Abstract
The X-ray crystal structure of Cowpea chlorotic mottle bromovirus (CCMV) revealed a unique tubular structure formed by the interaction of the N-termini from six coat protein subunits at each three-fold axis of the assembled virion. This structure, termed the β-hexamer, consists of six short β-strands. The β-hexamer was postulated to play a critical role in the assembly and stability of the virion by stabilizing hexameric capsomers (Speir et al., 1995). Mutational analyses of the β-hexamer structure, utilizing both in vitro and in vivo assembly assays, demonstrate that this structure is not required for virion formation devoid of nucleic acids in vitro or for RNA-containing virions in vivo. However, the β-hexamer structure does contribute to virion stability in vitro and modulates disease expression in vivo. These results support a model for CCMV assembly through pentamer intermediates.
Keywords: Virus structure, Virus assembly, Virus stability, Symptom expression
Introduction
Cowpea chlorotic mottle bromovirus (CCMV) serves as a model system for understanding the protein-protein and protein-RNA interactions that dictate icosahedral virus assembly, stability, and disassembly. CCMV is a member of the Bromoviridae virus family (alphavirus-like superfamily). The viral genome consists of four (+) single-stranded RNA molecules that are encapsidated into three morphologically identical 280 Å icosahedral virus particles. Viral RNA 1 and RNA 2, which encode for proteins involved in RNA-dependent RNA replication, are each packaged into separate virions. Viral RNA 3 (an mRNA for the 32 kDa viral movement protein) and RNA 4 (a subgenomic RNA expressed from RNA 3, which serves as an mRNA for the 20 kDa coat protein) are co-packaged into a third virion in an approximate 1:1 molar ratio (for review see: Ahlquist, 1992).
We have previously demonstrated that the CCMV coat protein expressed in E. coli can be assembled in vitro into either empty virions or, in combination with viral RNA, into RNA containing virions that are indistinguishable from plant purified virus (Zhao et al., 1995). Under low pH (<5.5) and moderate ionic strength conditions (i = 0.2–1.0), the purified coat protein self-assembles into T = 3 virions lacking RNA (empty particles). Assembly of empty particles has not been observed to occur in vivo. Under appropriate in vitro assembly conditions (pH 7.0, i < 0.2), the coat protein and RNA assemble together to form T = 3, RNA-containing particles. This facile CCMV in vitro assembly system allows the creation of coat protein mutants that facilitate a systematic analysis of CCMV assembly (Bancroft and Hiebert, 1967; Bancroft et al., 1973; Fox et al., 1997; Fox et al., 1996; Zhao et al., 1995).
The high resolution X-ray crystal structure of CCMV demonstrates that the virion is comprised of 180 copies of the coat protein subunit arranged on a T = 3 icosahedral lattice, consisting of 12 pentamer and 20 hexamer capsomers (Speir et al., 1995). Most of the coat protein is folded in a manner quite similar to that found in other viral structural proteins that have a core structure consisting of an eightstranded, antiparallel β-barrel motif (residues 51–181; Fig. 1A) (for reviews see: Johnson and Speir, 1997; Rossmann and Johnson, 1989). The N-terminus and the C-terminus of the coat protein subunit extend in opposite directions from the base of the β-barrel. Both termini are thought to play a critical role in virion assembly and stability. Reciprocal interactions between adjacent C-termini result in the formation of a non-covalent coat protein dimer that is essential for initiating virion assembly in vitro and in vivo (Zhao et al., 1995). The first 25 N-terminal residues of the coat protein are disordered in the X-ray crystal structure. However, this region contains a high proportion of basic residues (8 out of 25 residues) that are required for in vitro assembly of RNA-containing virus but not for empty particles (Vriend et al., 1986; Zhao et al., 1995).
Fig. 1.
Structural features of CCMV coat protein and virus particles. (A) Ribbon diagram of the CCMV coat protein. The β-strand involved in the β-hexamer formation (amino acids 29–33) is labeled. (B) Enlarged views of a hexamer viewed down the three-fold axis with the β-hexamer emphasized. (C) Side view of the β-hexamer motif.
Analysis of the crystal structure of CCMV revealed that residues near the N-terminus of the coat protein (residues 29–33) form a unique intersubunit β-barrel structure that occurs at each three-fold axis but at none of the five-fold axes (Fig. 1B and 1C). This β-hexamer structure consists of six short β-strands that form underneath the eight-stranded, antiparallel β-barrel motif of the subunit. This structure was postulated to stabilize hexameric capsomers and thereby play an essential role in the assembly and stability of the virion (Speir et al., 1995). It was proposed that an early step in virion formation was the assembly of six non-covalent dimers into a hexamer of dimers stabilized by this β-hexamer structure. Subsequent addition of non-covalent dimers to the hexamer of dimers accompanied by the binding of divalent metal cations and viral RNA induced protein shell curvature along with the formation of pentamers and recruitment of additional hexamer capsomers to form the T = 3 icosahedral virion (Johnson, 1996; Johnson and Speir, 1997). Such an assembly model suggests an important role for the β-hexamer structure in initiating virion assembly. However, a recent study of CCMV capsid assembly indicates that nucleation occurs via a pentamer rather than a hexamer of dimers (Zlotnick et al., 2000). Here we report the results of a mutational analysis of the β-hexamer structure designed to test effects on virion assembly in vitro and in vivo, and implications for CCMV assembly.
