Abstract
Dopamine, acting through D1 receptors, is thought to play an important role in cognitive functions of the frontal cortex such as working memory. D1 receptors are widely expressed in fast-spiking (FS) interneurons, a prominent class of inhibitory cells that exert a powerful control of neuronal firing through proximal synapses on their postsynaptic targets. FS cells are extensively mutually interconnected by both GABAA receptor-mediated synapses and gap junction-mediated electrical synapses, and networks of FS cells play a crucial role in the generation of rhythmic synchronous activity. Although recent studies have documented the effects of dopamine modulation of neocortical synaptic connections among excitatory cells and between excitatory and various inhibitory cells, the effects of dopamine receptor activation on GABAergic and electrical interactions among FS cells is not known. To resolve this, we recorded from pairs of FS cells in the infragranular layers of mouse neocortical slices and tested the effects of D1-like (D1/D5) receptor activation on these connections. We found that D1-like receptor activation modulated GABAergic but not electrical connections between them. A D1-like receptor agonist preserved the strength of electrical coupling but reduced the amplitude of IPSPs and IPSCs between FS cells. Our results suggest that D1-like receptor activation has synapse-specific effects within networks of FS cells, with potential implications for the generation of rhythmic activity in the neocortex.
Keywords: dopamine, D1, electrical coupling, neocortex, FS interneurons, IPSPs
Introduction
Mesocortical dopaminergic projections have an important modulatory role on circuits of the cerebral cortex with implications for the formation of working memory and other cognitive processes (Brozoski et al., 1979; Goldman-Rakic, 1995). Despite growing information regarding the effects of dopamine on excitatory synaptic transmission between pyramidal cells (Gao et al., 2001; Seamans et al., 2001; Gonzalez-Islas and Hablitz, 2003; Onn et al., 2006), on synaptic excitation of fast-spiking (FS) interneurons (Gao and Goldman-Rakic, 2003), and inhibitory inputs from various interneuron types onto pyramidal cells (Gonzalez-Islas and Hablitz, 2001; Gao et al., 2003; Kroner et al., 2007), its effect on GABAergic transmission between FS cells has not been studied. FS cells include parvalbumin (PV)-expressing basket cells that exert a powerful control of firing and synchronization of activity in many postsynaptic targets (Cobb et al., 1995; Sik et al., 1995; Tamas et al., 1997). They are extensively mutually interconnected, by both GABAergic and gap junction-mediated electrical synapses (Kawaguchi and Kubota, 1993, 1997, 1998; Galarreta and Hestrin, 1999, 2002; Gibson et al., 1999; Tamas et al., 2000). These properties endow FS cell networks with the ability to generate gamma frequency (20–80 Hz) population oscillations (Whittington et al., 1995; Hormuzdi et al., 2001; Traub et al., 2001). Such rhythms, prevalent in the EEG of humans and animals during attentive states, may provide a temporal structure for the integration of sensory information (Gray and Singer, 1989). Because the properties of GABAergic interactions within networks of interneurons influences the dynamic and temporal characteristics of oscillatory activity, discovering whether dopamine modulates synaptic interactions between them is of considerable interest (Traub et al., 1996). Much work also suggests that gap junction-mediated electrical coupling between interneurons has an additional supporting role in enhancing the synchrony of gamma activity (Deans et al., 2001; Hormuzdi et al., 2001; Buhl et al., 2003) and can also allow networks of FS cells to be sensitive to the level of coherence of excitatory inputs (Galarreta and Hestrin, 2001). Electrical coupling in several different brain structures is sensitive to modulation by a variety of neurotransmitters (O'Donnell and Grace, 1993; Rorig and Sutor, 1996; Velazquez et al., 1997; Landisman and Connors, 2005). Dopamine in particular can modulate electrical coupling between retinal cells in which gap junctions are composed of the same connexin constituents as neocortical FS cells (Hampson et al., 1992; Belluardo et al., 2000). Thus, dopamine modulation of the extensively coupled FS interneuron network might have a profound influence on neocortical circuits and oscillating neuronal ensembles (Galarreta and Hestrin, 1999; Amitai et al., 2002).
The aim of this study was to examine the modulation of both inhibitory synaptic transmission and gap junction-mediated electrical coupling between FS interneurons in the frontal cortex using a selective D1-like dopamine receptor agonist. We discovered that D1-like receptor activation has differential effects on each type of FS–FS connection. Whereas electrical coupling remained unaffected, GABAergic inhibition between FS cells was depressed. Thus, D1-like dopamine receptor activation could affect the properties of rhythmic synchronous activity in the neocortex.
Materials and Methods
Slice preparation and cell identification.
FS interneurons were identified in a mouse line (G42) expressing enhanced green fluorescent protein (EGFP) under the control of the promoter for glutamic acid decarboxylase 67 (GAD67) (Chattopadhyaya et al., 2004). Juvenile mice of both sexes (14–20 d old) were anesthetized by inhaled isoflurane and were decapitated. Coronal slices (30° angle), 300 μm thick, were cut in an ice-cold extracellular solution containing the following (in mm): 18.8 sucrose, 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 26 NaHCO3, 25 glucose, and 0.05 Na2S2O5. After dissection, the slices were incubated in the same extracellular solution at 31–33°C for 25 min. For the subsequent incubation period, slices were maintained at room temperature (20–22°C) in a solution containing the following (in mm): 125 NaCl, 3.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 26 NaHCO3, 20 glucose, 4 lactic acid, 2 pyruvic acid, 0.05 Na2S2O5, and 0.4 ascorbic acid. Kynurenic acid (1 mm) was included in all solutions during the dissection and incubation periods. For recording, slices were kept in a submersion-type recording chamber bathed by an extracellular solution containing the following (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 26 NaHCO3, 20 glucose, 4 lactic acid, 2 pyruvic acid, 0.05 Na2S2O5, and 0.4 ascorbic acid, pH 7.4 (315 mOsm). All extracellular solutions were continuously bubbled with a gas mixture of 95% O2 and 5% CO2 and applied using a gravity-feed superfusion system with a flow rate of ∼5 ml/min.
