Abstract
Salicinoids are well-known defense compounds in salicaceous trees and careful screening at the population level is warranted to fully understand their diversity and function. European aspen, Populus tremula, is a foundation species in Eurasia and highly polymorphic in Sweden. We exhaustively surveyed 102 replicated genotypes from the Swedish Aspen collection (SwAsp) for foliar salicinoids using UHPLC-ESI-TOF/MS and identified nine novel compounds, bringing the total to 19 for this species. Salicinoid structure followed a modular architecture of a salicin skeleton with added side groups, alone or in combination. Two main moieties, 2′-cinnamoyl and 2′-acetyl, grouped the SwAsp population into four distinct chemotypes, and the relative allocation of salicinoids was remarkably constant between different environments, implying a highly channeled biosynthesis of these compounds. Slightly more than half of the SwAsp genotypes belonged to the cinnamoyl chemotype. A fraction synthesized the acetyl moiety alone (∼7%) or in combination with cinnamoyl (∼2%), and close to forty percent lacked either of the two characteristic moieties, and thus resemble P. tremuloides in their salicinoid profile. The two most abundant chemotypes were evenly distributed throughout Sweden, unlike geographical patterns reported for SwAsp phenology traits, plant defense genes, and herbivore community associations. Here we present the salicinoid characterization of the SwAsp collection as a resource for future studies of aspen chemical ecology, salicinoid biosynthesis, and genetics.
Introduction
Salicinoids, also known as phenolic glycosides [1] or salicylates [2], are dominant bioactive natural products in the Salicaceae (Populus and Salix) [3]. Salicinoid diversity ranges from simple structures like salicin to higher order compounds, such as cinnamoylsalicortin (Fig. 1). The relationship between herbivores and salicinoids has mostly involved studies of a few dominant compounds that repel generalist insect defoliators [3]–[6] and mammalian browsers [7], [8], attract specialist herbivores [9], [10], or have ambiguous effects on herbivore presence and abundance [11]–[13]. Salicinoids vary in their toxicity, alone and in combination [14], and it has been suggested that they increase in toxicity with greater molecular complexity [6].
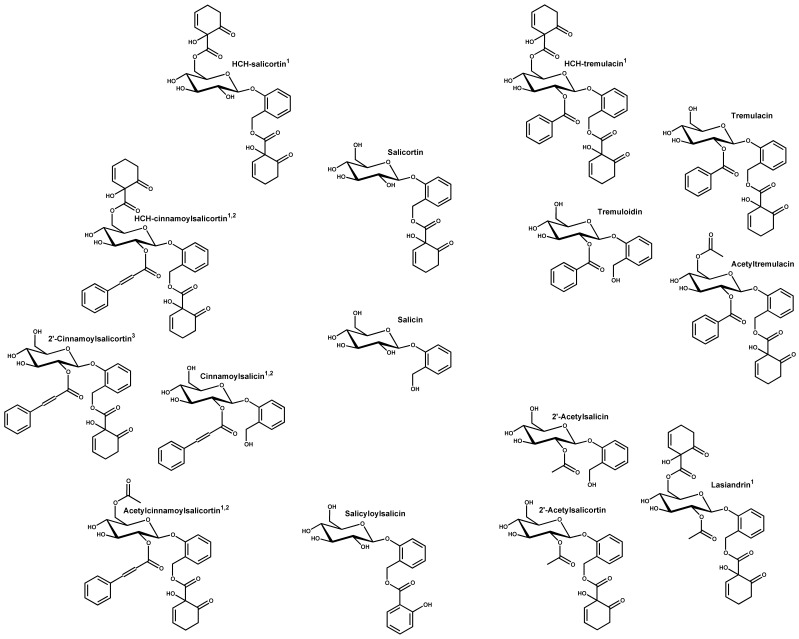
Figure 1. Structural relationship of 19 salicinoids found in the foliage of Populus tremula from the SwAsp collection grouped similar to the loading plot (Fig. 2b).
1 = new compounds for P. tremula, 2 = compounds with two isomers present but the conformation of the cinnamoyl group double bond is ambiguous. 3 = 2′-(E)- and 2′-(Z)-cinnamoylsalicortin.
