Abstract
Objective
To determine if consumption of a reduced-carbohydrate (CHO) diet would result in preferential loss of adipose tissue under eucaloric conditions, and whether changes in adiposity were associated with changes in postprandial insulin concentration.
Methods
In a crossover-diet intervention, 30 women with PCOS consumed a reduced-CHO diet (41:19:40%energy from CHO:protein:fat) for 8 weeks and a standard diet (55:18:27) for 8 weeks. Body composition by DXA and fat distribution by CT were assessed at baseline and following each diet phase. Insulin AUC was obtained from a solid meal test (SMT) during each diet phase.
Results
Participants lost 3.7% and 2.2% total fat following the reduced-CHO diet and STD diet, resp. (p<0.05 for difference between diets). The reduced-CHO diet induced a decrease in subcutaneous-abdominal, intra-abdominal, and thigh-intermuscular adipose tissue (−7.1%, −4.6%, and −11.5%, resp.), and the STD diet induced a decrease in total lean mass. Loss of fat mass following the reduced CHO diet arm was associated with lower insulin AUC (p<0.05) during the SMT.
Conclusions
In women with PCOS, consumption of a diet lower in CHO resulted in preferential loss of fat mass from metabolically harmful adipose depots, whereas a diet high in CHO appeared to promote repartitioning of lean mass to fat mass.
Keywords: macronutrient composition, obesity, intra-abdominal adipose tissue
1. Introduction
Polycystic Ovary Syndrome (PCOS) is the most common endocrine disorder affecting nearly 8–10% of premenopausal women 1. Primary features of the syndrome include biochemical or clinical hyperandrogenism, ovulatory dysfunction and polycystic ovaries 2. Other common features include insulin resistance, elevated circulating insulin, and metabolic dysfunction 3, which may be mechanistically linked to weight gain and obesity in this population4;5. In PCOS, elevated insulin may contribute to excess adiposity by limiting fat mobilization and oxidation, thus making weight maintenance and weight loss a significant challenge. Obesity, specifically abdominal or ectopic adiposity, may contribute to the severity of symptoms and progression of comorbidities associated with PCOS. Non-pharmacological interventions to limit adiposity in the PCOS population are needed.
A number of studies have demonstrated that weight loss and diet quality may contribute to improvement of symptoms associated with PCOS 6–9. However, whether qualitative aspects of the diet affect weight or body composition and fat distribution independent of energy restriction among women with PCOS is unclear. Diets reduced in carbohydrate (CHO) with a low glycemic load may be more likely to lower postprandial glucose and the subsequent insulin secretory response when compared to diets higher in CHO content10;11. We previously reported a significant reduction in intra-abdominal adipose tissue among women in response to a diet reduced in CHO content during weight maintenance conditions, and a preferential loss of fat mass during weight loss conditions 12. These findings suggest that a diet reduced in CHO may be an optimal dietary approach to reducing total and abdominal adiposity among women with PCOS, considering their prevailing hyperinsulinemia.
The objective of the present study was to determine if, in women with PCOS, consumption of a diet moderately reduced in CHO would reduce total and regional adipose tissue during weight maintenance conditions, and whether changes in adiposity would be associated with lower postprandial insulin concentrations.
2. Material and Methods
2.1 Participants
Thirty women with PCOS defined using the NIH 1990 criteria were recruited. The diagnostic criteria included i) hyperandrogenism and/or hyperandrogenemia, (ii) oligo-ovulation, and (iii) the exclusion of other existing disorders such as Cushing’s syndrome, hyperprolactinemia, or congenital (non-classic) adrenal hyperplasia. Specific inclusion and exclusion criteria have been described elsewhere13. In brief, exclusion criteria included structured exercise >2 hours per week, pregnancy, current breastfeeding, use of medication that could affect body composition or glucose metabolism (including oral contraceptives, cholesterol medications, and blood pressure medications), current use of tobacco, use of illegal drugs in last 6 months, major food allergies or food dislikes, and a medical history that contra-indicated inclusion in the study. Participants had a BMI under 45 kg/m2 and were weight stable 6-months prior to enrolling in the study. Prior to enrollment in the study, participants were informed of the protocol and engaged in oral and written consent. The protocol for this study was approved by the Institutional Review Board for Human Use at UAB, and all participants signed informed consent prior to initiation of testing.