Fluorescent neurons in layers V and VI of the motor cortex (M1 and M2) were identified using an upright Axioskop microscope (Zeiss, Thornwood, NY) equipped with a 40× water immersion lens and a filter set (500DF25, 525DRLP, and 545AF35) appropriate for EGFP (XF104; Omega Optical, Brattleboro, VT). Once a fluorescent neuron was selected, it was visualized using infrared differential interference contrast video microscopy and recorded using conventional patch-clamp techniques (Stuart et al., 1993). EGFP-positive cells that discharged high-frequency nonadapting spikes in response to threshold current injection were classified as fast-spiking cells (Kawaguchi and Kubota, 1997). The input resistance of FS cells was determined by injecting pulses of hyperpolarizing current (50 pA, 300 ms). Spike amplitudes and afterhyperpolarization potentials (AHPs) were measured relative to the spike threshold.
Paired recordings and data analysis.
Simultaneous somatic whole-cell patch-clamp recordings were performed at 32°C using patch electrodes (3–4 MΩ). For experiments in which evoked IPSPs/IPSCs were elicited between FS cells, the intracellular solution contained the following: 95.4 mm K-methylsulphate, 36 mm KCl, 9 mm HEPES, 4 mm MgATP, 3 mm GTP, 20 mm creatine phosphate, 0.18 mm EGTA, and 0.3% biocytin, pH 7.3, 295 mOsm. For experiments in which miniature IPSCs (mIPSCs) were recorded in FS cells, the composition of the intracellular solution was the following: 117 mm KCl, 9 mm HEPES, 4 mm MgATP, 3 mm GTP, 20 mm creatine phosphate, 0.18 mm EGTA, and 0.3% biocytin, pH 7.3, 295 mOsm. The series resistance (<25 MΩ) was left uncompensated. Cells were recorded using two Axopatch 200B amplifiers (Molecular Devices, Sunnyvale, CA). The voltage and current output were filtered at 5 kHz and digitized at 16 bit resolution (ITC-18; InstruTech, Port Washington, NY), with a sampling frequency of 10 kHz. Data acquisition and analysis were performed using Igor software (WaveMetrics, Lake Oswego, OR). Recordings of IPSPs/IPSCs and electrical connections were made after bath application of the AMPA/kainate receptor antagonist DNQX (10 μm; Sigma, St. Louis, MO). The connectivity of pairs of FS cells via GABAergic synapses was tested in current-clamp mode by injecting brief (3 ms) current pulses to induce action potentials in each cell. Because of the high chloride intracellular solution (estimated Cl− reversal potential, approximately −34 mV), IPSPs appeared as depolarizing voltage deflections. The presence of electrical coupling between pairs of FS cells was determined by injecting pulses of hyperpolarizing current into each cell and detecting a change in the membrane voltage of the non-injected neuron. The coupling coefficient (the ratio of the amplitude of the voltage change in the non-injected cell to that in the injected cell) was used to assess the strength of electrical coupling under baseline conditions. The effects of dopamine receptor activation on the synaptic properties of FS cells were determined by bath applying the D1-like receptor agonist SKF81297 (6-chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide) (Tocris Bioscience, Ellisville, MO) for 10–20 min. SKF81297 was prepared in DMSO and stored as frozen aliquots before use. Sodium metabisulphite (Na2S2O5 at 50 μm) was included in all extracellular solutions to reduce oxidation of the D1-like agonist (Sutor and ten Bruggencate, 1990). Two sets of experiments were performed to test the effects of the drug on GABAergic inhibition between FS cells. In the first, both cells were recorded in current-clamp mode and postsynaptic GABAergic events were recorded as depolarizing IPSPs. In the second set of experiments, the postsynaptic cell in a pair was held in voltage clamp, and IPSCs were elicited in response to injection of current pulses in the presynaptic cell. The percentage of failures of synaptic transmission was determined for each recording by visual inspection. When testing for the effects of D1-like receptor modulation on the strength of electrical coupling between FS cells, both cells were held in voltage-clamp mode at −70 mV. A −30 mV, 300 ms voltage step was applied to cell 1. The current response in the non-injected cell (cell 2) was measured, and the strength of the electrical coupling was expressed as conductance (in nanosiemens) (Gesyn = I/V). Data are given as mean ± SEM. Statistical significance was tested using a two-tailed paired t test (Student's paired t test), and differences were considered significant if p < 0.05. mIPSCs were recorded in voltage-clamp mode, after bath application of DNQX (10 μm) and tetrodotoxin (TTX) (1 μm).
Histology and morphology.
To study the morphology of the recorded neurons, biocytin (0.3%) was included in the pipette solution. Slices containing biocytin-filled cells were fixed with 4% paraformaldehyde in 0.01 m phosphate buffer and 0.2% picric acid for at least 24 h at 4°C. Standard avidin–biotin–horseradish peroxidase complex (ABC; Vector Laboratories, Burlingame, CA) and the 3,3′-diaminobenzidine reaction procedure were used to visualize the neurons. The slices were mounted in Mowiol 40-88 medium. Three-dimensional reconstructions of the neurons were done with Neurolucida (MicroBrightField, Willinston, VT) using a 100× oil immersion objective (numerical aperture 1.4; Zeiss) on an Axioskop 40 microscope. No correction was made for tissue shrinkage.
