Abstract
The composition and sources of fine particulate matter (PM2.5) were investigated in rural and urban locations in Iowa, located in the agricultural and industrial Midwestern United States from April 2009 to December 2012. Major chemical contributors to PM2.5 mass were sulfate, nitrate, ammonium, and organic carbon. Non-parametric statistical analyses demonstrated that the two rural sites had significantly enhanced levels of crustal materials (Si, Al) driven by agricultural activities and unpaved roads. Meanwhile, the three urban areas had enhanced levels of secondary aerosol (nitrate, sulfate, and ammonium) and combustion (organic and elemental carbon). The heavily industrialized Davenport site had significantly higher levels of PM2.5 and trace metals (Fe, Pb, Zn), demonstrating the important local impact of industrial point sources on air quality. Sources of PM2.5 were evaluated by the multi-variant positive matrix factorization (PMF) source apportionment model. For each individual site, seven to nine factors were identified: secondary sulfate (accounting for 29–30% of PM2.5), secondary nitrate (17–24%), biomass burning (9–21%), gasoline combustion (6–16), diesel combustion (3–9%), dust (6–11%), industry (0.4–5%) and winter salt (2–6%). Source contributions demonstrated a clear urban enhancement in PM2.5 from gasoline engines (by a factor of 1.14) and diesel engines (by a factor of 2.3), which is significant due to the well-documented negative health impacts of vehicular emissions. This study presents the first source apportionment results from the state of Iowa and is broadly applicable to understanding the differences in anthropogenic and natural sources in the urban-rural continuum of particle air pollution.
1. Introduction
Air pollution presents a major health risk and contributes to acute and chronic disease including respiratory infections, heart disease, and cancer.1, 2 Respirable particulate matter (PM) has a greater impact on human health outcomes than other ambient air pollutants, including ozone, sulfur dioxide, and nitrous oxides.3 Decades of epidemiological have documented the association between exposure to ambient PM10 and mortality. For example, mortality was strongly and significantly correlated with increases in daily PM10 concentrations in the urban area of St. Louis, Missouri and in surrounding communities.3 A study covering twenty cities in the United States (US) established the connection between elevated PM10 and cardiovascular and respiratory illnesses, in particular.4 Within a region, the health effects of aerosols measured in vitro by cytotoxicity, inflammation, and oxidative stress endpoints differ across urban, rural, and remote locations due to differences in PM composition and sources.5 Due to this underlying connection between ambient PM and health, the United States Environmental Protection Agency has established National Ambient Air Quality Standards (NAAQS). The primary standard for PM2.5 on an annual average basis was set at 15 μg m−3 in 1997 and was reduced to 12 μg m−3 in 2012.6
Particulate mass has historically been the metric for regulation, because its relationship to health effects is well documented. However, the majority of the particle mass is comprised of low-toxicity minerals derived from soil dust and inorganic salts like ammonium sulfate, ammonium nitrate, and sodium chloride.7 Transition metals that have low atmospheric abundance, but are capable of redox cycling, contribute significantly to adverse health effects.8–10 Thus, the chemical composition of PM is expected to be an important determinant in its health outcomes.
Reductions in ambient PM2.5 are consistent with longer life expectancies, yet the benefits of PM reductions are greater in urban areas in comparison to rural areas.11 Rural locations are inherently understudied, although 19.3% of Americans, or over 59 million people in the United States live in rural communities.12 This percentage is much higher in Midwestern states such as Iowa where 43.5% of the population lives in rural areas.13 Spatial differences in PM composition are driven by different sources across urban and rural locations. Toxic metal levels are enhanced in polluted urban and industrial locations3, 4 and near-roadways.14, 15 In agricultural areas bioaerosols are generated by harvesting, transporting, processing, or storing grain, and livestock operations and these bioaerosols enriched in biological material of microbial, plant, or animal origin16 and are typically greater than 2.5 μm in diameter.17 Dusts in agricultural areas can be distinguished from urban dusts by their metal composition.16, 18 Urban dusts generally contain higher concentrations of metals emitted from industry and vehicles,18, 19 whereas agricultural dusts are more enriched in crustal metals.16
Receptor-based source apportionment modeling is a technique used to evaluate the sources of pollution and to maintain air quality standards.20 Positive matrix factorization (PMF) is a receptor-oriented source apportionment model which can resolve factors, factor profiles and factor contributions to ambient measurements.21 PMF analyzes the co-variation of chemical species over time and identifies factors based on a constrained weighted least-squares matrix.21, 22 PMF has been successfully used as a source apportionment tool of PM2.5 in many air quality studies in the USA.23–26 Prior PMF studies in the USA have resolved in the range of 7–11 sources at different locations, including secondary sulfate, secondary nitrate, wood combustion, and fossil fuel (gasoline, diesel and coal) combustion and minor sources such as dust, sea salt, and point sources. While these prior studies have focused on one or two receptor locations, there is need to understand the differences in PM sources across broader spatial scales and understand the differences in PM composition across urban and rural locations.
This study investigates the composition and sources of PM2.5 across urban and rural locations in the agricultural and industrial Midwestern United States. Using measurements of PM2.5 from three cities in Iowa and two background locations obtained from USEPA Air Quality Database (USEPA AQS Data Mart), seasonal and spatial differences were investigated with statistical methods and PM2.5 sources were evaluated with PMF modeling. This study presents the first source apportionment of PM2.5 in Iowa. With a focus on = quantifying regional and local pollution sources, we present new information on the spatial variability in PM composition and sources across the rural-urban continuum.
2. Material and methods
2.1. Sampling sites, aerosol sampling and chemical analysis
Five USEPA PM2.5 monitoring sites in Iowa were considered in this study (Fig. 1). Cedar Rapids (42.0051° N, 91.6793° W), Des Moines (41.6032°N, 93.6431°W), and Davenport (41.5300° N, 90.5876° W) are cities with populations (and number of houses) of 126,326 (57,217), 203,433 (88,729), and 99,685 (44,087), respectively.27 These three urban sites are part of the EPA Chemical Speciation Network (CSN). Rural sites included Viking Lake State Park in Montgomery County (40.9691° N, 95.0450° W) and Lake Sugema State Park in Van Buren county (40.6951° N, 92.0063° W), where populations (and numbers of houses) are 10,740 (5,239) and 7,570 (3,670), respectively.27 These two sites are part of the EPA Interagency Monitoring of Protected Visual Environments (IMPROVE) network. During the spring and summer, winds are predominantly southerly and north-westerly at the study sites. During winter and fall, winds are predominately north-westerly. It is important to note that summertime southerly winds may transport air masses from the St. Louis, MO urban area to the rural Van Buren site and westerly may transport air masses from the Omaha, NE urban area to the Montgomery site.
Fig. 1.
Map of the five sampling sites in Iowa, with urban sites marked by stars and rural sits marked by circles.
