Abstract
The present study examined age-related differences in saccade curvature as older and younger adults looked to an “X” target that appeared concurrently with an “O” distractor. A fixation gap procedure was used to introduce variance into the saccadic latencies of both groups. Consistent with earlier findings, younger adults’ early-onset saccades curved towards the distractor (as the distractor competed with the target for response selection), while late-onset saccades curved away from the distractor (as the distractor location became inhibited over time). In contrast, older adults’ saccades gradually decreased in curvature towards the distractor, but at no point along the latency continuum did they show deviations away. These results suggest that while the local inhibitory mechanisms responsible for decreases in curvature towards distractors may be preserved with age, aging may lead to a selective decline in the frontal inhibitory mechanisms responsible for deviations away from distractors.
Keywords: aging, inhibition, eye movements, saccades, curvature
Objects in the environment rarely occur in isolation and as such, successfully gazing to a stimulus of interest necessarily requires one to avoid looking at other distracting stimuli in the visual scene. Saccades, the rapid ballistic eye movements that are used to reorient gaze, are often influenced by distractors, and several studies have shown that saccadic trajectories tend to curve towards distractors before the eyes reach their target destination (e.g., McPeek & Keller, 2001; Godijn & Theeuwes, 2002; Walker, McSorley, & Haggard, 2006). There is also evidence, however, of circumstances under which saccadic trajectories curve away from distractors (for a review, see Van der Stigchel, Meeter, & Theeuwes, 2006). For instance, Doyle and Walker (2001) showed that an irrelevant distractor presented to the left or right of fixation could cause both voluntary and reflexive saccades to veer in the opposite direction from the distractor’s location.
Whether saccades deviate towards or away from distractors depends on an interplay between top-down and bottom-up processes within the neural oculomotor map thought to reside in the superior colliculus (SC). Trajectory deviations towards distractors are thought to result from an averaging process within that map, through which the disparate peaks of neural activity that correspond to individual saccade goals are combined to form a single vector directed towards an intermediate location (McPeek & Keller, 2001; Tipper, Howard, & Paul, 2001). If circumstances allow, top-down inhibition can be applied to the non-target regions of the map in an effort to reduce the influence of distractor-related activity on the generated saccade. In some cases, inhibition may reduce activity at distractor locations to below baseline levels, leading the saccade to curve away from the distractor location (Godijn & Theeuwes, 2002). This top-down inhibitory process, likely projected from the frontal eye fields (FEFs) onto the SC (Godijn & Theeuwes, 2002; Guitton, Buchtel, & Douglas, 1982; Schlag-Rey, Schlag, & Dassonville, 1992), takes time to exert its effects on saccade programming. Hence, there is a clear time course to saccadic trajectory deviations that can be observed by comparing the curvature for saccades of varying latencies. When little time has passed between the appearance of a distractor and the initiation of a saccade, the saccade will curve towards the distractor. As inhibition is applied over time, however, longer latency saccades will show decreasing curvature towards the distractor, and eventually begin to curve away (McSorley, Haggard, & Walker, 2006; Walker et al., 2006).
If saccade trajectory deviations away from distractors largely depend on frontally mediated inhibitory mechanisms, then one population who may be expected to not show this effect is older adults. A wide body of work suggests that older adults are less able than younger adults to inhibit unwanted distraction (for a review, see Lustig, Hasher, & Zacks, 2007). This inhibitory deficit appears to be remarkably pervasive, as not only has it been observed on tasks relating to ‘higher order’ functions such as memory (e.g., Zacks, Radvansky, & Hasher, 1996), attention (e.g., Gazzaley, Cooney, Rissman, & D’Esposito, 2005), reading (e.g., Connelly, Hasher, & Zacks, 1991) and problem solving (e.g., May, 1999), but also on more ‘low level’ tests of motor control, such as the stop signal task (May & Hasher, 1998), antisaccade task (Munoz, Broughton, Goldring, & Armstrong, 1998), and studies of attentional capture in visual search (e.g., Kramer, Hahn, Irwin, & Theeuwes, 1999). Of particular relevance to the discussion of trajectory deviations is older adults’ reduced ability to restrain prepotent responses when those responses are deemed incorrect (Hasher, Zacks, & May, 1999). Thus, older adults may be less able than younger adults to inhibit distractor locations when producing a saccade to a target and therefore they may not show saccadic deviations away from those distractors.