Salicinoids derive from the shikimate-phenylpropanoid pathway [3], which produces other phenolic compounds, such as flavonoids, lignins, tannins, and anthocyanins [15], [16]. Several of the genes responsible for specific phenylpropanoid classes have already been described, including those involved in the biosynthesis of monolignols [17], [18], flavonoids [15], [16], and condensed tannins [19], [20]. Salicinoid biosynthesis, however, remains poorly understood [15], [16], [21], [22]. Several evolutionary theories argue that selection should favor a diverse, random, or unpredictable mix of defense compounds in plants [23], [24]. For compounds, such as terpenoids, it has been demonstrated that individual profiles and high diversity at the population level results from only a few key genes, that are expressed consistently within a genotype but vary greatly among genotypes [25], [26]. Although Populus and Salix species share many salicinoid compounds, their profiles can also separate salicaceous trees both inter- [3], [27] and intraspecifically [28], [29]. Furthermore, studies of hybridizing species suggest that inheritance may often be additive with intermediate levels of phenolic compounds in hybrids [30]–[32], although examples of transgressive inheritance also exist in which hybrids express extreme levels of specific phenolics, including elevated levels of salicinoid compounds [33]. In a recent study, Abreu et al. [29] described an unexpected diversity of salicinoids from five genotypes of European aspen (Populus tremula L.). Besides salicortin, tremulacin, salicin, and tremuloidin, the signature salicinoids of the North American sister species P. tremuloides [1], [28], P. tremula also contained complex salicinoids like 2′-cinnamoylsalicortin, previously only reported for Salix sericea [34], and 2′-acetylsalicin and 2′-acetylsalicortin, also found in S. pentandra [35]. Abreu et al. [29] showed that concentrations of some of these novel salicinoids in P. tremula matched levels in other salicaceous systems that share the same compounds and predicted a minimum of three chemical phenotypes (hereafter chemotypes) of Swedish aspen [29], [36].
Historically, screening work of salicinoids used only qualitative methods, such as thin-layer chromatography [27], [37], [38], as opposed to liquid chromatography-mass spectrometry (LC-MS) techniques presently used for identification and quantification [2], [29], [33], [39]. In addition, species specific profiles have often been based on analyses of a limited number of individuals (e.g., [27], [37], [38]). At the population level, surveying only a few individuals increases the risk of underestimating natural product diversity and abundance. In salicaceous species, hidden salicinoids could thus potentially be a source of reported ambiguous associations found between phytochemistry and biotic stress agents [11]–[13].
With aspen's highly diverse salicinoid assemblage [29], the large genetic diversity in Sweden [40], and its complex associated arthropod communities [41], a careful chemical mapping of a larger population of P. tremula is warranted. This study presents an exhaustive identification of salicinoid diversity in 319 individual Populus tremula trees replicating 102 genotypes from the Swedish Aspen (SwAsp) collection and evaluates the frequency and distribution of the chemotypes across the landscape. To assess the expression in individual trees, salicinoid profiles of the same genotype were also compared across extreme environments.
Materials and Methods
Salicinoid standards
Salicortin, tremuloidin, and HCH-salicortin standards were supplied by Prof. R. L. Lindroth and salicyloylsalicin by Prof. S. D. Mansfield. Salicin and tremulacin standards were purchased from Sigma-Aldrich (St. Louis, MO, USA), and 2′-(E)-, 2′-(Z)-cinnamoylsalicortin, and tremulacin were isolated from P. tremula [39].
Plant material
Two sets of samples were used for this study, consisting of about ten haphazardly chosen, fully expanded leaves from each of 319 individuals of 102 P. tremula genotypes from two different environments, belonging to the Swedish Aspen (SwAsp) collection [29], [36], [41]. SwAsp consists of aspen trees from a range of latitudes (56–67°N) throughout Sweden, collected as roots in 2003 and propagated as genotype replicates. The first greenhouse sample set included leaves from 98 genotypes with 1–2 individuals per genotype that had been growing in standard greenhouse conditions on the Umeå University campus in Umeå, Sweden, since 2005. In 2007, leaves were picked from the greenhouse trees, flash-frozen in liquid nitrogen, and stored at −80°C until chemical analysis (see [29] for details). The second field set, collected in the summer of 2010, consisted of foliage from 41 genotypes from the outdoor SwAsp garden at Sävar (∼20 km NW of Umeå), 37 of which were also represented in the greenhouse population. These trees had been planted in 2004 and at the time of sampling had reached an average height of 220 cm (ranging from 65–405 cm) and every genotype was present in the field in replicates of 2–8. Leaves were harvested and immediately placed in separate glassine envelopes, frozen in liquid nitrogen while in the field, brought to a −80°C freezer, lyophilized in a pre-chilled chamber, and then stored at −20°C until chemical analysis. Replicate individuals of all genotypes currently grow in two common gardens (Ekebo 55.9°N, 13.1°E and Sävar 63.4°N, 20.6°E; see also [36] or [41] for location details of original populations). In addition, most of the genotypes are kept in tissue culture at Umeå Plant Science Centre in Umeå, Sweden, and can be propagated upon request.