All aerosol samples were collected and analyzed by the United States EPA; all data analyzed in this study were retrieved from the AQS Datamart.6 Details of sample collection and filter analysis are described in detail elsewhere.28 Briefly, PM samples were collected using commercial air samplers. PM2.5 mass concentrations were measured gravimetrically on Teflon filters. Elemental composition was measured by the energy dispersive X-ray fluorescence (EDXRF) on Teflon filters for Na, K, Al, Ca, Mg, Si, Ti, Zr, Rb, Sb, Fe, Cr, Cu, Zn, Mn, Pb, As, Br, Cl, Se, Ni, V, Cd and Sr. Water-soluble inorganic ions (SO42−, NO3− and NH4+)were measured by ion chromatography on either nylon or Teflon filters; however, NH4+ data were not available for Montgomery and Van Buren. Fractions of organic carbon (OC1, OC2, OC3 and OC4) and elemental carbon (EC1, EC2, and EC3) were measured using the thermal-optical OC/EC analyzer (DRI Model 2001)on quartz fiber filters.29 Organic carbon was operationally-defined into four fractions evolving at 120 °C, 250 °C, 450 °C and 550° C, respectively whereas elemental carbon was defined by three fractions evolving at 550 °C, 700 °C and 800 °C, respectively.30 Thermal optical reflectance (TOR) was used to account for the conversion of OC to pyrolytic organic carbon (OP) during the thermal gradient.31 The EC1 fraction was corrected for OP and is denoted EC1-OP.
Sample data were drawn from April 2009 to December 2012 after the CSN and IMPROVE networks adopted consistent methods of sample collection and analysis, ensuring data are comparable across networks. The Cedar Rapids and Des Moines sites followed the one-in-six day sampling frequency whereas Davenport and rural locations followed the one-in-three day sampling frequency. Sample data from 3–5 July were excluded because of outlying concentrations of trace metals and potassium, due to fireworks and local activities, which biased results. For the discussion of seasonal variations, winter is defined as December-February, spring as March–May, summer as June–August, and fall as September–November.
2.2. Statistical analysis of data
PM2.5 mass and speciation data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (version 21) in order to evaluate the statistical significance of differences (at the 95% confidence interval) in PM mass and species concentrations across the study sites. The Kolmogorov-Smirnov and Shapiro-Wilk tests indicated that chemical speciation data was not normally distributed and the Levene test indicated that there was no homogeneity of variance (homoscedasticity) in the data.32 Consequently, non-parametric statistical tests (i.e. the U of Mann Whitney and the Z of Kolmogorov-Smirnov) were used.32
2.3. PMF analysis and modeling parameters
Positive matrix factorization (EPA PMF version 3.0) is a multivariate factor analysis tool that decomposes a matrix X (n × m) of chemically-speciated samples into two matrices including source contributions (G, n × p) and source profiles (F, p × m),21 for number of samples (n), number of chemical species (m), and the number of factors (p).33 PMF ensures that all of the species profiles (matrix F) are non-negative, each sample has a non-negative source contribution (matrix G), and the sum of the predicted elemental mass contributions for each source must be less than or equal to the total measured mass for each element. The mass balance equation (1) in the PMF can be written in the following way:
| (1) |
where xij is the concentration of jth chemical species in the i number of samples, gik is the mass concentration in the ith sample from the kth source, fkj is fraction of chemical species j from the source k, and eij is the residual of species j in the ith sample.
PMF allows the adjustment of each data point based on the uncertainty measurements. In this way, the influence of data points with lower or more uncertain concentrations can be minimized. The PMF uncertainty function is
| (2) |
where uij is the user defined uncertainty in the jth species for ith sample. The PMF model solves for the Q minima using the input provided by the user to the program.33
In this study, PM2.5 mass concentrations were supplied as the total variable34 and fitting species included OC1, OC2, OC3, OC4, EC1-OP, EC2, EC3, OP, SO42−, NO3−, NH4+, Na, K, Al, Ca, Mg, Si, Ti, Zr, Rb, Fe, Cr, Cu, Zn, Mn, Pb, As, Br, Cl, Se, Ni, V, Cd and Sr, when data met the model criteria.35 If the 80% of data of a chemical species were below detection limit, data were not considered. Data were processed following the recommendations of Polissar et al.36. The uncertainty input to the model was the sum of the measurement uncertainty and one-third of the method detection limit (MDL), both supplied by the AQS Data base.6 When the measured concentrations fell below the MDL, the data were replaced by 1/3 of MDL and their uncertainties were assigned as 5/6 of MDL. Species were then categorized following the recommendations of Paatero and Hopke.37 If the signal to noise ratio for a chemical species was less than 2, the uncertainty of the data were increased by three times. If the signal to noise ratio was less than 0.2, the data were not considered.
The “base run” was executed with different factor solutions to find the global minima Q.36 The stability and uncertainty of the base PMF results with the lowest Q value were analyzed by bootstrapping and Fpeak analysis, respectively.38 In the bootstrapping stage, the PMF model synthesized new datasets from non-overlapping block of samples; 95–100% of the bootstrapped factors were mapped to the corresponding base factor and the concentrations of dominant species in the base solution were within the interquartile range of the bootstrapped factors, both indicating stable model result. Across the base run and five Fpeak analyses (ranging −1 to +1), neither significant changes in calculated Q-values nor significant changes in factor contributions were observed. The error in factor profiles was determined as the standard deviation of the base result and the Fpeak result at a strength of 0.5.38
3. Results and discussion
3.1. Spatial variability of PM2.5
Annual-average PM2.5 concentrations across the five study sites are shown in Figure 2. In general, rural locations generally exhibited lower PM2.5 concentrations (8.4–10.4 μg m−3) compared to the urban sites (9.5–11.6 μg m−3). Non-parametric statistical analyses demonstrate that Davenport PM2.5 loadings are significantly higher (p < 0.005) than the other four sites, while PM2.5 mass concentrations were not significantly different across Cedar Rapids, Davenport, Montgomery, and Van Buren (Table 1). Annual average PM2.5 concentrations exceeded neither the primary EPA NAAQS of 15 μg m−3 set in 2006 nor the revised primary standard of 12 μg m−3 set in 2012.39 The study sites were also in compliance with the 24-hour average PM2.5 NAAQS standard of 35 μg m−3. The elevated PM2.5 levels in Davenport are driven by local activities and are discussed in the context of chemical tracers section 3.2.
Fig. 2.