Results
Physiological and anatomical characterization of FS cells
The experiments in this study were performed using acute coronal slices (300 μm thick) prepared from juvenile mice (14–20 d old) in which EGFP was expressed under the control of the promoter for GAD67. EGFP-positive cells in the neocortex of these animals are PV positive, and they comprise a subset (∼50%) of all PV-positive neurons (Chattopadhyaya et al., 2004). Cells were visualized under infrared video microscopy and somatic recordings were made using standard patch-clamp techniques. We recorded from 48 EGFP-positive cells in layers V and VI and characterized them electrophysiologically. EGFP cells had a mean resting membrane potential of −71.8 ± 0.6 mV and input resistance of 82.6 ± 3.0 MΩ (n = 48). In response to depolarizing current injection, most EGFP-positive cells displayed a firing pattern characteristic of FS cells (Kawaguchi and Kubota, 1997). After just suprathreshold current injection, they fired short epochs of mostly rhythmic action potentials at high frequency (Fig. 1B). The mean discharge frequency for bursts of action potentials at threshold was 99.4 ± 9.9 Hz and ranged from 30.4 to 219.0 Hz (n = 27). The mean burst interspike interval (ISI) was 13.1 ± 1.4 ms, and the coefficient of variation (CV) of the ISI was 0.112 ± 0.018 (n = 27). Periods of high-frequency discharge were often followed by a quiescent period devoid of spiking, in which a rhythmic subthreshold membrane potential oscillation was sometimes present (Fig. 1B,D). Single action potentials of EGFP cells had a mean width (at half-height) of 0.24 ± 0.01 ms, an amplitude of 54.9 ± 1.0 mV, and were followed by AHPs with a mean amplitude of 24.0 ± 1.0 mV (n = 48) (Fig. 1C). To examine the morphology of FS cells, EGFP cells were filled with biocytin and reconstructed (n = 18) (Fig. 1A). All FS cells were found to resemble basket cells. They possessed aspiny, radial dendrites and elaborate axonal arbors confined mainly to layers V and VI (Galarreta and Hestrin, 1999).
Figure 1.

Morphological and physiological characteristics of EGFP-expressing FS interneurons in mouse neocortex. A, Neurolucida reconstruction of a pair of biocytin-filled FS cells that were reciprocally connected by GABAergic synapses and electrically coupled via gap junctions. Cell 1 axon, Light blue; dendrites, dark blue; cell 2 axon, light red; dendrites, dark red. B, Firing patterns of each cell in response to low- and high-intensity suprathreshold current injection. C, Single short-duration FS cell action potential, followed by prominent afterhyperpolarization. D, Expanded trace from hatched box in B showing rhythmic subthreshold membrane potential oscillations typical of quiescent periods between epochs of high-frequency discharge. E, ISI for cell 1 from the middle trace in B.
D1-like receptor activation alters the intrinsic properties of FS cells
Dopaminergic projections to the rodent motor cortex show a distinct topography, with prominent innervation of layers V and VI (Descarries et al., 1987; Berger et al., 1991). In keeping with this pattern, the proportion of GABAergic interneurons expressing dopamine receptors (both D1 and D2 types) is much higher in the infragranular than the supragranular layers (Le Moine and Gaspar, 1998). D1-like and D2-like dopamine receptors mediate opposing effects on the intracellular signaling cascade through their stimulation of Gs- and Gi-proteins, respectively (Kebabian et al., 1984). Furthermore, each receptor subtype possesses a different affinity for dopamine, and the effects of dopamine on synaptic interactions of cortical neurons vary according to the concentration used (Kebabian et al., 1984; Seeman and Van Tol, 1994; Missale et al., 1998; Zheng et al., 1999; Trantham-Davidson et al., 2004). Therefore, the use of dopamine or a nonspecific agonist in these experiments would activate both types of receptors, perhaps resulting in the physiological effects from activation of one receptor type canceling out those caused by agonism of the other (Trantham-Davidson et al., 2004). Parvalbumin-containing fast-spiking interneurons are particularly sensitive to D1-like receptor activation compared with calbindin-expressing, low-threshold-spiking cells, reflecting the preferential expression of this receptor in the former (Le Moine and Gaspar, 1998; Muly et al., 1998; Gorelova et al., 2002; Paspalas and Goldman-Rakic, 2005; Kroner et al., 2007). The proportion of PV-immunoreactive neurons that express the D1 receptor is particularly high (>60%) in the infragranular layers. We therefore tested the effects of D1-like dopamine receptor activation on the synaptic and electrical interactions of FS cells in layers V and VI using the D1-like receptor agonist SKF81297. Bath application of 10 μm SKF81297 depolarized FS cells by 4.2 ± 0.5 mV (p < 0.001; n = 26) (Fig. 2A,B) (Gorelova et al., 2002). In keeping with previous studies, the SKF-induced depolarization showed no sign of recovery during the course of washout in these experiments (Gorelova et al., 2002). We found that SKF81297 application also increased the input resistance of the cell from 100.6 ± 5.1 to 110.2 ± 6.8 MΩ (p < 0.001; n = 26) (Fig. 2C,D). Although the depolarization and increased input resistance increases their excitability, the threshold for action potential generation in FS cells did not change (p > 0.05; n = 5).