Chemical analysis
Samples were analyzed using ultra high performance liquid chromatography (UHPLC) with UV and electro-spray ionization time-of-flight mass spectrometry (ESI-TOF/MS) detectors, using the same instrumental conditions as Abreu et al. [29]. Sample preparation differed slightly for the two sample sets, using fresh weight material for the greenhouse and lyophilized foliage for the field samples. Frozen greenhouse leaf material was ground in liquid nitrogen using a mortar and pestle. For the 2010 field samples, we ground the lyophilized leaves on a Retsch (Verder Group, Haan, Germany) ball mill, placing crushed foliage into 20 ml plastic vials along with two 12.5 mm carbide balls and shaking them at 60 Hz for 1 minute. For both sample sets, 10.00±1.00 mg powder was extracted in 1 ml of cold (4°C) methanol: chloroform: water (v:v:v), containing deuterated salicylic acid as an internal standard [29]. After centrifugation in a chilled centrifuge, 200 µl of the extract supernatant from greenhouse samples and 100 µl from the field samples was dried in a speedvac. Just before analysis, the dried greenhouse samples were reconstituted with 20 µl of methanol and 20 µl of a 0.1% v/v aqueous formic acid solution and 25 µl of each for the field samples. Differences in the initial amounts of sample dried and final reconstitution volumes served to equalize the final salicinoid concentrations between fresh-frozen and lyophilized leaf tissue, assuming a ∼60 percent difference in water content. Compounds in the reconstituted plant extracts were separated on a C18 UPLC™ column (2.1×100 mm, 1.7 µm) and analyzed by an Acquity photodiode array detector coupled in line with a LCT Premier TOF/MS (all from Waters, Milford, MA, USA) as described in Abreu et al. [29].
MassLynx 4.1 software package (Waters Corp.) allows extraction of single ion chromatograms (±0.15 exact mass unit) from the total ion chromatogram using the QuanLynx module, and was used to search for known and theoretical salicinoids using both the deprotonated ([M-H] -) and formate adduct ([M-H+FA] -) ions (Table S1 in Material S1). QuanLynx software was used to integrate the single ion chromatograms and obtain peak areas, which were normalized with internal standard peak area and individual sample weight. All peak areas represented the formate adduct ion, except for salicyloylsalicin, which included both the deprotonated and formate adduct ion peaks summed, as in Abreu et al. [29].
Salicinoid identification
Salicortin, tremulacin, salicin, tremuloidin, salicyloylsalicin, HCH-salicortin, 2′-(E)-, and 2′-(Z)-cinnamoylsalicortin were determined using retention times and molecular weight information of purified standards injected on the UHPLC-ESI-TOF/MS [29], [39]. The compounds 2′-acetylsalicin, 2′-acetylsalicortin, and acetyltremulacin were identified with LC-MS molecular weights and retention times from previous work [29]. The retention times of lasiandrin (HCH-2′-acetylsalicortin) and HCH-tremulacin were confirmed with molecular mass and with Salix species known to have these compounds that were included in the present LC runs [35], [42].
UHPLC with high-resolution tandem mass spectrometry (MS/MS) was used to determine the structure of novel salicinoids, using the same chromatographic conditions as Abreu et al. [29]. Peaks were separated on a Hypersil C18 GOLD column (2.1×50 mm, 1.9 µm) using a Thermo Accela LC system coupled to a LTQ Orbitrap MS (all from Thermo Fisher Scientific, Bremen, Germany) and centroid mass spectra of negative ions were collected after collision-induced dissociation (CID) in the LTQ cell at 35 eV. The deprotonated ion was used for all compounds, except for cinnamoylsalicin, which was fragmented using the formate adduct ion.
Statistical analyses
Salicinoid chemotypes were identified using principal component analysis (PCA; SIMCA-P+ v. 12.0 [43]). Due to differences in sampling environment, we used percentages derived from the normalized peak areas of the 19 salicinoids. To further statistically examine the differences between compound profiles, SAS software version 9.1 [44] was used to perform a two-factor multivariate analysis of variance (MANOVA; PROC GLM function with the MANOVA statement) using the same data as above, with sample set (greenhouse or field grown trees) and chemotype (four chemotypes) as factors. Significant MANOVA tests were followed up with ANOVAs for individual compounds.
Amounts of the most common and abundant salicinoids (salicortin and tremulacin) from the field samples were correlated (PROC CORR) separately for trees either low (TL chemotypes) or high in 2′-cinnamoylsalicortin (CN chemotypes), followed by Fisher's Z to determine if the two correlation coefficients differed.
To compute salicinoid clonal repeatability (H 2, broad-sense heritability), we used the R statistical package as described by Robinson et al. [44], [45]. Clonal repeatability was calculated the greenhouse and the field samples separately, and for the combined population, when they occurred in replicate of two or more. For all statistical analyses we insured that variables met assumptions of normality, applying transformations where necessary.