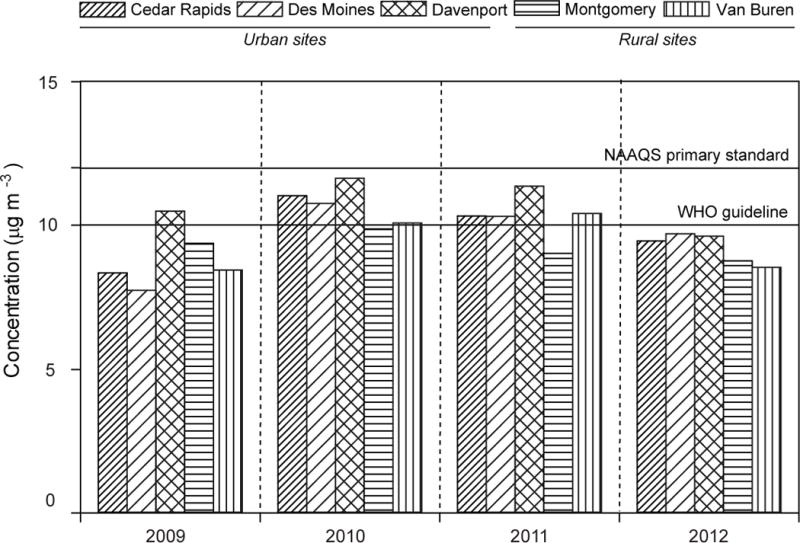
Annual average PM2.5 concentrations at five locations in Iowa, with Cedar Rapids, Des Moines, and Davenport representing urban areas and Montgomery and Van Buren representing rural areas.
Table 1.
Statistical significance (p-value) of the non-parametric tests of U of Mann-Whitney (upper matrix) and Z of Kolmogorov-Smirnov (lower matrix) tests for PM2.5 mass. Results in bold correspond to significant differences between the two sites (i.e. the null hypothesis is rejected) with p > 0.05.
| Sites | Cedar Rapids | Des Moines | Davenport | Montgomery | Van Buren |
|---|---|---|---|---|---|
| Cedar Rapids | 0.861 | 0.005 | 0.810 | 0.596 | |
| Des Moines | 0.779 | 0.001 | 0.962 | 0.771 | |
| Davenport | 0.008 | 0.002 | 0.001 | 0.001 | |
| Montgomery | 0.326 | 0.296 | 0.001 | 0.706 | |
| Van Buren | 0.785 | 0.907 | 0.001 | 0.768 |
3.2. Seasonal and spatial variations of chemical components in PM2.5
Monthly-averaged concentrations of PM2.5 species are shown in Figure 3, including secondary ions (NH4+, NO3−, SO42−), organic carbon (OC), elemental carbon (EC), crustal elements (Si, Ca, Al) and trace metals (Fe, Pb, Zn and Cu) and demonstrate significant differences across urban and rural areas.
Fig. 3.
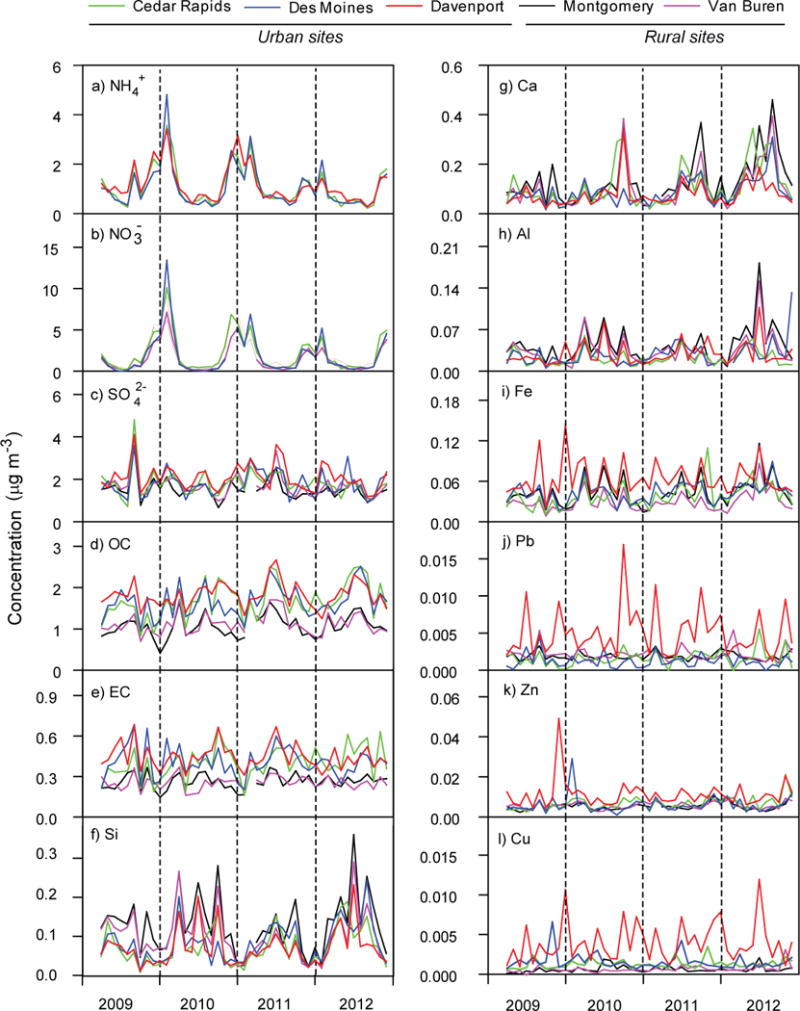
Seasonal variations of secondary tracers (NH4+, NO3−, SO42− and OC), combustion tracers (EC), crustal tracers (Si, Ca, and Al) and anthropogenic tracers (Fe, Pb, Zn and Cu) at the sampling sites in Iowa.
Crustal elements (Si,Ca, Al) are elevated in spring, summer, and fall periods and lowest in winter. The seasonal variation is driven by groundcover, seasonal differences in agricultural activities, and long-range transport.26, 40 The monthly averaged Si concentrations at the two rural sites ranged 0.02–0.36 μg m−3 and were significantly higher (p < 0.039) than urban concentrations (0.01–0.24 μg m−3) (Table S1). Likewise, Al concentrations at rural sites were ranged from 0.10–0.13 μg m−3 were significantly higher (p < 0.031) compared to urban site levels of 0.07–.08 μg m−3 (Table S1). The enrichment of crustal metals at rural sites indicates a greater influence of resuspended dusts on PM2.5 in rural locations.
Urban locations had enhanced concentrations of trace metals, particularly those associated with industrial activity. Average urban levels of Fe, Pb, and Zn ranged from 0.04–0.07 μg m−3, 0.001–0.005 μg m−3, and 0.006–0.011 μg m−3 while average rural concentrations were 0.03–0.04 μg m−3, 0.002 μg m−3, and 0.006 μg m−3, respectively. Maximum trace metal concentrations were observed at Davenport, where heavy industry includes manufacturing of agricultural machinery, heavy weapons, and ammunition, and associated truck and ship traffic. Statistical analysis (Table S2) shows that Davenport metal data are significantly different from the rural and other two urban sites (p < 0.001). With the exception of Zn, the Cedar Rapids and Davenport sites are also statistically different from the rural sites. These data provide conclusive evidence of enhanced exposure to toxic elements in urban areas of heavy industry compared to rural locations.