The present study aimed to determine if saccade trajectory deviations differ between older and younger adults. More specifically, we asked whether the two groups differ in terms of the time-course of their trajectory deviations. In order to address this question, we adapted the paradigm used by McSorley et al. (2006). Older and younger participants fixated a central fixation dot and then moved their eyes to an “X” target which appeared simultaneously with an “O” distractor. Participants were aware that targets would always be located on the vertical or horizontal axes and distractors would always be located to the left or right of targets on the diagonal axes (see Figure 1). In order to observe trajectory deviations over an extended time course, saccade latency was experimentally manipulated using a fixation gap procedure (e.g., Ross & Ross, 1980; Saslow, 1967). Namely, the fixation dot was removed from the display at varying stimulus offset asynchronies (SOAs) relative to target onset (−200, −100, −50, 0, 50, 100, and 200 ms). Removing the fixation dot 200 ms before the target and distractor appear (−200 ms SOA) should produce a robust gap effect (very short saccade reaction times, or SRTs). As the SOA increases, the gap effect should be gradually reduced, and SRTs should gradually increase. Importantly, older adults exhibit a comparable gap effect to that of younger adults (Pratt, Abrams, & Chasteen, 1997) and thus, the SOA manipulation was expected to have a similar effect on the latencies of both groups.
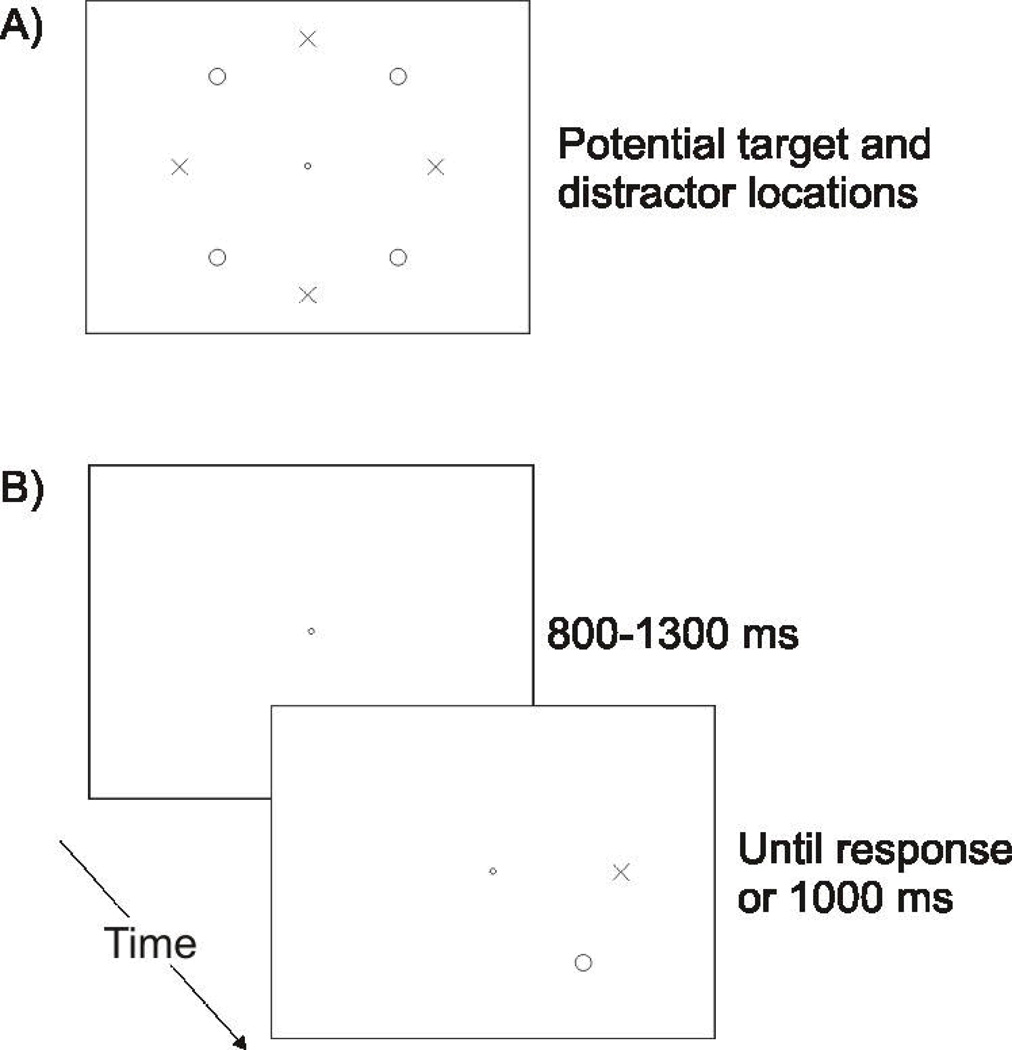
Figure 1.