2.2 Protocol
The study design was a crossover dietary intervention. Participants consumed a reduced-CHO diet for 8 weeks and a standard (STD) diet for 8-weeks separated by a 4-week washout period. Participants completed a 4-day food record (3 weekdays, 1 weekend day) for assessment of typical nutrient intake prior to beginning the dietary intervention, and again following the 4-week washout period. Diet order (reduced-CHO first then STD diet, or STD diet first then reduced-CHO diet) was assigned using a randomization scheme following baseline testing. Dual-energy X-ray absorptiometry (DXA) and computed tomography (CT) scans were acquired for all participants at baseline of each diet arm and following completion of each diet arm. A solid meal test (SMT) to determine glucose and insulin response to the study meal was performed at the 4-week midpoint of each diet arm. Sample size calculations were based on our previous data from a weight maintenance study in a population of obese men and women12. In this study, we detected a decrease in IAAT of 11.0 ±9.7 cm2 after 8 weeks of consumption of a eucaloric reduced-CHO diet. Assuming a change of 11.0 ± 9.7 cm2, a two-sided paired t-test, and a significance alpha level of 0.05, we would have over 80% power to detect a significant change in IAAT with 17 participants per diet group. In the present study, we enrolled 30 women to allow for attrition; 23 completed both arms of the intervention, and 27 completed the reduced-CHO arm.
2.3 Diets
All food was provided to the participants for each 8-week diet arm by the UAB Clinical Research Unit (CRU) Metabolic Kitchen. For the duration of the study, participants reported to the CRU several times each week to be weighed and to collect food for off-site consumption. Participants were blinded to diet order, and either first consumed a reduced CHO diet (41% CHO, 19% protein, and 40% fat) for 8-weeks or the STD (55% CHO, 18% protein, and 27% fat) diet for 8-weeks. The glycemic index of the reduced CHO diet was ~50 and that of the STD diet was ~60. Details of the two diets were previously reported 13. Intervention diet menus were designed using Nutrition Data System for Research (NDSR) software versions 2009 and 2011 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Examples of meals provided from each diet are shown in Table 1. Total energy requirements were determined by using each individual’s measured resting energy expenditure from indirect calorimetry with an activity factor of 1.35. Participants were asked to maintain their baseline level of physical activity throughout the study period.
Table 1.
Example day of meals for the reduced CHO diet and the standard (STD) diet
| Reduced-CHO diet (1800 calorie) | STD diet (1800 calorie) | ||
|---|---|---|---|
| Breakfast | |||
| Pear, fresh | 1 medium | White bagel | 1 medium |
| Oatmeal, instant | 1 packet (28 g) | Cream cheese, regular | 28.4 g |
| Butter, regular | 10 g | Boiled egg | 2 large |
| Sugar | 1 packet (4 g) | Orange juice | 118.3 mL |
| Egg, boiled | 1 large | ||
| 2% Milk | 236.6 mL | ||
| Bacon, regular | 2 slices (28.4 g) | ||
| Lunch | |||
| Chicken Vegetable Soup | 396.9 g | White bread | 2 slices |
| Black bean vegetarian burger | 1 each (67 g) | Peanut butter, regular | 21.3 g |
| Hamburger bun | 1 medium | Jelly, regular | 2 packets (28 g) |
| American cheese | 1 slice (42 g) | Light canned peaches | 227.2 g |
| Mustard | 1 packet (6 g) | Baby carrots, fresh | 10 medium |
| Mayonnaise, regular | 1 packet (12 g) | Ranch dressing, fat free | 12.4 g |
| Ketchup | 1 packet (10 g) | ||
| Bugles, original1 | 15 g | ||
| Orange, fresh | 1 small | ||
| Dinner | |||
| Chicken breast | 1 breast (85 g) | Breaded chicken patty, pre-cooked | 1 each (129 g) |
| Green beans, frozen | 1 cup | Hamburger bun, white | 1 medium |
| Lima beans, frozen | 1 cup | Ketchup | 1 packet (10 g) |
| Butter, regular | 10 g | Mustard | 1 packet (6 g) |
| Graham crackers, plain | 2 squares | Broccoli, frozen | 1 cup |
| Peanut butter, regular | 21.3 g | Hard candy | 3 each (18 g) |
| Pretzels | 28.4 g | ||
| Orange juice | 118.3 mL | ||
Bugles, Generals Mills, Minneapolis, MN, USA
2.4 Body composition and fat distribution
