Abstract
Dysregulation of hepatic glucose uptake (HGU) and inability of insulin to suppress hepatic glucose production (HGP), both contribute to hyperglycemia in patients with type 2 diabetes (T2D). Growing evidence suggests that insulin can inhibit HGP not only through a direct effect on the liver, but also via a mechanism involving the brain. Yet the notion that insulin action in the brain plays a physiological role in the control of HGP continues to be controversial. Although studies in dogs suggest that the direct hepatic effect of insulin is sufficient to explain day-to-day control of HGP, a surprising outcome has been revealed by recent studies in mice investigating whether the direct hepatic action of insulin is necessary for normal HGP: when hepatic insulin signaling pathway was genetically disrupted, HGP was maintained normally even in the absence of direct input from insulin. Here we present evidence that points to a potentially important role of the brain in the physiological control of both HGU and HGP in response to input from insulin as well as other hormones and nutrients.
Keywords: hepatic glucose production, hepatic glucose uptake, obesity, type 2 diabetes, pancreas, hypothalamus
Introduction
The alarming epidemic of obesity and type 2 diabetes (T2D) in the US and worldwide has heightened the need for new strategies for improving glycaemic control and preventing diabetes complications [1–4]. Defective control of hepatic glucose production (HGP) is an important contributor to hyperglycaemia in patients with diabetes [5], but mechanisms underlying this defect are incompletely understood. Here we review evidence that in addition to the direct action of islet hormones on hepatocytes, HGP can be regulated by the brain in response to input from a variety of hormones and nutrients. Similarly, a role for the brain is suggested in the control of hepatic glucose uptake (HGU), a major determinant of post-prandial glycaemia [6]. An improved understanding of the roles of the brain and islet in the control of HGP will inform our understanding of the pathogenesis of diabetes and may one day yield improved approaches to its treatment.
The role of the brain in the control of glucose metabolism
Evidence of a role for the brain in both glucose homeostasis and diabetes pathogenesis originated with the seminal observation by the renowned physiologist Claude Bernard that in rabbits, puncturing the floor of the fourth ventricle results in glycosuria [7]. A century later, Jean Mayer proposed the “glucostatic hypothesis,” which introduced the concept that hypothalamic glucoreceptors sense fluctuations in circulating glucose levels (or, more precisely, neuronal glucose availability) and translate them into a neural signal [8]. The subsquent discovery of glucose-sensing neurons within the hypothalamus [9] and their role in glucose counterregulation (the response to hypoglycemia that restores blood glucose levels to normal) [10,11] further established a role for the brain in control of glucose metabolism. Despite these advances, the question of whether the brain plays a physiological role in the day-to-day control of glucose homeostasis remains a matter of active debate. In this review, we highlight recent findings that point to a role for the brain in the physiological control of both HGU and HGP in response to input from circulating hormones and nutrients.
The role of the liver in the control of glucose metabolism
The liver plays a pivotal role in whole-body glucose homeostasis. Following a meal, ingested nutrients are absorbed from the gastrointestinal (GI) tract into the portal vein and therefore pass through the liver before entering the systemic circulation. Indeed, the liver is a major determinant of oral glucose tolerance, since it removes and stores a large fraction of ingested glucose following a meal, while simultaneously reducing its release back into the circulation. Conversely, when food is no longer being absorbed from the GI tract, an increase of HGP is required to meet the body’s need for glucose and avert hypoglycemia. Thus, the liver changes from being a net consumer to a net producer of glucose on a daily basis. How does this occur?
In the fasted state, plasma glucose levels begin to decline as nutrients are no longer absorbed from the GI tract. Consequently, insulin secretion from pancreatic islets decreases, whereas glucagon secretion tends to increase. Since blood from the pancreas drains into the portal vein, the liver is normally exposed to levels of islet hormones considerably higher than in the systemic circulation. Also important is the fact that glucagon acts on hepatocytes to increase HGP (both by mobilizing hepatic glycogen stores through glycogenolysis and by converting the non-carbohydrate precursors, lactate, glycerol, non-esterified fatty acids (NEFA), and amino acids into glucose through the process of gluconeogenesis), whereas insulin has the opposite effect. Fasting is also associated with activation of the hypothalamic-pituitary-adrenal (HPA) axis and thus increases of circulating glucocorticoids, which also serve to augment HGP. Speaking metaphorically, it is therefore as if one has stepped on the accelerator pedal (increased glucagon and glucocorticoids) while simultaneously taking one’s foot off the brake pedal (reduced insulin) where HGP is concerned, a combination designed to ensure that fasting plasma glucose concentrations do not fall below the normal range.