A) Depiction of the potential target (X’s) and distractor (O’s) locations. B) A typical trial sequence. Participants fixated the central circle until a target appeared, at which point they were required to move their eyes to the target using a single saccade. The target always appeared concomitantly with a distractor in of the two target-adjacent locations.
We expected to replicate the findings of McSorley et al. (2006) with our younger group. That is, younger adults were expected to demonstrate a linear relationship between saccade latency and trajectory deviations, with faster saccades deviating towards the distractor and slower saccades deviating away. If older adults simply require more time to inhibit distractor locations, then we would expect them to demonstrate a different time-course to that of younger adults, with deviations away from distractors only occurring at the longest saccade latencies. If, however, older adults are incapable of inhibiting distractors before moving their eyes, then we would expect them to show few deviations away from distractors and instead produce deviations towards distractors across the entire range of saccade latencies.
Method
Participants
Participants were 8 younger adults (17–25; M = 20.13, SD = 2.59) and 8 older adults (60–74; M = 68.50, SD = 6.07). Younger adults were undergraduate students at the University of Toronto and received partial course credit for their participation. Older adults were recruited from the community and received monetary compensation for their participation. Two younger adults and one older adult were replaced because their eyes could not be tracked reliably (yielding a total n of 8 for each group). All participants reported having normal or corrected to normal vision.
Younger adults had an average of 14.25 (SD = 1.67) years of education and a mean score of 28.75 (SD = 5.20) on the Shipley Vocabulary Test (Shipley, 1946). Older adults did not differ from younger adults in years of education (M = 15.50, SD = 5.01); however, and as is common in the literature, they did score higher on the vocabulary test (M = 35.63, SD = 2.13), t(14) = 3.46, p < .01.
Apparatus
Eye movements were recorded by monitoring pupil position and corneal reflectance using a camera-based eye tracker (SR Research Eyelink 1000) with a temporal resolution of 1000 Hz and an RMS spatial resolution of 0.01° of visual angle. Gaze position was established using a nine-point calibration and validation. The beginning and end of saccadic eye movements were determined using a 30°/s threshold with the additional criteria that the eye exceeded an acceleration of 8000°/s/s during the movement. Experimental displays were presented on a 19-in. flat CRT at a refresh rate of 85 Hz and a resolution of 1024 × 768 pixels. A chin rest was used to fix participants’ heads 80 cm from the monitor.
Procedure
Older participants were tested in the morning (9–11am) and younger participants were tested in the afternoon (12–5pm). Previous work has shown that inhibitory control follows a circadian pattern, with older adults experiencing peak control in the morning and younger adults experiencing peak control in the afternoon (Hasher et al., 1999). Thus, in order to maximize older adults’ ability to inhibit distractors on the eye movement task, participants were tested at their respective age group’s optimal time of day.
Each experimental session began with eye-tracker setup during which a calibration and validation were performed repeatedly until a minimum average accuracy of 0.5° was attained. Participants then completed one block of 8 practice trials, followed by eight blocks of 41 experimental trials. Between blocks, the experimenter could elect to recalibrate the eye tracker.
Every trial began with a fixation stimulus (a white ring with an outer diameter of 0.35° and an inner diameter of 0.16°) that was presented in the center of the display on a light-grey background (see Figure 1 for a typical trial sequence). Once participants moved their gaze to within 1.5° of the fixation stimulus (all reported distances are from the center of a stimulus), they were required to maintain fixation within this region for a randomly determined duration between 800 and 1300 ms, after which both the target and distractor stimuli appeared simultaneously. The target was a white cross and always appeared 8.0° above, below, to the left of, or to the right of the fixation stimulus. The distractor was a white circle that could appear in the four locations that were 8° from the fixation stimulus and equidistant from adjacent target locations. The distractor was always presented in one of the two locations directly adjacent to the target. Both the target and the distractor subtended 1.0° horizontally and vertically, and were drawn with line widths of 0.1°.