Results and discussion
Role of β-hexamer motif in T = 3 particle assembly
A β-hexamer deletion mutant (NΔβ27–35) and wild-type controls expressed and purified from E. coli were analyzed for their ability to assemble empty and viral RNA-containing particles in vitro. Western blot analysis confirmed the presence of the CCMV coat protein in E. coli extracts for both the NΔβ27–35 mutant and wild-type controls. As expected, the full-length coat protein expressed in BL21DE3 E. coli migrated at a position corresponding to a molecular mass of 20 kDa, which is equivalent to wild-type coat protein isolated from virus. The E. coli expressed protein of the NΔβ27–35 mutant migrated with the expected downward shift in mobility on SDS–PAGE gels (data not shown). The wild-type and NΔβ27–35 coat proteins were both partially purified (50–80% pure) from E. coli and used for in vitro assembly studies.
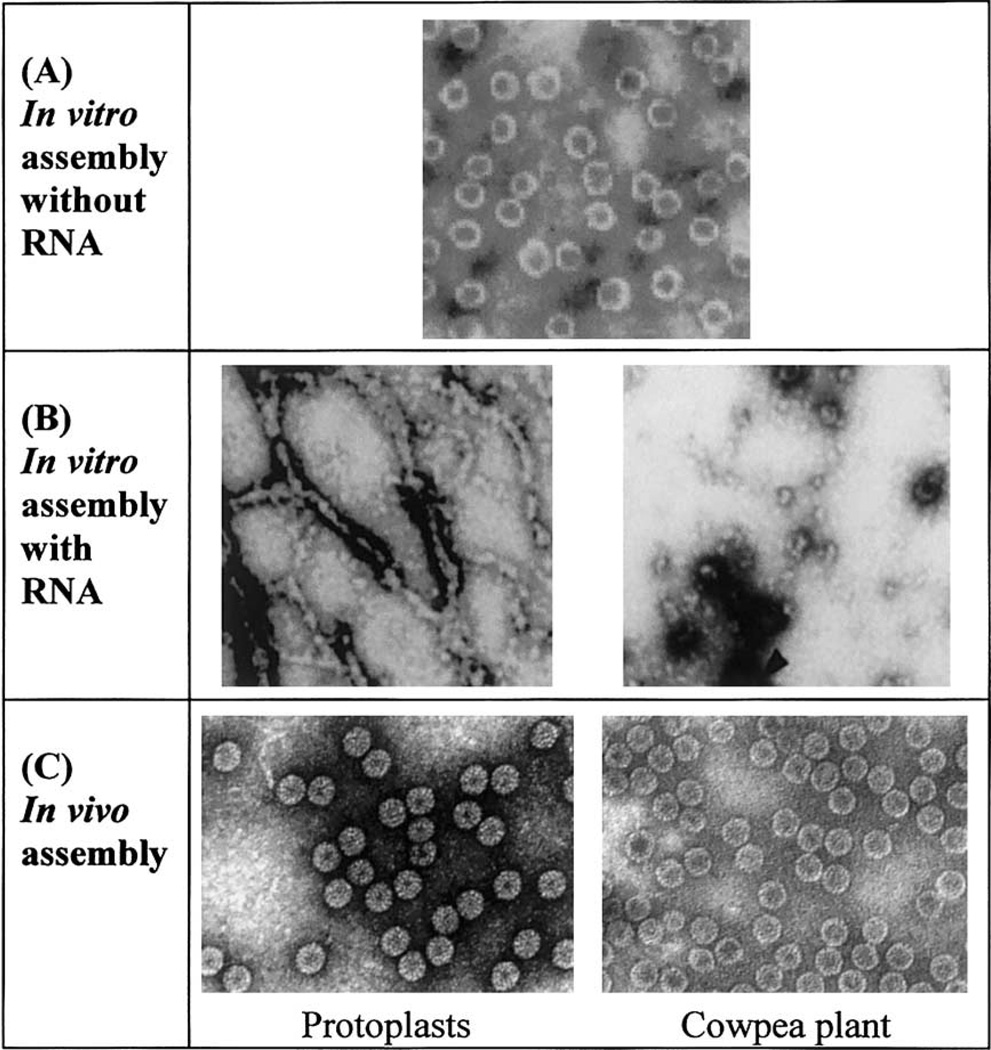
Empty particle assembly was examined under high salt conditions (1.0 M NaCl, pH 4.8). Negative stain transmission electron microscopy (TEM) of particle samples demonstrated that the wild type and NΔβ27–35 coat proteins each assemble into empty particles of diameter 280 Å and with T = 3 quasi-symmetry (Fig. 2). In addition, both of the assembly products sedimented at similar velocities on 5–40% sucrose gradients. However, the NΔβ27–35 assembly products tended to yield a broader peak, and analysis of this peak by TEM revealed that its leading edge consisted of broken and irregular empty particles, whereas its lagging edge contained a population of homogeneous intact particles of 280 Å diameter. The 52S sedimentation value for the intact particles corresponds to the same sedimentation value previously reported for empty T = 3 particles and is in contrast to an 88S value previously determined for viral RNA containing T = 3 particles (Bancroft et al., 1968; Fox et al., 1998; Zhao et al., 1995). As previous studies have demonstrated that coat protein dimers are essential building blocks for T = 3 particle assembly (Zhao et al., 1995), our results show that removal of the β-hexamer sequence is not detrimental to assembly and therefore conclude that proper dimer formation has occurred.