Organic carbon is derived primary combustion sources (such as biomass burning or fossil fuel use) as well as secondary organic aerosol (SOA formation). OC was a dominant component of PM2.5 contributing 2.3–67.3% of PM2.5. Daily OC concentrations ranged from 0.3–5.2 μg m−3 at urban sites compared to 0.1–4.6 μg m−3 at the rural sites. OC levels were significantly elevated at urban sites compared to rural locations (p < 0.001). Also, OC concentrations were significantly different between Davenport and other two urban locations (p < 0.043; Table S1). These results are consistent with prior studies that reported urban enhancements of OC levels in the range of 2–5 compared to rural sites in the US.40, 41 These data reveal the importance of local sources of carbonaceous aerosol loadings in urban areas and that OC loadings are reduced in rural areas.
Elemental carbon is the product of incomplete combustion of carbonaceous material and is considered a tracer for diesel engine emissions.42 Like OC, EC levels were enhanced in urban areas with average concentrations of 0.03–1.5 μg m−3 compared to rural sites with average concentrations of 0.01–1.0 μg m−3. EC levels are significantly enhanced in urban locations (p < 0.001), indicating the greater role of combustion-derived PM in urban airsheds.
Secondary ammonium nitrate (NH4NO3) is a major contributor to PM2.5 across urban and rural locations. Secondary sources are generally considered to be regional in nature. The monthly average NO3− and NH4+ concentrations have similar seasonal variations and are well-correlated (r2 = 0.79–0.85) across the five study sites. The highest NO3− concentrations were recorded in winter (4.1–5.0 μg m−3) and lowest in summer (0.2–0.5 μg m−3). Likewise, The NH4+ concentration was lowest in in summer (0.5–0.7 μg m−3) and peaked during winter (1.9–2.0 μg m−3). It is well-established that winter conditions (including long nights, lower temperatures and boundary layer height, and higher humidity) promote the formation of secondary NH4NO3.43,44 The summertime minimum may be partially enhanced to negative sampling artifacts whereby ammonium nitrate volatilizes as ammonia and nitric acid from Teflon filters during periods of elevated temperatures.45 These seasonal variations of NH4+ and NO3− are consistent with a greater trend across the US.46, 47 The daily concentrations of NO3− were statistically different (p < 0.011) between rural and urban locations (except for Des Moines) (Table S1). Higher NO3− concentrations in the urban atmospheres are associated with the higher amount of NOx emissions from the combustion sources, particularly automobiles.40 The daily concentrations of NO3− are also significantly different between urban sites, indicating the importance local combustion contributions to NO3−.
Secondary SO42− was a major contributor to PM2.5 mass, but showed little seasonal variation, at levels ranging from 1.5–2.1 μg m−3 in spring, 1.7–2.7 μg m−3 in summer, 1.3–1.7 μg m−3 in fall and1.6–2.2 μg m−3 in winter (Fig. 3c). Similar concentrations have been reported for the rural and urban areas in the central US, Central Great Plains, and Northeastern US.40, 41, 48 Daily SO42− concentrations were significantly enhanced at Davenport (0.01–10.3 μg m−3) when compared to Cedar Rapids (0.3–9.3 μg m−3, p < 0.003), Des Moines (0.01–7.5 μg m−3, p < 0.003) and the rural sites (Montgomery: 0.04 to 8.1 μg m−3, p < 0.001, and Van Buren: 0.2 to 9.3 μg m−3, p < 0.001, Table S1). Higher concentrations of SO42− at Davenport could be related to greater emissions of SO2 from industry and boat-engines on the nearby Mississippi River, which burn fuels with higher sulfur content. The combination of secondary ion data demonstrates that local combustion contributes to atmospheric SO2 and NOx contribute to local secondary aerosol, giving rise to spatial differences across the urban-rural continuum.
3.3. Source identification and factor profiles
With the expectation of differences across urban and rural areas, the PMF model was run separately for each study site. Base model results, with lowest Q value, are summarized in Table 2 and were obtained for a 9-factor solution for Cedar Rapids, an 8-factor solution at Des Moines, and 7-factor solutions for Davenport, Montgomery and Van Buren sites. Representative chemical profiles of PMF factors for the Cedar Rapids site are shown in Fig. 4. The left axis shows the log-transformed PM2.5 mass fraction of each species, whereas the right axis shows the percent of species attributed to that factor. The factor contributions to ambient PM2.5 across urban and rural sites are shown in Table 2.
Table 2.
Absolute and relative contributions of PMF factors to PM2.5 at urban and rural sites in Iowa.
| Source | Source characteristics | Contribution in μg m−3 (%) | Enhancement factor | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Cedar Rapids | Des Moines | Davenport | Montgomery | Van Buren | Urban | Rural | ||
| Secondary sulfate | SO42−, NH4+, OC | 2.7 (30) | 3.1 (44)a | 3.2 (31) | 3.0 (35) | 3.2 (38) | 1.08 | – |
| Secondary nitrate | NO3−, NH4+ | 2.1 (23) | 1.5 (17) | 2.1 (21) | 2.1 (24) | 1.8 (22) | – | 1.03 |
| Biomass burning | K, OC | 1.5 (16) | 0.9 (9) | 1.6 (16) | 1.8 (21) | 1.0 (12) | – | 1.05 |
| Gasoline combustion | OC, EC1, Zn | 0.5 (6) | 0.7 (7) | 1.7 (16)b | 0.5 (6)c | 1.2 (13)b | 1.14 | – |
| Diesel combustion | EC2, OC, Pb | 0.9 (9) | 0.6 (7) | 0.9 (8) | 0.4 (5) | 0.3 (3)b | 2.29 | – |
| Dust | Si, Al, Ca, Fe, Ti | 0.9 (11)d | 1.0 (11) | 0.6 (6) | 0.5 (6) | 0.5 (6) | 1.67 | – |
| Industry | Mn, Cr, Cu, Ni | 0.4 (4) | 0.4 (5) | 0.04 (0.4)b | – | – | nae | – |
| Winter salt | Na or Cl | 0.2 (2) | – | 0.3 (3) | 0.3 (4) | 0.5 (6) | – | nae |
Sum of two resolved secondary sulfate factors, see section 3.3 for details.
Vehicular and industrial sources co-varied, see text for details.
Vehicular factors include chemical characteristics of road dust (e.g. Ca, Mg).
Sum of Ca and Al-Si enriched dust factors.
Enrichment factor not calculated due to unresolved factor(s).
Fig. 4.