Once the target was present, participants were required to move their gaze to within 3° of the target stimulus using a single saccade. If participants failed to maintain fixation before the target was presented, a 200 Hz error tone sounded for 100 ms, the display items were extinguished for 750 ms, and then the trial recommenced. If fixation failed three times consecutively, the experimenter could choose to recalibrate the eye tracker. After the target was presented, if participants failed to initiate a saccade within 1000 ms, or failed to move their eyes to the target location first, the error tone sounded and the trial was counted as an error. At the end of a trial, the display items remained on the display for 250 ms and were then extinguished for an inter-trial interval of 600 ms. In order to produce a range of SRTs, the fixation stimulus was offset during each trial at different times relative to the onset of the target. The SOA between the onset of the target and the offset of the fixation stimulus on each trial was randomly determined to have one of seven possible values: −200, −100, −50, 0, 50, 100, and 200 ms. Therefore, this experiment had one within-subject factor (SOA) that had seven levels, and one between-subject factor (Age) that had two levels.
Measures
Two dependent measures were used to evaluate age-related changes in the time-course of saccadic trajectory deviations: SRT and saccadic curvature. SRT was calculated as the latency between the onset of the target stimulus and the onset of the target directed saccade. Saccadic curvature was calculated using the quadratic method outlined by Ludwig and Gilchrist (2002). Namely, the trajectory of each saccade was scaled and translated to travel a common absolute distance, and the best-fitting quadratic polynomial to the trajectory was determined. The coefficient of the quadratic term of the resulting polynomial provides the measure of the amplitude of curvature, which is reported in hundredths of a degree of visual angle. To reveal the time-course of inhibition, each participant’s responses were collapsed across the SOA conditions, and then vincentized into five SRT bins (Vincent, 1912). Each bin contained one quintile of a participant’s SRTs, and the mean curvature for the responses in each bin was calculated. Changes in mean curvature across bins were used to infer changes in saccadic curvature over time (McSorley et al., 2006). The number of bins was chosen to balance variability in the mean curvature for each bin against accuracy in the depiction of the curvature time course.
Results
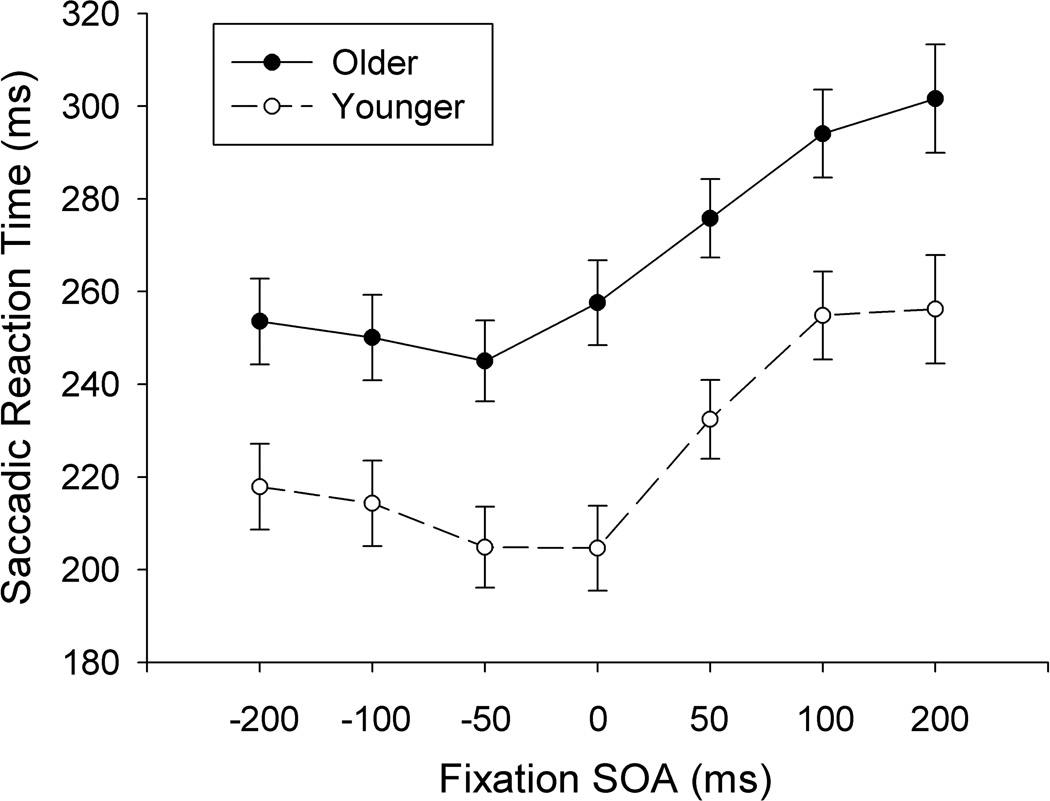
Error trials of younger (M = 8.61%, SD = 8.16) and older (M = 16.65%, SD = 5.52) participants were excluded from the reported analyses. As well, trials were recursively trimmed from each participant’s data set using a three standard-deviation cut-off, first based on SRT and then curvature, for both younger (6.44%) and older (7.01%) participants. Before investigating whether there are age-related changes in trajectory deviations, the effect of the SOA manipulation was first evaluated using a 2 (Age) × 7 (SOA) mixed analysis of variance (ANOVA) on SRT. As can be seen in