The situation is essentially reversed following the ingestion of glucose or a mixed meal, with rising plasma levels of glucose and insulin effectively suppressing HGP while simultaneously increasing HGU, a combination that effectively limits the post-prandial blood glucose excursion. Interestingly, insulin plays a crucial role to inhibit HGP, whereas HGU is by comparison much less sensitive to insulin. Instead, an increased concentration of glucose in the portal vein relative to the systemic circulation, referred to as the “portal signal,” is sufficient to explain the increase of HGU that occurs following an oral glucose load, with subsequent storage of glucose as glycogen in hepatocytes [6]. In this way, the liver is responsible for disposing one-third or more of an oral glucose load in normal individuals [6]. Importantly, diabetes is associated with impairments affecting both sides of hepatic glucose metabolism, characterized by the failure to increase HGU and suppress HGP normally following a meal [5].
Insulin inhibits HGP by both direct and indirect pathways
The hypothesis that insulin inhibits HGP solely through a direct action on hepatocytes is widely held and supported by a large body of literature. Following its entry into the portal vein, some 60% of secreted insulin is extracted by binding to hepatocyte insulin receptors (IR) prior to entering the systemic circulation, such that the liver is exposed to insulin concentrations some 3-fold higher than non-hepatic tissues. Further, unlike the vasculature in adipose, muscle and most other tissues, the capillary endothelium within the liver is highly fenestrated, such that hepatocytes are exposed to sinusoidal blood that does not have to transverse an endothelial barrier, and the binding of insulin to hepatic insulin receptors activates a well-characterized signal transduction cascade that potently inhibits both glycogenolysis and gluconeogenesis (described below).
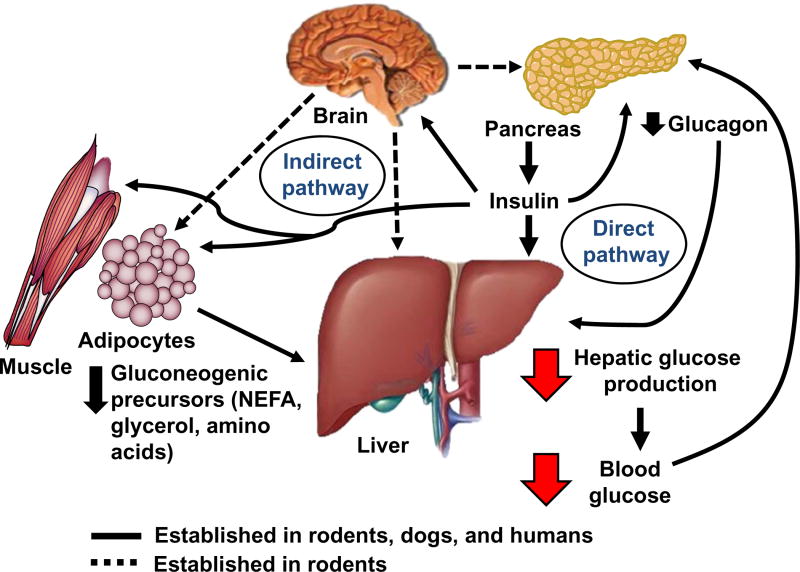
Yet the primacy of insulin action directly on the liver in the control of HGP has been effectively challenged, and a strong argument can be made in support of an indirect (or extra-hepatic) pathway to explain the insulin effect. Among seminal findings is the fact that insulin is similarly effective with respect to inhibition of HGP whether it is infused systemically or into the portal vein [12–14]. To explain regulation of HGP by insulin action at a remote site, several proposed mechanisms (summarized in Figure 1) have been advanced: 1) suppression of glucagon secretion from the pancreatic α-cell [15–17], 2) reduced availability of circulating gluconeogenic precursors (through inhibition of both adipocyte lipolysis (glycerol and NEFA) and muscle proteolysis (amino acids) [18–21], and 3) modulation of neural input to the liver via insulin action in the central nervous system (CNS) [22]. In addition, brain insulin signaling has been suggested to inhibit both adipose tissue lipolysis [23,24] and pancreatic glucagon secretion [25]. Despite these and several additional observations, however, the notion that insulin action in the brain plays a physiological role in this process continues to be a source of controversy [26].
Figure 1. Mechanisms of direct vs. indirect effects in the control of hepatic glucose production by insulin.
Insulin inhibits hepatic glucose production (HGP) via direct action at the liver (binding to hepatic insulin receptors) as well as indirectly at remote (extra-hepatic) sites. The indirect mechanisms of insulin on the liver include suppression of pancreatic α-cell glucagon secretion, inhibition of both adipocyte lipolysis (NEFA and glycerol) and skeletal muscle proteolysis (amino acids) to reduce the availability of circulating gluconeogenic precursors to the liver, and an increase in vagal efferent signaling to the liver via an action in the brain. In addition, insulin action in the brain has been shown to suppress pancreatic glucagon secretion and lipolysis which may reinforce insulin’s peripheral effects on these tissues.