Total body fat mass and lean mass were measured by DXA (iDXA, GE-Lunar Corporation, Madison, WI). Intra-abdominal adipose tissue (IAAT), subcutaneous abdominal adipose tissue (SAAT), thigh muscle, thigh subcutaneous adipose tissue(SAT), thigh perimuscular adipose tissue (PMAT), and thigh intermuscular adipose tissue(IMAT) were determined by computed tomography (CT) scanning. A five millimeter axial scan at the level of the umbilicus (approximately the L4–L5 intervertebral space) and another at midthigh were taken. Scans were later analyzed for cross-sectional area (cm2) of adipose tissue and muscle tissue using SliceOmatic image analysis software (version 4.3: Tomovision, Montreal, Canada). The abdomen scan was used to analyze IAAT and SAAT as previously described14. Thigh IMAT and PMAT were separated from thigh SAT by manually drawing a line along the fascia lata surrounding the thigh muscle. Subsequently, IMAT was partitioned from PMAT by manually drawing a line around the muscle itself to capture adipose tissue located directly between and within muscle groups. This technique has been previously described by us and others15;16. All scans were analyzed by the same image analyst (AG). The coefficient of variation (CV) for repeat cross-section analysis of scans among 40 subjects in our laboratory is <2%17
2.5 Solid meal test
A SMT was used to determine the glucose and insulin response over 4-hours following consumption of a study meal. At the 4-week midpoint of each diet arm, participants consumed one breakfast at the CRU following a 12-hour fast. The breakfast consumed was composed of the assigned menu items for that day and differed by diet arm. Participants were required to consume all food items included in the assigned breakfast meal and complete the meal in less than 20 minutes. To perform the test, a flexible intravenous catheter was placed in the antecubital space of the left arm. Blood samples were collected prior to meal consumption at baseline (time “zero”), and at times 15, 60, 90, 120,180, and 240 minutes. Sera were stored at −85°C. Samples were analyzed for insulin and glucose to determine glucose and insulin area-under-the-curve (AUC) using the trapezoidal method 18;19
2.6 Assays for glucose and insulin
Analyses were conducted in the Core Laboratory of the Center for Clinical and Translational Science, Nutrition and Obesity Research Center, and Diabetes Research Center. Glucose was measured using the glucose oxidase method (Stanbio Laboratory, Boerne, TX). In our laboratory, this assay had an intra-assay coefficient of variation (CV) of 1.2%, and an interassay CV of 3.1%. Insulin was assayed with immunofluorescence technology on a TOSOH AIA-II analyzer (TOSOH, South San Francisco, CA). ). In our laboratory, this assay had an intra-assay CV of 1.5%, and an interassay CV of 4.4 %.
2.7 Statistical analysis
Descriptive statistics were computed for all study variables of interest. Variables known to deviate from a normal distribution were log 10 transformed prior to statistical analysis. All statistical tests were two-sided and were performed using a type I error rate of 0.05. Statistical analyses were performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC). Paired t-test was used to determine the difference in baseline and 8-wk post intervention body composition, fat distribution, and serum analytes by diet arm. Analysis of covariance (ANCOVA) was used to determine the effect of diet on main outcome measures. Outcome measures at week 8 (postintervention) were used as the dependent variables, and baseline outcome measures were used as covariates. ANCOVA was also used to determine the effect of diet on change in total fat mass independent of change in total lean mass. Change in total fat mass was the dependent variable, and baseline total fat mass and change in total lean mass were covariates. Diet sequence was tested as a confounding variable in statistical models, and was found to not affect outcomes of interest. Thus, diet sequence was not included in the final models. Multiple linear regression modeling was used to determine independent associations of change in fat mass with insulin AUC derived from the solid meal test adjusted for glucose AUC and baseline fat mass.
3. Results
Twenty-three women completed both arms of the study, 27 women completed only the reduced-CHO arm, and four women discontinued the intervention for various reasons unrelated to the study. The study participants were an ethnically diverse sample of women (43%, 4%, and 53% were Caucasian, Hispanic and African-American, resp.) with an average age of 31± 5.8 years and a body mass index (BMI) of 31.8±5.7 kg/m2 at baseline. Although diets were designed to be weight-maintaining, participants lost weight across the intervention. On average, weight change was −1.3±2.3 kg for women on the STD diet arm, and −1.4±2.0 kg for women on the reduced-CHO diet arm, and did not differ by diet arm (p=0.56).