Figure 2, although older adults generally responded more slowly than younger adults, both groups showed a SOA effect, and the magnitude of the effect did not change with age. These three observations were confirmed by significant main effects of age (F(1, 14) = 5.19, MSE = 48,899.73, η2 = .270, p = .039) and SOA (F(6, 84) = 71.03, MSE = 7,752.14, η2 = .835, p < .001), but a non-significant two-way interaction (F(6, 84) = 1.36, MSE = 148.46, η2 = .089, p = .240), respectively. As such, the gap effect was comparable between younger and older adults, allowing us to collapse across SOA, and evaluate the time-course of trajectory deviations by observing the measure of curvature vincentized by SRT.
Figure 2.
Saccadic reaction time by SOA condition. Error bars are 1 SE of SRT for each SOA condition.
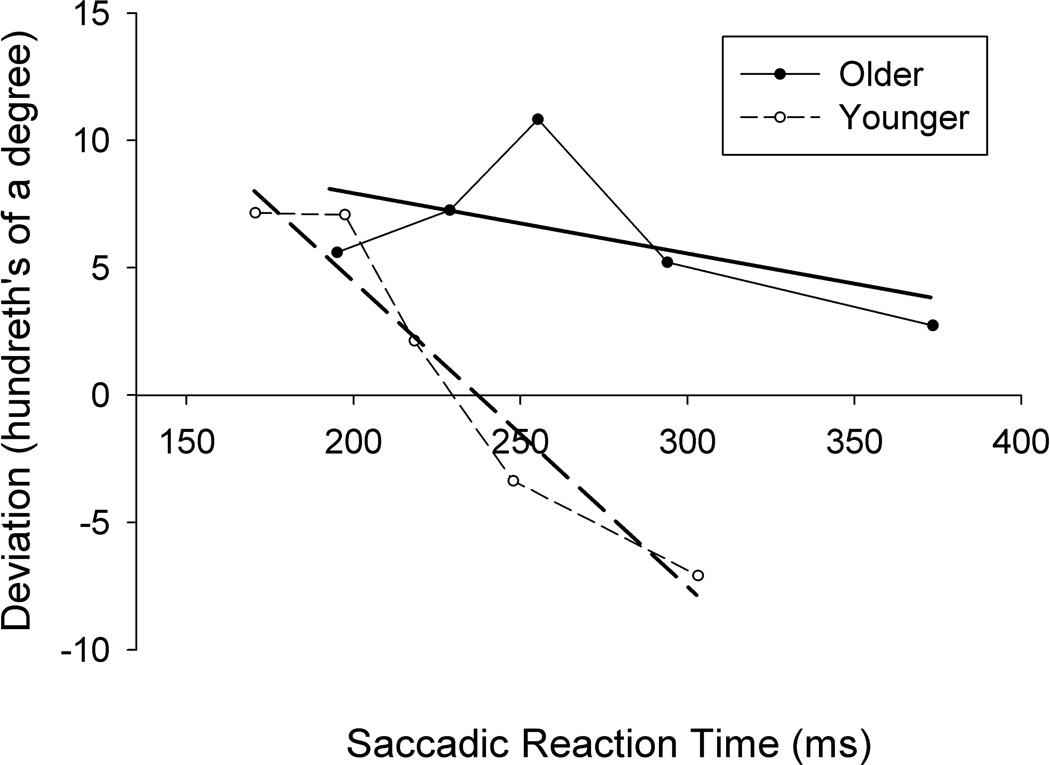
The time-course for older and younger adults is presented in Figure 3. As can be seen, there is little difference in saccadic curvature between older and younger adults at short latencies (i.e., < 200 ms), with the trajectories of both age groups curving towards the distractor. In contrast, there are marked differences in curvature between older and younger adults at longer latencies. While the trajectories of younger adults’ saccades change quickly from being curved towards the distractor to being curved away, this change never fully occurred in older adults, even at the longest latencies. To evaluate the significance of this difference, a linear regression of curvature onto SRT was performed for each participant using the vincentized means, and the mean slope of these lines was compared between age groups. In accordance with Figure 3, the mean slope (in hundredths of a degree per ms) was significantly steeper for younger adults (M = −0.12, SD = 0.09) than older adults (M = −0.03, SD = 0.05), as revealed using a two-tailed independent samples t test (t(14) = 2.50, d = 1.10, p = .025). Even at the longest SRT bin, the curvature of older adults’ saccades did not differ from zero (i.e., a straight trajectory; t(7) = 0.98, d = .35, p = .361), whereas younger adults’ saccades for this time bin showed significant deviations away from the distractor (t(7) = 2.58, d = .91, p = .037). Thus, on the average, older adults failed to show trajectory deviations away from distractors, even when they took longer than 350 ms to move their eyes.
Figure 3.
Trajectory deviations vincetized by saccadic reaction time. Each subject’s trials were divided into five bins based on reaction time (RT), one for each quintile of the subject’s RT distribution. Each point reflects the mean RT (x-axis) and mean curvature (y-axis) for one bin. Also plotted for each age group is the linear regression line of curvature onto saccadic reaction time.
Discussion
