Abstract
One of the earliest events in amyloid β-protein (Aβ) self-association is nucleation of Aβ monomer folding through formation of a turn at Gly25–Lys28. We report here the effects of structural changes at the center of the turn, Gly25-Ser26, on Aβ42 conformational dynamics and assembly. We used “click peptide” chemistry to quasi-synchronously create Aβ42 from 26-O-acyliso-Aβ42 (iAβ42) through a pH jump from 3→7.4. We also synthesized Nα-acetyl-Ser26iAβ42 (Ac-iAβ42), which cannot undergo O→N acyl chemistry, to study the behavior of this ester form of Aβ42 itself at neutral pH. Data from experiments monitoring increases in β-sheet formation (ThT, CD), hydrodynamic radius (RH), scattering intensity (QLS), and extent of oligomerization (IMS-MS), were quite consistent. A rank order of Ac-iAβ42 > iAβ42 > Aβ42 was observed. Photochemically cross-linked iAβ42 displayed an oligomer distribution with a prominent dimer band that was not present with Aβ42. These dimers also were observed selectively in iAβ42 in ion mobility spectrometry experiments. The distinct biophysical behaviors of iAβ42 and Aβ42 appear to be due to the conversion of iAβ42 into “pure” Aβ42 monomer, a nascent form of Aβ42 that does not comprise the variety of oligomeric and aggregated states present in pre-existent Aβ42. These results emphasize the importance of the Gly25-Ser26 dipeptide in organizing Aβ42 monomer structure and thus suggest that drugs altering the interactions of this dipeptide with neighboring side-chain atoms or with the peptide backbone could be useful in therapeutic strategies targeting formation of Aβ oligomers and higher-order assemblies.
INTRODUCTION
Alzheimer’s disease (AD) is a fatal neurodegenerative disorder linked particularly strongly to the pathologic assembly of a 42-residue form of the amyloid β-protein (Aβ), Aβ42 (1, 2). Pathognomonic features of AD include extracellular amyloid plaques containing fibrillar Aβ and intracellular neurofibrillary tangles containing tau protein (3). A prominent working hypothesis of AD pathogenesis focuses on the role(s) of oligomeric Aβ assemblies (4). If a particular Aβ oligomer is the proximate neurotoxin in AD, then knowledge-based design of therapeutic agents requires elucidation of the structural biology of Aβ monomer folding and oligomerization.
Biochemical, nuclear magnetic resonance spectroscopy (NMR), and computational studies of Aβ monomer dynamics have revealed a 10-residue segment, Ala21-Glu-Asp-Val-Gly-Ser-Asn-Lys-Gly-Ala30, that forms a turn-like structure nucleating Aβ monomer folding (5–10). Structural changes in this region caused by familial AD (FAD)- or cerebral amyloid angiopathy-linked amyloid β-protein precursor (AβPP) mutations have been shown to destabilize this turn nucleus, facilitating Aβ assembly (6, 9, 11). Computational studies have revealed that hydrogen bond formation can occur between the oxygen atoms of the Asp23 carboxylate anion and the amide hydrogens of Gly25, Ser26, Asn27, and Lys28. The Asp23:Ser26 hydrogen bond had the highest occurrence frequency (8), suggesting that the interaction of these two amino acids could be particularly important in organizing Aβ structure. In addition, Ser26 formed a 310 helix with Asn27 and Lys28 (8). Interestingly, Ser26 also appears to be important in controlling the structure of the APP juxtamembrane region (25Gly-Ser-Asn-Lys28). This turn region, which includes Lys28, mediates interaction with the γ-secretase complex and affects the peptide bond specificity of the complex, resulting in alterations in the distribution of Aβ peptide lengths produced (12–15). The structural dynamics involving Ser26 thus have relevance not only for understanding Aβ assembly, but also for understanding de novo Aβ production. For these reasons, we sought to elucidate more fully the role of Ser26 in this dynamics.
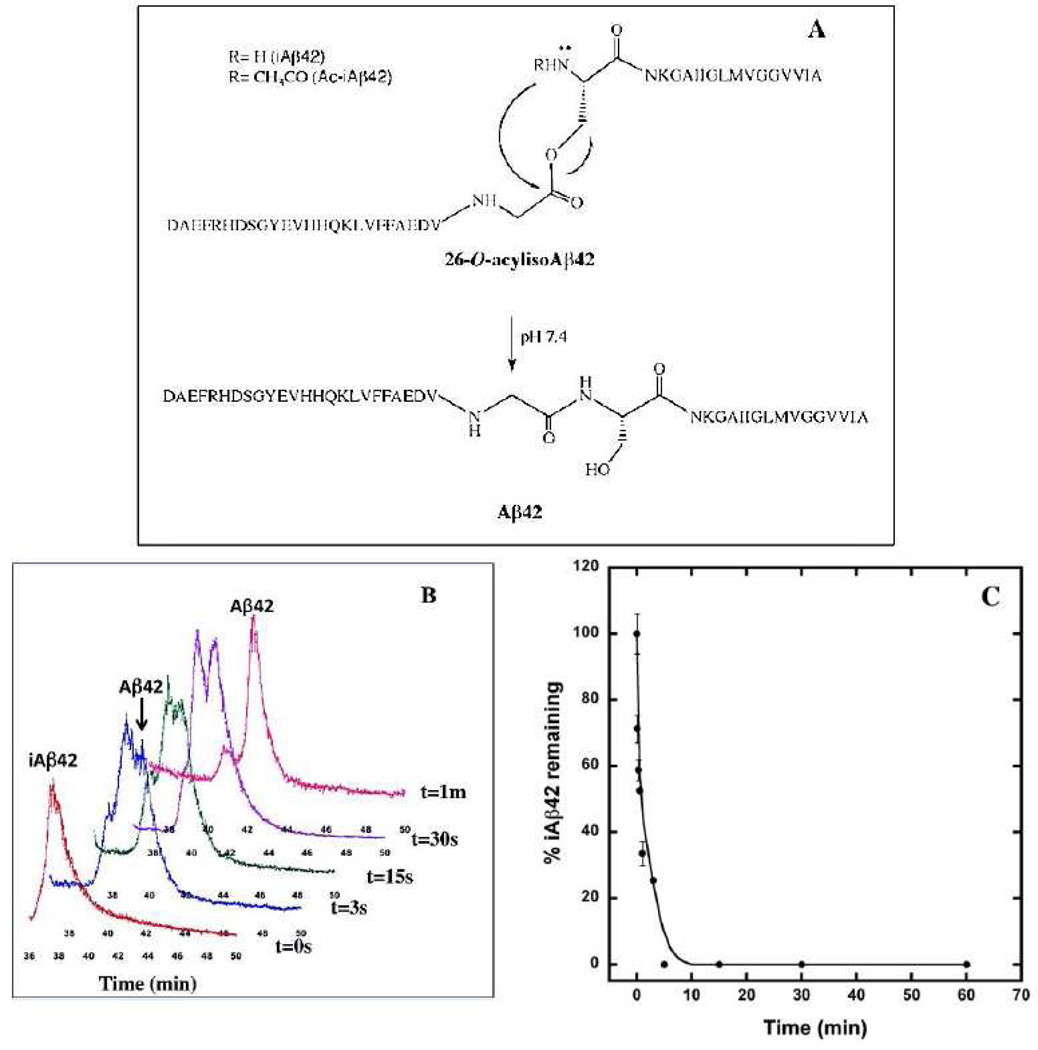
Fortuitously, concurrent with our studies of Aβ structural dynamics, an improved method for the solid phase peptide synthesis of Aβ42, which presents a number of synthetic and preparative challenges, was developed. This method involved the synthesis of an Aβ42 isomer as a “click peptide” (16). This strategy, originally developed by Sohma et al. (17, 18), involves synthesis of 26-O-acylisoAβ42 (iAβ42), which is identical in primary structure to normal human Aβ, except that Gly25 and Ser26 are linked through an ester bond (Fig. 1A). This ester form of Aβ42 displays significantly decreased on-resin β-sheet formation, which increases synthetic efficiency, and produces a crude product that is ≈100-fold more soluble than Aβ42, which increases yields during peptide purification. To form Aβ42 from iAβ42, all that is required is a pH shift from a strongly acidic regime to a neutral or basic one. In the basic pH regime, iAβ42 rapidly (t1/2≈30s) isomerizes into Aβ42, yielding the native Gly25–Ser26 peptide bond (17, 19).
Figure 1. “Click peptide” chemistry.
(A) iAβ42 to Aβ42 conversion scheme. (B) Conversion of iAβ42 to Aβ42. The figure shows the RP-HPLC conversion of iAβ42 to Aβ42 upon incubation at pH 7.5 monitored on a RP-HPLC C18 column. (C) Kinetics of conversion of iAβ42 to Aβ42 at pH 7.5. Half time (t1/2) of the conversion reaction in iAβ42 was determined using RP-HPLC followed by area under the peak quantitation at the different time intervals using Peak Simple chromatographic analysis software.
The substantial differences in chemical synthesis and purification behavior of iAβ42 relative to Aβ42 suggested that this peptide would be especially useful for evaluating the role of the Gly25-Ser26 dipeptide region in controlling Aβ assembly. Importantly, such studies are facilitated by the ability to produce native Aβ42 peptide quasi-synchronously from iAβ42 through a simple increase in pH. This latter ability would mitigate problems with pre-assay aggregation of Aβ42, problems that have complicated the interpretation of much experimental data (20). We report and discuss here the results of such studies.
RESULTS
Kinetics of O→N acyl migration
The in vitro study of Aβ assembly is complicated by technical problems related to peptide preparation and use (for a review, see (32)). These problems are especially relevant to studies of Aβ42, which is thought to be the key Aβ isoform linked to AD pathogenesis (4). To circumvent this problem, we utilized a novel “click peptide” chemistry (17, 19) to produce Aβ42 quasi-synchronously in situ through pH-induced O→N acyl migration within iAβ42 (Fig. 1A).
To determine the half time (t1/2) for conversion of iAβ42 to Aβ42, lyophilized iAβ42 was dissolved at pH 8.0 and Aβ42 production was monitored by RP-HPLC. An ≈1.5 min shift in peak position is indicative of conversion (Fig. 1B). Analysis of the conversion kinetics revealed t1/2 ≈ 30 s (Fig. 1C). Aβ42 monomer production from iAβ42 thus may be considered quasi-synchronous relative to the much longer half times for the evolution of ordered secondary structure, β-sheet formation, protofibril formation, and fibril formation (t1/2 ≈ 2–13 days) (32, 33). Quasi-synchronous production of Aβ42 in situ should decrease interpretive complications caused by the structural heterogeneity that usually exists in starting Aβ42 populations (32).