The case for direct action of insulin to control HGP
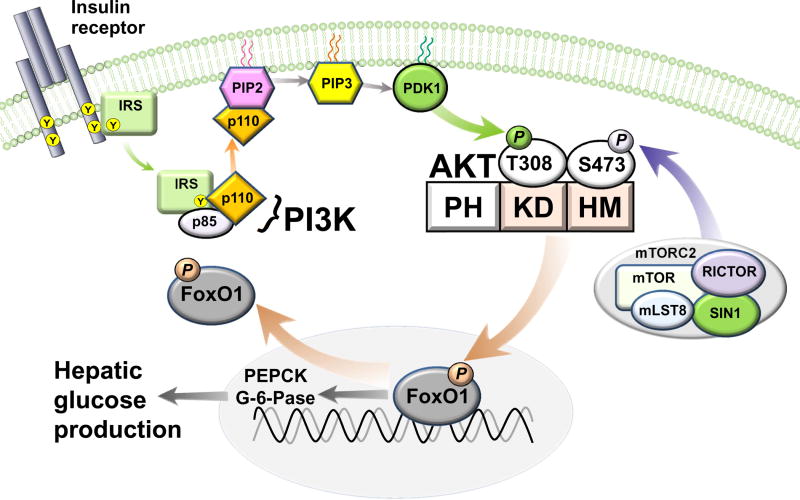
There is little question that insulin can regulate HGP via a direct action on the liver. Following binding to its receptor on hepatocytes, insulin activates the canonical insulin receptor substrate (IRS)-phosphatidylinositol 3-OH kinase (PI3K) pathway. This signal transduction pathway mediates insulin suppression of both glycogenolysis and gluconeogenesis via molecular mechanisms that are now well understood. Specifically, generation of phosphoinositide intermediates by PI3K activates phosphoinositide-dependent kinase-1 (PDK-1), which in turn phosphorylates the serine (Ser)/threonine (Thr) kinase AKT (also known as protein kinase B; PKB) [27]. In addition to PDK1-mediated phosphorylation at Thr308, AKT is also activated by phosphorylation at Ser473 by the rictor containing mammalian target of rapamycin complex 2 (mTORC2) [28,29], and AKT is a crucial mediator of many of insulin’s metabolic effects in liver, brain, muscle, adipose and other tissues [30,31].
Of particular relevance to the control of HGP is AKT-directed phosphorylation of the transcription factor forkhead box O1 (FoxO1). This phosphorylation event causes FoxO1 exclusion from the nucleus, thereby terminating the transcription of two rate-controlling enzymes for gluconeogenesis, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) (Figure 2) [32]. Consistent with this mechanism, mice with liver-specific deletion of either the insulin receptor (LIRKO) or the two hepatic AKT isoforms (DLKO) exhibit glucose intolerance and liver insulin resistance [33,34], owing to constitutive activation of FoxO1 and hence, unregulated and unconstrained HGP. These observations, together, offer compelling evidence that the direct action of insulin on hepatocytes is indispensable for normal control of HGP, and hence, normal glucose homeostasis.
Figure 2. Insulin signal transduction in hepatocytes.
Insulin regulates HGP by activating the IRS-PI3K pathway, which is coupled to the recruitment and activation of AKT, the key insulin signaling intermediary that inhibits the transcription factor FoxO1. Full activation of AKT requires PDK-1 directed phosphorylation at threonine 308 (T308) in the kinase domain (KD) followed by phosphorylation at serine 473 (S473) in the hydrophobic motif (HM) of AKT by the rictor containing complex, mammalian target of rapamycin (mTOR) complex 2 (mTORC2). Subsequently, the phosphorylation of FoxO1 by AKT leads to its nuclear exclusion, thereby terminating the transcription of two rate-controlling enzymes for gluconeogenesis (PEPCK and G-6-Pase). PH, pleckstrin homology domain; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate.
The gold standard for measuring liver glucose metabolism involves computing the arterio-venous glucose gradient across the liver by simultaneous sampling from the hepatic artery, hepatic portal vein, and hepatic vein [35]. Such studies require a large animal model and hence have been performed primarily in dogs, including extensive work by Cherrington and colleagues showing the expected changes of HGP in response to changes of portal vein insulin levels. These studies were undertaken with the pancreatic clamp technique, in which an infusion of somatostatin is used to inhibit islet hormone secretion, and insulin and glucagon are replaced at basal levels [18,19]. Using this approach, a selective rise of portal insulin levels was shown to reduce HGP by ~50% within a 3 h period, despite no change in levels of arterial insulin, liver sinusoidal glucagon, arterial plasma glucose or NEFA levels, or hepatic uptake of gluconeogenic precursors. This rapid, insulin-mediated suppression of HGP appears to be explained by the effect of increased hepatic sinusoidal insulin levels to inhibit glycogenolysis [19]. Conversely, a selective decrease of portal vein (hepatic sinusoidal) insulin levels led to a rapid and sustained increase of HGP, owing to a marked stimulation of glycogenolysis despite no change of arterial insulin levels [18]. In the canine model, therefore, HGP is highly sensitive to selective changes in portal vein insulin levels, even when systemic concentrations of insulin and glucose are held constant [18]. These data, based on direct measures of hepatic glucose flux in a large animal model, add to evidence in support of the primacy of insulin’s direct hepatic action in the control of HGP.