The present study asked whether the nature of saccadic trajectory deviations differs between older and younger adults. Saccade reaction time was experimentally manipulated using a fixation gap procedure (e.g., Ross & Ross, 1980; Saslow, 1967) and this manipulation successfully introduced similar amounts of variance into the SRTs of both older and younger adults. Despite an overlapping range of saccadic latency, the two groups clearly differed in terms of the time course of their trajectory deviations. While both groups’ saccades deviated towards distractors at shorter latencies, there were notable differences in curvature between older and younger adults at longer latencies. Replicating the findings of McSorley et al. (2006), young adults quickly transitioned from deviating towards distractors to deviating away. Older adults, however, demonstrated a shallower relationship between SRT and saccade curvature: as they took longer to move their eyes, their saccadic trajectories gradually became less curved, but at no point along the SRT continuum did older adults’ saccades significantly deviate away from the distractor. Thus, the current findings not only suggest a difference in the time course of saccade curvature between older and younger adults, but they also point to the stronger conclusion that older adults do not show this inhibitory eye movement effect, at least within the typical range of SRTs found in this study.1
Although the older group did not show deviations away from the distractor, both groups showed an initial decline in deviations towards it with increasing saccade latency, albeit at markedly different rates. This initial decline in curvature towards the distractor is thought to result from lateral inhibition within the oculomotor map, which gradually leads to the suppression of distractor-related activity in favor of the target location (McSorley et al., 2006; Port & Wurtz, 2003). Our results suggest that this local inhibitory mechanism is preserved in older adults, although it may decline in efficiency with age, as evidenced by the slower time course of this effect in the older group. In contrast, deviations away from distractors are thought to result from below baseline levels of activity at distractor locations and most likely require top-down inhibition from a source external to the motor map itself, possibly the FEFs (Godijn & Theeuwes, 2002; Guitton et al., 1982; Schlag-Rey et al., 1992). This top-down inhibitory process takes time to exert its effects and, thus, is only evident at longer saccade latencies in the younger group. The lack of deviations away from distractors in the older group suggests that it is this cortically-generated inhibitory mechanism that suffers most with age. Thus, local competitive inhibition between SC neurons may be relatively preserved with age, while cortically-generated inhibition, via connections projected from the frontal lobes, may decline (Colcombe, Kramer, Erickson, & Scalf, 2005; Head et al., 2004; Raz, 2000). Although future work is needed to determine the relative contribution of these inhibitory mechanisms to saccade curvature and how their weightings change with age, this hypothesis certainly fits with current theories of age-related frontal decline (e.g., West, 1996). At a behavioural level, older adults’ lessened ability to dampen down irrelevant information via frontally-mediated inhibitory control mechanisms (e.g. Gazzaley et al., 2005) ultimately leads to their greater knowledge of distracting information that is irrelevant to the task at hand (for a review, see Healey, Campbell, & Hasher, 2008).