We also synthesized Nα-acetyl-Ser26-iAβ42 (Ac-iAβ42) because the O→N acyl shift necessary to produce Aβ42 does not occur in this peptide. As predicted, the amount of Ac-iAβ42 observed during 60 min incubation at pH 7.5 remained constant (data not shown). The Ac-iAβ42 peptide was used throughout our experiments as a “non-clickable” control, i.e., a peptide in which an O→N acyl shift could not occur and thus one that remained in an ester form. Importantly, this peptide also allowed us to study how an acetyl group, instead of a hydrogen atom, on the Nα atom of Ser26 affected the peptide’s conformational and assembly properties.
Time evolution of ThT fluorescence
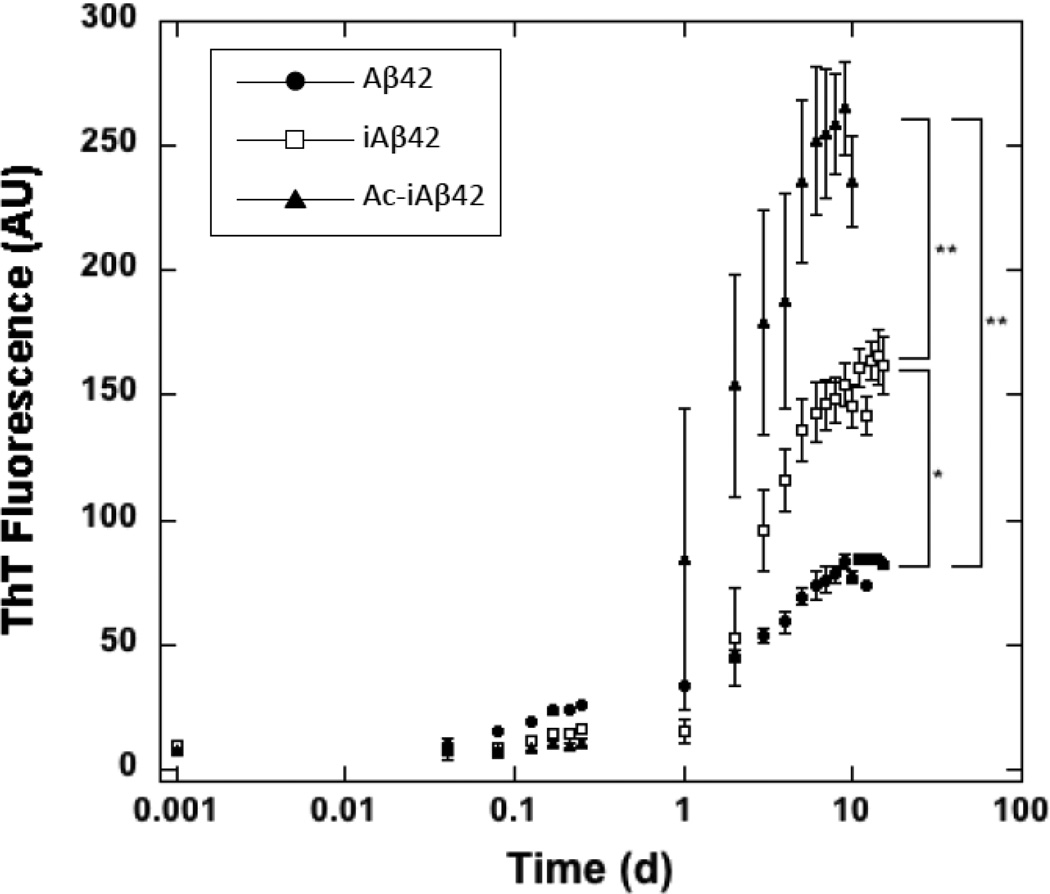
To begin comparative analysis of Aβ42, iAβ42, and Ac-iAβ42 assembly, we sought first to monitor the temporal development of β-sheet-rich fibrils. To do so, we used the technique of ThT fluorescence, which in the Aβ system has been shown to correlate highly with β-sheet formation (34–36). As shown in Fig. 2, lag phases for Aβ42, iAβ42, and Ac-iAβ42 were ≈1 h, ≈1 d, and ≈8 h. Ac-iAβ42 then showed a rapid increase in ThT fluorescence that plateaued at ≤10 d. iAβ42 had a slower rate of assembly and a fluorescence plateau at ≈10 d. Aβ42 displayed the slowest rate of ThT fluorescence increase and a plateau also at ≈10 d. The relative rates of increase in ThT fluorescence thus were Ac-iAβ42 > iAβ42 >Aβ42 (Fig. 2).
Figure 2. Kinetics of ThT fluorescence development. iAβ42, Ac-iAβ42 and Aβ42.
20 µM of the individual peptides were incubated with 40 µM ThT at pH 7.5 and 37°C with shaking and read in a 96 well microtiter plate reader at excitation wavelength 450 nm and emission wavelength 482 nm. Figure shows logarithmic plot of ThT fluorescence (arbitrary units) versus time (days). Error bars refer to SD. Statistical analyses were performed using Sigmastat statistical analysis software. (* and ** are p=0.002 and p<0.001 respectively relative to Aβ42).
Monitoring oligomerization using quasielastic light scattering spectroscopy (QLS)
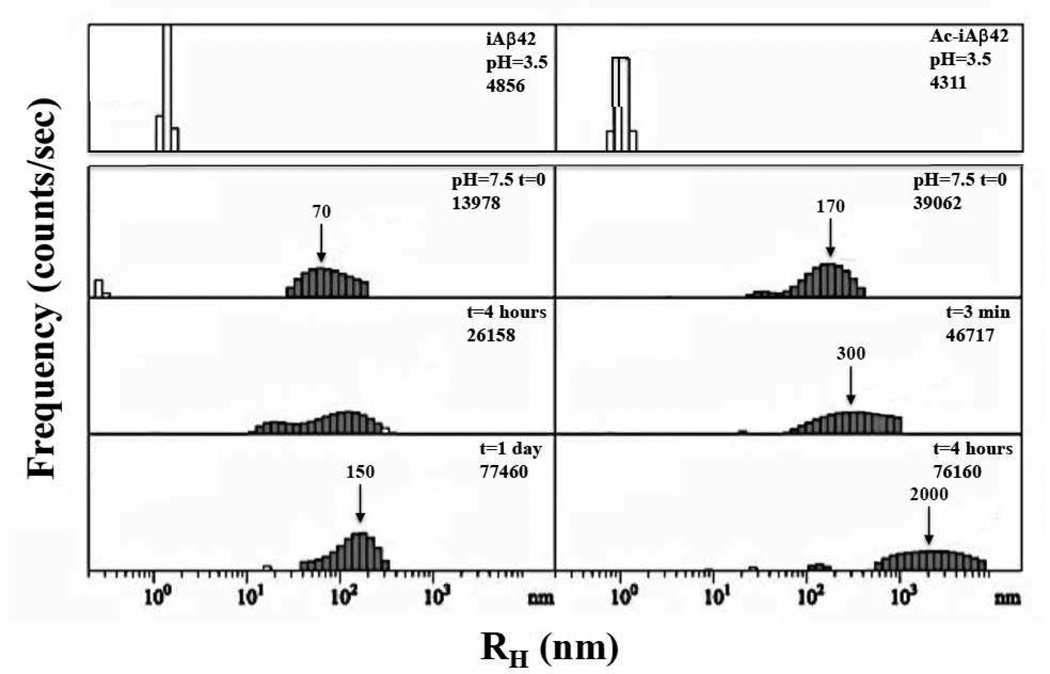
We used QLS as an orthogonal method to non-invasively monitor Aβ assembly (for a review of QLS applied to the Aβ system, see (37–39)). We first monitored samples of iAβ42 and Ac-iAβ42 in 0.2 mM sodium acetate, pH 3.5, at concentrations of approximately 77 µM and 154 µM, respectively. Only background scattering was detected throughout the initial observation period (See Figs. S1A and S1B). Such low scattering intensity at these concentrations indicates that the peptide is predominately in a monomeric state. A pH jump to 7.5 then was executed at 74 h for iAβ42 and 75.2 h for Ac-iAβ42 (Figs. 3, S1A (arrow), and S1B (arrow)). The iAβ42 samples immediately showed substantial scattering from particles with a wide distribution of sizes centered at ≈70 nm. The particles continued to increase in size, with the average size of the particles roughly doubling every day of incubation (Fig. S1A). Ac-iAβ42 showed immediate, even greater, aggregation. The initial aggregation rate was so high that no transition from low intensity to higher intensity was observed (Fig. S1B), as had been seen with iAβ42 (Fig. S1A). Indeed, in the first 3 min of measurement, the particle distribution was centered at RH≈170 nm, whereas in the second 3 min, the distribution maximum was centered at RH≈300 nm. After 4 h, particles of ≈2000 nm were observed (Fig. 3, right panel).
Figure 3. Quasielastic light scattering spectroscopy (QLS).
QLS was used to monitor the kinetics of assembly of iAβ42 (left) and Ac-iAβ42 (right) at pH 3.5 (top panel) and 7.5 (lower panels). Plot of intensity (counts/sec) versus hydrodynamic radius (RH in nm). Arrows indicate increasing particle size over time.
We then conducted a series of experiments in which Aβ samples were dissolved directly in 20 mM sodium phosphate, pH 7.5, at concentrations of 0.5 mg/ml, and then filtered using a 20 nm pore size Anotop filter. These samples initially produced only background scattering (Fig. 4, left panels), but scattering from particles was observed after several hours. The lag times1, during which no scattering from the peptides was observed, are listed in Table 1. Following this time, aggregation was observed and the rates of aggregation, dRH/dt, for the different peptides were found to vary substantially (Table 1, Fig. S2). Aβ42 assemblies increased in size at the rate of 2 nm/h, whereas iAβ42 and Ac-iAβ42 aggregates increased in size 4–5 times faster (8.5 and 10.0 nm/h, respectively; Fig. S2). The intensity of scattering from aggregates of all three samples remained small compared to the background scattering for several more hours, but eventually increased abruptly, displaying a third-order dependence on particle size (Fig. 4). Because iAβ42 and Ac-iAβ42 aggregated much faster than did Aβ42, the lag time (Table 1) for Aβ42 is significantly longer than for iAβ42 and Ac-iAβ42. These data are consistent with the previously determined rank order of β-sheet formation rates determined by ThT fluorescence, namely Ac-iAβ42 > iAβ42 >Aβ42.
Figure 4. Kinetics of scattering intensity increases.
QLS was used to monitor the pH-induced conversion of Aβ42, iAβ42 and Ac-iAβ42. Left column: Plots of intensity (counts/sec) versus elapsed time (h). Right column: Plots of Ln of Intensity (Ln (I)) versus Ln of hydrodynamic radius (Ln (RH)). Note that the time scales for each peptide differ from 100h (Aβ42) to 50h (iAβ42) to 30 h (Ac-iAβ42).
Table 1.