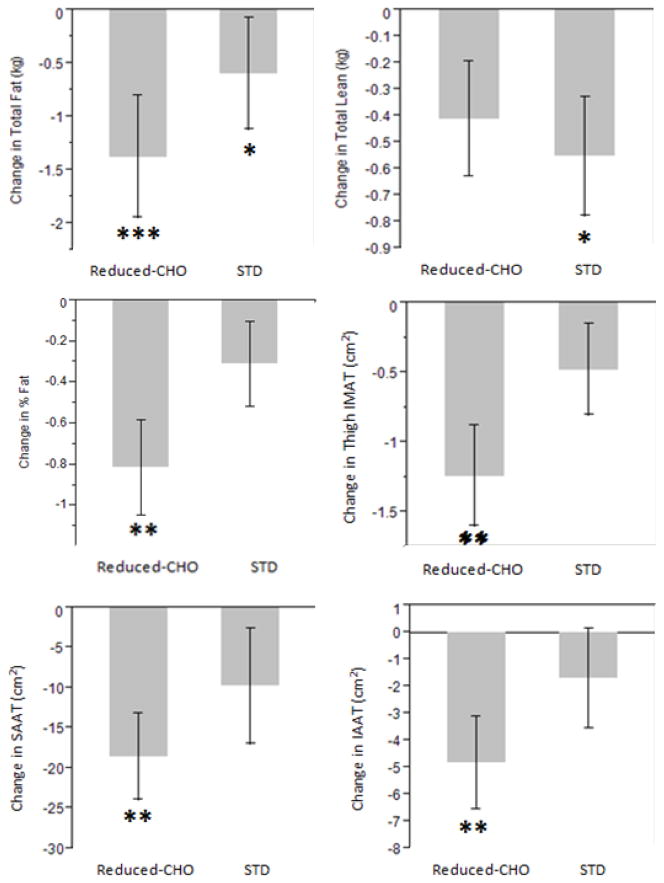
Changes in body composition, fat distribution, and serum analyte variables over the 16-week intervention period are reported by diet arm in Table 2 and Figure 1. Following both diet arms, paired t-tests indicated there were significant reductions in weight, total fat mass, and thigh-SAT. Participants lost 3.7% total body fat mass following the 8-week reduced-CHO diet arm and 2.2% total fat mass following the 8-week STD diet (p<0.001 and p<0.05, resp. ) arm. However, only the reduced-CHO diet resulted in significant decreases in IAAT, SAAT, and thigh-IMAT (−7.1%, −4.6%, and −11.5%, resp., p<0.01).
Table 2.
Baseline and 8-week outcomes by diet arm
| STD diet (n=23) | Reduced-CHO diet (n=27) | P for diett | |||
|---|---|---|---|---|---|
| Week 0 | Week 8 | Week 0 | Week 8 | ||
| Weight (kg) | 83.7±15.2 | 82.6±15.4* | 84.4±15.9 | 82.8±16.0** | 0.50 |
| Total fat (kg) | 35.9±11.9 | 35.1±10.6* | 37.6±11.3 | 36.2±11.6*** | 0.29 |
| % Body fat | 41.6±7.6 | 41.3±6.4 | 42.5±6.0 | 41.6±6.4** | 0.13 |
| Total Lean (kg) | 46.0±5.6 | 45.4±5.7* | 46.4±6.6 | 46.1±6.7 | 0.53 |
| IAAT (cm2) | 61.6±48.9 | 59.9±47.8 | 65.2±48.6 | 60.3±45.8** | 0.23 |
| SAAT (cm2) | 390.3±128.6 | 380.5±124.1 | 410.0±131.5 | 391.1±128.8** | 0.38 |
| Thigh SAT (cm2) | 317.1±102.7 | 305.2±103.5* | 308.8±105.9 | 297.3±102.0*** | 0.99 |
| Thigh PMAT (cm2) | 21.6±8.1 | 21.2±8.7 | 21.3±8.5 | 20.1±8.9 | 0.31 |
| Thigh IMAT (cm2) | 11.2±5.8 | 10.7±6.2 | 12.2±7.7 | 10.8±7.7** | 0.13 |
Data presented as mean±SD.
p<0.05,
p<0.01,
p<0.001 vs. week 0 of the same diet by paired t-test.