The results reported here are also in accordance with several other studies reporting age-related declines in the inhibitory control of eye movements. For instance, compared to younger adults, older adults are impaired on the antisaccade task (Munoz et al., 1998), are more distracted by irrelevant onsets during visual search (Kramer et al., 1999), and spend more time looking at objects that they were previously told to ignore (Ryan, Leung, Turke-Brown, & Hasher, 2006). Interestingly, work on aging and inhibition of return (the observation of slowed responses to previously attended objects and regions of space; IOR) presents a very different picture, with older adults demonstrating similar IOR effects to those of younger adults (e.g., Hartley and Kieley, 1995), even with multiple sequential cues (Pratt & Chasteen, 2007). Although trajectory deviations and IOR occur under similar circumstances, the two effects may ultimately rely on separate inhibitory processes (Godijn & Theeuwes, 2004). Saccadic deviations are most likely caused by inhibition projected from FEFs, while IOR may depend on a secondary inhibitory process arising from the left inferior parietal lobe and the supramarginal gyrus bilaterally (Lepsien & Pollman, 2002; Bowles, Ferber, & Pratt, 2005). While these parietal regions have also been associated with the inhibition of involuntary eye movements (i.e., antisaccades; Connolly, Goodale, DeSouza, Menon, &Vilis, 2000; Matsuda et al., 2004; although see Merriam et al., 2001), the fact that older adults demonstrate one inhibitory effect (IOR) and not the other (trajectory deviations away) reinforces the notion that these effects are tied to different underlying mechanisms. Taken together, these results also suggest that aging may selectively disrupt the inhibition stemming from frontal areas, but not the inhibition arising from parietal or subcortical areas.
Acknowledgments
This work was supported by Natural Sciences and Engineering Council of Canada Grant 482547 to Jay Pratt and by National Institute on Aging Grant R37 AGO4306 to Lynn Hasher.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/pag/
We cannot rule out the possibility that older adults may show this effect at extraordinarily long SRTs. However, even when the SRTs are vincentized into 8 bins (pulling older adults’ longest average RT out to 400 ms), older adults still do not show significant deviations away from the distractor, t(7) = 0.79, p = .457.
References
- Bowles B, Ferber S, Pratt J. Letter processing interferes with inhibition of return: Evidence for cortical involvement. Cognitive Brain Research. 2005;25:1–7. doi: 10.1016/j.cogbrainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychology and Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Connelly SL, Hasher L, Zacks RT. Age and reading: The impact of distraction. Psychology and Aging. 1991;6:533–541. doi: 10.1037//0882-7974.6.4.533. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, DeSouza JF, Menon RS, Vilish TT. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. Journal of Neurophysiology. 2000;84:1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Doyle MC, Walker R. Curved saccade trajectories: Voluntary and reflexive saccades curve away from irrelevant distractors. Experimental Brain Research. 2001;139:333–344. doi: 10.1007/s002210100742. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. Programming of endogenous and exogenous saccades: Evidence for a competitive integration model. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:1039–1054. doi: 10.1037//0096-1523.28.5.1039. [DOI] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. The relationship between inhibition of return and saccade trajectory deviation. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:538–554. doi: 10.1037/0096-1523.30.3.538. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Disturbances of voluntary saccadic eye movement mechanisms following discrete unilateral frontal lobe removals. In: Lennerstrand G, Zee DS, Keller EL, editors. Functional basis of ocular motility disorders. New York: Pergamon Press; 1982. pp. 497–499. [Google Scholar]