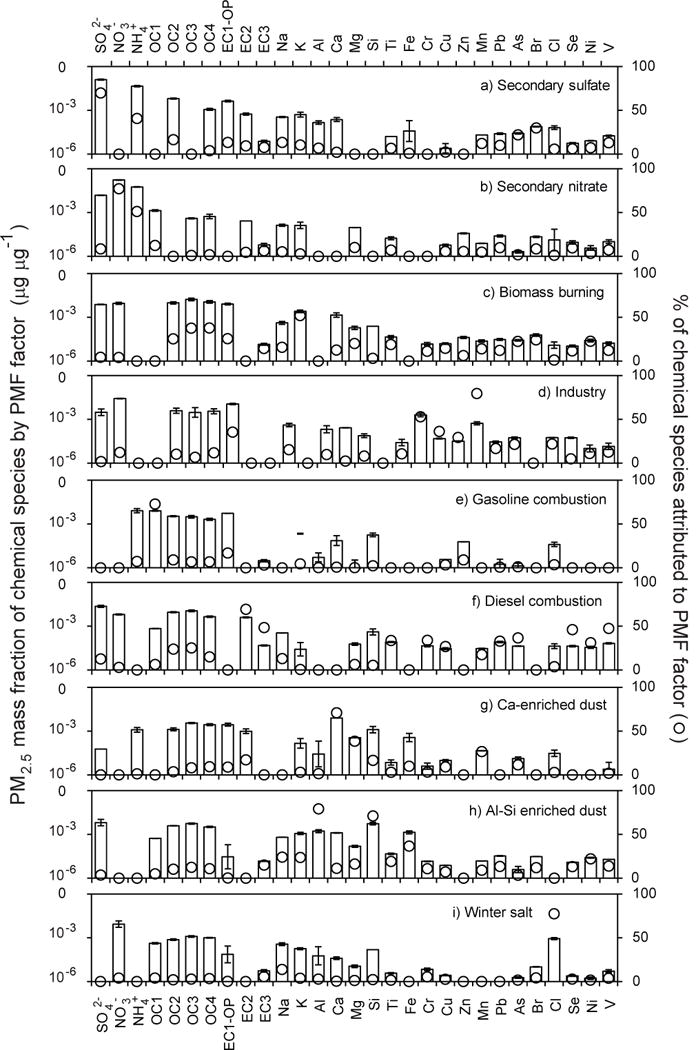
A representative set of PMF factors deduced from PM2.5 speciation data from Cedar Rapids. The left axis shows the log-transformed PM2.5 mass fraction of each species, whereas the right axis shows the percent of species attributed to that factor. The error bar shows the model uncertainty as one standard deviation.
The PMF model resolved factors that were common to all five sites: secondary sulfate, secondary nitrate, biomass burning, gasoline engines, diesel engines, and dust. Industrial factors were only resolved at the three urban sites. Winter salt, associated with the use of salt as a road deicer23 was not resolved at the Des Moines site. At the Davenport site, diesel and gasoline engine factors were mixed with trace metals, suggestive of mixed engine and industrial factors, leading to a low industrial source contribution despite the greatest trace metal concentrations. Gasoline and diesel engine factors at the rural sites co-varied with road dust, giving rise to combined factors. Two dust factors, one enriched in Ca and the other in Al-Si were resolved at Cedar Rapids and were summed for discussion. It is also notable that the industrial factor at Des Moines included minor amounts of crustal elements (Al, Fe and Si). Varying the number of factors did not resolve these co-variances.
The first secondary sulfate (I) factor is characterized by high concentrations SO42− and NH4+ and accounted for 66–68% of the observed SO42− and 41–54% of the observed NH4+ (Fig. 4a, Table 2). A second secondary sulfate factor (II) with higher concentration of SO42− and carbonaceous fractions were also detected for Des Moines and rural sites, consistent enhanced SOA formation in the presence of sulfate.49 This factor does not show any characteristic seasonal variation (Fig. 5a), although prior studies have documented summertime secondary sulfate production.23 The secondary sulfate factor accounted for 30–44% of the PM2.5 mass concentration at the urban and rural sites (Table 2). Similar contributions were also obtained for this source in some other rural and urban sites in the Midwest USA such as East St. Louis, IL; Detroit, MI; and Bondville, IL,23, 25, 26 but were approximately half the level detected in the northeastern United States.50 There was a modest enhancement by a factor of 1.08 in secondary sulfate at urban sites relative to rural locations.
Fig. 5.
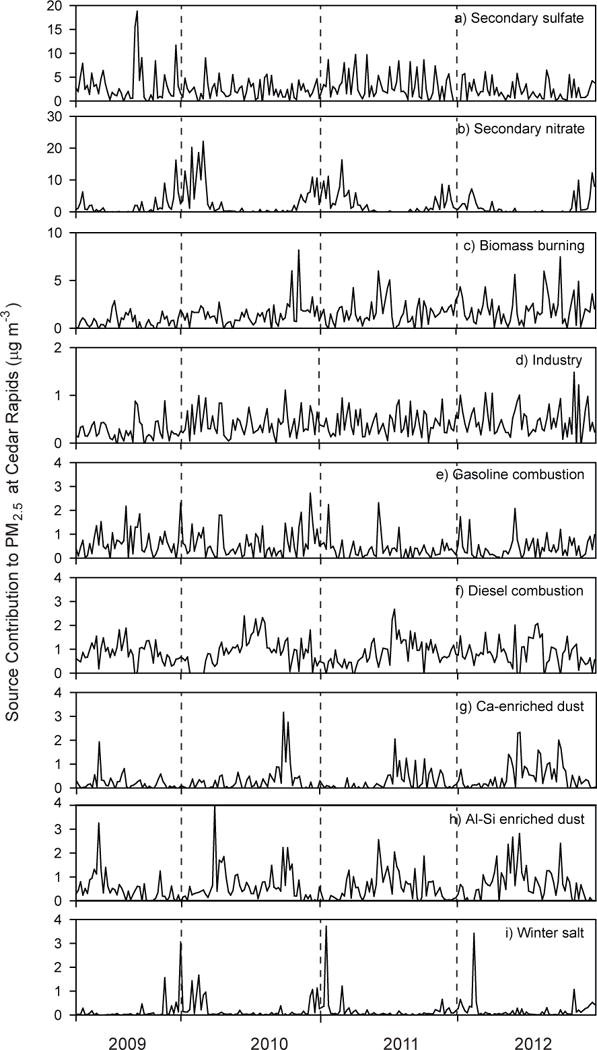
Temporal variations of PMF-resolved factor contributions to PM2.5.
The secondary nitrate factor is characterized by high concentrations of NO3−, accounting for 84–90% of observed nitrate (Fig. 4b). Seasonally, secondary nitrate peaked winter (Fig. 5b) when low temperatures and longer nights promote the formation of secondary NH4NO3.43, 44 This factor accounted for 17–24% of PM2.5 mass at the monitoring sites with maximum contributions occurring in winter (Table 2). A minor rural enhancement (1.03) in the secondary nitrate PMF factor was detected in PMF model results, but is not consistent with the urban enhancement in ambient nitrate concentrations. This anomaly is expected to be an artifact of missing NH4+ data at the rural sites. PMF-resolved secondary sulfate and nitrate factors account for a significant portion of ambient PM2.5 loadings, indicating the important role of secondary aerosol in ambient PM in the Midwestern US.