Results
New salicinoids from P. tremula
In addition to the ten salicinoids described from P. tremula by Abreu et al. [29], we found nine new compounds after searching the TOF/MS chromatograms of greenhouse and field foliage samples for 55 known and theoretical ions (Fig. 1, Material S1). The new salicinoids included five molecules similar to existing P. tremula compounds but with an additional HCH (hydroxycyclohexen-on-oyl) moiety, including HCH-salicortin, HCH-tremulacin, lasiandrin (HCH-2'-acetylsalicortin), and two isomers tentatively identified as HCH-cinnamoylsalicortin. In addition, we found the newly described 2′-(Z)- and 2′-(E)-cinnamoylsalicortin isomers [29], [39] and two salicinoid isomer pairs tentatively identified as cinnamoylsalicin and acetylcinnamoylsalicortin. Considering the structure of many other salicinoids [3], [39], the additional acetyl and HCH groups are most likely attached to C-6′ of glucose and the new isomer pairs probably contain 2′-(Z)- and 2′-(E)-cinnamoyl groups, respectively. The UV profiles of the new compounds showed typical salicinoid spectra (Table 1, Fig S1a page 4–8 in Material S1). As with these other studies, all new salicinoids with a cinnamoyl moiety had higher second maxima (274–279 nm) compared to those without this functional group (270–274 nm).
Table 1. UV maxima, theoretical and experimental exact masses, molecular formulas, and main high-resolution MS/MS fragments of the new salicinoids from Populus tremula.
| Compound | λmax | m/z [M-H]- | MS/MS fragments (relative intensity)a | ||
| Theoreticalmass | LTQ Orbitrap | ||||
| Mass | Formula | ||||
| HCH-salicortin | 218, 271 | 561.1614 | 561.1611 | C27H29O13 | 423 (100), 477 (58.3), 405 (40.0), 299 (13.9), 437 (9.7), 339 (7.3), 293 (6.2), 231 (5.5) |
| HCH-tremulacin | 221, 272 | 665.1876 | 665.1859 | C34H33O14 | 527 (100), 509 (33.6), 543 (28.6), 405 (14.4), 581 (8.4), 403 (2.8), 389 (1.5), 553 (1.0) |
| Cinnamoylsalicin | 218, 279b, 219, 279c | 415.1398 | 415.1385 | C22H23O8 | 415 (100), 147 (50.9), 309 (39.9), 414 (10.2), 285 (6.6), 509 (6.5), 252 (6.4), 515 (6.4) |
| Acetylcinnamoylsalicortin | 220, 274b, 218, 278c | 595.1821 | 595.1793 | C31H31O12 | 447 (100), 423 (15.9), 567 (5.2), 213 (4.3), 285 (4.1), 267 (4.0), 471 (3.5), 341 (2.9) |
| HCH-cinnamoylsalicortin | 222, 277b 219, 277c | 691.2032 | 691.2023 | C36H35O14 | 553 (100), 535 (28.7), 543 (19.5), 405 (10.2), 484 (6.6), 509 (6.5), 252 (6.4), 515 (6.4) |
| Lasiandrin | 219, 270 | 603.1719 | 603.1722 | C29H31O14 | 465 (100), 447 (22.4), 519 (8.5), 561 (5.8), 543 (4.5), 405 (3.6), 341 (3.0), 423 (2.1) |
See Fig. S1a in Material S1 for MS/MS spectra.
a = MS/MS performed on deprotonated isomer 2 of cinnamoyl compounds, except for cinnamoylsalicin, which used the formate adduct [M+FA-H]- (experimental mass m/z 461.1433) of isomer 2; b = isomer 1, c = isomer 2 by UHPLC retention times.
The MS/MS spectra of the new P. tremula salicinoids produced predictable fragments due to similar disassociation mechanisms (Figure S1b page 9–11 in Material S1). The spectra of all compounds with an additional HCH group [HCH-salicortin, and HCH-tremulacin, lasiandrin (HCH-2′-acetylsalicortin), and HCH-cinnamoylsalicortin] had a single dominant fragment due to the loss of the HCH group (neutral loss of 138) from either the core salicyl group or from the glucose. For all compounds, the m/z of this remaining fragment corresponded to their respective deprotonated salicinoid precursors (salicortin, tremulacin, 2′-acetylsalicortin, and 2′-cinnamoylsalicortin, respectively). The next most common fragment for all HCH-containing salicinoids, except for HCH-salicortin, also involved loss of the HCH group. In this case however, the C-O bond of the ether linkage broke proximal to the HCH moiety. For all compounds, these fragments were m/z 18 less than breakage of the distal C-O ether bond, indicating dehydration. While this fragmentation mechanism also produced a significant ion for HCH-salicortin (m/z 405), its second most abundant MS/MS fragment arose from cleavage and loss of most of the cylcohexeneone ring of either of the compound's HCH groups. The other HCH salicinoids also had relatively abundant fragments due to this breakage pattern. In addition, the spectra of all compounds contained a m/z 405 ion; a secondary fragment resulting from a combination of a loss of an HCH group combined with the cleavage of any moiety present at C-2′ of the sugar. A single ion dominated the MS/MS spectra of acetylcinnamoylsalicortin, resulting from the loss of the cinnamoyl group. Subsequent loss of the acetyl moiety led to the appearance of a deprotonated salicortin ion as the second most abundant ion (m/z 423). Lastly, fragmentation of cinnamoylsalicin with a formate adduct yielded the deprotonated compound as the primary fragment. Further dissociation of this structure created ions corresponding to a cinnamoyl group and to cinnamoyl-β-D-glucopyranose with subsequent loss of the salicyl moiety.