Quasielastic light scattering spectroscopy. Kinetic parameters of iAβ42, Ac-iAβ42 and native Aβ42 during quasi-synchronous assembly. Lag time is defined as the period between initial sample preparation/monitoring and the beginning of continuous increases in RH. This time is determined by establishing the point of intersection of two lines, one fitted to the initial quasi-constant portion of the progress curve and the other to that portion in which persistent increases in RH are observed. This latter curve fit also is used to establish dRH/dt, the change in hydrodynamic radius per unit time.
| Sample | Lag Time (h) | dRH/dt (nm/h) |
|---|---|---|
| Aβ42 | 24.5 | 2.0 |
| iAβ42 | 10.5 | 8.5 |
| Ac-iAβ42 | 6.5 | 10 |
Probing protein conformation using limited proteolysis
We next sought to probe the initial conformational states of the three peptides to determine if any relationship existed between these states and the assembly process, as determined by ThT and QLS. To do so, limited proteolysis experiments were performed using porcine pepsin and proteinase K. Limited proteolysis experiments previously revealed a structurally stable Aβ folding nucleus (10) and were used to compare turn stabilities (ΔΔGf) among Aβ peptides containing cerebral amyloid angiopathy- or AD-linked amino acid substitutions (6).
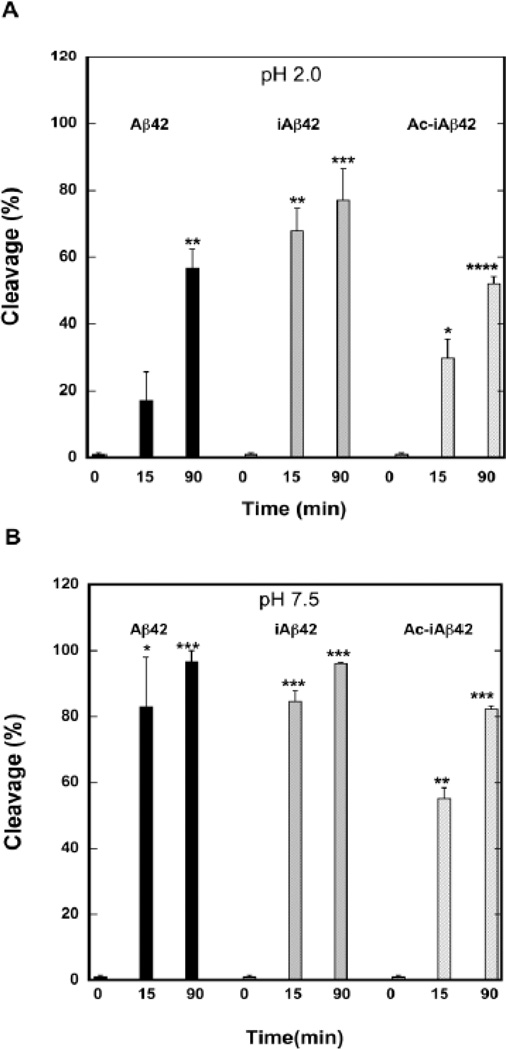
Here, we began our experiments at pH 2.0, a condition under which iAβ42 conversion cannot occur. We used the endoproteinase pepsin, a relatively non-specific protease with maximal activity at pH 2.0 that cleaves at hydrophobic and aromatic residues in the P1 position (40) (Phe, Val, Ala, Ile, Tyr, Trp, and Leu) if a hydrophobic residue is present at the P12 position. Time-dependent increases in proteolysis were readily apparent in the RP-HPLC chromatograms with Aβ42 displaying levels of cleavage of ≈15% at 15 min and ≈55% at 90 min (Fig. 5A). In contrast, ≈70% cleavage of iAβ42 was observed at 15 min and ≈80% cleavage was observed at 90 min. Ac-iAβ42 was cleaved similarly to Aβ42 (≈30% at 15 min and ≈50% at 90 min). The differences in cleavage levels among the peptides at 15 min were highly significant. The data suggest that pepsin-sensitive peptide bonds within iAβ42 are more accessible initially than are those same bonds in Aβ42 or Ac-iAβ42.
Figure 5. Probing peptide structure using limited proteolysis.
Aβ42, iAβ42, and Ac-iAβ42 were subjected to limited proteolysis with pepsin at pH 2.0 (A) or with proteinase K at pH 7.5 (B). Aliquots of the reaction mixture were removed at 0, 15, and 90 min. and then analyzed by RP-HPLC. The area of the peak representing uncleaved Aβ was estimated using the peak integration feature in Peak Simple chromatographic data analysis software. This area was used to calculate cleavage percent according to the formula Ct=(A0 − At) × 100/A0 ; where Ct is cleavage percent, A0 is initial area of Aβ HPLC peak, and At is area of Aβ peak at time t. Statistical analyses, performed using Sigmastat statistical analysis software, determined the significance of differences between samples at t=0 and subsequent time points. Top panel (A): * p=0.03, ** p=0.01, *** p<0.02, ****p=0.002; and bottom panel (B): * p=0.03, **p=0.02, ***p<0.001.
To determine if differences in protease sensitivity existed among Aβ42, Aβ42 formed by conversion of iAβ42, and Ac-iAβ42, we repeated the protease digestion experiments at pH 7.5. Pepsin is inactive at pH 7.5, so we used proteinase K because of its pH optimum (pH 8) and wide substrate specificity, which increases the sensitivity of the system to conformational differences. Aβ42 and iAβ42 were cleaved similarly, with 80–90% cleavage observed at 15 min and almost complete cleavage seen at 90 min (Fig. 5B). Ac-iAβ42 was more resistant to cleavage, displaying 60% cleavage at 15 min and ≈80% cleavage at 90 min.
Conformational dynamics determined by CD spectroscopy
We used CD spectroscopy to monitor temporal changes in peptide backbone conformation, (Fig. 6A–C). The spectra for Aβ42, iAβ42, and Ac-iAβ42 at pH 7.5 show clear differences in assembly kinetics. Aβ42 exists as a statistical coil at t=0 h. A transition to a mixed α/β conformer occurs between 60–180 min, before a predominately β-sheet population is observed at ≈6 h (Fig 6A). iAβ42 showed a much slower transition to β-sheet (Fig. 6B), displaying substantial statistical coil for ≈9 h, at which time a transition to β-sheet was observed. The mixed α/β conformation seen in Aβ42 was not prominent in this experiment, although some mixed conformation was observed at 19 h. Ac-iAβ42, in contrast to both Aβ42 and iAβ42, displayed a mixed α/β conformation at the initial time point (t=0 h) and converted rapidly (90 min) to β-sheet (Fig. 6C). The rapid conformational conversion of Ac-iAβ42 to β-sheet is consistent with its high aggregation propensity. The fact that Aβ42 converts faster than does iAβ42 (Fig. 6D) is consistent with the interpretation of the low pH limited proteolysis results, namely that Aβ42 initially is more folded or aggregated than is the newly formed iAβ42. (Parenthetically, these data demonstrate in a practical manner the theoretical value of the click peptide strategy for producing Aβ42.)
Figure 6. Circular dichroism spectroscopy.
Secondary structure dynamics was characterized using CD during incubation of peptides at pH 7.5, 37°C, with gentle shaking. Peptides were (A) Aβ42; (B) iAβ42; and (C) Ac-iAβ42 (D) β-sheet ellipticities of Aβ42, iAβ42 and Ac-iAβ42 at 215 nm versus time.
Determination of the Aβ oligomer size distribution by ion mobility spectroscopy-mass spectrometry (IMS-MS)
Mass spectra and arrival time distributions (ATDs) for Aβ42, iAβ42, and Ac-iAβ42 are shown in Figs. S3 and 7, respectively. Aβ42 has been characterized previously by IMS-MS (14, 27) and some of these data were included here for the purpose of direct comparison. The negative ion spectra of iAβ42, 20 min and 2 h after dissolution at pH 7.4, are shown in Figs. S3A and S3B, respectively. At 20 min, only the −3 and −4 monomer charge states are present. After 2 h of incubation, a new peak appears at z/n = −5/2 that must be due to oligomers (14) and indicates that early aggregation states of Aβ42 are being observed in real time. The mass spectrum of Ac-iAβ42 is shown in Fig. S3C. Unlike the Aβ42 and iAβ42 spectra, that of Ac-iAβ42 is dominated by a broad collection of unresolved peaks, indicative of rapid aggregation. To observe a resolved mass spectrum, the ammonium acetate concentration had to be reduced to 0.1 mM. This drop in buffer concentration dramatically reduced the rate of aggregation and yielded the spectrum shown in Fig. S3D, which is similar to that of iAβ42 (Fig. S3B).
Figure 7. Ion mobility spectrometry-mass spectrometry.
(A) Arrival time distributions (ATDs) of the z/n = −3 charge state of Aβ42 and iAβ42 at 100 eV and 30 eV. (B) ATDs of the z/n = −5/2 charge state of Aβ42 and iAβ42 → Aβ42 and at 100, 50 and 30 eV injection voltages.
Arrival time distributions (ATDs) for iAβ42 were obtained for each charge state in the 2 h mass spectrum of Fig. S3B and compared with ATDs of Aβ42 (Fig.7A and 7B). The ATDs for the z/n = −3 ions of Aβ42 and iAβ42 are shown in Fig. 7A. In previous studies of Aβ42, the −3 charge state ATD revealed two distinct features that were unambiguously assigned to two different monomeric structures (M1 and M2) (27, 41). The analysis of these results showed that M1 is a gas phase structure dominated by exposed hydrophobic residues and M2 is a dehydrated solution-like structure (8).
The two dominant features observed in the ATDs of iAβ42, labeled M1 and M2 in Fig. 7A, are clearly similar to those previously reported for Aβ42. What is unique is the small feature at 450 µs observed in the 100 eV ATD of iAβ42 (Fig. 7A). This feature became more intense at lower injection energy (30 eV) and thus most likely is the −6 dimer (labeled D). This peak is not observed in the Aβ42 ATD, thus it may be due to the dimerization of iAβ42 prior to isomerization or to the formation of the iAβ42:Aβ42 heterodimer concurrent with iAβ42 conversion to Aβ42. The cross section for this dimer is much larger than the z/n = −5/2 dimer (Table 2) and is consistent with it having a significantly different structure.
Table 2.
