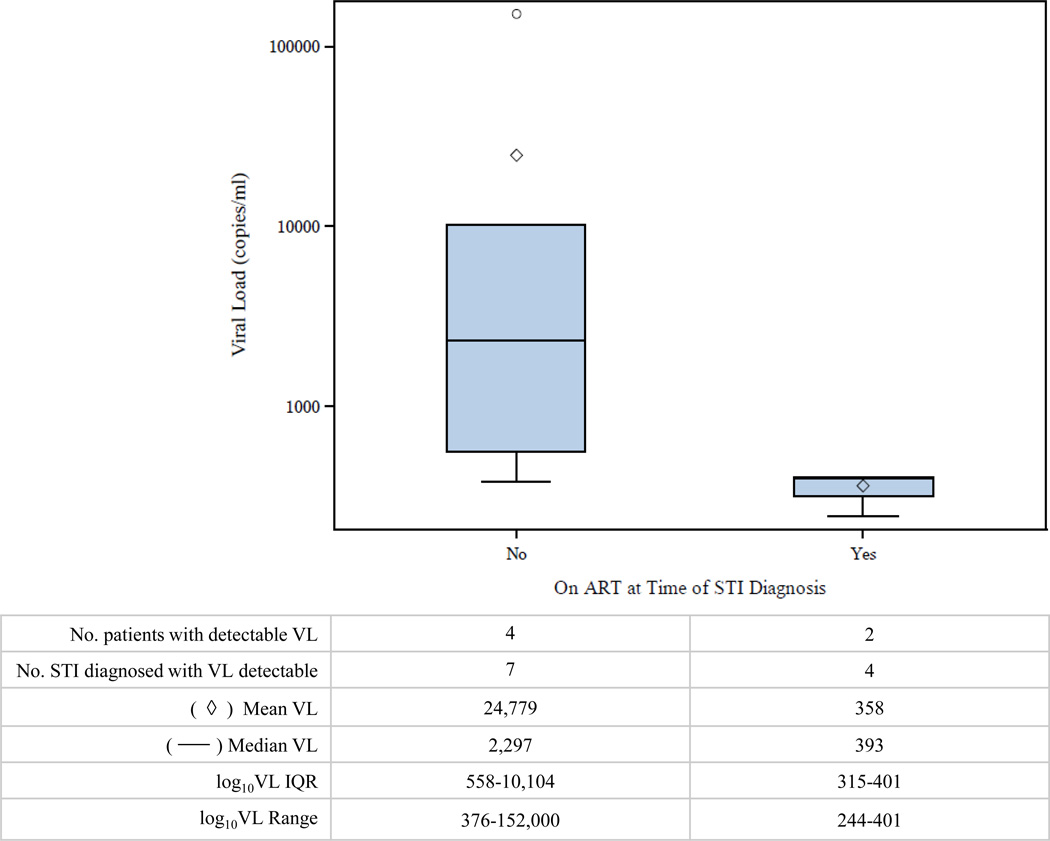
The most proximate VL to the date of incident STI diagnosis was above detectable limits (>50 copies/mL) at the time of 11 STI diagnoses in 6 patients (median=2 STIs/person; range 1–4 STIs/person). All detectable VLs were within 1 month of STI diagnoses. The median detectable VL at the time of incident STI diagnosis was 15,898 copies/mL (range 244–152,000). A total of 7 (64%) STIs diagnosed with a detectable VL occurred while the patient was not on ART. In 4 of 7 STI diagnoses in patients not on ART, an increase in VL was observed at the time of the STI diagnoses from the previous VL. The median detectable VL was lower for STIs diagnosed in patients on ART compared to patients not on ART (393 versus 2,297 copies/mL) (Figure 2). For patients on ART at the time of incident STI diagnosis, the median time from ART initiation to STI diagnosis was 278 days (range 208 to 939).
Figure 2.
Viral load (VL) values of patients with detectable HIV RNA (>50 copies/mL) at the time of STI diagnosis by antiretroviral treatment status.