Chemotypes and compound relationships
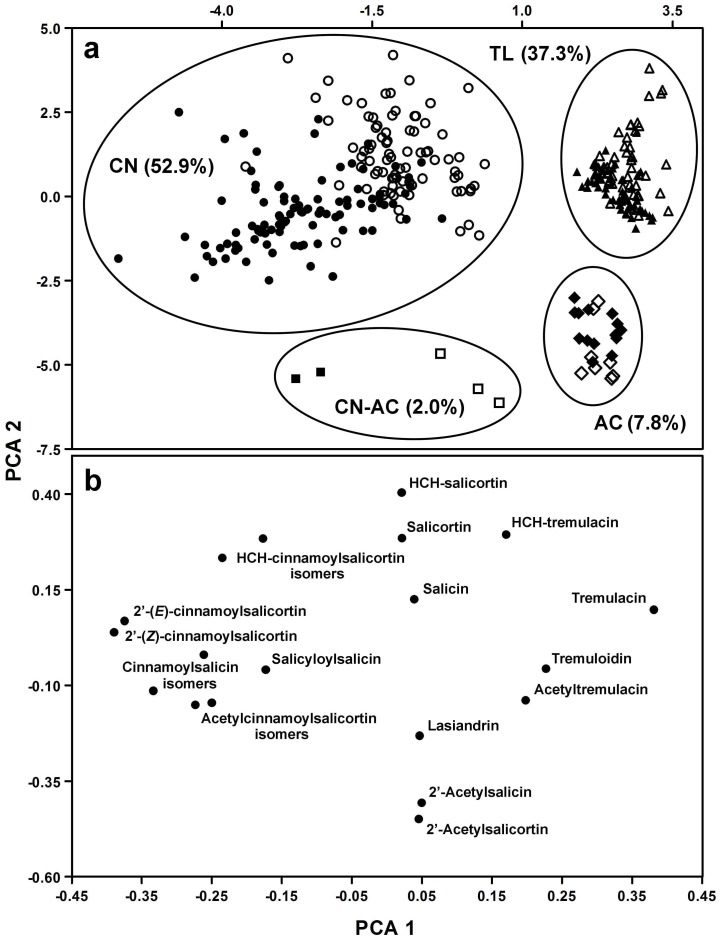
All samples contained the signature set of salicinoids in aspen (salicortin, tremulacin, salicin, and tremuloidin) [1], [3], [27], and in agreement with Abreu et al. [29] a subset of the samples also included novel salicinoids. The salicinoid profiles of SwAsp are presented by genotype and environment in Material S2. PCA analysis separated SwAsp trees into four distinct chemotype groups on the basis of 19 salicinoids with 31.2% of the variation explained by PC1 and 18.4% by PC2 (49.6% cumulative; Fig. 2). These chemotypes were mainly defined by the presence of high amounts of salicinoids with either cinnamoyl moieties (CN, 53% of all genotypes), 2′-acetyl moieties (AC, 8%), both of these moieties (CN-AC, 2%), or very low amounts of either (37%; Fig. 2a). The salicinoid profile of the last “tremuloides-like” (TL) chemotype resembles that of P. tremuloides [46]. Most of the AC chemotype trees had very low levels of cinnamoyl-containing salicinoids, however, five individuals from two genotypes had relatively high levels of compounds with both of these moieties, resulting in the separate chemotype designated CN-AC (Fig. 2a). While trees from particular chemotypes generally grouped together in greenhouse and field grown trees, the PCA showed some divergence between the two environments. This was especially evident for individuals from the CN and CN-AC chemotypes and less so for AC and TL trees (Fig. 2a).
Figure 2. PCA results for chemotypes and compound relationship of 19 salicinoids found in the foliage of Populus tremula from the SwAsp collection. a.
Score scatter plot of the first two principle components for 319 individuals from 102 genotypes. Solid symbols = greenhouse grown trees, open symbols = field grown trees; circles = CN (2′-cinnamoyl), diamonds = AC (2′-acetyl), squares = CN-AC (2′-cinnamoyl/2′-acetyl), and triangles = TL (tremuloides-like) chemotypes. Percentages of clones of the different salicinoid chemotypes in parentheses. b. Loading scatter plot of the first two principle components for 19 salicinoids.
The loading scatter plot of the 19 salicinoids from P. tremula showed that most compounds grouped according to specific chemical moieties (Fig. 2b). The first component (PC1) separated compounds with a cinnamoyl side chain [2′-(E)- and 2′-(Z)-cinnamoylsalicortin and the cinnamoylsalicin, acetylcinnamoylsalicortin, and HCH-cinnamoylsalicortin isomers] from compounds with a benzoyl group (tremulacin, tremuloidin, acetyltremulacin, and HCH-tremulacin). The second component (PC2) separated compounds with an 2′-acetyl, (2′-acetylsalicin, 2′-acetylsalicortin, and lasiandrin) from those with an additional HCH group (HCH-salicortin, HCH-tremulacin, and HCH-cinnamoylsalicortin).