Comparison of the collision cross section data for Aβ42, iAβ42 conversion and Ac-iAβ42 at pH 7.4 in negative ion mode.
| System | Aβ42 | iAβ42→Aβ42 | Ac-iAβ42 | |||||
|---|---|---|---|---|---|---|---|---|
| Oligomer | Charge State |
σ(Å2) | σ/n | σ(Å2) | σ/n | σ(Å2) | σ/n | |
| Monomers | ||||||||
| solvent free | −3 | 635 | 635 | 657 | 657 | 652 | 652 | |
| solution-like | −3 | 702 | 702 | 718 | 718 | 712 | 712 | |
| Dimers | −5 | 1256 | 628 | 1282 | 641 | 1318 | 659 | |
| −6 | - | - | 1587 | 743 | ||||
| Tetramer | −10 | 2332 | 583 | 2384 | 596 | 2204 | 551 | |
| Hexamer | −15 | 2898 | 483 | 2970 | 495 | 3024 | 504 | |
| Dodecamer(H2) | −30 | 4307 | 359 | 4428 | 369 | 4440 | 370 | |
The ATDs for the z/n = −5/2 ions of iAβ42 were acquired at three different injection energies, ranging from 30–100 eV, and are compared directly with the ATDs of Aβ42 in Fig. 7B. A detailed discussion of injection energy methods and assignment of the features is given in Bernstein et al. (27). Using the same analytical methods, the following oligomerization states are assigned to the features shown in the ATD of Fig. 7B: D = dimer, Te = tetramer, H = hexamer, and (H)2 = dodecamer (likely formed from stacking two planar hexamers) (14). A shoulder to the right of the (H)2 peak most likely corresponds to the decamer (P)2, where P = pentamer. No octamer was observed. The features observed for iAβ42 were assigned by analogy to Aβ42 (Fig. 7B).
The ATDs for Aβ42 and iAβ42 are very similar at high and medium injection voltages. However at low injection voltages, where solution oligomer distributions are most closely retained, they are quite different. Both have a significant dodecamer peak, but Aβ42 has a strong hexamer peak, while iAβ42 has essentially no hexamer peak and strong tetramer and dimer peaks. These differences must reflect differences in assembly. The dimer and tetramer peaks in the iAβ42 ATD likely are due to Aβ42:iAβ42 heterooligomers (as discussed above) and these mixed oligomers do not further aggregate.
The ATDs allow collision cross sections (σ) to be determined. The ATD for the Ac-iAβ42 z/n = −5/2 charge state initially was broad and comprised 3 distinct features (data not shown). After several hours of incubation, new features appeared. Assignments of these features were made by direct comparison to the ATDs of Aβ42 and iAβ42 (Figs. S4A and B). The ATDs are plotted here as a function of σ/n to normalize the experimental differences of pressure and temperature between experiments. As in Aβ42 and iAβ42, features corresponding to H2, P2, H, Te, and some D appear to be present in Ac-iAβ42 (Fig. S4C), although resolution of the D, Te, and H species is not clearly obtained. The σ/n values and the absolute cross sections are listed in Table 2 for Aβ42, iAβ42, and Ac-iAβ42.
Determination of the Aβ oligomer size distribution by PICUP
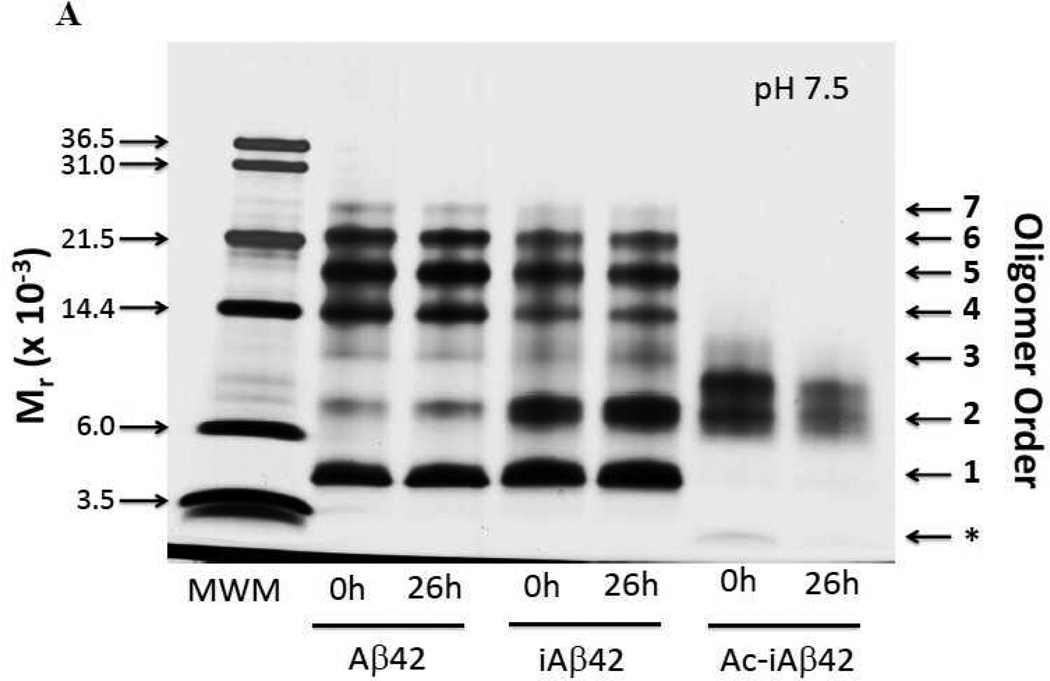
To monitor oligomer size distributions in hydro, we used PICUP followed by SDS-PAGE and silver staining (Fig. 8A). The three study peptides were cross-linked immediately after dissolution and filtration (t=0 h) and also after incubation at RT for 26 h without shaking (to monitor changes in oligomerization detectable with PICUP chemistry). At t=0 h and pH 7.5, Aβ42 displayed an intense monomer band, weak dimer and trimer bands, and intense bands corresponding to tetramer, pentamer and hexamer. A faint heptamer band also was observed. The distribution at 26 h was identical, within experimental error. iAβ42 displayed a similar distribution to Aβ42 at t=0 h, except that an intense dimer band also was observed. The iAβ42 distribution at t=26 h was similar to that at t=0 h. The oligomer distribution of Ac-iAβ42 was distinct from those of Aβ42 or iAβ42. This distribution included a very faint monomer band, an intense dimer band, an additional band at a position just above dimer, and in the case of the t=0 h time point, a faint band visible slightly above the position of trimer. The distributions of Ac-iAβ42 also changed little between 0 and 26 h. Quantification and normalization of band intensities was done to allow quantitative comparisons among the oligomer distributions (Table 3).
Figure 8. Photochemical cross-linking.
Oligomer distributions of Aβ42, iAβ42, and Ac-iAβ42 were determined using photo-induced cross-linking of unmodified proteins (PICUP), SDS-PAGE, and silver staining at the start of incubation at RT and after 26 h of incubation. (A) pH 7.5; and (B) pH 3.0. Aβ42, iAβ42 and Ac-iAβ42 were dissolved at a concentration of 1 mg/ml in pH 7.5 or pH 3.0 buffer. The samples were filtered using YM-50 Centricon (pH 7.5) and or Anotop 0.2 µm (pH 3.0) filter and incubated at RT for 0 and 26 h. At each time point, an aliquot of the reaction was removed and cross-linked and samples analyzed on a 10–20% Tricine gradient gel followed by silver staining. Densitometric analysis of the gels was performed using Image J. Arrows on right indicate oligomer order within the Aβ42 lanes. In panel (B), white numbers overlaying bands in the Ac-iAβ42 lanes indicate nominal oligomer order for these specific bands.
Table 3.
Densitometric analysis of the silver stained SDS-PAGE gel following PICUP at pH 7.5 using Image J. The area of each band in a particular lane was normalized by division by the sum of the areas of all the bands in that particular lane. This quotient was multiplied by 100 to yield the percentages in the table. Oligomer order 1–7 in the table refers to monomer through heptamer.
|
Oligomer Order (n) |
Aβ42 | iAβ42 | Ac-iAβ42a | |||
|---|---|---|---|---|---|---|
| 0h | 26h | 0h | 26h | 0h | 26h | |
| 1 | 19.4 | 21.2 | 23.1 | 22 | 2.1 | 3.5 |
| 2 | 13.1 | 13.4 | 25 | 25.6 | 38.4 | 49.1 |
| 2+ | NP | NP | NP | NP | 38.4 | 42.7 |
| 3 | 5.9 | 7.3 | 7.8 | 12.7 | 13.2 | 2.6 |
| 4 | 20.6 | 19.4 | 12.4 | 11.3 | 3.5 | NP |
| 5 | 21.9 | 20.8 | 18.3 | 15 | NP | NP |
| 6 | 14.7 | 14.7 | 11.3 | 11 | NP | NP |
| 7 | 4.2 | 3.2 | 2.0 | 2.4 | NP | NP |
1= <3.5 kDa, 2= runs as if it were dimer, 3= between dimer and trimer, 4= > trimer.
= bands not quantified (see main text). NP refers to Not Present.
iAβ42 does not convert to Aβ42 at pH 3.0. Although this pH is not physiologic, we were curious whether the different primary structures would produce different oligomerization patterns in this system. We found that the distribution of Aβ42 at t=0 h at pH 3.0 differed considerably from that seen at pH 7.5. The pH 3.0 distribution displayed an intense monomer band along with a series of bands appearing to range from dimer to heptamer, each of which had an intensity that was inversely proportional to its order (Table 4). A smaller band below the monomer (* in Fig. 8B) is seen, suggesting the presence of two closely related conformers. This type of distribution is characteristic of systems in which simple diffusion-limited cross-linking occurs, as opposed to the system at pH 7.5 in which preformed oligomers exist (31). No difference in the distribution pattern was seen at 26 h. iAβ42, in contrast, displayed a faint band migrating at a position between that of monomer and dimer and a more intense band at a position slightly above dimer. It was not possible to determine if a trimer band existed or whether the dimer electrophoresed as an intense band with some protein trailing behind. The iAβ42 distributions at 0 and 26 h were similar. Ac-iAβ42, in contrast to both Aβ42 and iAβ42, produced a distribution at 0 h with a relatively weak doublet monomer band, followed by intense dimer, trimer, and tetramer bands. A light pentamer band also was observed (Fig. 8B). This distribution was identical, within experimental error, at 26 h.
Table 4.
Densitometric analysis of the silver stained SDS-PAGE gel following PICUP at pH 3.0 using Image J. The area of each band in a particular lane was normalized by division by the sum of the areas of all the bands in that particular lane. This quotient was multiplied by 100 to yield the percentages in the table. Oligomer order 1–7 in the table refers to monomer through heptamer.
|
Oligomer Order (n) |
Aβ42 | iAβ42 | Ac-iAβ42 | |||
|---|---|---|---|---|---|---|
| 0h | 26h | 0h | 26h | 0h | 26h | |
| * | 6.5 | 8.0 | NP | NP | NP | NP |
| 1 | 21.7 | 21.8 | 20.1 | 21.9 | 17.3 | 18.2 |
| 2 | 25.9 | 28.0 | 59.4 | 58.6 | 28.4 | 29.0 |
| 3 | 20.9 | 18.3 | 20.5 | 19.4 | 29.1 | 29.0 |
| 4 | 11.7 | 13.4 | NP | NP | 18.4 | 18.8 |
| 5 | 10.5 | 9.0 | NP | NP | 6.8 | 4.8 |
| 6 | 2.5 | 1.3 | NP | NP | NP | NP |
| 7 | 0.2 | 0.3 | NP | NP | NP | NP |
= bands not quantified (see main text). NP refers to Not Present.