The case for brain insulin action in the control of HGP
In addition to the well-established role of insulin in the central regulation of energy homeostasis [36], pancreatic hormone secretion [25,37,38], and fat metabolism [23,24], insulin action in the brain can also affect hepatic glucose metabolism. While few would dismiss this claim, the extent to which brain insulin action participates in normal, day-to-day control of glucose homeostasis is poorly understood and remains controversial. Literature regarding hypothalamic insulin action and control of HGP began with studies in a rat model by Obici et al. [39], who showed that knockdown of hypothalamic insulin receptors via infusion of an IR anti-sense oligodeoxynucleotide directly into the third ventricle impairs the suppression of HGP during a hyperinsulinaemic-euglycaemic clamp, an effect interpreted as hepatic insulin resistance. Subsequent studies by the same group [40] used the pancreatic clamp method to show that in absence of changes in basal plasma insulin and glucagon levels, intracerebroventricular (icv) infusion of either insulin or an insulin mimetic increased hepatic insulin sensitivity and robustly suppressed HGP (by ~40%), whereas disruption of neuronal insulin signaling had the opposite effect. These and other findings support a model in which reduced hypothalamic insulin action, causes the liver to become unresponsive to the direct action of insulin.
Extending this work are studies implicating activation of neuronal ATP-sensitive potassium (KATP) channels in the mechanism underlying control of HGP by hypothalamic insulin action. In some neurons, KATP channels are downstream targets of the IRS-PI3K signaling pathway, and icv administration of drugs that activate KATP channels (such as diazoxide) exert hepatic effects similar to those of icv insulin [22]. Conversely, central administration of drugs that inactivate KATP channels (such as sulfonylureas) negate the effect of icv insulin, as does surgical resection of the hepatic branch of the vagus nerve [22]. Lastly, clamp studies in mice lacking the KATP channel subunit SUR1 exhibit both hepatic insulin resistance and increased gluconeogenesis [22]. Together, the above studies suggest that insulin signaling via the IRS-PI3K pathway (the same signal transduction pathway activated by insulin in hepatocytes) activates KATP channels in a subset of hypothalamic neurons. These neurons are proposed in turn to engage a neuro-circuit that ultimately enhances hepatocyte insulin sensitivity via a mechanism that is dependent on vagal innervation of the liver.
Recent studies by Filippi et al. [41] suggest that extra-hypothalamic brain areas can also mediate indirect control of HGP by insulin. They showed that HGP is inhibited by administration of insulin into the dorsal vagal complex (DVC) of the hindbrain and, surprisingly, that the intracellular signal transduction mechanism involved is distinct from that utilized by insulin in hypothalamic control of HGP. Specifically, insulin activation of the extracellular signal-regulated kinase (ERK) 1/2 signaling, rather than PI3K, appears to mediate the effect of DVC insulin to inhibit HGP. This work implies a distributed network of brain areas in the indirect control of HGP by insulin [41].
The efferent mechanism linking brain to liver in the indirect control of HGP appears to involve vagal efferent fibres, as selective hepatic vagotomy blocks the effect of central insulin, whereas selective vagal deafferentation (resection of only the vagal afferents) does not [22]. This does not exclude a potentially important role for other mechanisms (e.g., reduced sympathetic nervous system outflow to liver), however, and much additional work is needed to achieve a clear understanding of effector mechanisms involved. Some investigators report that neural inhibition of HGP involves activation of the transcription factor signal transducer and activator of transcription 3 (STAT3) in hepatocytes following interleukin-6 (IL-6) release from adjacent Kupffer cells (the resident macrophage of the liver) [42,43]. STAT3 opposes the action of FoxO1 and thus inhibits gluconeogenic gene expression, but how neural input to the liver might stimulate IL-6 release from Kupffer cells is unclear.
A completely separate set of conclusions regarding CNS mediated glucose lowering derives from work in rats with uncontrolled (insulin-deficient) diabetes induced by the β-cell toxin streptozotocin (STZ). In these animals, the ability of systemic insulin to lower blood glucose levels is substantially attenuated by infusion of a PI3K inhibitor directly into the third ventricle [44], implying that glucose lowering by systemic insulin requires intact neuronal PI3K signaling. Perhaps more important insights are derived from studies of leptin action in rats with STZ-induced diabetes.
Interestingly, adenoviral or pharmacological induced hyperleptinaemia fully ameliorates hyperglycaemia in STZ-induced diabetic rats.[45, 46] Unger and colleagues [46] identified suppression of glucagon as a potential mechanism to explain this finding. Work by Morton and colleagues subsequently demonstrated that when leptin is infused directly into the brain at low doses, it reproduces the surprising, complete normalization of hyperglycaemia in STZ-induced diabetic rats, despite persistent, severe insulin deficiency [47]. Under the influence of leptin, therefore, the brain has the previously unrecognized capacity to restore euglycaemia to diabetic animals via mechanisms that are entirely insulin-independent. Moreover, this effect of icv leptin involves normalization of elevated HGP [47], despite ongoing, severe insulin deficiency. Clearly, this action of leptin cannot be explained by simply increasing the sensitivity of hepatocytes to endogenous insulin, as there is not enough insulin present to mediate such an effect. An alternative explanation is that central leptin normalizes elevated plasma levels of glucagon and corticosterone. Although these effects do indeed occur, available data suggest that they are insufficient to explain the restoration of euglycaemia [48].