The biomass burning factor was characterized by higher OC to EC ratio and K (Fig. 4c).51–53 This factor showed no discernable seasonal variations or point source impacts (Fig. 5c). Although the nature of biomass burning varies seasonally, from home heating in the winter to open burning in the summer, these data suggest relatively consistent, year-round contributions to PM2.5. The biomass burning factor made important contributions to PM2.5, averaging 16% in Cedar Rapids, 9% in Des Moines, 16% in Davenport, 21% in Montgomery, and 12% in Van Buren (Table 2). The relative contribution of biomass to PM2.5 in Iowa aerosols is several times higher than other Midwestern cities, including Detroit, MI; and Chicago and East St. Louis, IL, while it was not detected at a rural site in Bondville, IL.23, 25,26 Biomass burning contributions to PM2.5 were slightly enhanced in rural locations (by a factor of 1.05) relative to urban sites.
The industrial emission factor is characterized by trace elements including Mn, Cr and EC1-OP along with Cu, Zn, Pb, Cl, As, Ni, and V (Fig. 4d).23 Industrial contributions were not affected by season (Fig. 5d). The industrial emission accounted for 0.4–5% of PM2.5 at urban sites, but was not resolved at rural sites. While industrial sources have impacts locally, they are observed not to have far-reaching consequences on air quality that lead to background levels of trace elements at the rural sites.
The gasoline engine factor was characterized by higher concentrations of OC and EC1 along with trace metal Zn (Fig. 4e). No seasonal variation was observed for gasoline engine factor (Fig. 5e), consistent with observations in the Detroit, MI.23 The diesel engine factor was characterized by high concentrations of EC relative to OC. In addition, this factor had the greatest contributions to select metals (including Pb, Ni, V) (Fig. 4f).30 However, the diesel engine factor exhibited summertime maxima (Fig 5f), which may be associated with vehicle-enhanced secondary organic aerosol (SOA) production. The combination of vehicular tracers with crustal elements indicates that gasoline and diesel engine factor incorporate non-tailpipe emissions and resuspended road dust. Prior source profiling efforts have reported Cu emissions from brake wear, Zn from the erosion of brake linings,54 and Pb from resuspension of road dust55. The relative contributions of gasoline and diesel engines to PM2.5 are reported in Table 2 and are consistent with prior source apportionment studies in the region for which motor vehicles accounted for 19% of PM2.5 in urban East St. Louis, IL26 and 11% of PM2.5 at a rural monitoring site in Bondville, IL25. The PMF results demonstrate a substantial urban enhancement in diesel engine contributions to PM2.5 (by a factor of 2.29) and gasoline vehicles (by a factor of 1.14), which is significant due to the toxic and carcinogenic properties of vehicular emissions.56
Two soil factors were resolved (Figs. 4g, h), with one enriched in Ca, Mg and Si (termed Ca-enriched dust) and the other enriched in Al and Si (termed Al-enriched dust). Together, these factors explain majority of the observed crustal elements (Ca: 83%, Al: 92% and Si: 80%). Both soil factors exhibit maxima in spring and fall which seasonally coincide with planting and harvesting of agricultural lands (Figs. 5g, h), however fine-scale temporal differences are responsible for the two separate factors that likely have different geological origins. Together, the two dust factors accounted for 6–11% of PM2.5 concentrations in urban locations and 6% of PM2.5 concentrations in rural locations. Lower contributions at rural sites are due to the inclusion of road dusts with vehicular factors. These results are consistent with prior studies that reported PM2.5 dust contributions of 4% at a rural location at Bondville, IL and 4–8% in urban areas at Detroit, MI.23, 24
The winter salt factor was characterized by maximum concentrations of Na or Cl (Figure 4i) and maximum contributions in the wintertime (Figure 5i), which coincides with the use salt to deice roadways. This factor contributed to 2–3% of PM2.5 at urban locations and 4–6% of PM2.5 at rural locations, consistent with prior studies that report this source contributing 5–8% of PM2.5 contribution in the US.23, 24
Representative chemical profiles of PMF factors at a rural site (Van Buren) are shown in Fig. S1. Seven factors were identified following the almost similar approach described above: secondary sulfate (38% of PM2.5), secondary nitrate (22%), biomass burning (12%), gasoline combustion (13%), diesel combustion (3%), dust (6%), and winter salt (6%). Minor differences were observed between the gasoline and diesel factors for the Cedar Rapids and Van Buren sites. Both gasoline factors were characterized by OC and EC1 and Zn, with a stronger road dust influence at the Van Buren Site. Both diesel factors were enhanced in EC relative to OC and contained Pb, Ni, V and other trace metals. In addition, the diesel factor at Van Buren included higher mass fractions of Ca and Mg, which is caused by the covariance of diesel emissions and resuspended dust in rural locations.
4. Summary and Conclusions
This study elucidates differences in PM2.5 composition and its sources at three urban locations—Cedar Rapids, Des Moines, and Davenport—and two rural locations—Montgomery and Van Buren Counties—in Iowa. Air quality in the heavily industrial city of Davenport is worse than other locations in terms of PM2.5 loadings and higher levels of trace metals, including Fe, Zn and, Pb, which demonstrate the important role of local industrial activity in urban air quality. Significant differences in OC and EC loadings, biomass burning contributions, and vehicular sources across urban and rural sites are driven by local emissions. These local emissions also give rise to spatial differences in secondary sulfate and ammonium nitrate across the urban-rural continuum. Higher levels of crustal elements (Ca, Al and Si) at Montgomery and Van Buren sites demonstrate the importance of wind-blown soil and dust in rural air quality, that are driven by agricultural activities and unpaved roadways in rural environments. This study demonstrates that regional efforts are needed to reduce secondary PM in the Midwestern USA with incremental benefits from local reductions. Meanwhile, local pollution controls on industry, vehicles, and biomass burning are needed to improve urban air quality. Reductions in PM loadings will require targeting different sources in urban and rural areas.
Supplementary Material
Environmental Impact Statement.
Particulate matter in the atmosphere presents a human health risk and contributes to mortality and morbidity. Improvement of air quality requires understanding the nature of pollution sources. In this study, the chemistry and sources of PM2.5 at urban and rural sites in Iowa were quantitatively evaluated with source apportionment modeling. Significant enhancements in diesel and gasoline vehicular and industrial sources were found in urban areas, while biomass burning was enhanced in rural areas. Secondary sources were dominant across the urban-rural continuum, largely driven by regional atmospheric with detectable local source influences. Efforts to improve air quality will require targeting different primary sources in urban and rural areas.