Figure 2.
Effect of D1-like receptor activation on intrinsic properties of FS cells. A, Experiment showing the effect of the D1-like receptor agonist SKF81297 (SKF) on the resting membrane potential of an FS interneuron in layer V of the motor cortex. B, Graph of population data showing mean resting membrane potential of FS cells under baseline conditions and after SKF81297 (n = 26; **p < 0.001). C, Voltage responses to injection of a 100 pA hyperpolarizing current step reveal a modest increase in input resistance of FS cells during SKF81297 application. D, Graph of group data displaying input resistance during SKF81297 application normalized to the baseline (**p < 0.001; n = 26).
Effect of D1-like receptor activation on electrical coupling between FS cells
Dopamine receptor activation has been shown to reduce dye-coupling between connexin 36-containing retinal amacrine cells, between pyramidal neurons of the juvenile frontal cortex, and between cells in the nucleus accumbens (Hampson et al., 1992; O'Donnell and Grace, 1993; Rorig et al., 1995). Previous work from our laboratory and others has demonstrated that FS cells are selectively and highly interconnected by gap junction-mediated electrical synapses in both juvenile and adult mice (Galarreta and Hestrin, 1999, 2002; Gibson et al., 1999). To test whether pairs of FS cells were electrically coupled, both cells were initially recorded in current-clamp mode. In response to a brief injection of hyperpolarizing current into one cell, a change in the membrane potential of the non-injected cell was apparent in pairs that were coupled. We found that 40.5% of the pairs examined (32 of 79 pairs) were electrically coupled. Electrical coupling was always bidirectional. The coupling coefficient ranged from 1.9 to 20.4%, with a mean value of 7.7 ± 1.0% (n = 32). To test whether D1-like receptor activation modulated electrical coupling between FS cells, we recorded from both cells in voltage-clamp mode at −70 mV. We generated a voltage step of −30 mV alternately in each cell, measured the resulting current in the coupled cell and calculated the electrical conductance (Fig. 3). The strength of electrical coupling was tested both before and during application of SKF81297 (Fig. 3). D1-like receptor activation had no effect on the strength of electrical coupling. After 15–20 min application, the coupling conductance was 95.6 ± 8.4% of the control value (baseline, 770.6 ± 157.2 pS, p > 0.05, n = 9; SKF81297, 743.6 ± 165.5 pS) (Fig. 3C,D).
Figure 3.
Effect of D1-like receptor activation on electrical coupling conductance (Gesyn) between FS cells. A, Individual experiment in which both FS cells were held in voltage clamp, and the strength of electrical coupling was assessed by producing a −30 mV voltage step in cell 1 and measuring the resulting current response in cell 2. The postsynaptic current responses are an average of 76 trials. The amplitude of the postsynaptic current response was unaffected by SKF81297 (SKF) application. B, Time course of Gesyn with each point representing the mean of nine experiments normalized to the baseline period. C, Graph of group data showing no significant change in Gesyn between FS cells during SKF application. D, Group data showing the effects of 15 min SKF81297 on the mean Gesyn for each of nine pairs of FS cells.
Activation of D1-like receptors reduces inhibitory synaptic strength between FS cells
We next asked whether activation of D1-like receptors can modulate the strength of GABAergic synaptic inhibition between FS cells. To study the connectivity of FS cells via GABAergic synapses, we recorded from pairs of FS cells in current-clamp mode and evoked unitary IPSPs by generating action potentials with brief suprathreshold current injections in the presynaptic cell. We found that 30.4% of the pairs examined (24 of 79) were connected by GABAergic synapses. The GABAergic connections were unidirectional in eight pairs and bidirectional in 16 pairs. In addition to inhibitory synaptic connections, many pairs of FS cells were also electrically coupled. Of all the pairs of FS cells tested, 40.5% (32 of 79) were electrically coupled. However, 75% (six of eight pairs) of pairs connected by unidirectional GABAergic synapses and 100% (16 of 16 pairs) connected by bidirectional synapses were electrically coupled. Thus, the incidence of electrical coupling is higher among GABAergically connected FS interneurons than among those that are not connected. In the first series of experiments, D1-like receptor activation (5–10 min) reduced the amplitude of IPSPs in 9 of 11 pairs. Overall, SKF81297 reduced the IPSP amplitude by 14.9 ± 6.0% of baseline (p < 0.05; n = 11) from 1.90 ± 0.34 to 1.76 ± 0.41 mV.
Both the D1-like receptor-mediated changes in neuronal input resistance and membrane potential would affect the amplitude of IPSPs recorded in FS cells. To reduce the confounding effect of these changes, we made recordings from pairs of FS cells in which action potentials were generated in the presynaptic cell under current clamp and IPSCs were recorded in the postsynaptic cell that was held in voltage-clamp mode at −70 mV (Fig. 4A). The intracellular filling solution contained 36 mm Cl−, and thus the IPSCs appeared as inward currents. Because each of the pairs of FS cells were also electrically coupled, a brief inward current associated with the spikelet was seen 0.13 ± 0.03 ms after the presynaptic action potential (Fig. 4A, marked by an asterisk). In addition, the appearance of a negative deflection in the average baseline current trace before the spikelet reflected the fact that the presynaptic action potentials were aligned and that the FS cells discharged ∼0.5 ms earlier during application of the drug. This previous firing probably reflected their more depolarized membrane potential (Fig. 4A). The average latency between the peak of the presynaptic action potential and the peak of the postsynaptic IPSC was 1.17 ± 0.10 ms (n = 6 pairs). Unitary IPSCs evoked by FS cells onto other FS cells had a peak amplitude of 60.7 ± 12.1 pA (conductance, 1.48 ± 0.30 nS) and a decay time constant of 2.52 ± 0.33 ms (n = 6 pairs).