Physiological relevance of direct and indirect control of HGP
To address whether insulin action in the brain is required for normal control of HGP, several groups have used mice in which neuronal insulin receptors are deleted either throughout the brain [24,49], selectively in the hypothalamus [39], or in defined hypothalamic neuronal subsets [42]. In each case, albeit to varying degrees, neuronal insulin receptor deletion is associated with glucose intolerance and/or systemic insulin resistance, suggesting that neuronal insulin action is required for normal glucose homeostasis. However, Cherrington and colleagues have argued cogently against the physiological relevance of hypothalamic insulin action in the control of HGP [26]. Their concern stems in part from reliance on data obtained using the glucose clamp method, the gold standard for measuring HGP in rodents. At issue is the fact that under normal conditions, insulin levels are higher in the portal vein than in the arterial blood (since blood from pancreatic islets drains into the portal vein), and that this gradient is not maintained during the clamp (since hyperinsulinaemia is achieved by administering insulin into the systemic venous circulation and endogenous insulin secretion is often suppressed by somatostatin infusion as part of the protocol). Accordingly, the “clamped” liver is subject to relative insulin deficiency. Furthermore, as some studies do not replace basal glucagon (to prevent an elevation in HGP secondary to relative hepatic hypoinsulinaemia), the net effect is that brain insulin action is measured under conditions in which the liver is exposed to relatively low levels of insulin and glucagon, and it is this unique situation that enables the detection of extra-hepatic insulin effects (e.g., in the hypothalamus) that would not be observed under more physiological conditions. Under usual circumstances, it is therefore argued, insulin control of HGP can and should be attributed to its direct action on hepatocytes.
As a proof of concept, Cherrington’s group carried out a series of sophisticated pancreatic clamp experiments (somatostatin plus intraportal infusion of basal insulin and glucagon to ensure that the liver is exposed to normal levels of these hormones) in overnight-fasted conscious dogs. The goal of these studies was to isolate insulin’s direct (hepatic) effect from its indirect ones, and determine which predominates in the control of HGP [50]. Following a 40 min control period, the route of basal insulin delivery was switched from the portal vein to a peripheral vein, thereby leading to the combination of arterial hyperinsulinaemia (at non-hepatic tissues) and relative hepatic insulin deficiency. Despite hyperinsulinaemia at the non-hepatic tissues (including the CNS), HGP increased by 2-fold relative to dogs that continued to receive intraportal insulin infusion. In a separate study [50], switching the portal vein insulin infusion to the head (carotid and vertebral) arteries to bring about a physiologic 4-fold rise of brain insulin levels over the last 2-h of the clamp failed to suppress HGP relative to that of the peripheral insulin infusion group. Thus, the authors concluded that insulin’s direct effect on the liver dominates over its indirect effects in the regulation of HGP in dogs.
In a follow-up study, Ramnanan et al. [51] sought to determine whether the insulin-brain-liver signaling axis described in rodents also exists in canines. During a basal pancreatic clamp, icv insulin or vehicle was infused into the third ventricle of a small number of dogs at a rate previously observed to suppress HGP in rats [40]. Central insulin had unambiguous effects in dogs: it induced AKT activation in the hypothalamus and STAT3 signaling in the liver, effects that were associated with suppression of liver gluconeogenic genes (PEPCK and G-6-Pase). In addition, net hepatic glucose output (NHGO) trended downward, as predicted from rodent studies, but the effect was not statistically significant. The above experiment therefore confirmed the existence of a mechanism for hepatic regulation by hypothalamic insulin signaling in dogs, but did not reveal major effects on HGP, leading the authors to conclude that the physiologic relevance of this insulin-brain-liver signaling axis is unclear. In a second study by the same group, insulin was infused into the head arteries to create a marked rise in brain insulin (by ~10-fold) in 42 h fasted dogs. This intervention had the predicted effect of reducing NHGO, but the effect was attributable to increased HGU and glycogen synthesis, rather than to reductions of either gluconeogenesis or HGP [51]. In parallel, concurrent icv administration of a PI3K inhibitor (LY294002) was shown to negate the suppressive effect of head insulin infusion on NHGO.