Chemotype and environment differences, main salicinoid correlations, and tree origin
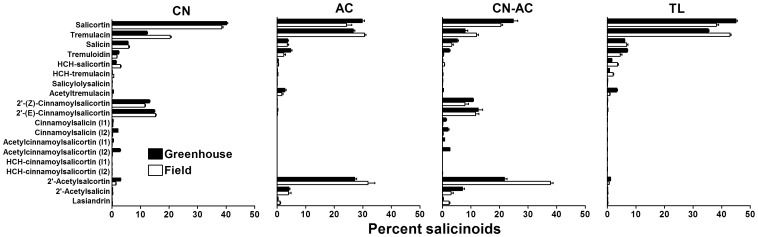
The two-factor MANOVA test showed that salicinoid profile differed for both environment (Wilks' λ = 0.32, F 19, 292 = 32.3, P<0.001), chemotype (Wilks' λ = 0.001, F 57, 871 = 154.5, P<0.001), and their interaction (Wilks' λ = 0.09, F 57, 871 = 18.5, P<0.001; Fig. 3). Individual ANOVA results showed that all salicinoids differed with chemotype, and all compounds, except salicin, 2′-(E)-cinnamoylsalicortin, isomer 2 of HCH-cinnamoylsalicortin, and 2′-acetylsalicin, also differed between environment (Fig. 3; Table 2). In general, greenhouse grown trees contained more salicortin, tremuloidin, acetyltremulacin, and 2′-(Z)-cinnamoylsalicortin, and field trees contained more tremulacin. For isomer 2 of HCH-cinnamoylsalicortin the difference between environments was only apparent due to the interaction of environment with chemotype. Many other salicinoids interacted between these two factors, usually due to differing ratios of particular salicinoids in samples of the different chemotypes, as in the case of both salicortin and tremulacin. In some instances, the compound patterns were reversed between chemotypes. For instance, AC and CN-AC chemotypes had much more 2'-acetylsalicortin in field grown trees.
Figure 3. Percentages (± SE) of 19 salicinoids from greenhouse (solid bars) and field (open bars) grown Populus tremula trees from the SwAsp collection of the four identified chemotypes, CN (2′-cinnamoyl), AC (2′-acetyl), CN-AC (2′-cinnamoyl/2′-acetyl), and TL (tremuloides-like)].
I1 and I2 = isomer 1 and 2, respectively, based upon UHPLC retention times.
Table 2. F and P values from the two-factor ANOVA comparing percentages of individual leaf extract salicinoids from Populus tremula trees of four chemotypes (CN, AC, CN-AC, and TL), grown in two environments (greenhouse and field), and their interaction (E*Ct).
| Compound | Environment | Chemotype | E*Ct | |||
| df = 1 | df = 3 | df = 3 | ||||
| F | P | F | P | F | P | |
| Salicortin | 17.0 | <0.001 | 90.1 | <0.001 | 8.2 | <0.001 |
| Tremulacin | 60.5 | <0.001 | 436.8 | <0.001 | 3.8 | 0.011 |
| Salicin | 0.4 | 0.508 | 10.0 | <0.001 | 0.8 | 0.497 |
| Tremuloidin | 22.6 | <0.001 | 71.8 | <0.001 | 3.8 | 0.010 |
| HCH-salicortin | 10.9 | 0.001 | 22.6 | <0.001 | 2.3 | 0.081 |
| HCH-tremulacin | 25.8 | <0.001 | 67.2 | <0.001 | 1.1 | 0.335 |
| Salicyloylsalicin | 26.9 | <0.001 | 14.8 | <0.001 | 2.4 | 0.066 |
| Acetyltremulacin | 22.0 | <0.001 | 35.2 | <0.001 | 1.5 | 0.225 |
| 2'-(Z)-Cinnamoylsalicortin | 4.9 | 0.028 | 28.5 | <0.001 | 4.5 | 0.004 |
| 2'-(E)-Cinnamoylsalicortin | 0.2 | 0.645 | 456.4 | <0.001 | 0.9 | 0.458 |
| Cinnamoylsalicortin (I1) | 17.4 | <0.001 | 282.1 | <0.001 | 6.0 | 0.001 |
| Cinnamoylsalicortin (I2) | 178.2 | <0.001 | 317.2 | <0.001 | 288.7 | <0.001 |
| Acetylcinnamoylsalicortin (I1) | 14.9 | 0.000 | 21.3 | <0.001 | 16.7 | <0.001 |
| Acetylcinnamoylsalicortin (I2) | 17.4 | <0.001 | 38.7 | <0.001 | 30.5 | <0.001 |
| HCH-cinnamoylsalicortin (I1) | 7.6 | 0.006 | 607.4 | <0.001 | 21.9 | <0.001 |
| HCH-cinnamoylsalicortin (I2) | 3.2 | 0.073 | 855.5 | <0.001 | 2.7 | 0.048 |
| 2'-Acetylsalcortin | 17.0 | <0.001 | 90.1 | <0.001 | 8.2 | <0.001 |
| 2'-Acetylsalicin | 2.4 | 0.126 | 123.5 | <0.001 | 1.5 | 0.223 |
| Lasiandrin | 18.8 | <0.001 | 27.2 | <0.001 | 2.7 | 0.048 |
I1 and I2 indicate isomers 1 and 2, respectively, designated by UHPLC retention times. See Figure 3 for corresponding data.