Acknowledgments
Funding was provided by the University of Iowa Environmental Health Sciences Research Center (EHSRC) through the National Institutes of Health (NIH, P30 ES05605).
References
- 1.Beckett WS. Current concepts: Occupational respiratory diseases. N Engl J Med. 2000;342:406–413. doi: 10.1056/NEJM200002103420607. [DOI] [PubMed] [Google Scholar]
- 2.Karnae S, John K. Source apportionment of fine particulate matter measured in an industrialized coastal urban area of South Texas. Atmospheric Environment. 2011;45:3769–3776. [Google Scholar]
- 3.Dockery DW, Pope CA, Xu XP, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An Associated Between Air Pollution and Mortality in 6 United States Cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 4.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 US Cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 5.Perrone MG, Gualtieri M, Consonni V, Ferrero L, Sangiorgi G, Longhin E, Ballabio D, Bolzacchini E, Camatini M. Particle size, chemical composition, seasons of the year and urban, rural or remote site origins as determinants of biological effects of particulate matter on pulmonary cells. Environmental Pollution. 2013;176:215–227. doi: 10.1016/j.envpol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 6.USEPA, Technology Transfer Network. National Ambient Air Quality Standards (NAAQS) Available from: http://www.epa.gov/ttn/naaqs/standards/pm/s_pm_index.html (Accessed February 2013)
- 7.Borm PJA, Kelly F, Kunzli N, Schins RPF, Donaldson K. Oxidant generation by particulate matter: from biologically effective dose to a promising, novel metric. Occup Environ Med. 2007;64:73–74. doi: 10.1136/oem.2006.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly FJ. Oxidative stress: Its role in air pollution and adverse health effects. Occup Environ Med. 2003;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafer MM, Perkins DA, Antkiewicz DS, Stone EA, Quraishi TA, Schauer JJ. Reactive oxygen species activity and chemical speciation of size-fractionated atmospheric particulate matter from Lahore, Pakistan: an important role for transition metals. J Environ Monit. 2010;12:704–715. doi: 10.1039/b915008k. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen M, Autrup H, Moller P, Hertel O, Jensen SS, Vinzents P, Knudsen LE, Loft S. Linking exposure to environmental pollutants with biological effects. Mutat Res-Rev Mutat Res. 2003;544:255–271. doi: 10.1016/j.mrrev.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Correia AW, Pope CA, Dockery DW, Wang Y, Ezzati M, Dominici F. Effect of Air Pollution Control on Life Expectancy in the United States An Analysis of 545 US Counties for the Period from 2000 to 2007. Epidemiology. 2013;24:23–31. doi: 10.1097/EDE.0b013e3182770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UCSB. 2010 Census Urban and Rural Classification and Urban Area Criteria. United States Cencus Bureau. 2010 [Google Scholar]
- 13.USDA, United States Department of Agriculture. Data Sets: State Fact Sheets: Iowa [Internet, updated January 17, 2012] 2012 http://www.ers.usda.gov/statefacts/IA.HTM.
- 14.Nitta H, Sato T, Nakai S, Maeda K, Aoki S, Ono M. Respiratory Health Associated with Exposure to Automobile Exhaust. 1. Results of Cross-Sectional Studies in 1979, 1982, and 1983. Arch Environ Health. 1993;48:53–58. doi: 10.1080/00039896.1993.9938393. [DOI] [PubMed] [Google Scholar]
- 15.Ntziachristos L, Ning Z, Geller MD, Sheesley RJ, Schauer JJ, Sioutas C. Fine, ultrafine and nanoparticle trace element compositions near a major freeway with a high heavy-duty diesel fraction. Atmospheric Environment. 2007;41:5684–5696. [Google Scholar]
- 16.Donham KJ. Hazardous Agents in Agricultural Dusts and Methods of Evaluation. Am J Ind Med. 1986;10:205–220. doi: 10.1002/ajim.4700100305. [DOI] [PubMed] [Google Scholar]
- 17.Lammel G, Schneider F, Bruggemann E, Gnauk T, Rohrl A, Wieser P. Aerosols emitted from a livestock farm in southern Germany. Water Air Soil Poll. 2004;154:313–330. [Google Scholar]
- 18.Jancsek-Turoczi B, Hoffer A, Nyiro-Kosa I, Gelencser A. Sampling and characterization of resuspended and respirable road dust. Journal of Aerosol Science. 2013;65:69–76. [Google Scholar]
- 19.de Foy B, Smyth AM, Thompson SL, Gross DS, Olson MR, Sager N, Schauer JJ. Sources of nickel, vanadium and black carbon in aerosols in Milwaukee. Atmospheric Environment. 2012;59:294–301. [Google Scholar]
- 20.Bachmann J. Will the circle be unbroken: A history of the US national ambient air quality standards. Journal of the Air & Waste Management Association. 2007;57:652–697. doi: 10.3155/1047-3289.57.6.652. [DOI] [PubMed] [Google Scholar]
- 21.Paatero P, Tapper U. Positive Matrix Factorization – A Nonnegative Factor Model With Optimal Utilization Of Error-Estimates Of Data Values. Environmetrics. 1994;5:111–126. [Google Scholar]
- 22.Paatero P. Least squares formulation of robust non-negative factor analysis. Chemometrics Intell Lab Syst. 1997;37:23–35. [Google Scholar]
- 23.Gildemeister AE, Hopke PK, Kim E. Sources of fine urban particulate matter in Detroit, MI. Chemosphere. 2007;69:1064–1074. doi: 10.1016/j.chemosphere.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Gao N, Gildemeister AE, Krumhansl K, Lafferty K, Hopke PK, Kim E, Poirot RL. Sources of fine particulate species in ambient air over Lake Champlain Basin, VT. J Air Waste Manage. 2006;56:1607–1620. doi: 10.1080/10473289.2006.10464557. [DOI] [PubMed] [Google Scholar]
- 25.Kim E, Hopke PK, Kenski DM, Koerber M. Sources of fine particles in a rural Midwestern US area. Environmental Science & Technology. 2005;39:4953–4960. doi: 10.1021/es0490774. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Hopke PK, Turner JR. Source identification of airborne PM2.5 at the St. Louis-Midwest Supersite. J Geophys Res-Atmos. 2006;111 [Google Scholar]
- 27.US Census Bureau. Available from: http://quickfacts.census.gov/qfd/states/19000.html (accessed February 2013)
- 28.EPA. Technology Transfer Network Ambient Monitoring Technology Information Center. 2013. [Google Scholar]