Although the above studies in dogs suggest that direct hepatic effect of insulin is sufficient to explain day-to-day control of HGP, they do not address the extent to which this effect is required, and a surprising outcome has been revealed by recent studies in mice that investigate this question. In these studies, the hepatic insulin signaling pathway was genetically disrupted and yet HGP appeared to remain more or less unaffected. Buettner et al. [52] demonstrated that when hepatic insulin receptors were ablated using an IR anti-sense oligodeoxynucleotide in otherwise normal mice, liver insulin signaling was markedly attenuated, as expected, but the ability of systemic insulin to regulate HGP during a hyperinsulinaemic-euglycaemic clamp was not. This study also revealed that although the acute disruption of insulin’s direct action on the liver stimulated glycogenolysis, the effect was offset by a parallel decrease of gluconeogenesis. The authors concluded that in the setting of acute hepatic insulin deficiency, insulin action at an extra-hepatic site is sufficient to control HGP, a conclusion seemingly in conflict with work performed in mice with lifelong, liver-specific IR deletion (LIRKO) [34].
Additional insight comes from Okamoto et al., who studied mice lacking insulin receptors in brain and liver [53]. They found that partial restoration of insulin receptor expression in the liver of these mice did not restore insulin’s ability to suppress HGP during a hyperinsulinaemic-euglycaemic clamp [53], whereas restoration of insulin receptors to both brain and liver did [54]. Perhaps the compelling evidence of an indirect pathway through which insulin can regulate HGP comes from comparison of the phenotypes of mice with liver-specific disruption of insulin signaling alone (by genetic, liver-specific deletion of the two AKT isoforms (DLKO)) to mice with liver-specific deletion of FoxO1 as well as the two AKT isoforms (TLKO) [33]. Despite the fact that insulin cannot act directly on hepatocytes to regulate HGP via the canonical AKT-FoxO1 pathway in TLKO mice, both HGP and systemic glucose homeostasis are controlled normally, even in response to systemic insulin. These findings indicate that genetic disruption of liver insulin action increases HGP because of excessive FoxO1 activity, removal of which enables normal control of HGP via the indirect pathway. These observations collectively suggest that insulin can regulate HGP indirectly via a FoxO1-independent mechanism, but that the effect is blocked by excessive FoxO1 signaling (as occurs in DLKO and LIRKO mice [33,34]). Perhaps more importantly, the data indicate that in mice, the direct hepatic action of insulin is dispensable for normal control of HGP and glucose homeostasis, as long as the indirect pathway is able to function effectively. Stated differently, the collective data suggest that both the direct and the indirect pathways are sufficient to control HGP, and that when one does not function properly, the other can compensate.
Still other possibilities warrant consideration in the analysis of these findings [26,55]. For one, the role played by the liver in overall glucose metabolism differs in several respects between rodents and larger mammals. Unlike dogs and humans that maintain hepatic glycogen even after 42 h of fasting [56,57], rodents deplete glycogen stores quickly owing to their relatively much higher metabolic rate and greater capacity for glycogenolysis [58]. Accordingly, basal HGP in rodents (~13 mg/kg/min for rats and ~30 mg/kg/min for mice) [40,42] is considerably higher on a weight basis compared with dogs and humans (~3 mg/kg/min) [5,50]. Since basal glucagon levels are similar in these species, it is possible that rodent liver is more actively driven by neural input than occurs in larger animals, which may explain how insulin action in the brain more effectively controls HGP (even when hepatic insulin signaling is disrupted). Lastly, the time-course that has been chosen to study CNS insulin action differs markedly between rodents and dogs. While clamp studies are usually performed in rodents in the setting of chronic (4-h) or life-long changes in brain insulin signaling [22,39–43,53,54], insulin action in the brain of dogs was acutely modified for 1-h prior to, or concurrently with a systemic insulin challenge [50,51,59]. Studies to date have yet to examine long-term changes in brain insulin signaling in the control of HGP in canines, and such studies may reveal different effects on HGP.
Hepatic glucose uptake
Hepatic glucose uptake is largely an insulin-independent process that, like HGP, contributes importantly to whole-body glucose homeostasis, and the brain is clearly implicated in this process [6]. Specifically, the delivery of glucose into the portal vein, such that portal vein glucose levels exceed those in the systemic circulation, is a key stimulus to HGU and subsequent glycogen synthesis. This negative arterial-portal venous glucose gradient is referred is referred to as the “portal signal”, and this signal must be processed in the brain for HGU to increase, presumably by stimulating liver glucokinase activity [6,60]. Interestingly, the capacity of the brain to enhance liver glucose uptake is augmented by insulin action in the hypothalamus of dogs [51].