Discussion
Our results suggest that despite regular assessment and counseling on risk behaviors during routine study and care visits, STI incidence was high, at a rate of 6.8 diagnoses/100 person-years, among this primarily young, MSM cohort diagnosed with AHI. Only 7 of 46 STIs were diagnosed in the first year after AHI diagnosis. The median time to the first STI diagnosis in this population was over 1 year, which suggests a change in sexual behavior in the period immediately following AHI diagnosis. STI incidence >1 year after entering care was approximately double that of patients who had been in care for ≤1 year. The results suggest IRs increased yearly after entering care, peaking at approximately 3 years. However, CIs were wide and overlapped at each time period. The decrease in STI IRs observed in this cohort ≥4 years after enrollment is at least partially due to fewer patients being followed for longer time points.
Previous studies have shown similar risk behavior modifications upon notification of HIV diagnosis, including a decline in unprotected anal intercourse, an increase in serosorting practices, and a reduction in the number of partners.10–12,17 Persons newly-diagnosed with AHI often experience illnesses related to seroconversion and/or a period of psychological adjustment to their diagnosis. This phase is temporary, and presumably any behavior modifications associated with becoming aware of one’s HIV diagnosis could attenuate over time.11
As evidenced by high rates of STI diagnoses prior to AHI diagnosis, patients in this cohort appear to return to risk behaviors they engaged in prior to their AHI diagnosis, despite consistent, directed risk-reduction messaging during HIV care visits. Interventions to maintain longer-term behavior change and prevent onward HIV transmission are needed in persons with a history of STI diagnoses, and may need to focus on different contextual and situational factors that influence young MSM, such as increased use of the internet to meet partners and high rates of STIs in sexual networks.18–20
Approximately one-quarter of patients diagnosed with a STI during follow-up were viremic within 1 month of STI diagnosis. Viremia was expectedly higher among those not on ART as compared to those on treatment. Given that STIs have been found to increase HIV concentrations in genital lesions and semen, thereby amplifying the potential of transmission,21 it is important to ensure all HIV-infected patients, regardless of CD4 cell count, are treated with ART in a timely manner.22 ART during AHI can rapidly suppress viremia and protect sexual partners from becoming infected with HIV despite on-going high risk sexual behavior.
This study has several limitations. First, both the sample size and the number of patients with a STI during follow-up were relatively small, limiting our power to assess the association of time since AHI diagnosis and incident STI. Second, the study may not be generalizable beyond the Southeastern United States. Third, this study was conducted as a retrospective, secondary analysis of an observational and open-label treatment study whose primary aims did not include the assessment of incident STI diagnoses. STI screening at follow-up visits was not standardized, but rather ordered as clinically determined by individual HIV providers. Further, differential screening rates among young MSM with a history of STI diagnoses may have contributed to higher STI IRs observed in this sub-population. Because patients can screen and be diagnosed with STIs at locations other than Duke University and UNC Infectious Disease Clinics, we could not achieve complete case ascertainment. Despite our efforts to include all lab verified STI testing and diagnoses performed at outside sites, it is likely that the estimates provided underestimate the true IR in this population. A similar assessment performed on the entire HIV patient population (both acutely- and chronically-infected patients) receiving care at UNC estimated the STI incidence as 2.7 cases/100 person-years (95% CI 2.3–3.1) (unpublished data). This was markedly lower than the likely underestimated IRs provided in this analysis, suggesting STIs cause considerable morbidity in persons diagnosed during AHI. As recommended by the Centers for Disease Control,23 regular screening of STIs among patients diagnosed during AHI and in care is important, as this population continues to engage in risk behaviors at a higher rate than persons diagnosed at later stages of disease.