Assembly Morphology
To determine the morphologies of the peptide assemblies, electron microscopy was performed on days 0, 7, and 14, at both pH 7.5 and 3.5. At pH 7.5, day 0 (Fig. 9A and Table 5), Aβ42 showed mainly small, globular assemblies ranging in diameter from 9–47 nm. A few assemblies were seen that were oblong, with lengths ranging from 15–28 nm and diameter ranging from 8–23 nm. iAβ42 displayed similar globular structures, but their size distribution was skewed toward larger sizes (diameters ranging from 30–73 nm). Ac-iAβ42 produced assemblies similar to those of Aβ42.
Figure 9. Morphology of Aβ assemblies.
Electron microscopy of Aβ42, iAβ42, and Ac-iAβ42 was performed after 0, 7 and 14 d of assembly at (A) pH 7.5 or (B) pH 3.5. Inset in panel A is a magnified region. Fibril twisting is noted by red arrows (narrow or on-edge structure) and blue arrows (wide, multi-filar structures). Scale bars are 200 nm.
Table 5.
Dimensions of assemblies observed by EM. Peptides were incubated at either pH 7.5 or 3.5. Following incubation, different classes of assemblies were observed, including globules, short fibrils, and long fibrils. If present, the numbers represent the size range, in units of nm, of each assembly type. Assembly lengths are reported in nm within parentheses.
| Peptide | pH | Day 0 | Day 7 | Day 14 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| globules |
short fibrils |
long fibrils |
globules |
short fibrils |
long fibrils |
globules |
short fibrils |
long fibrils |
||
| Aβ42 | 7.5 | 9–47 8–23 (15–28) |
- | - | - | 6–13 (117–178) | 6–13 | - | 9–12 (33–157) | 7–12 |
| iAβ42 | 7.5 | 12–56 | - | - | 9–16 | 5–11 | 5–11 | 8–30 | - | 5–14 |
| Ac-iAβ42 | 7.5 | 11–36 | - | - | 7–12 10–14 (36–73) |
5–8 (52–208) | 6–11 | - | - | 4–8 |
| Aβ42 | 3.5 | (15–35) | 5–12 | - | - | - | 6–13 | - | - | 5–11 |
| iAβ42 | 3.5 | (17–47) | - | - | - | - | 3–8 13–26 | 3–10 | 7–9 (42–78) | 5–11 |
| Ac-iAβ42 | 3.5 | 4–8 | 5–10 (17–66) | 6–10 (82–239) | 5–14 | 7–11 (34–366) | 5–9 | 9–15 12–32 (21–52) |
9–13 (42–110) | 5–8 |
At day 7, all three peptides had formed fibrils. Aβ42 displayed short and long fibrils ranging in diameter from 6–13 nm. The iAβ42 fibrils were long and relatively uniform in structure, with diameter of 5–11 nm. Some fibrils appeared to comprise twisted filaments with pitches of ≈120–180 nm (Fig. 9A, blue and red arrows). A small number of globular assemblies of diameter 9–16 nm also were present. Ac-iAβ42, in contrast to the other two peptides, formed a structurally heterogeneous population comprising predominately relatively straight fibrils with diameters of ≈5–11 nm and lengths ranging from ≈50–200 nm. At day 14, dense meshes of fibrils were formed by each of the peptides.
Analogous experiments were performed at pH 3.5 (Fig. 9B and Table 5). Aβ42 formed short, often worm-like, structures at day 0. Globular or oblong structures also were observed. iAβ42, in contrast, formed predominately globular structures, similar to but of lesser diameter than those formed at pH 7.5. Occasionally, a short, straight or curved fibril was seen. Ac-iAβ42 formed a heterogeneous population of assemblies that included globular or oblong structures as well as numerous short, usually curved, fibrils.
At day 7, fibrils were observed in each peptide population. Aβ42 formed predominately long fibrils, but with some short fibrils and globules as well. iAβ42 fibrils comprised two populations, one thicker (13–26 nm) than the other (3–8 nm). Ac-iAβ42 formed numerous short fibrils of variable length as well as some small globules.
At day 14, Aβ42 fibril morphology remained similar to that at day 7. iAβ42 displayed a more heterogeneous population of fibrils than that observed at day 7. Both short and long fibrils were seen, and bright small globules often were found associated with them. Whether these globules were an intrinsic part of the fibril structure, or simply adherent to the fibrils, cannot be ascertained. Ac-iAβ42 formed fibrils similar to those of iAβ42, although the average fibril length appeared shorter and the electron bright globules were more numerous and found both associated with and not associated with fibrils. There was greater heterogeneity among the assemblies formed by Ac-iAβ42 relative to those formed by Aβ42 or iAβ42.
DISCUSSION
The etiology of AD remains enigmatic. However, a number of viable working hypotheses exist, including those focusing on the role(s) of Aβ oligomers (reviewed in (4, 42, 43)). In the work reported here, we studied a region of the Aβ molecule thought critical in controlling monomer folding, oligomerization, and higher-order assembly, namely Ala21-Glu22-Asp23-Val24-Gly25~Ser26-Asn27-Lys28-Gly29-Ala30 (the tilde (~) signifies either an ester or peptide bond) (6, 10). The tetrapeptide segment Gly25~Ser26-Asn27-Lys28 forms a turn-like structure stabilized by an extensive H bond network involving Ser26 (5–10). This turn nucleates Aβ monomer folding (10), affects APP processing (12–15), and is a site for amino acid substitutions causing FAD and CAA (6, 9, 11). We used seven complementary methods, in two different pH regimes, to study the structural dynamics and assembly of Aβ42 peptides containing either a peptide (Aβ42), ester (iAβ42), or Nα-acetyl ester (Ac-iAβ42) Gly25~Ser26 inter-amino acid bond. We also were able to examine the behavior of “nascent” Aβ42 formed quasi-synchronously (t1/2≈30s) in situ through O→N acyl migration within iAβ42.
In discussing our results, we abstract key points from the large data set obtained, consider the significance of these points to in vitro studies of Aβ structural biology, and opine on how the data contribute to our understanding of the molecular pathogenesis of AD.
We found, as expected, that pH-induced O→N acyl migration in iAβ42 occurs rapidly, with a t1/2≈30 s. The iAβ42→Aβ42 conversion thus is quasi-synchronous relative to the time constants for peptide secondary structure changes, oligomerization, or fibril formation, which are measured in hours and days. The rapid conversion allowed us to monitor structural features and dynamics of Aβ42 monomers created ab initio in situ, a capability that avoids much of the confounding effects of Aβ peptide lyophilizate solvation and preparation for assay, e.g., pre-existing β-sheets and intra-preparation aggregation (44).
We observed a remarkable agreement among data from experiments monitoring rates of increase in β-sheet formation (ThT, CD), RH, and scattering intensity (QLS). This kinetics showed a rank order of Ac-iAβ42 > iAβ42 > Aβ42. Why? A reasonable supposition is that the rank order reflects the relative abilities of each peptide to fold and self-associate into ordered (in this case, β-sheet-rich) assemblies. Ac-iAβ42 could display a greater area of solvent accessible hydrophobic surface due to a lower propensity to form the Gly25-Lys28 turn, which prevents intramolecular interactions between hydrophobic peptide segments adjacent to the turn (the “legs” in a β-hairpin). The result would be a concomitant increase in intermolecular interactions among these exposed hydrophobic regions, and a rapid hydrophobic collapse producing either off pathway aggregates or molten globule-like structures. In the former case, conversion to ordered oligomers or fibrillar structures would not occur, whereas in the latter case, ordered assembly into higher-order structures, including protofibrils and fibrils, might be facilitated (Fig. 10).
Figure 10. Pathways of Aβ assembly.
The assembly of Aβ into fibrils (“on pathway”) may follow a linear pathway from Aβ monomer to oligomer to protofibril to fibril (4). Monomers may assemble into a variety of non-fibrillar structures, including annuli (pores) (46), quasi-spherical or oblong structures, close-packed globules, and “off pathway” oligomers. Monomers also may aggregate into relatively amorphous structures the size of oligomers or into larger aggregates or precipitates. These aggregates may undergo structural rearrangement and reenter the pathway of fibril formation. iAβ42 converts into nascent Aβ42 that has a high propensity for dimerization and facile entry and movement down the fibril formation pathway. Aβ42, depending on its starting assembly state(s), may form off pathway assemblies or also traverse the fibril formation pathway. Ac-iAβ42 appears first to undergo rapid aggregation followed by structural reorganization and eventual fibril formation. [The annulus in the figure is reprinted by permission from Macmillan Publishers Ltd: [Nature Protocols] (Vol. 5: No. 6: 1186–1209), copyright (2010).]
This latter argument is consistent with the increased rate of conformational change in the iAβ42 sample. A reasonable supposition is that the rate difference between iAβ42 and Aβ42 is due to the conversion of iAβ42 into “pure” Aβ42 monomer, i.e., nascent Aβ42 that exists as a monomer, absent pre-existent “off-pathway” aggregates that could retard movement along the pathway of oligomers→protofibrils→fibrils (Fig. 10). The idea of a nascent Aβ monomer, as discussed above, may explain why limited proteolysis experiments at pH 2 demonstrated a rank order of protease sensitivity of iAβ42 > Aβ42 ≈ Ac-iAβ42. Among the three peptides, iAβ42 is least able to fold/collapse to sequester protease-sensitive peptide bonds. Results at pH 7.5 are also consistent with this proposition. In this pH regime, where iAβ42 converts rapidly to Aβ42 and where protease action is very rapid, similar proteinase K digestion sensitivities were observed for the two peptides. In contrast, Ac-iAβ42 was significantly (p<0.005) less sensitive to proteinase K than were Aβ42 or iAβ42, likely due to rapid aggregation (as was shown in QLS studies), which sequestered pepsin-sensitive peptide bonds.
IMS-MS experiments were particularly useful in monitoring the oligomerization phases of Aβ assembly. Injection energy-dependent IMS studies revealed both the existence and stabilities of different oligomers. ATDs of the −5/2 (z/n) ions of Aβ42 and iAβ42 differed. This was particularly true of the ATDs acquired at low injection energies (23 eV and 30 eV for Aβ42 and iAβ42, respectively). Only di-hexamer and hexamer were observed in the Aβ42 sample, whereas di-hexamer, tetramer and dimer were observed with iAβ42. The ATDs at 50 eV showed that the di-hexamers and di-pentamers formed from nascent Aβ42 were more prominent than those formed by pre-existent Aβ42. This observation was consistent with the ATDs of the −3 ions of each isoform, which demonstrated that converted iAβ42 forms stable dimers at 30 eV injection energy whereas Aβ42 does not. Taken together, these data are consistent with our prior supposition that nascent Aβ42 (i.e., iAβ42 immediately after pH-induced conversion to Aβ42) exists in a monomer state that more readily forms low-order oligomers than does Aβ42, which exists ab initio in a variety of oligomeric and aggregated states. It should be noted that our data also are consistent with the formation of mixed iAβ42/Aβ42 dimers in the −6 and −5 charge states, and these mixed systems may contribute to formation of higher-order oligomers in the iAβ42 system at high pH. This may be so because dimerization of iAβ42 and nascent Aβ42 occurs intra-experimentally before iAβ42 is able to convert completely to Aβ42.