Brain mechanisms controlling insulin-independent glucose disposal
Recent evidence implicates the brain in the control of insulin-independent glucose disposal, also referred to as glucose effectiveness (GE). Our group recently reported that following icv administration to genetically obese, leptin-deficient ob/ob mice, the gastrointestinal hormone fibroblast growth factor-19 (FGF19) improves glucose tolerance by rapidly increasing GE [61]. Specifically, within 2-h of a low dose icv injection of FGF19 that has no glucose-lowering effects when given peripherally, ob/ob mice displayed a 3-fold increase of GE despite no change of either insulin secretion or insulin sensitivity. The mechanism underlying this increase of GE is unclear, but may involve increased metabolism of glucose to lactate, since the improvement of glucose tolerance was associated with a robust increase of plasma lactate levels. Since the glucose lowering effect of FGF19 in ob/ob mice, whether given icv or systemically, was reduced by prior icv administration of an FGF receptor inhibitor (PD173074), a major role for the brain in the anti-diabetic effect of the circulating hormone is implied [61]. These findings, combined with the aforementioned effect of icv leptin to normalize diabetic hyperglycaemia in rats with STZ-induced diabetes, suggest that in response to diverse hormonal and nutrient input, the brain has the inherent capacity to potently and rapidly engage insulin-independent mechanisms that promote glucose lowering.
Concluding remarks
Growing evidence shows that the control of hepatic glucose metabolism is both complex and highly redundant, with both direct and indirect mechanisms playing an important role. Although studies in dogs suggest that the direct hepatic effect of insulin is sufficient to explain day-to-day control of HGP, genetic studies in mice indicate that HGP can be regulated normally even in the absence of a direct input from insulin. Species differences may contribute to these divergent outcomes, but there can be little question that the brain can exert potent effects on virtually all aspects of hepatic glucose metabolism. One hypothetical model for viewing the interaction between the islet and brain is that the hepatic response to the direct action of insulin is continuously – and sometimes powerfully – influenced by the brain; e.g., that input to the liver from the brain “sets the gain” for the hepatic response to the direct action of islet hormones.
Importantly, data from TLKO mice [33] as well as from rats with STZ-induced diabetes [47] suggest that this indirect mechanism appears to effectively control HGP even when the liver cannot sense insulin at all. Extending these observations, it is possible that the dysregulation of HGP characteristic of T2D involves defects in both the direct and indirect pathways. Combined with new evidence that the brain can powerfully control GE [61], and because gradually declining GE is a major contributor to impaired glucose tolerance in the progression of T2D [6,62], a compelling rationale exists for studies that clarify the role of and mechanisms underlying CNS control of glucose homeostasis. This information is critical to future studies that will determine whether defects in this system contribute to obesity-associated glucose intolerance and its progression to T2D.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK083042, DK090320; by a Fellowship Training grant (DK007247); by the Nutrition Obesity Research Center (DK035816), and the Diabetes Research Center (DK17047) at the University of Washington, and by funding from the American Diabetes Association grant: 1-14-BS-182.
Footnotes
CONFLICT OF INTEREST: The authors have declared that no conflict of interest exists.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21 (Suppl 3):C11–14. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- 4.Hossain P, Kawar B, EL Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 6.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012;3:286–294. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard C. Leçons de physiologie expérimentale appliquées á la médecine. Paris: Baillère et Fils; 1854. [Google Scholar]
- 8.Mayer J. Glucostatic mechanism of regulation of food intake 1953. Obes Res. 1996;4:493–496. doi: 10.1002/j.1550-8528.1996.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 9.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 10.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borg MA, Tamborlane WV, Shulman GI, Sherwin RS. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes. 2003;52:663–666. doi: 10.2337/diabetes.52.3.663. [DOI] [PubMed] [Google Scholar]
- 12.Mittelman SD, Fu YY, Rebrin K, Steil G, Bergman RN. Indirect effect of insulin to suppress endogenous glucose production is dominant, even with hyperglucagonemia. J Clin Invest. 1997;100:3121–3130. doi: 10.1172/JCI119867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ader M, Bergman RN. Peripheral effects of insulin dominate suppression of fasting hepatic glucose production. Am J Physiol. 1990;258:E1020–1032. doi: 10.1152/ajpendo.1990.258.6.E1020. [DOI] [PubMed] [Google Scholar]
- 14.Lewis GF, Zinman B, Groenewoud Y, Vranic M, Giacca A. Hepatic glucose production is regulated both by direct hepatic and extrahepatic effects of insulin in humans. Diabetes. 1996;45:454–462. doi: 10.2337/diab.45.4.454. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Maruyama H, Hirose H, et al. Exogenous insulin dose-dependently suppresses glucopenia-induced glucagon secretion from perfused rat pancreas. Metabolism. 1995;44:358–362. doi: 10.1016/0026-0495(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 16.Lewis GF, Vranic M, Giacca A. Glucagon enhances the direct suppressive effect of insulin on hepatic glucose production in humans. Am J Physiol. 1997;272:E371–E378. doi: 10.1152/ajpendo.1997.272.3.E371. [DOI] [PubMed] [Google Scholar]