Finally, this study is limited by the lack of data on the HIV status of the partners of AHI patients, preventing any conclusions about onward transmission of HIV. It is possible that some patients were practicing serosorting behaviors and acquired STIs from HIV-positive partners. The majority of STI diagnoses occurred in patients on treatment with undetectable VLs. Previous research indicates an association between a person’s belief that an undetectable VL reduces infectiousness and engaging in unprotected anal intercourse, regardless of the partner’s HIV status.24 This phenomenon could reduce transmission of HIV, but fail to protect against the transmission of other STIs. Additional messaging is needed during HIV care visits to inform patients about continued risk of STI acquisition with serosorting and unprotected sex while undetectable, despite reductions in HIV transmission risk.
Our results suggest a persistence of high risk sexual behavior in this young, primarily MSM cohort, despite receipt of regular prevention messages at study and HIV care visits. Healthcare providers should take advantage of each care encounter to address STI assessment, risk-reduction messaging, and HIV management. We believe annual STI screenings for syphilis, GC and CT (at a minimum) are critical to monitor STI acquisition and risk behavior present in this patient population.25 Approximately 92% of the AHI patients diagnosed with a STI during follow-up were MSM, suggesting tailored risk prevention interventions are needed to address ongoing risk behaviors in this group.26,27 Finally, these data strongly support the importance of ART initiation in those diagnosed with AHI, as specifically recommended in revised guidelines,22 to reduce the risk of onward HIV transmission in the presence of continued high risk sexual behavior.
Acknowledgements
We would like to acknowledge the generous contributions of Bristol Myers Squibb and Gilead Science Inc., the UNC Clinical and Translational Research Center, and the following NIH-funded programs: The UNC Center for AIDS Research (1P30AI 50410-04) and a grant (RR00046) from the General Clinical Research Centers program of the Division of Research Resources. We greatly appreciate the support of all study staff members, HIV care providers, and particularly the individuals who participated in this study. Additionally the authors are appreciative of work of the DIS Officers of the North Carolina Department of Health and Human Services.
Source of Funding Statement: Cynthia Gay has received research support from Abbot Laboratories, Janssen Research & Development, Bristol Myers Squibb, Gilead Sciences, and Argos Therapeutics. Kara McGee is an advisor with Gilead Sciences Focused Opinions, Research, Consultation, and Exchange Advisory Board. Joseph Eron is an ad hoc consultant to Bristol Myers Squibb, Gilead Sciences, Janssen, Merck and GSK/ViiV. Joseph Eron has received research support through UNC from Merck, BMS and GSK/ViiV. Charles Hicks receives grant/research support from Gilead Sciences, Merck, Janssen Virology, Argos, ViiV, and Bristol Myers Squibb and participates on the Scientific Advisory Board for Gilead Sciences, Merck, Janssen Virology, ViiV, and Bristol Myers Squibb.
Footnotes
Conflict of Interest: For the remaining authors, none were declared.
References
- 1.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BG, Roger M, Routy JP, et al. High rates of forward transmission events after acute/early HIV-1 infection. The Journal of infectious diseases. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 4.Pao D, Fisher M, Hue S, et al. Transmission of HIV-1 during primary infection: relationship to sexual risk and sexually transmitted infections. AIDS. 2005;19:85–90. doi: 10.1097/00002030-200501030-00010. [DOI] [PubMed] [Google Scholar]
- 5.Pilcher CD, Eron JJ, Jr, Vemazza PL, et al. Sexual transmission during the incubation period of primary HIV infection. JAMA : the journal of the American Medical Association. 2001;286:1713–1714. doi: 10.1001/jama.286.14.1713. [DOI] [PubMed] [Google Scholar]