Fig. 2.
Electron micrographs of CCMV β-hexamer deletion mutants assembled under in vitro and in vivo conditions. (A) Purified NΔβ27–35 coat protein in vitro assembled into T = 3 particles in the absence of viral RNA. (B) Purified NΔβ327–35 coat protein in vitro assembled in the presence of viral RNA showing aberrant assembly products. (C) β-hexdel T = 3 particles purified from protoplasts or plants.
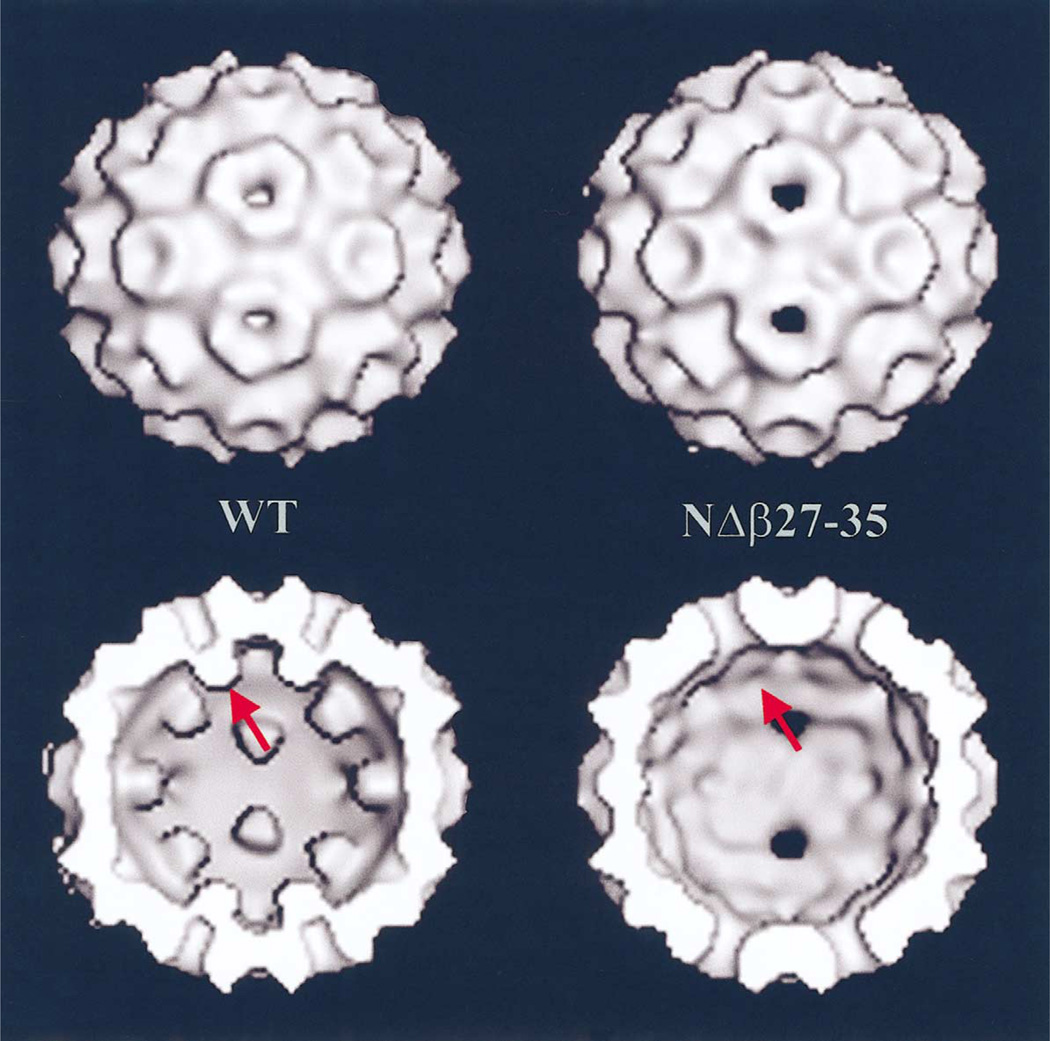
Electron cryo-microscopy (cryo-EM) and three-dimensional image reconstruction of wild-type and mutant T = 3 empty particles assembled in vitro revealed only one obvious difference corresponding to loss of β-hexamer density in the NΔβ27–35 particles (Fig. 3). Except for this difference, the NΔβ27–35 and wild-type empty particles have nearly identical structures at 28 Å resolution. Close inspection of the NΔβ27–35 particle reconstruction reveals an absence of density at the three-fold (quasi-six-fold) axes inside the capsid that corresponds to the β-hexamer region present in wild-type particles.