Figure 4.

D1-like receptor activation reduced GABAergic IPSCs between FS interneurons. A, Individual experiment in which SKF81297 (SKF) reduced the amplitudes of IPSCs between a pair of FS cells. “Pre” denotes the averaged presynaptic action potentials and “post” the averaged IPSCs recorded during baseline conditions and after 15 min application of SKF81297 (76 traces averaged for each). The asterisk indicates the spikelet resulting from electrical coupling between the two cells. The presynaptic spike traces were aligned at their peak in the time axis. SKF81297 depolarized FS cells by several millivolts as can be seen by the offset of averaged baseline and SKF81297 spikes in the ordinate. As a result of the depolarized membrane potential, FS cells fired action potentials ∼0.5 ms earlier during drug application. This explains the apparent negative deflection before the spikelet in the averaged baseline current trace. B, The time course of the reduction of IPSC amplitudes after application of the drug. Each point represents the mean of six experiments normalized to the baseline period. Bar denotes SKF81297 application period. C, Graph showing the effects of SKF81297 on the mean IPSC amplitudes for each of six pairs of FS cells. D, Population data displaying the mean IPSC amplitude during SKF81297 application, normalized to the baseline amplitude. **p < 0.0005. E, The time course of experiments in which IPSCs were elicited after blockade of D1-like receptors with SCH23390. Each point represents the mean of four experiments normalized to the “baseline” period. Bar denotes SKF81297 application period. SCH23390 was applied throughout the experiment. F, Population data displaying the mean IPSC amplitude during SKF81297 + SCH23390 application, normalized to the amplitude under baseline conditions in SCH23390.
Similar to the current-clamp experiments, SKF81297 depolarized the FS cells as indicated by a mean −116.0 ± 53.3 pA increase in the current required to hold the postsynaptic cells at −70 mV (n = 6). SKF81297 application for 15–20 min reduced the IPSC amplitude by 31.8 ± 3.9% (n = 6). The IPSC amplitude was reduced from 60.7 ± 12.1 pA (conductance, 1.48 ± 0.30 nS) to 41.9 ± 8.6 pA (conductance, 1.02 ± 0.21 nS; n = 6; p < 0.01) (Fig. 4B,D). A reduction in the inhibitory postsynaptic conductance was obtained in each of six pairs tested (range of reduction, 18.9–43.2%) (Fig. 4C). The reduction in IPSC conductance appeared to take effect after only a few minutes with a plateau apparent after 15–20 min application (Fig. 4B). Despite the reduction in IPSC amplitude, the decay time constant of the inhibitory events remained unchanged (baseline, 2.52 ± 0.33 ms; SKF81297, 2.58 ± 0.27 ms; n = 6; p > 0.05). In addition to reducing the peak IPSC amplitude, a significant increase in the CV was also apparent during SKF81297 application, suggestive of a presynaptic mechanism of action (32.6 ± 19.3%; n = 6; p < 0.05). To further probe the mechanism of GABAergic depression, we examined the percentage of synaptic failures in response to presynaptic spikes for each pair of FS cells under baseline conditions and during SKF81297 application. As has been demonstrated previously, synapses made by fast-spiking interneurons are highly reliable. Of six pairs of FS cells tested, only one pair exhibited synaptic failures under baseline conditions (failure rate, 5.3%). During D1-like receptor activation, three of six pairs showed failures. Of the three pairs that exhibited failures during SKF81297 application, the failure rate was 18.1 ± 8.3% (range, 2.0–29.8%). The increased incidence of synaptic failures between pairs of FS cells during SKF81297 application is further suggestive of a presynaptic mechanism underlying the D1-like receptor-mediated depression of FS–FS GABAergic inhibition.
To discount the involvement of non-receptor-mediated effects of the D1-like agonist in IPSC depression, we performed an additional series of experiments in which we applied the D1-like agonist SKF91287 during blockade of D1-like receptors with the D1-like antagonist SCH23390 [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride] (10 μm) (Fig. 4E,F). SCH23390 application abolished the SKF81297-mediated depression in IPSCs between FS cells (IPSC amplitude: SCH23390, 25.2 ± 5.2 pA; SCH23390 + SKF81297, 22.0 ± 4.9 pA; p > 0.05; n = 4).