- 6.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. The Journal of infectious diseases. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 7.McCoy SI, Eron JJ, Kuruc JD, et al. Sexually transmitted infections among patients with acute HIV in North Carolina. Sexually transmitted diseases. 2009;36:372–374. doi: 10.1097/OLQ.0b013e3181997252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zetola NM, Bernstein KT, Wong E, Louie B, Klausner JD. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. J Acquir Immune Defic Syndr. 2009;50:546–551. doi: 10.1097/qai.0b013e318195bd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore ZS, McCoy S, Kuruc J, Hilton M, Leone P. Number of named partners and number of partners newly diagnosed with HIV infection identified by persons with acute versus established HIV infection. J Acquir Immune Defic Syndr. 2009;52:509–513. doi: 10.1097/QAI.0b013e3181ac12bf. [DOI] [PubMed] [Google Scholar]
- 10.Colfax GN, Buchbinder SP, Cornelisse PG, Vittinghoff E, Mayer K, Celum C. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS. 2002;16:1529–1535. doi: 10.1097/00002030-200207260-00010. [DOI] [PubMed] [Google Scholar]
- 11.Steward WT, Remien RH, Higgins JA, et al. Behavior change following diagnosis with acute/early HIV infection-a move to serosorting with other HIV-infected individuals. The NIMH Multisite Acute HIV Infection Study: III. AIDS and behavior. 2009;13:1054–1060. doi: 10.1007/s10461-009-9582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettifor A, MacPhail C, Corneli A, et al. Continued high risk sexual behavior following diagnosis with acute HIV infection in South Africa and Malawi: implications for prevention. AIDS and behavior. 2011;15:1243–1250. doi: 10.1007/s10461-010-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008;49:212–218. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 14.Gay CL, Mayo AJ, Mfalila CK, et al. Efficacy of NNRTI-based antiretroviral therapy initiated during acute HIV infection. AIDS. 2011;25:941–949. doi: 10.1097/QAD.0b013e3283463c07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodowanec A, Nayak S, Charurat M, Vaughan L, Kanno M, Fantry L. Prevalence of asymptomatic bacterial sexually transmitted infections in hospitalized HIV patients in Baltimore City. J Int Assoc Physicians AIDS Care (Chic) 2012;11:16–19. doi: 10.1177/1545109711418509. [DOI] [PubMed] [Google Scholar]
- 16.McKellar MS, Cope AB, Gay CL, et al. Acute HIV-1 infection in the Southeastern United States: a cohort study. AIDS research and human retroviruses. 2013;29:121–128. doi: 10.1089/aid.2012.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 18.Hurt CB, Beagle S, Leone PA, et al. Investigating a sexual network of black men who have sex with men: implications for transmission and prevention of HIV infection in the United States. J Acquir Immune Defic Syndr. 2012;61:515–521. doi: 10.1097/QAI.0b013e31827076a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muessig KE, Pike EC, Fowler B, et al. Putting prevention in their pockets: developing mobile phone-based HIV interventions for black men who have sex with men. AIDS Patient Care STDS. 2013;27:211–222. doi: 10.1089/apc.2012.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hightow-Weidman LB, Pike E, Fowler B, et al. HealthMpowerment.org: feasibility and acceptability of delivering an internet intervention to young Black men who have sex with men. AIDS Care. 2012;24:910–920. doi: 10.1080/09540121.2011.647677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed on March 27, 2014]; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 23.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2010;59:1–110. [PubMed] [Google Scholar]
- 24.Bruce D, Harper GW, Suleta K. Sexual risk behavior and risk reduction beliefs among HIV-positive young men who have sex with men. AIDS and behavior. 2013;17:1515–1523. doi: 10.1007/s10461-012-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RT, Hoare A, Prestage GP, Donovan B, Kaldor JM, Wilson DP. Frequent testing of highly sexually active gay men is required to control syphilis. Sexually transmitted diseases. 2010;37:298–305. doi: 10.1097/OLQ.0b013e3181ca3c0a. [DOI] [PubMed] [Google Scholar]
- 26.Bolu OO, Lindsey C, Kamb ML, et al. Is HIV/sexually transmitted disease prevention counseling effective among vulnerable populations?: a subset analysis of data collected for a randomized, controlled trial evaluating counseling efficacy (Project RESPECT) Sexually transmitted diseases. 2004;31:469–474. doi: 10.1097/01.olq.0000135987.12346.f2. [DOI] [PubMed] [Google Scholar]
- 27.Rietmeijer CA. Risk reduction counselling for prevention of sexually transmitted infections: how it works and how to make it work. Sexually transmitted infections. 2007;83:2–9. doi: 10.1136/sti.2006.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]