Fig. 3.
(Top) Shaded-surface representations of the image reconstructions of in vitro assembled CCMV wild-type (WT) and NΔβ27–35 empty particles. (Bottom) Same as (Top) but with front half of particles removed to show the particle interiors indicating the presence (WT) or absence (NΔβ27–35) of β-hexamer density at the pseudo-six-fold axes (arrow).
Assembly of NΔβ27–35 under low salt conditions (0.2 M) failed to yield T = 3 particles, but instead only gave small aggregates of protein. Under such conditions proper coat protein dimer formation may be adversely influenced by the β-hexamer deletion. In the presence of similar low salt conditions, wild-type coat protein assembles into a heterogeneous mixture of assembly products, including T = 3 particles (data not shown). It appears therefore, that the absence of the β-hexamer sequence promotes the formation of non-productive in vitro assembly products when the salt concentration is low.
In the presence of viral RNA at pH 7.0, a limited fraction of the NΔβ27–35 coat protein assembled into T = 3-like particles. However, these appeared among a large background of aberrant assembly products such as tubes and double-shelled particles (Fig. 2). The wild-type coat protein conversely assembled into T = 3-like RNA-containing particles. Many of the same types of aberrant NΔβ27–35 assembly products have been previously observed in vitro upon assembly of wild-type protein at elevated pH in the absence of nucleic acid (Adolph and Butler, 1974; Bancroft et al., 1969). Hence, removal of the β-hexamer region may alter associations with the viral nucleic acid and thereby discourage T = 3 virion assembly. Removal of β-hexamer sequences could cause the basic N-terminus of the coat protein to lie closer to the base of the β-barrel central motif, and retracted somewhat from the RNA core. The N-terminus is believed to have direct coulombic interactions with the viral RNA. Removal of the β-hexamer structure may therefore alter protein-protein and/or protein-RNA interactions in a manner that may interfere with the assembly process.
In vivo particle assembly and symptom expression
Two classes of mutants were constructed to test the importance of the β-hexamer structure. The first was a simple deletion of this region (pβ-hexdel). The second was a substitution mutation where the amino acids involved in the β-strand were replaced with glycines. Glycines were chosen because they are unlikely to participate in a structural formation but will maintain a similar distance between the N-terminal amino acids and the base of the β-barrel fold. Tobacco protoplasts electroporated with pβ-hexdel (a construct that results in deletion of coat protein residues 27–35 that comprise the β-hexamer structure), pβ-hexsub (a construct that replaces wild-type β-hexamer amino acids with glycine residues), and wild-type control RNAs were confirmed positive for the presence of CCMV coat protein using polyclonal serum raised against purified virions in ELISA assays (positives were indicated if the ELISA values were greater than 3 × background controls). TEM analysis of protoplast extracts inoculated with pβ-hexdel and pβ-hexsub demonstrated the presence of T = 3 particles indistinguishable from wild-type particles (Fig. 2).
In vivo, the β-hexamer region can modulate the host response to viral infection. β-hexamer deletion and polyglycine substitution both resulted in dramatic symptom expression. Nicotiana benthamiana and cowpea plants inoculated with pβ-hexdel, pβ-hexsub, and wild-type viral RNAs, all transcribed in vitro, showed significant virus accumulation when evaluated by ELISA. The N. benthamiana plants inoculated with the pβ-hexdel RNA did not show any mosaic symptoms which is common in this host, but the infected plants were extremely stunted and died shortly after ELISA analysis. Cowpea plants inoculated with pβ-hexdel RNA displayed severe stunting, mottling, and chlorosis unlike the controls inoculated with wild-type viral RNA (Fig. 4). The β-hexsub mutant exhibited wild-type levels of virus expression as shown by ELISA with the same mottling of cowpea but less detrimental effects on N. benthamiana. TEM analysis of cowpea plant extracts inoculated with pβ-hexdel and pβ-hexsub demonstrated the presence of particles indistinguishable from wild-type T = 3 particles (Fig. 2). These purified virions contain a full complement of the viral RNAs based on their ability to reinfect host plants and by spectroscopic analysis (A260/A280 = 1.7).
Fig. 4.
Typical symptom expression of cowpea infected with the β-hexdel mutant virus as compared to wild-type virus.
Virus from cowpea plants that were ELISA positive for β-hexdel and β-hexsub particles could be passaged onto cowpea plants through inoculation with a sap extract. These plants were also evaluated by ELISA 10 days post inoculation (p.i.). In each instance, the ELISA values for the β-hexdel and β-hexsub mutations were similar to wild-type values (data not shown). Symptom expression of severe stunting, mottling, and chlorosis was again observed in both β-hexamer mutants. Cowpea plants infected with either of the β-hexamer mutants failed to produce seed when grown to maturity. Restriction endonuclease analysis and DNA sequencing of the RT-PCR products of β-hexdel and β-hex-sub infected plants confirmed the entire coat protein sequence including the presence of the mutations. Purified β-hexdel mutant virus had identical sedimentation velocities as viral RNA-containing particles on sucrose gradients and showed a decrease in mass as expected on SDS–PAGE (data not shown).