By ANCOVA, effect of diet on 8-wk body composition and fat distribution measures after adjustment for baseline body composition and fat distribution measures.
IAAT, intra-abdominal adipose tissue; IMAT, intermuscular adipose tissue; PMAT, perimuscular adipose tissue; SAAT, subcutaneous abdominal adipose tissue; SAT, subcutaneous adipose tissue.
Fig. 1.
Mean (±SE) difference in 8-wk total fat mass, total lean mass, % fat, intra-abdominal adipose tissue (IAAT), subcutaneous abdominal adipose tissue (SAAT), and thigh intermuscular adipose tissue (IMAT) by diet. *p<0.05, **p<0.01, ***p<0.001 for paired t-test. Reduced CHO, reduced carbohydrate; STD. standard.
The STD diet, but not the reduced-CHO diet, resulted in a significant decrease in total lean mass (−1.3%, p<0.05). Change in fat mass was significantly greater following the reduced-CHO diet arm compared to the STD diet arm after adjustment for baseline total fat mass and change in lean mass (p<0.05 for difference between diets, Figure 2). Loss of fat mass was associated with lower insulin AUC (Standardized β=0.50, p<0.05, Figure 3) during the solid meal test, whereas there was no association during the STD diet arm.
Fig. 2.
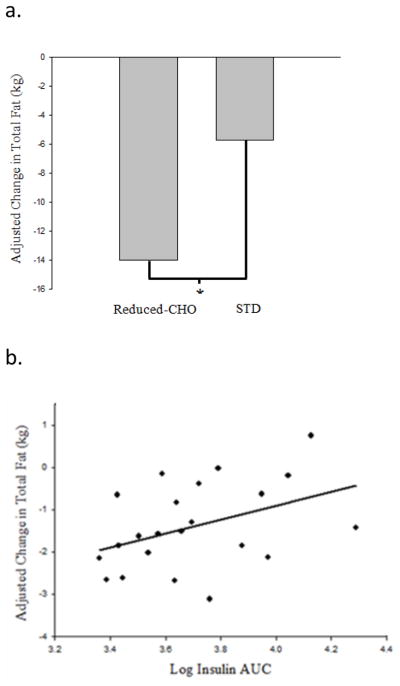
Change in fat mass, adjusted for baseline total fat (kg) and change in total lean (kg) by ANCOVA, was significantly greater following the reduced carbohydrate (CHO) diet arm compared to the standard (STD) diet arm; *p<0.05 for diet effect (a.). Change in fat mass following the reduced CHO diet arm was associated with insulin area-under-the-curve (AUC) derived from the reduced CHO solid meal test (model adjusted for baseline total fat mass; standardized β=0.50, p<0.05) (b)
4. Discussion
Weight gain and obesity are particularly problematic among women with PCOS. It is likely that common features of the syndrome, such as hyperinsulinemia, metabolic dysfunction and hyperandrogenemia, are mechanistically linked to accumulation of excess adiposity, specifically to the abdominal region. Regardless of BMI, a fat distribution pattern characterized by proportionally greater abdominal adiposity may further exacerbate inherent metabolic abnormalities associated with the syndrome. To date, the optimal dietary strategy to reduce propensity for weight gain in this population remains equivocal. In the current study, we tested the hypothesis that a diet reduced in CHO content would result in greater loss of fat mass compared to a higher-CHO diet matched for caloric content. Although the diets were designed to be weight maintaining, women lost some weight across both arms of the study. On average, this weight change was small (−1.6? kg) and did not differ with diet arm. Thus, it was unlikely to have influenced the results. However, results indicate that the reduced-CHO diet had more favorable effects on body composition than the STD diet by inducing greater loss of fat mass while preserving lean mass. Following the reduced-CHO diet, loss of fat mass appeared to be due primarily to loss of adiposity from the abdominal region and other metabolically harmful depots, such as thigh-IMAT. These data suggest that a diet lower in CHO may be effective in improving body composition and fat distribution among women with PCOS.