- 29.Chow JC, Watson JG, Pritchett LC, Pierson WR, Frazier CA, Purcell RG. The Dri Thermal Optical Reflectance Carbon Analysis System – Description, Evaluation and Applications in United-States Air-Quality Studies. Atmos Environ a-Gen. 1993;27:1185–1201. [Google Scholar]
- 30.Watson JG, Chow JC, Lowenthal DH, Pritchett LC, Frazier CA, Neuroth GR, Robbins R. Differences in the carbon composition of source profiles for diesel-powered and gasoline-powered vehicles. Atmospheric Environment. 1994;28:2493–2505. [Google Scholar]
- 31.Chow JC, Chen LWA, Watson JG, Lowenthal DH, Magliano KA, Turkiewicz K, Lehrman DE. PM2.5 chemical composition and spatiotemporal variability during the California Regional PM10/PM2.5 Air Quality Study (CRPAQS) J Geophys Res-Atmos. 2006;111 [Google Scholar]
- 32.Diaz AM, Diaz JP, Exposito FJ, Hernandez-Leal PA, Savoie D, Querol X. Air masses and aerosols chemical components in the free troposphere at the subtropical Northeast Atlantic region. Journal of Atmospheric Chemistry. 2006;53:63–90. [Google Scholar]
- 33.U. S. EPA. EPA-PMF 3.0. 2008. [Google Scholar]
- 34.Kim E, Hopke PK. Comparison between conditional probability function and nonparametric regression for fine particle source directions. Atmospheric Environment. 2004;38:4667–4673. [Google Scholar]
- 35.Kim E, Hopke PK, Edgerton ES. Improving source identification of Atlanta aerosol using temperature resolved carbon fractions in positive matrix factorization. Atmospheric Environment. 2004;38:3349–3362. [Google Scholar]
- 36.Polissar AV, Hopke PK, Paatero P. Atmospheric aerosol over Alaska – 2. Elemental composition and sources. J Geophys Res-Atmos. 1998;103:19045–19057. [Google Scholar]
- 37.Paatero P, Hopke PK. Discarding or downweighting high-noise variables in factor analytic models. Anal Chim Acta. 2003;490:277–289. [Google Scholar]
- 38.Paatero P, Hopke PK, Song XH, Ramadan Z. Understanding and controlling rotations in factor analytic models. Chemometr Intell Lab. 2002;60:253–264. [Google Scholar]
- 39.EPA. National Ambient Air Quality Standards. 2013. [Google Scholar]
- 40.Hand JL, Schichtel BA, Pitchford M, Malm WC, Frank NH. Seasonal composition of remote and urban fine particulate matter in the United States. J Geophys Res-Atmos. 2012;117 doi: 10.1029/2024JD042579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malm WC, Schichtel BA, Pitchford ML, Ashbaugh LL, Eldred RA. Spatial and monthly trends in speciated fine particle concentration in the United States. J Geophys Res-Atmos. 2004;109 [Google Scholar]
- 42.Schauer JJ. Evaluation of elemental carbon as a marker for diesel particulate matter. Journal Of Exposure Analysis And Environmental Epidemiology. 2003;13:443–453. doi: 10.1038/sj.jea.7500298. [DOI] [PubMed] [Google Scholar]
- 43.Alexander B, Hastings MG, Allman DJ, Dachs J, Thornton JA, Kunasek SA. Quantifying atmospheric nitrate formation pathways based on a global model of the oxygen isotopic composition (delta O-17) of atmospheric nitrate. Atmos Chem Phys. 2009;9:5043–5056. [Google Scholar]
- 44.Stanier C, Singh A, Adamski W, Baek J, Caughey M, Carmichael G, Edgerton E, Kenski D, Koerber M, Oleson J, Rohlf T, Lee SR, Riemer N, Shaw S, Sousan S, Spak SN. Overview of the LADCO winter nitrate study: hourly ammonia, nitric acid and PM2.5 composition at an urban and rural site pair during PM2.5 episodes in the US Great Lakes region. Atmospheric Chemistry and Physics. 2012;12:11037–11056. [Google Scholar]
- 45.Hering S, Cass G. The magnitude of bias in the measurement of PM2.5 arising from volatilization of particulate nitrate from teflon filters. Journal Of The Air & Waste Management Association. 1999;49:725–733. doi: 10.1080/10473289.1999.10463843. [DOI] [PubMed] [Google Scholar]
- 46.Pitchford ML, Poirot RL, Schichtel BA, Malm WC. Characterization of the Winter Midwestern Particulate Nitrate Bulge. J Air Waste Manage. 2009;59:1061–1069. doi: 10.3155/1047-3289.59.9.1061. [DOI] [PubMed] [Google Scholar]
- 47.Walker JM, Philip S, Martin RV, Seinfeld JH. Simulation of nitrate, sulfate, and ammonium aerosols over the United States. Atmos Chem Phys. 2012;12:11213–11227. [Google Scholar]
- 48.Hand JL, Schichtel BA, Malm WC, Pitchford ML. Particulate sulfate ion concentration and SO2 emission trends in the United States from the early 1990s through 2010. Atmos Chem Phys. 2012;12:10353–10365. [Google Scholar]
- 49.Tolocka MP, Turpin B. Contribution of Organosulfur Compounds to Organic Aerosol Mass. Environmental Science & Technology. 2012;46:7978–7983. doi: 10.1021/es300651v. [DOI] [PubMed] [Google Scholar]
- 50.Polissar AV, Hopke PK, Poirot RL. Atmospheric aerosol over Vermont: Chemical composition and sources. Environmental Science & Technology. 2001;35:4604–4621. doi: 10.1021/es0105865. [DOI] [PubMed] [Google Scholar]
- 51.Andreae MO, Merlet P. Emission of trace gases and aerosols from biomass burning. Glob Biogeochem Cycle. 2001;15:955–966. [Google Scholar]
- 52.Wu CF, Larson TV, Wu SY, Williamson J, Westberg HH, Liu LJS. Source apportionment of PM2.5 and selected hazardous air pollutants in Seattle. Sci Total Environ. 2007;386:42–52. doi: 10.1016/j.scitotenv.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 53.Watson JG, Chow JC. Estimating middle-, neighborhood-, and urban-scale contributions to elemental carbon in Mexico City with a rapid response aethalometer. J Air Waste Manage. 2001;51:1522–1528. doi: 10.1080/10473289.2001.10464379. [DOI] [PubMed] [Google Scholar]
- 54.Lough GC, Schauer JJ, Park JS, Shafer MM, Deminter JT, Weinstein JP. Emissions of metals associated with motor vehicle roadways. Environmental Science & Technology. 2005;39:826–836. doi: 10.1021/es048715f. [DOI] [PubMed] [Google Scholar]
- 55.Deocampo DM, Reed PJ, Kalenuik AP. Road Dust Lead (Pb) in Two Neighborhoods of Urban Atlanta, (GA, USA) Int J Env Res Pub He. 2012;9:2020–2030. doi: 10.3390/ijerph9062020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lighty JS, Veranth JM, Sarofim AF. Combustion aerosols: Factors governing their size and composition and implications to human health. Journal Of The Air & Waste Management Association. 2000;50:1565–1618. doi: 10.1080/10473289.2000.10464197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