- Hartley A, Kieley JM. Adult age difference in the inhibition of return of visual attention. Psychology and Aging. 1995;10:670–683. doi: 10.1037//0882-7974.10.4.670. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance, XVII. Cambridge, MA: MIT Press; 1999. pp. 653–675. [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: Costs and potential benefits. In: Sossin WS, Lacaille J-C, Castellucci VF, Belleville S, editors. Progress in brain research. Vol. 169. Amsterdam: Elsevier; 2008. pp. 353–363. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Irwin DE, Theeuwes J. Attentional capture and aging: Implications for visual search performance and oculomotor control. Psychology and Aging. 1999;14:135–154. doi: 10.1037//0882-7974.14.1.135. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Pollman S. Covert reorienting and inhibition of return: An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Ludwig CJ, Gilchrist ID. Measuring saccade curvature: a curve-fitting approach. Behavioural Research Methods, Instruments, & Computers. 2002;34(4):618–624. doi: 10.3758/bf03195490. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Zacks RT. Inhibitory deficit theory: Recent developments in a “new view”. In: Gorfein DS, MacLeod CM, editors. Inhibition in cognition. Washington, DC: American Psychological Association; 2007. pp. 145–162. [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, Taira M, Kojima T. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: Cortical and subcortical networks. Psychiatry Research: Neuroimaging. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- May CP. Synchrony effects in cognition: The costs and a benefit. Psychonomic Bulletin and Review. 1999;6:142–147. doi: 10.3758/bf03210822. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L. Synchrony effects in inhibitory control over thought and action. J Exp Psychol Human. 1998;24:263–279. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Short-term priming, concurrent processing, and saccade curvature during a target selection task in the monkey. Vision Research. 2001;41:785–800. doi: 10.1016/s0042-6989(00)00287-x. [DOI] [PubMed] [Google Scholar]
- McSorley E, Haggard P, Walker R. Time course of oculomotor inhibition revealed by saccade trajectory modulation. Journal of Neurophysiology. 2006;96:1420–1424. doi: 10.1152/jn.00315.2006. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA. Stimulus-response incompatibility activates cortex proximate to three eye fields. Neuroimage. 2001;13:794–800. doi: 10.1006/nimg.2000.0742. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. Journal of Neurophysiology. 2003;79:1887–1903. doi: 10.1152/jn.01151.2002. [DOI] [PubMed] [Google Scholar]
- Pratt J, Abrams RA, Chasteen AL. Initiation and inhibition of saccadic eye movements in younger and older adults: An analysis of the gap effect. Journal of Gerontology: Psychological Sciences. 1997;52B:P103–P107. doi: 10.1093/geronb/52b.2.p103. [DOI] [PubMed] [Google Scholar]
- Pratt J, Chasteen AL. Examining inhibition of return with multiple sequential cues in younger and older adults. Psychology and Aging. 2007;22:404–409. doi: 10.1037/0882-7974.22.2.404. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Ross LE, Ross SM. Saccade latency and warning signals: stimulus onset, offset, and change as warning events. Perception & Psychophysics. 1980;27:251–257. doi: 10.3758/bf03204262. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Leung G, Turke-Brown NB, Hasher L. Assessment of age-related changes in inhibition and binding using eye movement monitoring. Psychology and Aging. 2007;22:239–250. doi: 10.1037/0882-7974.22.2.239. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Latency for saccadic eye movement. Journal of the Optical Society of America. 1967;57:1030–1033. doi: 10.1364/josa.57.001030. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Institute of Living Scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- Schlag-Rey M, Schlag J, Dassonville P. How the frontal eye field can impose a saccade goal on superior colliculus neurons. Journal of Neurophysiology. 1992;67:1003–1005. doi: 10.1152/jn.1992.67.4.1003. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Howard LA, Paul MA. Reaching affects saccade trajectories. Experimental Brain Research. 2001;136:241–249. doi: 10.1007/s002210000577. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel, Meeter M, Theeuwes J. Eye movement trajectories and what they tell us. Neuroscience and Biobehavioral Reviews. 2006;30:666–679. doi: 10.1016/j.neubiorev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Vincent SB. The function of vibrissae in the behavior of the white rat. Behavior Monographs. 1912;1(No. 5) [Google Scholar]
- Walker R, McSorley E, Haggard P. The control of saccade trajectories: Direction of curvature depends on prior knowledge of target location and saccade latency. Perception and Psychophysics. 2006;68:129–138. doi: 10.3758/bf03193663. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Radvansky G, Hasher L. Studies of directed forgetting in older adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:143–156. doi: 10.1037//0278-7393.22.1.143. [DOI] [PubMed] [Google Scholar]