To collaborate the in vivo results with the in vitro assembly results using E. coli purified coat protein, purified in vivo β-hexdel and wild-type particles were disassembled and reassembled using in vitro conditions. Disassembled coat protein was reassembled either into empty particles at low pH (in the absence of RNA) or at neutral pH in the presence of viral RNA. Like the assembly using protein purified from E. coli, 280 Å particles were observed by TEM under both empty and RNA-containing conditions. Some double-shelled particles were also evident during assembly with RNA.
Kinetics of virus assembly
We speculate that the dramatic effect of β-hexamer deletions on symptom expression may be a consequence of the influence of β-hexamer sequence alterations on the kinetics of virion assembly. Based on this model, a mutated β-hexamer sequence may reduce the rate of virion assembly and subsequently create an increase in the cellular concentrations of unassembled coat protein subunits and viral RNAs. The increased pool size of these products may then trigger a host response to viral infection. Consistent with this hypothesis, others have recently reported that mutations in the N-terminus of the bromovirus coat protein also result in enhanced symptom expression (Rao and Grantham, 1995, 1996). Therefore, all these N-terminal mutations possibly reduce the kinetics of viral RNA packaging within the virion and elevated levels of non-packaged viral RNA trigger the enhanced symptom expression.
Particle stability
All the β-hexamer mutant particles, assembled in vivo and in vitro, were less stable than wild-type particles. The β-hexamer deletion and substitution mutants were both susceptible to thermal disassembly at a temperature approximately 10°C below that required to destabilize wild-type virions. Empty particles assembled from coat protein purified from E. coli were incubated at increasing temperatures and analyzed by TEM for the presence of intact particles. The number of intact particles was inversely correlated to the temperature. At 37°C, greater than 50% of the empty NΔβ25–37 particles were dissociated. In contrast, greater than 50% of the empty wild-type particles remained intact with the temperature elevated to 55°C. At 70°C, some empty wild-type particles (<20%) were still present but no intact NΔβ25–37 particles were observed. The same β-hexamer mutant, purified from plants, also exhibited reduced thermal stability compared to wild-type virus. In a second assay, dynamic light scattering was used to distinguish between assembled virions and disassembled subunits. Dynamic light scattering showed that the β-hexamer mutant disassembled at a temperature 10°C lower than that required to disassemble wild-type CCMV. The β-hexamer mutant consists of a single population of particles with an average diameter of 29.6 nm until the temperature reaches 55°C at which point disassembly products begin to be detected. This is in contrast to wild-type particles that do not begin to dissociate into subunits until the temperature reaches 65°C. These observations suggest the β-hexamer region contributes to thermal stability of virions. Nonetheless, this contribution is not essential for virion formation or stability at temperatures typical for normal virus replication.
A recent model proposed that early formation of a hexamer of dimers was required for CCMV assembly (Speir et al., 1995). This model was based in part on the supposition that the β-hexamer would act as a strong stabilizing motif that would favor formation of a hexamer of dimers in the next step of assembly. Our results indicate that, while the β-hexamer motif does affect the assembly process, it is not essential for in vitro assembly of empty T = 3 particles. We have also shown that the β-hexamer region is dispensable for virion assembly and is not an essential switch that controls the ratio of hexamers and pentamers during the CCMV assembly process. These results are supported by our recent demonstration that assembly is initiated by formation of a pentamer of dimers rather than a hexamer of dimers (Zlotnick et al., 2000). Also, the high resolution crystal structure of the T = 3 cucumber mosaic virus (CMV) shows that the CMV and CCMV coat proteins have nearly identical structures, with the exception that CMV lacks a β-hexamer structure at the icosahedral three-fold axes. Instead, the N-terminus of the CMV coat protein forms an extended, six-helix bundle at each three-fold axis (Smith et al., 2000). Our experience with the CCMV system raises a cautionary note: that critical roles assigned to particular motifs based solely on structural analysis must be experimentally tested in vivo and in vitro to insure proper validation.
Materials and methods
Wild-type CCMV was propagated and purified from cowpea plants (Vigna unguiculata (L.) Var. California Blackeye) as previously described (Lane, 1974; Zhao et al., 1995). Purified CCMV was disassembled into its component parts and the coat protein was isolated as described (Zhao et al., 1995).
Site-directed mutagenesis and cloning of the β-hexamer mutants
Deoxyoligonucleotide site-directed mutagenesis protocols (Stratagene, La Jolla, CA) were used to introduce either amino acid deletions or substitutions into the β-hexamer structure of the CCMV coat protein (Table 1). Constructs NΔβ27–35 and pβ-hexdel delete nucleotides encoding for the β-hexamer region in plasmids for expression in E. coli or plant cells, respectively. Construct pβ-hexsub replaced the β-hexamer amino acids with glycine residues in a plasmid for expression in plant cells. All constructs were screened for the presence of the introduced restriction endonuclease site (where applicable) and confirmed by DNA sequencing of the entire coat protein gene. To further confirm that the constructs encoded for the expected size coat protein, coupled in vitro RNA transcription and translation was performed using a 5′ coat protein specific PCR primer that incorporated a T7 RNA polymerase promoter. SDS– PAGE was used to analyze estimates of 35S methionine labeled protein product size.