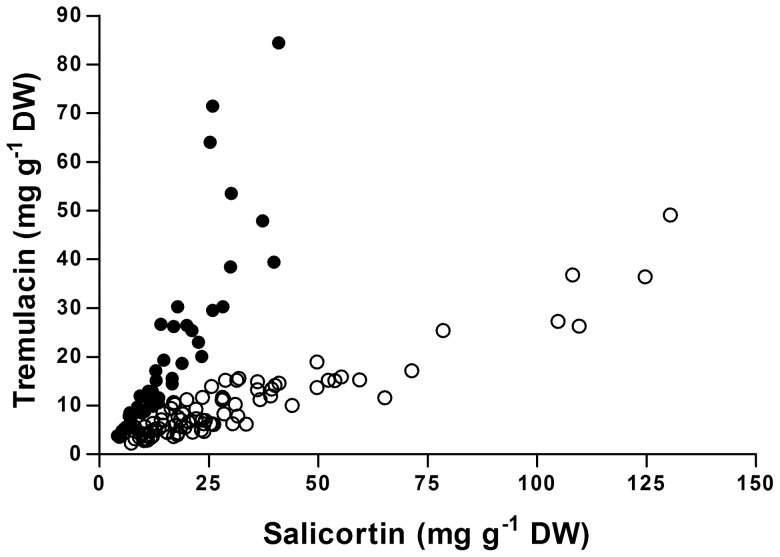
Correlations between the amounts of tremulacin and salicortin in field grown individuals with either low (TL chemotypes; r = 0.87, P<0.001, N = 49) or high (CN chemotypes; r = 0.93, P<0.001, N = 83) levels of 2′-cinnamoylsalicortin showed strong positive relationships in both cases (Fig. 4). The patterns were, however, notably different with CN trees containing considerably less tremulacin with increasing amounts of salicortin (Fisher's Z = 1.71, P<0.05). The relative amounts of these two salicinoids varied with chemotype from an approximately even relationship of 1 tremulacin: 1 salicortin (in mg g-1) in TL chemotypes to a 1∶4 relationship in the CN chemotype.
Figure 4. Relationship between the two most abundant salicinoids, tremulacin and salicortin, in field grown Populus tremula trees for chemotypes low (TL; solid circles) or high (CN; open circles) in 2′-cinnamoylsalicortin.
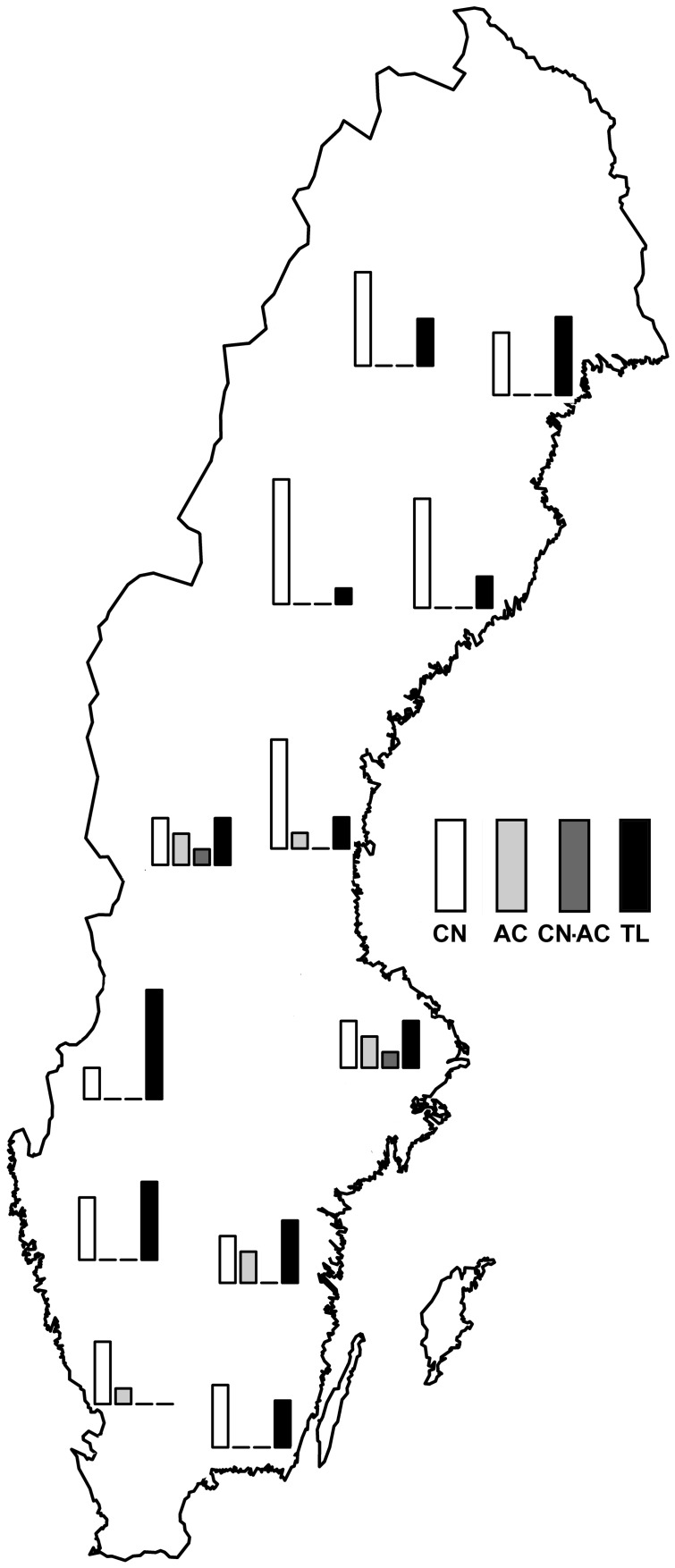
Trees belonging to the CN and TL chemotypes occurred at sites throughout Sweden (Fig. 5) and their distribution did not follow a simple geographic or clinal pattern. The genotypes containing 2′-acetyl compounds (AC and CN-AC) originated from central and southern Sweden.
Figure 5. Salicinoid chemotypes of Populus tremula trees (the SwAsp collection) collected from ten locations throughout Sweden.
White = CN (2′-cinnamoyl), light gray = AC (2′-acetyl), dark gray = CN-AC (2′-cinnamoyl/2′-acetyl), and black = TL (tremuloides-like). Bar height corresponds to the number of individuals of each chemotype.
Clonal repeatability of salicinoids