In the case of Ac-iAβ42, the very poorly resolved MS spectra suggested that substantial aggregation occurred rapidly following sample dissolution in 10 mM buffer. This hypothesis was confirmed by study of the same peptide in 100 µM buffer (a 100-fold lower buffer concentration), a concentration regime in which well-resolved spectra were produced that had predominant peaks at m/z values of −4, −3, and −5/2, similar to those produced by iAβ42. ATD experiments on the −5/2 ion of Ac-iAβ42 acquired at an injection energy of 50 eV displayed a peak distribution comprising di-hexamer and di-pentamer, as did those of Aβ42 and iAβ42 samples, but also a much more intense hexamer peak and essentially no dimer peak. These data are consistent with the fact that this isoform aggregates much faster than either Aβ42 or iAβ42. The high aggregation propensity of Ac-iAβ42 observed in the IMS-MS experiments was consistent with the high assembly/aggregation propensities observed in the prior ThT, CD, QLS, and proteolysis experiments.
The IMS-MS data confirm and extend the observation of assembly differences between pre-existent Aβ42 and nascent Aβ42 (formed from conversion of iAβ42). As mentioned above, these differences could involve formation of mixed dimers of the two isoforms. Another possibility is that conversion of the iAβ42 to Aβ42 produces a much more homogeneous population of Aβ42 monomers, as opposed to a pre-existent Aβ42 population that already contains monomers, oligomers, and aggregates. This monomer population self-associates through a smaller number of pathways relative to pre-existent Aβ42, which forms a much more heterogeneous population of conformers and pre-existent oligomers and thus accesses a more diverse set of assembly paths and products. The effects of starting state conformation are even more pronounced with the Ac-iAβ42 peptide. The acetylation of this peptide eliminates the possibility of native-like folding at Gly25 and Ser26 resulting in rapid aggregation, potentially due to the enthalpic gains of sequestering solvent exposed hydrophobic peptide regions or establishing Coulombic or hydrogen bond interactions (Fig. 10).
We used PICUP as one orthogonal method for determining Aβ oligomerization state. iAβ42 converts to Aβ42 during PICUP experiments done at pH 7.5. The experiment thus reveals features of the oligomer distribution of nascent Aβ42, i.e., a population of peptides initially comprising monomeric Aβ42. When iAβ42 was cross-linked, the most striking feature of the oligomer distribution, relative to pre-existent Aβ42, was an intense dimer band. Fewer tetramers and hexamers were observed, a result consistent with the "zero sum" nature of the system—namely, increases in dimer concentration had to be compensated for by decreases in the concentrations of other oligomers. The existence of higher numbers of dimers is consistent with the existence of greater numbers of monomers after conversion (through the law of mass action). This contrasts with pre-existent Aβ42, which has been shown to contain β-sheet structures even in its lyophilized state (44) and thus presents what one might conceptualize as a partially "pre-aggregated" state. As discussed above, a prominent −6 dimer was also observed in the IMS-MS experiments with iAβ42 (Fig. 7A), but not for Aβ42.
Ac-iAβ42 displayed a strikingly different pH 7.5 oligomer distribution, one characterized by essentially a single feature, two bands migrating with apparent molecular weights slightly lower and slightly higher, respectively, than that of Aβ42 dimer. The narrow distribution of oligomers is consistent with the SDS-induced dissociation of large Ac-iAβ42 aggregates, such as those observed in QLS and IMS-MS experiments. Rapid aggregation could sequester sites of cross-linking, explaining why Aβ42-like oligomer distributions were not observed.
Oligomer distributions in PICUP experiments at pH 3.0 were instructive. The “ladder-type” distribution of Aβ42 (monotonic decrease in band intensity) was consistent with simple diffusion-limited peptide:peptide interactions, in contrast to the discontinuous distribution characteristic of normal Aβ42 oligomerization. Nevertheless, the presence of bands up to the size of heptamer shows that the oligomer organization necessary for successful intermolecular cross-linking existed in Aβ42 at this pH. This was not the case with iAβ42, which displayed a single predominant band migrating between dimer and trimer (along with a faint band migrating between monomer and dimer). This distinct pattern, and the absence of a monomer band, suggests highly efficient cross-linking of a single predominant oligomer form, and by inference, the inability of the Gly25-Ser26 peptide ester to assume a conformation characteristic of the normal, peptide bond-containing Aβ42 isomer. It is possible that this predominant form is the dimer found so abundantly in IMS-MS work. The fundamental conformational basis for this cross-linking difference could be that monomers at pH 3.0 rapidly form dimers with adjacent Tyr10 residues. It also is possible that higher-order oligomers existed, but were not cross-linked, as evidenced by the lack of SDS-stable higher-order oligomer bands. A related mechanism could explain the broader distribution of Ac-iAβ42 oligomer types observed at pH 3.0 versus pH 7.5—whether as specific oligomers, or as oligomers within much larger assemblies, chemical accessibility is higher at pH 3.0 and thus a broader range of covalently associated (SDS-stable) oligomers is observed.
Finally, and not surprisingly, differences observed among the peptides in oligomerization (IMS-MS, PICUP), assembly kinetics (QLS, CD), β-sheet formation (ThT fluorescence and CD), and protease sensitivity were reflected in quaternary structure variations determined by EM. All peptides formed globular structures and fibrils, but the relative amounts of each of these structures, and their precise morphologies, differed depending on pH and time.
CONCLUSIONS
We observed a remarkable agreement among data from experiments monitoring β-sheet formation (ThT, CD), hydrodynamic radius (RH) and scattering intensity (QLS), and oligomerization (IMS-MS), namely a rank order of Ac-iAβ42 > iAβ42 > Aβ42. These data were consistent with high protease resistance of Ac-iAβ42. When iAβ42 was cross-linked, the most striking feature of the oligomer distribution, relative to pre-existent Aβ42, was an intense dimer band. IMS-MS experiments also showed that pre-existent Aβ42 did not form stable dimers, whereas iAβ42 did, a fact that could explain why this latter peptide could also readily form dodecamers and decamers. Effects of Gly25-Ser26 structure were reflected in the constellations of quaternary structures determined by EM. The distinct biophysical behaviors of iAβ42 and Aβ42 appear to be due to the conversion of iAβ42 into nascent (pure) Aβ42 monomer, which lacks the variety of oligomeric and aggregated states present in pre-existent Aβ42. It is intriguing to consider whether in situ creation of Aβ42 from iAβ42 in biological systems might yield results distinct from those obtained using preformed Aβ42 and thus challenge prevailing views of Aβ42 structure-activity relationships. In conclusion, our results emphasize the importance of the Gly25-Ser26 dipeptide in organizing Aβ42 monomer structure and thus suggest that drugs altering the interactions of this dipeptide with neighboring side-chain atoms or with the peptide backbone could be useful in therapeutic strategies targeting formation of Aβ oligomers and higher-order assemblies. Recent studies showing that iAβ42 (at pH 2) and [Nα-methyl-β-Ala26]Aβ42 [at pH 7.4] do indeed inhibit fibril formation augur well for this strategy (45).
MATERIALS AND METHODS
Chemicals and reagents
All chemicals and enzymes were purchased from Sigma Chemical Co. (Saint Louis, MO) and were of the highest purity available. Water was de-ionized and filtered using a Milli-Q system (Millipore Corp., Bedford, MA). YM-50 kDa filters were purchased from Millipore Corp. Xpress™ silver-staining kit was from Invitrogen (Carlsbad, CA). Solvents for LC-MS were HPLC grade (Fisher Scientific, Pittsburgh PA).
Peptide synthesis
26-O-acylisoAβ42 (iAβ42), 26-Nα-acetyl-O-acylisoAβ42 (Ac-iAβ42), and Aβ(1–42) (Aβ42) were synthesized using 9-fluorenylmethoxycarbonyl (Fmoc) chemistry and purified by reverse phase high performance liquid chromatography (RP-HPLC), essentially as described (21). The identity and purity (usually >97%) of the peptides were confirmed by amino acid analysis, mass spectrometry, and reverse phase high performance liquid chromatography (RP-HPLC). Ac-iAβ42 was synthesized as described above, except that Fmoc-Ser-OH, not Fmoc-Ser(tBu)-OH, was coupled to Asn27. Following coupling and washing with NMP, the Fmoc group of serine was removed with 20% (v/v) 4-methyl piperidine in NMP by incubating for 20 minutes at RT (23°C). Acetylation of the Ser Nα atom was accomplished using 0.5 M acetic anhydride, 0.125 M DIEA, 0.15 M HOBt in NMP. Following washing in NMP, Fmoc-Gly-OH then was coupled to the Ser 26 OHβ using the DIPCDI-DMAP method, as per Sohma et al (19).
Kinetics of production of Aβ42 from iAβ42
Lyophilizates of Aβ42, iAβ42, or Ac-iAβ42 were dissolved immediately prior to assay by gentle vortexing at concentrations of 20–30 µM in 100 mM ammonium bicarbonate, pH 8.0. Peptides were incubated at RT without agitation. Eight µl aliquots of the reaction volume were removed periodically and added to 5 µl of trifluoroacetic acid (TFA) (to stop conversion of the iAβ42 peptide samples). The samples then were placed on ice. Ten µl of HPLC solvent A (2% (v/v) acetonitrile, 0.1% (v/v) acetic acid, 0.02% (v/v) TFA, in water) was added to the sample and the mixture then was analyzed by RP-HPLC. A 2–100% gradient of solvent B (acetonitrile in 0.1 % (v/v) acetic acid and 0.02 % (v/v) TFA) was run over a 40 min time period using a C18 column (Nova-Pak 3.9 × 150 mm, 4 mm particle size, 60 Å pore size) eluted at a flow rate of 1 ml/min with UV peak detection at 215 nm (10, 22). Peak Simple 2000 Chromatography Integration Software (SRI Instruments, Torrance, CA) was used to determine peak areas in the resulting chromatograms.
Thioflavin T (ThT) binding
Peptides were prepared at a nominal concentration of 0.5–1 mg/ml by dissolving lyophilizates in 1 volume (v) 60 mM NaOH: 4.5 v milliQ water: 4.5 v 20 mM sodium phosphate buffer, pH 7.5, containing 0.002% (w/v) sodium azide. The solutions were sonicated for 1 min in a Branson 1200 bath sonicator (Branson Ultrasonics Corp, Danbury, CT). The peptide solutions then were centrifuged in 16,000 Å g for 10 min. The pH of the peptide solutions was confirmed using a micro pH electrode (Orion, Model 9810BN). After centrifugation and filtering, the concentration of the peptides was determined from their A280 values, using an extinction coefficient of 1280 cm−1M−1.