Table 1.
β-hexamer mutants
| Construct name | Base plasmid | Primer sequence | Amino acid positions |
|---|---|---|---|
| NΔβ27–35 | pCC4FL (Zhao et al., 1995) | Negative sense (Kunkel, 1987) 5′ GCC TGA AGC GAT ACG AGT GTT CCG CTT GTT 3′ |
27–35 deleted |
| pβ-hexdel | pCC3TP4 (Allison et al., 1988) | Positive sense (MluI) 5′GAA CAA GCG GAA CAC GCG TAT CGC TTC AGG CCA AGG C 3′ Negative sense 5′ GCC TTG GCC TGA AGC GAT ACG CGT GTT CCG CTT GTT C 3′ |
27–35 deleted |
| pβ-hexsub | pCC3TP4 (Allison et al., 1988) | Positive sense (ApaI) 5′ CGG AAC ACT CGT GTG GTC CAA GGA GGC GGG GGT GGG CCC ATC GCT TCA GGC CAA GGC 3′ Negative sense 5′GCC TTG GCC TGA AGC GAT GGG CCC ACC CCC GCC TCC TTG GAC CAC ACG AGT GTT CCG 3′ |
30–35 glycine substitution |
Expression and purification of CCMV coat protein from E. coli
Expression of full-length and deletion forms of the CCMV coat protein in E. coli was carried out using established protocols (Zhao et al., 1995). Both wild-type and NΔβ27–35 mutant forms of the coat protein were localized in the insoluble inclusion body fraction of E. coli lysates. This insoluble protein was solubilized in 8 M urea, followed by stepwise dialysis with a high salt buffer in order to remove the urea and refold the protein. The refolded protein was further purified by selective ammonium sulfate precipitation and FPLC size exclusion chromatography (Superose 6, Amersham Pharmacia Biotech, Uppsala, Sweden). The final yield of protein subunit was determined by absorbance (molar absorbtivity (280 nm) of 24,075 M−1 cm−1) to be 10 mg/l of induced culture media and 50–80% pure. The partially purified coat protein was analyzed by SDS–PAGE, Western blotting with polyclonal antibodies produced against purified CCMV, and by UV absorption spectrophotometry.
In vitro assembly of virus particles
Coat protein obtained from disassembled virions purified from plant tissue or purified from E. coli was assembled in vitro into either empty particles or RNA-containing particles as described (Zhao et al., 1995). In brief outline, empty particles were assembled by dialyzing purified coat protein against low salt buffer (0.1 M NaOAc pH 4.8, 0.1 M NaCl, 0.2 mM PMSF) or high salt buffer (0.1 M NaOAc pH 4.8, 0.9 M NaCl, 0.2 mM PMSF) for 10–12 h at 4°C. Viral RNA-containing particle assembly was carried out by mixing purified coat protein with in vitro transcribed CCMV RNA2 in a 4:1 (wt:wt) ratio in a total volume of 100 µ1. This reassembly mixture was dialyzed against assembly buffer (50 mM NaOAc pH 7.0, 50 mM NaCl, 10 mM KCl, 5 mM MgCl2, 1 mM DTT, 0.2 mM PMSF) for 2.5 h at 4°C.
RNA-containing and empty particle assembly mixtures were applied to 5–40% sucrose gradients in appropriate buffers and centrifuged at 38,000 rpm for three hours at 4°C in a Beckman SW41TI rotor. The gradients were fractionated while monitoring the absorbance at 260 nm. The desired fractions were collected and dialyzed against virus buffer (0.1 M NaOAc pH 4.8, 0.001 M EDTA). Virus particles were concentrated using Centricon-100 microcon-centrators (Millipore, Bedford, MA).
In vitro RNA transcription of CCMV cDNA
Xba I linearized plasmids pCC1TP1, pCC2TP2, and pCC3TP4 (plasmids containing wild-type full-length cDNAs corresponding to CCMV RNAs 1, 2, and 3 respectively, kindly provided by P. Ahlquist, University of Wisconsin, Madison (Allison et al., 1988)) and pβ-hexdel and pβ-hexsub (constructs corresponding to β-hexamer deletions and substitutions) were used as templates for viral RNA synthesis by in vitro transcription with T7 mMessage Machine (Ambion, Austin, TX). In vitro RNA transcription yields and quality were estimated by UV absorbance (1.0 OD = 44 µg/ml) and visualization on denaturing agarose gels (Sambrook et al., 1989).