- 17.Giacca A, Fisher SJ, McCall RH, Shi ZQ, Vranic M. Direct and indirect effects of insulin in suppressing glucose production in depancreatized dogs: role of glucagon. Endocrinology. 1997;138:999–1007. doi: 10.1210/endo.138.3.5025. [DOI] [PubMed] [Google Scholar]
- 18.Sindelar DK, Chu CA, Venson P, Donahue EP, Neal DW, Cherrington AD. Basal hepatic glucose production is regulated by the portal vein insulin concentration. Diabetes. 1998;47:523–529. doi: 10.2337/diabetes.47.4.523. [DOI] [PubMed] [Google Scholar]
- 19.Sindelar DK, Balcom JH, Chu CA, Neal DW, Cherrington AD. A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog. Diabetes. 1996;45:1594–1604. doi: 10.2337/diab.45.11.1594. [DOI] [PubMed] [Google Scholar]
- 20.Lewis GF, Vranic M, Harley P, Giacca A. Fatty acids mediate the acute extrahepatic effects of insulin on hepatic glucose production in humans. Diabetes. 1997;46:1111–1119. doi: 10.2337/diab.46.7.1111. [DOI] [PubMed] [Google Scholar]
- 21.Sindelar DK, Chu CA, Rohlie M, Neal DW, Swift LL, Cherrington AD. The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes. 1997;46:187–196. doi: 10.2337/diab.46.2.187. [DOI] [PubMed] [Google Scholar]
- 22.Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 23.Scherer T, O’Hare J, Diggs-Andrews K, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch L, Wunderlich FT, Seibler J, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paranjape SA, Chan O, Zhu W, et al. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes. 2010;59:1521–1527. doi: 10.2337/db10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramnanan CJ, Edgerton DS, Cherrington AD. Evidence against a physiologic role for acute changes in CNS insulin action in the rapid regulation of hepatic glucose production. Cell Metab. 2012;15:656–664. doi: 10.1016/j.cmet.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 31.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 32.Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Lu M, Wan M, Leavens KF, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 35.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 37.Chen M, Woods SC, Porte D., Jr Effect of cerebral intraventricular insulin on pancreatic insulin secretion in the dog. Diabetes. 1975;24:910–914. doi: 10.2337/diab.24.10.910. [DOI] [PubMed] [Google Scholar]
- 38.Woods SC, Porte D., Jr Effect of intracisternal insulin on plasma glucose and insulin in the dog. Diabetes. 1975;24:905–909. doi: 10.2337/diab.24.10.905. [DOI] [PubMed] [Google Scholar]
- 39.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 40.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 41.Filippi BM, Yang CS, Tang C, Lam TK. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 2012;16:500–510. doi: 10.1016/j.cmet.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Konner AC, Janoschek R, Plum L, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Inoue H, Ogawa W, Asakawa A, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Gelling RW, Morton GJ, Morrison CD, et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3:67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. PNAS. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.German JP, Thaler JP, Wisse BE, et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152:394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz MW, Seeley RJ, Tschop MH, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503:59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 50.Edgerton DS, Lautz M, Scott M, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest. 2006;116:521–527. doi: 10.1172/JCI27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramnanan CJ, Saraswathi V, Smith MS, et al. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest. 2011;121:3713–3723. doi: 10.1172/JCI45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buettner C, Patel R, Muse ED, et al. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115:1306–1313. doi: 10.1172/JCI23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest. 2005;115:1314–1322. doi: 10.1172/JCI23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin HV, Plum L, Ono H, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes. 2010;59:337–346. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cherrington AD. The role of hepatic insulin receptors in the regulation of glucose production. J Clin Invest. 2005;115:1136–1139. doi: 10.1172/JCI25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendrick GK, Frizzell RT, Williams PE, Cherrington AD. Effect of hyperglucagonemia on hepatic glycogenolysis and gluconeogenesis after a prolonged fast. Am J Physiol. 1990;258:E841–E849. doi: 10.1152/ajpendo.1990.258.5.E841. [DOI] [PubMed] [Google Scholar]
- 57.Nuttall FQ, Ngo A, Gannon MC. Regulation of hepatic glucose production and the role of gluconeogenesis in humans: is the rate of gluconeogenesis constant? Diabetes Metab Res Rev. 2008;24:438–458. doi: 10.1002/dmrr.863. [DOI] [PubMed] [Google Scholar]
- 58.Kokubun E, Hirabara SM, Fiamoncini J, Curi R, Haebisch H. Changes of glycogen content in liver, skeletal muscle, and heart from fasted rats. Cell Biochem Funct. 2009;27:488–495. doi: 10.1002/cbf.1602. [DOI] [PubMed] [Google Scholar]
- 59.Ramnanan CJ, Kraft G, Smith MS, et al. Interaction between the central and peripheral effects of insulin in controlling hepatic glucose metabolism in the conscious dog. Diabetes. 2013;62:74–84. doi: 10.2337/db12-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coate KC, Kraft G, Irimia JM, et al. Portal vein glucose entry triggers a coordinated cellular response that potentiates hepatic glucose uptake and storage in normal but not high-fat/high-fructose-fed dogs. Diabetes. 2013;62:392–400. doi: 10.2337/db12-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morton GJ, Matsen ME, Bracy DP, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123:4799–4808. doi: 10.1172/JCI70710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19:1018–1030. doi: 10.2337/diacare.19.9.1018. [DOI] [PubMed] [Google Scholar]