The primary finding in this tightly-controlled crossover dietary intervention was that the reduced-CHO diet resulted in preferential loss of fat mass compared to the STD diet. Loss of fat mass was achieved without caloric restriction, but rather with only a moderate reduction in CHO content and an increase in dietary fat. This shift in body composition to proportionally less fat mass relative to lean mass suggests that a reduced-CHO diet improves fat oxidation while preserving lean mass. These findings are supported by the results of our previous study, reporting significantly greater fat mass loss relative to lean mass following a hypocaloric moderately reduced-CHO diet in healthy overweight/obese men and women 12. Other studies have examined the effects of reduced-CHO or low glycemic load diets under hypocaloric conditions in women with PCOS without significant group differences in weight loss 6–8;20. In these studies, the weight loss design and lack of robust measures of body composition may have limited the ability to detect preferential loss of fat mass in the lower CHO groups. Change in gross weight did not significantly differ by diet arm in our study; thus, using this outcome alone would have masked the differences in changes in body composition that occurred. Inconsistencies in the literature regarding the effects of diets differing in macronutrient composition on weight loss in women with PCOS 6–8;20 may also be due to differences in study design and methodologies that affect diet adherence. In our study, all food was provided throughout both arms of our dietary intervention, likely minimizing the potential for non-compliance. Thus, we believe these results provide substantial evidence that reducing CHO consumption may be optimal for women with PCOS.
Other studies have reported favorable changes in body composition under the conditions of a reduced CHO intake in healthy populations 21–23. In a 10 week weight loss trial among healthy adult women, Layman et al observed favorable effects of a low CHO/high protein vs. high CHO/low protein diet on body composition despite similar weight loss between diet groups. These investigators held the fat content constant on both experimental diets and were unable to determine if the preferential loss of fat to lean mass among their subjects was due to limiting CHO or increasing protein content of the study diets 21. To our knowledge, the current study is the first conducted in women with PCOS to report significant loss of fat mass and preservation of lean mass in response to a moderately reduced CHO weight maintenance diet without manipulation of protein content on the compared experimental diets. Further research is needed to determine the specific metabolic effects of CHO consumption on energy partitioning in the context of a weight maintaining diet.
The differences in loss of fat mass between the reduced CHO diet and the STD diet appeared to be explained primarily by loss of adipose tissue from two major sub-compartments of the abdomen. Following the reduced-CHO arm, participants had a 7.1% reduction in intra-abdominal and a 4.6% reduction in subcutaneous abdominal fat. Numerous studies have reported that women with PCOS tend to demonstrate a fat distribution pattern synonymous with adverse metabolic health 4;24, and various reports suggest that both intra-abdominal and subcutaneous adipose tissue are associated with chronic low-grade inflammation, dyslipidemia, and insulin resistance in this population 24–26. Results from the present study, indicating loss of SAAT and IAAT under weight maintenance conditions, are analogous with the results of our previous study, during which healthy women consuming a reduced CHO weight maintenance diet had a significant reduction in IAAT. We believe this is the first study to report significant loss of IAAT and SAAT in response to a reduced CHO weight maintenance diet among women with PCOS.
During the reduced-CHO diet, participants also had a significant decrease in thigh IMAT, while there were comparable decreases in thigh SAT with each diet. Thigh IMAT is an ectopic adipose tissue depot located within and between muscle groups and fibers that has been associated with numerous diabetes and cardiovascular disease risk factors 27–29. Conversely, thigh SAT has been linked favorably with metabolic health 16 and it is thought this adipose tissue depot may be protective against development of metabolic disease. Altogether, our data suggests the reduced CHO diet primarily induced loss of adipose tissue from metabolically harmful depots, i.e. thigh IMAT, SAAT and IAAT, thus profoundly improving fat distribution pattern, and potentially metabolic health, in these women.
The physiological mechanism(s) underlying the greater loss of adipose tissue on the reduced-CHO diet, independent of caloric restriction, is unclear, but may be related to alterations in insulin secretion. Insulin greatly influences carbohydrate and lipid metabolism, promoting glycogen synthesis and glucose oxidation, and also promoting triglyceride storage 30. In addition, insulin itself promotes adipogenesis 30. By lowering fasting and postprandial insulin, a reduced CHO diet may enhance lipolysis, promoting use of lipid as a fuel source and reducing adipocyte size, and decreasing adipogenesis and the development of new adipose tissue.