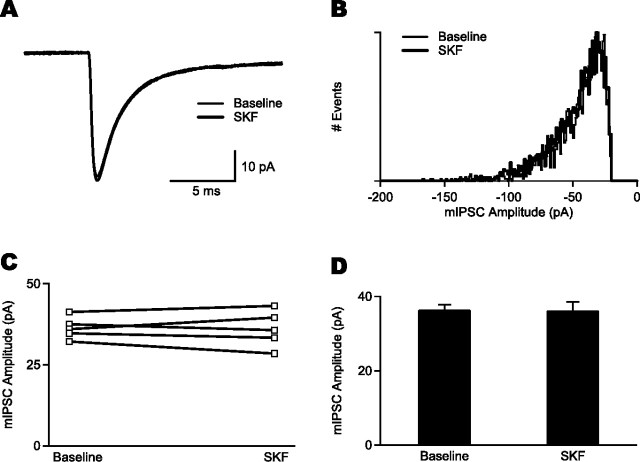
Activation of D1-like receptors does not affect mIPSCs in FS cells
Although the increased CV of peak IPSC amplitudes and failure rate of IPSCs during SKF81297 application suggested that the reduction in strength of GABAergic transmission was attributable to a presynaptic effect, it is possible that an additional postsynaptic mechanism, such as reduced sensitivity of GABAA receptors, may contribute to some degree (Flores-Hernandez et al., 2000). To test this mechanism, we recorded from FS cells using chloride-rich (117 mm Cl−) internal solution. The cells were held at −70 mV, and we examined action potential-independent mIPSCs in the presence of TTX and DNQX (Fig. 5A). Miniature IPSCs in FS cells had a mean amplitude of 36.3 ± 1.5 pA (conductance, 542.6 ± 22.6 pS; n = 5 cells), a single-exponential decay with an averaged time constant of 2.7 ± 0.4 ms (n = 5 cells), and could be blocked by picrotoxin (n = 2 cells). We found that 15–20 min of SKF81297 application did not produce a change in the peak amplitude of mIPSCs (SKF81297, 36.0 ± 2.5 pA; conductance, 538.4 ± 37.8 pS; p > 0.05; n = 5) (Fig. 5B–D). This result suggests that SKF81297 does not affect GABAA receptor-mediated events in FS cells through a postsynaptic mechanism and provides support for the idea that D1-like receptor-mediated depression of evoked inhibitory events between FS interneurons has a presynaptic locus. SKF81297 application also had no significant effects on the decay time (SKF81297, 2.8 ± 0.4 ms; p > 0.05; n = 5) or on the frequency (baseline, 4.0 ± 1.0 Hz; SKF81297, 5.2 ± 1.7 Hz; p > 0.05; n = 5) of mIPSCs in FS cells.
Figure 5.
Effect of D1-like receptor activation on mIPSCs in FS interneurons. A, Superimposed averaged mIPSC traces in baseline and in SKF81297 (SKF) (constructed from 1878 and 2820 events, respectively). The mean amplitude and decay time of mIPSCs was unchanged after drug application. B, Histogram showing amplitude distribution of mIPSCs (with number of events normalized) for the same cell shown in A. The amplitude distribution of mIPSCs was unaffected by SKF81297. C, Group data showing the effects of SKF81297 on the mean peak mIPSC amplitudes for each of five FS cells. D, Graph of population data displaying the mean mIPSC amplitude under baseline conditions and after SKF81297 application.
Discussion
Our work is the first to report on the dopaminergic modulation of synaptic and electrical interactions among FS cells. We found that D1-like dopamine receptor activation increased the excitability of FS interneurons, reduced the amplitude of inhibitory synaptic events between FS cells, but preserved FS–FS electrical coupling.
Electrical coupling between FS cells
Our finding that D1-like receptor activation neither reduced nor enhanced the strength of electrical coupling between FS cells in the frontal cortex was surprising. A number of previous studies have reported a dopamine and/or specifically D1-like receptor-mediated decrement in measures of electrical coupling between different cell types. A striking example of neurotransmitter modulation of gap junctions comes from dopamine modulation of retinal amacrine cells (Hampson et al., 1992). Reduced amacrine cell coupling has been shown to involve D1-like receptors, elevation in intracellular cAMP levels, activation of protein kinase A (PKA), and phosphorylation of the connexin constituents of the gap junctions (connexin 36) (Hampson et al., 1992; Urschel et al., 2006). In common with retinal amacrine cells, the main connexin in neocortical interneurons is also connexin 36 (Belluardo et al., 2000; Deans et al., 2001), so it might have been expected that D1-like receptor activation would bring about reduced FS–FS cell coupling in the present study. Despite their common expression of connexins and D1 receptors, however, it is possible that the properties of the receptors, phosphorylation state of the connexins, and activation of intracellular signaling pathways may differ in these cells. An example of dopamine receptor modulation of presumed connexin 36-mediated electrical coupling in the neocortex comes from a study in which dopamine reduced the extent of Neurobiotin-coupled pyramidal cells in the superficial layers at an early developmental stage (Rorig et al., 1995). An alternative explanation concerns the potential loss of an intracellular signaling component that is involved in connexin phosphorylation as a result of the intracellular dialysis inherent to whole-cell patch-clamp techniques. It should be noted, however, that D1-like receptor activation in our system affected both FS cell excitability and synaptic transmission. These data suggest, therefore, that two signaling mechanisms underlying these effects are not washed out by the patch-clamp technique.