In vivo analysis of β-hexamer mutations
Primary leaves of ten-day-old cowpea plants or four-leaf-stage N. benthamiana plants were mechanically inoculated with CCMV RNAs at a concentration of 0.5 µg/leaf. Seven to ten days p.i., plants were analyzed by ELISA using CCMV polyclonal antibodies and by transmission electron microscopy (TEM) analysis using 1% uranyl acetate as a negative stain. Equivalent amounts of total plant protein were analyzed by ELISA in a double sandwich method (Clark and Adams, 1977). All experiments were repeated at least three times. Plant tissue containing virus particles was subsequently passaged in another round of cowpea plants to determine if the particles were infectious. Plant material that was ELISA positive after passage was subjected to RT-PCR analysis and virus purification (Zhao et al., 1995). RT-PCR was performed according to manufacturer’s recommendations (Promega, Madison, WI) with primers that were designed to amplify the coat protein gene of CCMV. Restriction endonuclease digestion and direct DNA sequencing of the RT-PCR products determined the presence of each of the coat protein β-hexamer mutations.
Tobacco cell cultures (BY-2) were maintained in TSCM (Watanabe and Okada, 1986). The cell walls from five-day old cultures were removed by cellulase and pectolyase digestion in 0.4 M mannitol for two h. These tobacco protoplasts were then washed twice with 0.4 M mannitol and electroporated in the presence of CCMV in vitro transcribed RNAs. After electroporation, protoplasts were added to protoplast growth media and incubated for 48 h at 25°C. The cells were harvested by low speed centrifugation (750 g) and analyzed for the presence of virus particles by ELISA with CCMV polyclonal antibodies and visualized by TEM as described above. ELISA was used to analyze equivalent total protein for each construct and all experiments were repeated at least three times.
Electron cryo-microscopy and three-dimensional image reconstruction
The methods of cryo-EM and image processing that were used to obtain three-dimensional reconstructions of in vitro assembled empty wild-type and NΔβ27–35 CMV particles have been previously described (Baker et al., 1988; Cheng et al., 1995; Cheng et al., 1994; Fox et al., 1998; Zhao et al., 1995). In brief outline, CCMV samples were adhered to holey carbon films, vitrified in liquid ethane, inserted into a Gatan cold holder (Gatan Inc., Warrendale, PA), and transferred into a Philips EM420 TEM (Philips Electronic Instruments, Mahwah, NJ) where they were maintained at near liquid nitrogen temperature and micrographs were recorded. Micrographs for use in image processing were recorded under minimal dose conditions (~20e−/Å2), at a nominal magnification of 49,000× and at 80 kV and an objective lens defocus of 0.9 µm. The orientations and phase origins (particle centers) of the particles in the images were determined using a model-based approach that utilized a previously determined reconstruction of wild-type CCMV as the starting model (Cheng et al., 1994; Fox et al., 1998; Speir et al., 1995). Reconstruction of the wild type and NΔβ27–35 empty particles were computed to an effective resolution of 28 Å from 64 and 108 particle images, respectively.
Thermal stability assay
Empty wild-type and NΔβ27–35 particles assembled from E. coli purified coat protein were assayed for thermostability by incubation for 20 min at 4°C, 25°C, 37°C, 55°C, and 72°C, respectively. Incubation of particles at each temperature was immediately followed by negative stain TEM examination. To assess the reassembly competence of particles after 72°C treatment, particles were incubated overnight at 4°C and reexamined by TEM. Wild-type and β-hexdel virions purified from cowpea were analyzed by dynamic light scattering while incubating for 15 min at 25°C, 35°C, 45°C, 55°C, 60°C, 65°C, 70°C, and 75°C. Scattering was measured at 90° with a 661 nm diode laser and the correlation function was fit using CONTIN (Brookhaven 90Plus particle size analyzer). The change in size distribution of the particles is indicative of the particle stability.
Acknowledgments
This investigation was supported in part by grants from the NSF to M.Y. (CB-9723752) and T.S.B. (MCB-9206305) and from the Public Health Service to T.S.B. (GM33050). We thank Susan Brumfield for technical assistance.