Assays were conducted in 0.4 ml, 96-well, optical bottom, polymer based microtiter plates (Thermo Scientific Nunc, Rochester, NY). An aliquot of the Aβ42 stock solution (see above) was pipetted into each well, followed by 1.6 µL of 5 mM ThT in phosphate buffer. The total volume in each well was adjusted to 200 µL with phosphate buffer, yielding a final Aβ concentration of 20 µM and a ThT concentration of 40 µM. The wells were gently mixed by pipetting, sealed using an adhesive plate sealer, and incubated at 37°C with gentle shaking. The plate was read in a microplate reader (λex=450 nm, λem=482 nm) immediately and then at regular intervals. Blank wells contained ThT and buffer. Five or more replicates were done for each sample. The mean of the blank readings was subtracted from the mean of the sample readings at each time point and the corrected values, along with SD and mean, were plotted using KaleidaGraph (v 4.1, Synergy Software, Reading, PA). Statistical analyses on the data (t-test and Mann Whitney Rank test) were performed using SigmaStat (Jandel Scientific, San Jose, CA).
Quasielastic light scattering spectroscopy (QLS)
In experiments at neutral conditions, Aβ42, iAβ42 and Ac-iAβ42 were dissolved at a nominal concentration of 0.5 mg/ml (110 µM) in 20 mM sodium phosphate, pH 7.5, briefly vortexed, sonicated for 20 s, and filtered using a 20 nm Anotop filter (Whatman, Maidstone, England). Amino acid analysis was done post facto to determine the actual protein concentration (see Results). Samples were monitored at RT for 7–10 days. In experiments with initial acidic conditions, samples of iAβ42 and Ac-iAβ42 were dissolved in 0.2 mM sodium acetate, pH 3.5, at concentrations of 77 µM and 154 µM, respectively. Each sample then was vortex mixed briefly, sonicated for 20 s, and filtered using a 20 nm Anotop filter. Samples were monitored at RT for 3 days and then brought to neutral pH by addition of 0.5 v of 20 mM sodium phosphate, pH 7.5. Measurements were done using a custom optical setup comprising a 40 mW He-Ne laser (λ=633nm) (Coherent, Santa Clara, CA) and PD2000DLS detector/correlator unit (Precision Detectors, Bellingham, MA). Light scattering was measured at a 90° angle. The intensity correlation function and the diffusion coefficient (D) frequency distribution were determined using Precision Deconvolve software (Precision Detectors, Bellingham, MA). The hydrodynamic radius RH was calculated from D according to the Stokes-Einstein equation, where kB is Boltzmann's constant, T is Kelvin, and η is the solvent viscosity (23).
Limited proteolysis
Peptides (2 mg/ml) were digested using proteinase K or porcine pepsin. Proteinase K digestions were performed by adding the enzyme, at an E:S ratio of 1:1000 (w/w), to Aβ dissolved in 100 mM ammonium bicarbonate, pH 8.0, after addition of 10 % (v/v) 60 mM NaOH. Aliquots were removed at 0, 15, and 90 min, and then the reactions were quenched using 20 µl of 50% (v/v) TFA in water.
Pepsin digestion was performed by adding the enzyme to peptides dissolved directly in 10 mM HCl, pH 2.0, at an enzyme: substrate (E:S) ratio of 1:1000 (w/w). Digestion was allowed to proceed at RT for 0, 15, or 90 min. At each time point, a 20 µl aliquot was removed and the proteolysis was stopped by addition of 10 µl of 5% (w/v) ammonium hydroxide in water.
The resulting samples were analyzed by gradient RP-HPLC using a Nova-Pak 3.9 × 150 mm, 4 mm particle size, 60 Å pore size, C18 column. Solvent A was 0.02% (v/v) TFA, 0.1% (v/v) acetic acid, and 2% acetonitrile (v/v) in water. Solvent B was 90% (v/v) acetonitrile, 0.02% (v/v) TFA, 0.1% (v/v) acetic acid, in water. A linear (1.25% B/min) gradient from 0→100% B was run at a flow rate of 1.0 ml/min. Peak detection was done by UV absorbance at 215 nm. Peak quantitation was performed using Peak Simple 2000 Chromatography Integration Software. Statistical analyses on the data (t-test and Mann Whitney Rank test) were performed using SigmaStat (Jandel Scientific, San Jose, CA).
Circular Dichroism Spectroscopy
Aβ42, iAβ42 and Ac-iAβ42 peptide solutions were prepared as stated in “Thioflavin T (ThT) binding.” The peptides then were incubated at 37°C with gentle shaking in an Innova 4080 incubator shaker (New Brunswick Scientific, Edison, NJ). CD spectra were obtained every 30 min for the first 2 h, and subsequently every hour, using a JASCO J-810 spectropolarimeter (Tokyo, Japan). The CD parameters were: wavelength scan range, 190–260 nm; data pitch, 0.2 nm; continuous scan mode, 10 scans of each sample; scan speed, 100 nm/min; 1 sec response; and band width, 2 nm. The spectra were processed using the means movement smoothing parameter within the Spectra Manager software. The data were subsequently plotted using KaleidaGraph (v 4.1.3).
Ion Mobility Spectrometry-Mass Spectrometry (IMS-MS)
Standard mass spectra and ion mobility experiments were performed on an instrument built “in-house” that comprises a nano-electrospray ionization (N-ESI) source, an ion funnel, a temperature-controlled drift cell and a quadrupole mass filter followed by an electron multiplier for ion detection (24). The high-resolution 13C isotope distributions for each peak in the mass spectra were obtained on a Q-TOF mass spectrometer (Micromass, UK) equipped with an N-ESI source (25, 26). During ion mobility measurements, the ions were stored at the end of the ion funnel and then pulsed into the drift cell, which was filled with 5 Torr of helium gas, and drawn through the cell under the influence of a weak electric field (2–20 V/cm). The ion injection energy into the drift cell was varied from 20 to 100 eV. At low injection voltages, the ions were gently pulsed into the mobility cell and only needed a few "cooling" collisions to reach thermal equilibrium with the buffer gas helium. At high injection voltages, the larger collision energy led to internal excitation of the ions before cooling and equilibrium occurred. This transient internal excitation can lead to annealing, that is partial or complete isomerization, to give the most stable conformers, or can lead to dissociation of dimers and oligomers of higher order (27). The ions exit the drift cell and pass through a quadrupole mass filter, allowing a mass spectrum to be obtained. Alternatively, the quadrupole can be set to monitor a specific peak in the mass spectrum as a function of time, producing an arrival time distribution (ATD). The arrival time is related directly to the mobility constant K, which in turn is inversely proportional to the collision cross-section σ (26, 28). Accurate (±1%) collision cross sections are obtained. All Aβ42 samples were dissolved at 1 mg/mL (0.22 mM) in 25 mM ammonium acetate, pH 8.3, resulting in a final pH of 7.4. Immediately prior to mass spectrometry analysis, the stock solution was diluted to 20 µM in 25 mM ammonium acetate (or other desired buffer concentrations) and adjusted to the appropriate pH for the experiment. A 5–10 µL aliquot of sample was loaded into a metal-coated borosilicate glass capillary for N-ESI applications.
Oligomerization of Aβ42
Aβ oligomerization was monitored using Photo-Induced Crosslinking of Unmodified Proteins (PICUP), essentially as described (29). Peptide solutions at pH 7.5 were prepared essentially as stated in “Thioflavin T (ThT) binding.” Peptide solutions at pH 3.0 were prepared by dissolving lyophilizates directly in 0.1M glycine-HCl, pH 3.0, at concentrations of 0.5–1 mg/ml. The solutions were sonicated for 1 min in a Branson 1200 bath sonicator (Branson Ultrasonics Corp, Danbury, CT), after which they were filtered using a sterile 0.20 µm Anotop filter (Whatman International Ltd, Maidstone, England). The peptides then were incubated at RT.
Eighteen µl of sample were periodically cross-linked using the PICUP reaction (30). Briefly, 1 µl of 2 mM Tris (2,2′-bipyridyl) dichlororuthenium (II) hexahydrate (Ru(bpy)) was added to a 0.2 ml thin-walled PCR tube (Eppendorf AG, Hamburg, Germany) containing the sample, followed by addition of 1 µl of 40 mM ammonium persulfate (APS) in PBS. The tube then was irradiated for 1 s with incandescent light using a high intensity illuminator (Dolan-Jenner Industries Inc., Model 170-D). The reaction was quenched immediately with 1 µl 1M DTT in water and the sample was vortexed and placed on ice. To determine the oligomer size distribution, an equal volume of 2Å Tris-Tricine SDS sample buffer (Invitrogen, Carlsbad, CA) was added to each sample. The samples then were boiled in a 100°C water bath for 5–10 min and electrophoresed on a 10–20% T, 1 mm thick, Tris-Tricine SDS gel (Invitrogen, Carlsbad, CA). The gel was silver stained using a SilverXpress® Silver Staining Kit (Novex). For crosslinking at pH 3.0, all reagents were dissolved directly in 0.1M glycine-HCl, pH 3.0. The PICUP chemistry occurs at pH 3.0 as it does at other pH values (31).
Electron microscopy (EM)
Formvar 400 mesh grids were glow discharged on a Med010 mini-deposition system EM glow discharge attachment (model BU007284-T, Balzers Union Ltd, Hudson, NH) containing a cylindrical discharge compartment and an adjacent discharge control and timer unit. Samples were mixed thoroughly and then 8 µl was applied onto the grid. The grid was covered and incubated for 20 min at RT. Liquid was wicked off using a filter paper wick by gently touching the tip of the filter paper to the edge of the grid. Five µl of 2.5% (v/v) glutaraldehyde in water were applied to the grid, which was incubated for 3 min in the dark. The glutaraldehyde solution was wicked off and replaced with 5 µl of 1% (w/v) uranyl acetate in water, and incubated for 3 minutes in the dark. The grids then were wicked off and air-dried. A JEOL 1200 EX (JEOL Ltd., Tokyo, Japan) transmission electron microscope was used to visualize the samples.
Supplementary Material
Highlights.
Structural isomorphs/analogues of the Gly25-Ser26 dipeptide within Aβ42 cause substantial changes in Aβ structure and assembly.
The Gly25-Ser26 and Gly25-Nα-acetyl-Ser26 peptide ester forms of Aβ42 oligomerize and form fibrils more readily than does native Aβ42.
Formation of nascent Aβ42 from 26-O-acylisoAβ42 produces a “pure” Aβ42 population with enhanced abilities to dimerize and form higher order structures.