Depression of GABAergic inhibition between FS cells
The strength of GABAA receptor-mediated inhibition between FS cells was similar to that seen in other studies (Galarreta and Hestrin, 1999; Gibson et al., 1999; Galarreta and Hestrin, 2002). Our work is the first to report on the effects of dopamine receptor activation on the amplitude of IPSPs/IPSCs between FS cells. We found that application of a D1-like agonist reduced IPSCs by approximately one-third. D1 receptors are present on both presynaptic and postsynaptic compartments of FS cells (Muly et al., 1998; Paspalas and Goldman-Rakic, 2005), so it is conceivable that the D1-like receptor-mediated depression of IPSCs could have a presynaptic site of action, a postsynaptic mechanism, or a combination of both. Our finding that SKF81297 increased the coefficient of variation of IPSC amplitudes suggests that activation of presynaptic D1-like receptors affected the release of GABA and reduced the amplitude of GABAergic postsynaptic events. In keeping with previous studies, we found that synapses made by fast-spiking neocortical interneurons are highly reliable under normal conditions, perhaps because of the high number of synaptic contacts onto their postsynaptic targets (Xiang et al., 2002; Zaitsev et al., 2007). However, our finding that SKF81297 application increased the incidence of failures of synaptic transmission in some pairs of FS cells is suggestive of a presynaptic mechanism. To determine whether activation of postsynaptic D1-like receptors also contributed to the depression of FS–FS IPSCs, we tested the effects of D1-like activation on the amplitude of action potential-independent miniature IPSCs. Our finding that the mean amplitude of mIPSCs was unchanged leads us to reject the possibility that a postsynaptic effect such as altered sensitivity of the GABAA receptors contributed to the reduction in FS–FS inhibitory synaptic events. A previous study described a D1-like receptor-mediated increase in FS cell somatodendritic excitability that is accompanied by a depression of IPSCs between FS cells and pyramidal cells (Gao et al., 2003). It has also been shown that D1-like activation did not change mIPSC frequency (Kroner et al., 2007). The effects of dopamine receptor activation on GABAergic transmission in the neocortex are therefore complex, and it is likely that different mechanisms underlie the D1-like receptor modulation of evoked and miniature IPSCs. Thus, our observation of unchanged mIPSC frequency may not preclude a presynaptic effect. For example, if D1 receptor activation were to reduce the amplitude of evoked IPSCs by blocking presynaptic calcium channels and depressing GABA release, it is unlikely that the frequency of action potential-independent mIPSCs would be affected (Momiyama and Fukazawa, 2007; Zaitsev et al., 2007). Additional work will be required to determine the precise nature of the presynaptic mechanism underlying the suppression of evoked IPSCs between FS cells. In a variety of different brain regions, D1 receptor activation has been shown to influence P/Q-type calcium channels through the activity of PKA, with varying effects on synaptic transmission (Surmeier et al., 1995; Arias-Montano et al., 2007; Momiyama and Fukazawa, 2007). In the striatum, D1 receptor activation stimulates the calcium-dependent release of GABA through modulation of P/Q channels, whereas D1 activation in the basal forebrain blocks P/Q-type channels and depresses glutamate release (Girault et al., 1986; Floran et al., 1990; Harsing and Zigmond, 1997; Momiyama and Fukazawa, 2007). In contrast to other classes of interneurons, it is known that release of GABA from fast-spiking neocortical interneurons is mediated by P/Q-type calcium channels (Zaitsev et al., 2007). Thus, suppression of P/Q-type channels is a suitable candidate mechanism for the D1 receptor-mediated depression of FS–FS GABAergic inhibition that we observed.
Functional implications
GABAergic synaptic inhibition and gap junction-mediated electrical coupling have each been identified as mechanisms that can allow for the activity of connected neurons to be tightly synchronized with minimal phase lag (Whittington et al., 1995; Beierlein et al., 2000; Bartos et al., 2007). Synchronization within networks of FS cells benefits, however, from the presence of both mechanisms. Networks of FS cells are extensively interconnected by GABAergic synapses and almost exclusively coupled to each other via gap junctions. Because the involvement of FS interneurons in generating gamma frequency network activity is generally accepted, modulation of the strength of either of these connections may have important effects on neocortical rhythmogenesis (Traub et al., 1996; Wang and Buzsaki, 1996; Penttonen et al., 1998). D1-like receptor activation has been shown previously to decrease the incidence of coordinated network activity in the neocortex and to depress hippocampal gamma oscillations (Weiss et al., 2003; Ikegaya et al., 2004). In addition to the depression of IPSCs between FS cells, any reduction in synchronous neocortical activity resulting from D1-like receptor activation could be attributable to an effect on synapses between pyramidal neurons and/or between pyramidal neurons and FS cells (Gao et al., 2001, 2003; Gao and Goldman-Rakic, 2003). The values of FS–FS cell IPSC conductance that we discovered are generally similar to those used in computational models of gamma frequency network oscillations generated by networks of mutually interconnected interneurons (Traub et al., 1997, 2001). A key determinant of the frequency of gamma oscillations generated by isolated interneuron networks is thought to be the magnitude of synaptic inhibition between interneurons (Traub et al., 1996). We therefore suggest that, in addition to possibly diminishing the amplitude of rhythmic activity, a consequence of D1-like receptor activation might be an increase in the frequency of network oscillations as a result of reduced synaptic inhibition between FS cells.
The finding that electrical coupling between FS cells was essentially unaffected by D1-like receptor activation may have important functional consequences. Electrical coupling within networks of interneurons has been shown to act synergistically with GABAergic inhibition to enhance the precision of neuronal firing (Tamas et al., 2000; Galarreta and Hestrin, 2001; Bartos et al., 2007). At the network level, both experimental and theoretical studies have demonstrated that the presence of electrical synapses between interneuron dendrites can enhance the synchrony of gamma frequency oscillations generated by networks of interconnected interneurons (Hormuzdi et al., 2001; Traub et al., 2001). The stability and synchrony of gamma oscillations generated by interneuron networks is known to be affected by variations in the rate of interneuron discharge, and it is thought that the deleterious effects of such heterogeneity can be ameliorated by the ability of FS cells to share tonic excitation via gap junctions (White et al., 1998). The preservation of electrical coupling between FS cells during D1-like receptor activation, therefore, allows for the synchrony of network oscillations to be maintained despite possible changes in its dynamic or temporal characteristics arising from reduced mutual synaptic inhibition. To determine the effects of D1/D5 receptor activation on the output of neocortical circuits, much additional work is required. Our work provides an insight into the effects of dopamine receptor activation on the synaptic and electrical interactions of a network of interneurons with widespread influence on neocortical circuits.