References
- Adolph KW, Butler PJG. Studies on the assembly of a spherical plant virus. I. States of aggregation of the isolated protein. J. Mol. Biol. 1974;88:327–341. doi: 10.1016/0022-2836(74)90485-9. [DOI] [PubMed] [Google Scholar]
- Ahlquist P. Bromovirus RNA replication and transcription. Curr. Opin. Gen. and Dev. 1992;2(1):71–76. doi: 10.1016/s0959-437x(05)80325-9. [DOI] [PubMed] [Google Scholar]
- Allison RF, Janda M, Ahlquist P. Infectious in vitro transcripts from cowpea chlorotic mottle virus cDNA clones and exchange of individual RNA components with brome mosaic virus. J. Virol. 1988;62(10):3581–3588. doi: 10.1128/jvi.62.10.3581-3588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TS, Drak J, Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc. Natl. Acad. Sci. USA. 1988;85:422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft JB, Bracker CE, Wagner GW. Structures derived from cowpea chlorotic mottle and brome mosaic virus protein. Virology. 1969;38:324–335. doi: 10.1016/0042-6822(69)90374-2. [DOI] [PubMed] [Google Scholar]
- Bancroft JB, Hiebert E. Formation of an infectious nucleoprotein from protein and nucleic acid isolated from a small spherical virus. Virology. 1967;32:354–356. doi: 10.1016/0042-6822(67)90284-x. [DOI] [PubMed] [Google Scholar]
- Bancroft JB, Rees MW, Johnson MW, Dawson JRO. A salt-stable mutant of cowpea chlorotic mottle virus. J. Gen. Virol. 1973;21:507–513. [Google Scholar]
- Bancroft JB, Wagner GW, Bracker CE. The self-assembly of a nucleic-acid free pseudo-top component for a small spherical virus. Virology. 1968;36(1):146–149. doi: 10.1016/0042-6822(68)90126-8. [DOI] [PubMed] [Google Scholar]
- Cheng RH, Kuhn RJ, Olson NH, Rossmann MG, Choi HK, Smith TJ, Baker TS. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995;80(4):621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RH, Reddy VS, Olson NH, Fisher AJ, Baker TS, Johnson JE. Functional implications of quasi-equivalence in a T = 3 icosahedral animal virus established by cryo-electron microscopy and x-ray crystallography. Structure. 1994;2:271–282. doi: 10.1016/s0969-2126(00)00029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MF, Adams AN. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Fox J, Albert FG, Speir J, Young MJ. Characterization of a disassembly deficient mutant of cowpea chlorotic mottle virus. Virology. 1997;227(1):229–233. doi: 10.1006/viro.1996.8292. [DOI] [PubMed] [Google Scholar]
- Fox JM, Wang G, Speir JA, Olson NH, Johnson JE, Baker TS, Young MJ. Comparison of the native CCMV virion with in vitro assembled CCMV virions by cryoelectron microscopy and image reconstruction. Virology. 1998;244(1):212–218. doi: 10.1006/viro.1998.9107. [DOI] [PubMed] [Google Scholar]
- Fox JM, Zhao X, Speir JA, Young MJ. Analysis of a salt stable mutant of cowpea chlorotic mottle virus. Virology. 1996;222:115–122. doi: 10.1006/viro.1996.0402. [DOI] [PubMed] [Google Scholar]
- Johnson JE. Functional implications of protein-protein interactions in icosahedral viruses. Proc. Natl. Acad. Sci. USA. 1996 Jan;93:27–33. doi: 10.1073/pnas.93.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Speir JA. Quasi-equivalent viruses: a paradigm for protein assemblies. J. Mol. Biol. 1997;269(5):665–675. doi: 10.1006/jmbi.1997.1068. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Method Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lane LC. The bromoviruses. Adv. Virus Res. 1974;19:151–220. doi: 10.1016/s0065-3527(08)60660-0. [DOI] [PubMed] [Google Scholar]
- Rao ALN, Grantham GL. Biological significance of the seven amino- terminal basic residues of brome mosaic virus coat protein. Virology. 1995;211:42–52. doi: 10.1006/viro.1995.1377. [DOI] [PubMed] [Google Scholar]
- Rao ALN, Grantham GL. Molecular studies on brome mosaic virus coat protein. II. Functional analysis of the amino terminal arginine, rich motif and its role in encapsidation, movement and pathology. Virology. 1996;226:294–305. doi: 10.1006/viro.1996.0657. [DOI] [PubMed] [Google Scholar]
- Rossmann MG, Johnson JE. Icosahedral RNA virus structure. Annu. Rev. Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. 2nd ed. Plainview, NY: Cold Springs Harbor Laboratory Press; 1989. [Google Scholar]
- Smith TJ, Chase E, Schmidt T, Perry KL. The structure of cucumber mosaic virus and comparison to cowpea chlorotic mottle virus. J. Virol. 2000;74(16):7578–7586. doi: 10.1128/jvi.74.16.7578-7586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir JA, Munshi S, Wang G, Baker TS, Johnson JE. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure. 1995;3:63–78. doi: 10.1016/s0969-2126(01)00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend G, Verduin BJ, Hemminga MA. Role of the N-terminal part of the coat protein in the assembly of cowpea chlorotic mottle virus. A 500 MHz proton nuclear magnetic resonance study and structural calculations. J. Mol. Biol. 1986;191(3):453–460. doi: 10.1016/0022-2836(86)90140-3. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Okada Y. In vitro viral RNA synthesis by a subcellular fraction of TMV-inoculated tobacco protoplasts. Virology. 1986;149:64–73. doi: 10.1016/0042-6822(86)90087-5. [DOI] [PubMed] [Google Scholar]
- Zhao X, Fox JM, Olson NH, Baker TS, Young MJ. In vitro assembly of cowpea chlorotic mottle virus from coat protein expressed in Escherichia coli and in vitro-transcribed viral cDNA. Virology. 1995;207(2):486–494. doi: 10.1006/viro.1995.1108. [DOI] [PubMed] [Google Scholar]
- Zlotnick A, Aldrich R, Johnson JM, Ceres P, Young MJ. Mechanism of capsid assembly for an icosahedral plant virus. Virology. 2000;277(2):450–456. doi: 10.1006/viro.2000.0619. [DOI] [PubMed] [Google Scholar]