We previously reported, in this same population, that consumption of the reduced CHO diet compared to a higher CHO diet resulted in a decrease in fasting insulin, an increase in acute phase insulin response, and no change in overall insulin response to a liquid meal challenge (10), suggesting reduced chronic exposure to insulin. In this study, we observed that lower postprandial insulin during the lower CHO solid meal test was associated with greater loss of fat mass during the lower-CHO diet arm. Taken together, these observations suggest that by chronically limiting the insulin response to a meal and reducing fasting insulin, a reduced CHO diet may be permissive to increased fatty acid mobilization and oxidation from adipose tissue, and to reduced adipogenesis.
Preservation of lean mass during the reduced-CHO diet arm may also be the result of an overall reduction in insulin exposure. Participants had a significant loss of lean mass during the STD (i.e. higher CHO) diet, which may be explained by the effects of greater fasting and postprandial insulin levels on substrate oxidation and preferential use of metabolic fuel sources. Considering that one of the primary actions of insulin is to limit fatty acid mobilization and oxidation, thus protecting adipose tissue stores, greater insulin secretion in response to greater glucose stimuli from the higher CHO containing diet may have induced repartitioning of energy from lean tissue to adipose tissue.
The present study has several strengths. All food was provided throughout both arms of the study to control subject intake and study menus were composed of foods that could be practically incorporated into free-living intake. This is the first tightly controlled dietary intervention among women with PCOS to use a robust measure of body composition and fat distribution to examine changes to precise adipose tissue depots. Further, the weight maintenance design allowed observation of the effects of macronutrient composition independent of caloric restriction on the outcome of interest. In addition, the crossover design minimized potential inter-subject variability in response to the diets. Limitations of this study include the relatively small sample size and the inability to determine the independent effects of dietary fat vs. CHO content.
4.1 Conclusion
In conclusion, the primary finding in this tightly-controlled crossover dietary intervention was that in women with PCOS consumption of a weight-maintaining diet lower in CHO content resulted in profound improvements in body composition and fat distribution by inducing preferential loss of fat mass, specifically from ectopic, metabolically harmful, adipose depots. In contrast, a diet higher in CHO appeared to promote repartitioning of lean mass to fat mass. Additionally, overall reduced insulin exposure with the reduced-CHO diet may be in part responsible for the selective depletion of adipose tissue and preservation of lean body mass. Overall, our results provide substantial evidence that reducing CHO consumption may be ideal for women with PCOS.
Highlights.
Women with PCOS had significant improvements in body composition and fat distribution following consummation of a eucaloric reduced-CHO diet for 8 weeks.
The reduced-CHO diet induced preferential loss of fat mass, specifically from ectopic, metabolically harmful, adipose depots
The STD diet, but not the reduced-CHO diet, resulted in a significant decrease in total lean mass.
Acknowledgments
Supported by: R01HD054960, UL1RR025777, P30DK56336, P60DK079626.
The authors gratefully acknowledge the help of Maryellen Williams and Cindy Zeng of the UAB Metabolism/Human Physiology Core Laboratory (Nutrition Obesity Research Center, Diabetes Research Center, Center for Clinical and Translational Science) with laboratory analyses, and of Betty Darnell and Suzanne Choquette of the UAB Center for Clinical and Translational Science with experimental design and diet development.
Abbreviations
- PCOS
polycystic ovary syndrome
- CHO
carbohydrate
- NIH
National Institutes of Health
- BMI
body mass index
- STD
standard
- DXA
dual energy x-ray absorptiometry
- CT
computed tomography
- NDSR
Nutrition Data System for Research
- IAAT
intra-abdominal adipose tissue
- SAAT
subcutaneous abdominal adipose tissue
- IMAT
intermuscular adipose tissue
- SAT
subcutaneous adipose tissue
- PMAT
perimuscular adipose tissue
- CRU
Clinical Research Unit
- AUC
area-unser-the-curve
- CV
coefficient of variation
Footnotes
Disclosure Statement: The authors have nothing to disclose.
The authors’ responsibilities were as follows—AMG and BAG: analyzed the data, wrote the manuscript, and had primary responsibility for final content; LLG: conducted the research. All authors participated in the design of the research project and read and approved the final manuscript.