Compounds targeting the Gly25-Ser26 dipeptide within Aβ42 may have therapeutic potential.
ACKNOWLEDGEMENTS
This work was supported by NIH Grants NS038328 (DBT), AG047116 (MTB) and AG041295 (DBT), and by the Jim Easton Consortium for Drug Discovery and Biomarkers at UCLA (DBT). We acknowledge the use of instruments at the Electron Imaging Center for NanoMachines at the California NanoSystems Institute, UCLA (supported by NIH Grant 1S10RR23057). Waters Corp is also acknowledged for the donation of a prototype Synapt TWIMS spectrometer (to MTB).
ABBREVIATIONS
- Aβ
Amyloid β-protein
- Aβ42
Aβ(1–42)
- AD
Alzheimer’s disease
- ATD
Arrival Time Distribution
- CD
Circular Dichroism
- HPLC
High Performance Liquid Chromatography
- IMS-MS
Ion Mobility Spectroscopy-Mass Spectrometry
- 26-O-acylisoAβ42
iAβ42
- 26-Nα-acetyl-O-acylisoAβ42
Ac-iAβ42
- LP-MS
Limited proteolysis-mass spectrometry
- PICUP
Photo-Induced Cross-linking of Unmodified Proteins
- QLS
Quasielastic light scattering
- Ru (Bpy)
Tris (2,2′-bipyridyl) dichloro ruthenium (II) hexahydrate
- ThT
Thioflavin T
- TFA
Trifluoroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We define lag phase as the period between initial sample preparation/monitoring and the beginning of continuous increases in intensity. This time is determined by establishing the point of intersection of two lines, one fitted to the initial quasi-constant intensity portion of the progress curve and the other to that portion in which persistent increases in intensity are observed. This latter curve fit also is used to establish dRH/dt.
REFERENCES
- 1.Selkoe DJ. Biochemistry and molecular biology of amyloid β-protein and the mechanism of Alzheimer's disease. Handb Clin Neurol. 2008;89:245–260. doi: 10.1016/S0072-9752(07)01223-7. [DOI] [PubMed] [Google Scholar]
- 2.Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Roychaudhuri R, Yang M, Hoshi MM, Teplow DB. Amyloid β-protein assembly and Alzheimer disease. J Biol Chem. 2009;284:4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borreguero JM, et al. Folding events in the 21–30 region of amyloid β-protein (Aβ) studied in silico. Proc Natl Acad Sci U S A. 2005;102:6015–6020. doi: 10.1073/pnas.0502006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant MA, et al. Familial Alzheimer's disease mutations alter the stability of the amyloid β-protein monomer folding nucleus. Proc Natl Acad Sci U S A. 2007;104:16522–16527. doi: 10.1073/pnas.0705197104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz L, et al. Solvent and mutation effects on the nucleation of amyloid β-protein folding. Proc Natl Acad Sci U S A. 2005;102:18258–18263. doi: 10.1073/pnas.0509276102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumketner A, et al. Structure of the 21–30 fragment of amyloid β-protein. Protein Sci. 2006;15:1239–1247. doi: 10.1110/ps.062076806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krone MG, et al. Effects of familial Alzheimer's disease mutations on the folding nucleation of the amyloid β-protein. J Mol Biol. 2008;381:221–228. doi: 10.1016/j.jmb.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazo ND, Grant MA, Condron MC, Rigby AC, Teplow DB. On the nucleation of amyloid β-protein monomer folding. Protein Sci. 2005;14:1581–1596. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Mousseau N, Derreumaux P. The conformations of the amyloid-β (21–30) fragment can be described by three families in solution. J Chem Phys. 2006;125:084911. doi: 10.1063/1.2337628. [DOI] [PubMed] [Google Scholar]
- 12.Ren Z, Schenk D, Basi GS, Shapiro IP. Amyloid β-protein precursor juxtamembrane domain regulates specificity of γ-secretase-dependent cleavages. J Biol Chem. 2007;282:35350–35360. doi: 10.1074/jbc.M702739200. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, et al. A helix-to-coil transition at the ε-cut site in the transmembrane dimer of the amyloid precursor protein is required for proteolysis. Proc Natl Acad Sci U S A. 2009;106:1421–1426. doi: 10.1073/pnas.0812261106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein SL, et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer's disease. Nature Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtenthaler SF, et al. The intramembrane cleavage site of the amyloid precursor protein depends on the length of its transmembrane domain. Proc Natl Acad Sci U S A. 2002;99:1365–1370. doi: 10.1073/pnas.032395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutter M, et al. Switch peptides in statu nascendi: Induction of conformational transitions relevant to degenerative diseases. Angew Chem Int Ed Engl. 2004;43:4172–4178. doi: 10.1002/anie.200454045. [DOI] [PubMed] [Google Scholar]
- 17.Sohma Y, Kiso Y. “Click peptide”: chemical biology-oriented synthesis of Alzheimer’s disease-related amyloid β peptide (Aβ) analogues based on the “O-acyl isopeptide method". Chembiochem. 2006;7:1549–1557. doi: 10.1002/cbic.200600112. [DOI] [PubMed] [Google Scholar]
- 18.Sohma Y, Sasaki M, Hayashi Y, Kimura T, Kiso Y. Novel and efficient synthesis of difficult sequence-containing peptides through O-N intramolecular acyl migration reaction of O-acyl isopeptides. Chem Commun. 2004:124–125. doi: 10.1039/b312129a. [DOI] [PubMed] [Google Scholar]
- 19.Sohma Y, et al. Development of O-acyl isopeptide method. Biopolymers. 2007;88:253–262. doi: 10.1002/bip.20683. [DOI] [PubMed] [Google Scholar]
- 20.Teplow DB, et al. Elucidating amyloid β-protein folding and assembly: A multidisciplinary approach. Acc Chem Res. 2006;39:635–645. doi: 10.1021/ar050063s. [DOI] [PubMed] [Google Scholar]
- 21.Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 22.Sohma Y, et al. The 'O-acyl isopeptide method' for the synthesis of difficult sequence-containing peptides: application to the synthesis of Alzheimer's disease-related amyloid β peptide (Aβ) 1–42. J Pept Sci. 2005;11:441–451. doi: 10.1002/psc.649. [DOI] [PubMed] [Google Scholar]
- 23.Einstein A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Annalen der Physik. 1905;322:549–560. [Google Scholar]
- 24.Wyttenbach T, Kemper PR, Bowers MT. Design of a new electrospray ion mobility mass spectrometer. Int J Mass Spectrom. 2001:13–23. [Google Scholar]
- 25.Pringle SD, et al. An investigation of the mobility separation of some peptide and protein ions using a new hybrid quadrupole/travelling wave IMS/oa-ToF instrument. Int J Mass Spectrom. 2007;261:1–12. [Google Scholar]
- 26.Wyttenbach T, Bowers MT. Gas-phase conformations: The ion mobility/ion chromatography method. Top Curr Chem. 2003:207–232. [Google Scholar]
- 27.Bernstein SL, et al. Amyloid β-protein: monomer structure and early aggregation states of Aβ42 and its Pro19 alloform. J Am Chem Soc. 2005;127:2075–2084. doi: 10.1021/ja044531p. [DOI] [PubMed] [Google Scholar]
- 28.Gidden J, Ferzoco A, Baker ES, Bowers MT. Duplex formation and the onset of helicity in poly d(CG)n oligonucleotides in a solvent-free environment. J Am Chem Soc. 2004;126:15132–15140. doi: 10.1021/ja046433+. [DOI] [PubMed] [Google Scholar]
- 29.Bitan G, Teplow DB. Rapid photochemical cross-linking–a new tool for studies of metastable, amyloidogenic protein assemblies. Acc Chem Res. 2004;37:357–364. doi: 10.1021/ar000214l. [DOI] [PubMed] [Google Scholar]
- 30.Bitan G. Structural study of metastable amyloidogenic protein oligomers by photo-induced cross-linking of unmodified proteins. Meth Enzymol. 2006;413:217–236. doi: 10.1016/S0076-6879(06)13012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bitan G, Lomakin A, Teplow DB. Amyloid β-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J Biol Chem. 2001;276:35176–35184. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- 32.Teplow DB. Preparation of amyloid β-protein for structural and functional studies. Meth Enzymol. 2006;413:20–33. doi: 10.1016/S0076-6879(06)13002-5. [DOI] [PubMed] [Google Scholar]
- 33.Hartley DM, et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naiki H, Nakakuki K. First-order kinetic model of Alzheimer's β-amyloid fibril extension in vitro. Lab Invest. 1996;74:374–383. [PubMed] [Google Scholar]
- 35.LeVine H., 3rd Quantification of β-sheet amyloid fibril structures with thioflavin T. Meth Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 36.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils—current status. J Chem Biol. 2010;3 doi: 10.1007/s12154-009-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomakin A, Benedek GB, Teplow DB. Monitoring protein assembly using quasielastic light scattering spectroscopy. Meth Enzymol. 1999;309:429–459. doi: 10.1016/s0076-6879(99)09029-1. [DOI] [PubMed] [Google Scholar]
- 38.Lomakin A, Teplow DB, Benedek GB. Quasielastic light scattering for protein assembly studies. Meth Mol Biol. 2005;299:153–174. doi: 10.1385/1-59259-874-9:153. [DOI] [PubMed] [Google Scholar]
- 39.Lomakin A, Teplow DB. Quasielastic light scattering study of amyloid β-protein fibrillogenesis. Meth Mol Biol. 2012;849:69–83. doi: 10.1007/978-1-61779-551-0_6. [DOI] [PubMed] [Google Scholar]
- 40.Nomenclature E. IUBMB Enzyme Nomenclature: Pepsin. 1989 ( http://www.chem.qmul.ac.uk). [Google Scholar]
- 41.Baumketner A, et al. Amyloid β-protein monomer structure: a computational and experimental study. Protein Sci. 2006;15:420–428. doi: 10.1110/ps.051762406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: An emperor in need of clothes. Nature Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 43.Hayden EY, Teplow DB. Amyloid β-protein oligomers and Alzheimer's disease. Alz Res Ther. 2013;5:60. doi: 10.1186/alzrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fezoui Y, et al. An improved method of preparing the amyloid β-protein for fibrillogenesis and neurotoxicity experiments. Amyloid: Int J Prot Fold Dis. 2000;7:166–178. doi: 10.3109/13506120009146831. [DOI] [PubMed] [Google Scholar]
- 45.Kawashima H, et al. A new class of aggregation inhibitor of amyloid-β peptide based on an O-acyl isopeptide. Bioorg Med Chem. 2013;21:6323–6327. doi: 10.1016/j.bmc.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 46.Jan A, Hartley DM, Lashuel HA. Preparation and characterization of toxic Aβ aggregates for structural and functional studies in Alzheimer's disease research. Nat Protoc. 2010;5:1186–1209. doi: 10.1038/nprot.2010.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.