Footnotes
This work was supported by National Institutes of Health Grant EY12114 and by the National Alliance for Research on Schizophrenia and Depression. We thank Z. J. Huang for providing the EGFP mice, J. Li for excellent technical assistance, and S. Brown, D. Freir, and S. Pangratz-Fuehrer for comments on this manuscript.
References
- Amitai Y, Gibson JR, Beierlein M, Patrick SL, Ho AM, Connors BW, Golomb D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–4152. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Montano JA, Floran B, Floran L, Aceves J, Young JM. Dopamine D(1) receptor facilitation of depolarization-induced release of gamma-amino-butyric acid in rat striatum is mediated by the cAMP/PKA pathway and involves P/Q-type calcium channels. Synapse. 2007;61:310–319. doi: 10.1002/syn.20372. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. doi: 10.1016/s0006-8993(00)02300-3. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–824. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Floran B, Aceves J, Sierra A, Martinez-Fong D. Activation of D1 dopamine receptors stimulates the release of GABA in the basal ganglia of the rat. Neurosci Lett. 1990;116:136–140. doi: 10.1016/0304-3940(90)90399-t. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Hernandez S, Snyder GL, Yan Z, Fienberg AA, Moss SJ, Greengard P, Surmeier DJ. D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol. 2000;83:2996–3004. doi: 10.1152/jn.2000.83.5.2996. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Goldman-Rakic PS. Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci USA. 2003;100:2836–2841. doi: 10.1073/pnas.262796399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Girault JA, Spampinato U, Glowinski J, Besson MJ. In vivo release of [3H]gamma-aminobutyric acid in the rat neostriatum. II. Opposing effects of D1 and D2 dopamine receptor stimulation in the dorsal caudate putamen. Neuroscience. 1986;19:1109–1117. doi: 10.1016/0306-4522(86)90127-2. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II–III pyramidal neurons in rat prefrontal cortex. J Neurosci. 2003;23:867–875. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG, Jr, Zigmond MJ. Influence of dopamine on GABA release in striatum: evidence for D1–D2 interactions and non-synaptic influences. Neuroscience. 1997;77:419–429. doi: 10.1016/s0306-4522(96)00475-7. [DOI] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Beaulieu M, Itoh Y. Pharmacological and biochemical evidence for the existence of two categories of dopamine receptor. Can J Neurol Sci. 1984;11:114–117. doi: 10.1017/s0317167100046254. [DOI] [PubMed] [Google Scholar]
- Kroner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Gaspar P. Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Brain Res Mol Brain Res. 1998;58:231–236. doi: 10.1016/s0169-328x(98)00118-1. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Momiyama T, Fukazawa Y. D1-like dopamine receptors selectively block P/Q-type calcium channels to reduce glutamate release onto cholinergic basal forebrain neurones of immature rats. J Physiol (Lond) 2007;580:103–117. doi: 10.1113/jphysiol.2006.125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, III, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci. 1998;18:10553–10565. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993;13:3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, Wang XB, Lin M, Grace AA. Dopamine D1 and D4 receptor subtypes differentially modulate recurrent excitatory synapses in prefrontal cortical pyramidal neurons. Neuropsychopharmacology. 2006;31:318–338. doi: 10.1038/sj.npp.1300829. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci. 2005;25:1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Rorig B, Sutor B. Regulation of gap junction coupling in the developing neocortex. Mol Neurobiol. 1996;12:225–249. doi: 10.1007/BF02755590. [DOI] [PubMed] [Google Scholar]
- Rorig B, Klausa G, Sutor B. Dye coupling between pyramidal neurons in developing rat prefrontal and frontal cortex is reduced by protein kinase A activation and dopamine. J Neurosci. 1995;15:7386–7400. doi: 10.1523/JNEUROSCI.15-11-07386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Sutor B, ten Bruggencate G. Ascorbic acid: a useful reductant to avoid oxidation of catecholamines in electrophysiological experiments in vitro? Neurosci Lett. 1990;116:287–292. doi: 10.1016/0304-3940(90)90088-q. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol (Lond) 1997;500:715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol (Lond) 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci. 1997;4:141–150. doi: 10.1023/a:1008839312043. [DOI] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- Velazquez JL, Han D, Carlen PL. Neurotransmitter modulation of gap junctional communication in the rat hippocampus. Eur J Neurosci. 1997;9:2522–2531. doi: 10.1111/j.1460-9568.1997.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Veh RW, Heinemann U. Dopamine depresses cholinergic oscillatory network activity in rat hippocampus. Eur J Neurosci. 2003;18:2573–2580. doi: 10.1046/j.1460-9568.2003.02970.x. [DOI] [PubMed] [Google Scholar]
- White JA, Chow CC, Ritt J, Soto-Trevino C, Kopell N. Synchronization and oscillatory dynamics in heterogeneous, mutually inhibited neurons. J Comput Neurosci. 1998;5:5–16. doi: 10.1023/a:1008841325921. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer v of rat visual cortex. J Neurophysiol. 2002;88:740–750. doi: 10.1152/jn.2002.88.2.740. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Lewis DA, Krimer LS. P/Q-type, but not N-type, calcium channels mediate GABA release from fast-spiking interneurons to pyramidal cells in rat prefrontal cortex. J Neurophysiol. 2007;97:3567–3573. doi: 10.1152/jn.01293.2006. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-d-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91:527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]