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Ezeh U, Pall M, Mathur R, et al. Effects of endogenous androgens and abdominal fat distribution on the interrelationship between insulin and non-insulin-mediated glucose uptake in females. J Clin Endocrinol Metab. 2013;98:1541–1548. doi: 10.1210/jc.2012-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang RJ, Nakamura RM, Judd HL, et al. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–359. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A, Graf M, Mandeli J, et al. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987;65:499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- 6.Marsh KA, Steinbeck KS, Atkinson FS, et al. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92:83–92. doi: 10.3945/ajcn.2010.29261. [DOI] [PubMed] [Google Scholar]
- 7.Mehrabani HH, Salehpour S, Amiri Z, et al. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31:117–125. doi: 10.1080/07315724.2012.10720017. [DOI] [PubMed] [Google Scholar]
- 8.Moran LJ, Noakes M, Clifton PM, et al. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84:77–87. doi: 10.1093/ajcn/84.1.77. [DOI] [PubMed] [Google Scholar]
- 9.Barr S, Reeves S, Sharp K, et al. An isocaloric low glycemic index diet improves insulin sensitivity in women with polycystic ovary syndrome. J Acad Nutr Diet. 2013;113:1523–1531. doi: 10.1016/j.jand.2013.06.347. [DOI] [PubMed] [Google Scholar]
- 10.Brand-Miller JC, Thomas M, Swan V, et al. Physiological validation of the concept of glycemic load in lean young adults. J Nutr. 2003;133:2728–2732. doi: 10.1093/jn/133.9.2728. [DOI] [PubMed] [Google Scholar]
- 11.Walsh CO, Ebbeling CB, Swain JF, et al. Effects of diet composition on postprandial energy availability during weight loss maintenance. PLoS One. 2013;8:e58172. doi: 10.1371/journal.pone.0058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goss AM, Goree LL, Ellis AC, et al. Effects of diet macronutrient composition on body composition and fat distribution during weight maintenance and weight loss. Obesity(Silver Spring) 2012 doi: 10.1002/oby.20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gower BA, Chandler-Laney PC, Ovalle F, et al. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol(Oxf) 2013 doi: 10.1111/cen.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kekes-Szabo T, Hunter GR, Nyikos I, et al. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obes Res. 1994;2:450–457. doi: 10.1002/j.1550-8528.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 16.Goss AM, Gower BA. Insulin sensitivity is associated with thigh adipose tissue distribution in healthy postmenopausal women. Metabolism. 2012;61:1817–1823. doi: 10.1016/j.metabol.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goran MI, Kaskoun M, Shuman WP. Intra-abdominal adipose tissue in young children. Int J Obes Relat Metab Disord. 1995;19:279–283. [PubMed] [Google Scholar]
- 18.Le Floch JP, Escuyer P, Baudin E, et al. Blood glucose area under the curve. Methodological aspects. Diabetes Care. 1990;13:172–175. doi: 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- 19.Matthews JN, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamets K, Taylor DS, Kunselman A, et al. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81:630–637. doi: 10.1016/j.fertnstert.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Layman DK, Boileau RA, Erickson DJ, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 22.Volek JS, Sharman MJ, Love DM, et al. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism. 2002;51:864–870. doi: 10.1053/meta.2002.32037. [DOI] [PubMed] [Google Scholar]
- 23.Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 24.Karabulut A, Yaylali GF, Demirlenk S, et al. Evaluation of body fat distribution in PCOS and its association with carotid atherosclerosis and insulin resistance. Gynecol Endocrinol. 2012;28:111–114. doi: 10.3109/09513590.2011.589929. [DOI] [PubMed] [Google Scholar]
- 25.Tao T, Li S, Zhao A, et al. Expression of the CD11c gene in subcutaneous adipose tissue is associated with cytokine level and insulin resistance in women with polycystic ovary syndrome. Eur J Endocrinol. 2012;167:705–713. doi: 10.1530/EJE-12-0340. [DOI] [PubMed] [Google Scholar]
- 26.Huang ZH, Manickam B, Ryvkin V, et al. PCOS is associated with increased CD11c expression and crown-like structures in adipose tissue and increased central abdominal fat depots independent of obesity. J Clin Endocrinol Metab. 2013;98:E17–E24. doi: 10.1210/jc.2012-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams MJ, Hunter GR, Kekes-Szabo T, et al. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855–860. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 28.Yim JE, Heshka S, Albu JB, et al. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol. 2008;104:700–707. doi: 10.1152/japplphysiol.01035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoico E, Rossi A, Di FV, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65:295–299. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimitriadis G, Mitrou P, Lambadiari V, et al. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93 (Suppl 1):S52–S59. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]