Overall, clonal repeatabilities (H 2; broad-sense heritability) for salicinoids in this study were high with the greenhouse population showing slightly higher values compared to field grown trees (Table 3). The most notable examples that contributed to this trend were salicortin, tremuloidin, and the isomer pairs of cinnamoylsalicin and acetylcinnamoylsalicortin. Both acetyltremulacin and lasiandrin had substantially lower clonal repeatabilities in the greenhouse environment than in the field. In addition, all salicinoids with higher clonal repeatabilities in the field contained two HCH moieties.
Table 3. Clonal repeatabilities (H 2) of 19 salicinoids from 85 clones of Populus tremula from greenhouse (GH), field, and combined (All) populations. I1 and I2 indicate isomers 1 and 2, designated by UHPLC retention times.
| Compound | H 2 | ||
| GH | Field | All | |
| Salicortin | 0.87 | 0.70 | 0.67 |
| Tremulacin | 0.98 | 0.98 | 0.92 |
| Salicin | 0.46 | 0.45 | 0.29 |
| Tremuloidin | 0.81 | 0.59 | 0.60 |
| HCH-salicortin | 0.86 | 0.91 | 0.76 |
| HCH-tremulacin | 0.85 | 0.96 | 0.80 |
| Salicyloylsalicin | 0.90 | 0.84 | 0.69 |
| Acetyltremulacin | 0.95 | 0.90 | 0.83 |
| 2′-(Z)-Cinnamoylsalicortin | 0.98 | 0.96 | 0.96 |
| 2′-(E)-Cinnamoylsalicortin | 0.99 | 0.98 | 0.98 |
| Cinnamoylsalicin (I1) | 0.79 | 0.41 | 0.54 |
| Cinnamoylsalicin (I2) | 0.94 | 0.74 | 0.57 |
| Acetylcinnamoylsalicortin (I1) | 0.88 | 0.77 | 0.51 |
| Acetylcinnamoylsalicortin (I2) | 0.92 | 0.75 | 0.54 |
| HCH-cinnamoylsalicortin (I1) | 0.88 | 0.87 | 0.70 |
| HCH-cinnamoylsalicortin (I2) | 0.86 | 0.91 | 0.83 |
| 2′-Acetylsalicortin | 0.99 | 0.99 | 0.98 |
| 2′-Acetylsalicin | 0.96 | 0.95 | 0.92 |
| Lasiandrin | 0.76 | 0.89 | 0.73 |
Discussion
Salicinoid survey of P. tremula
On the basis of literature studies (i.e., [3]), previous salicinoid analyses [29], [39], and theoretical structures, we identified a total of 19 potentially bioactive salicinoid compounds from P. tremula, adding nine new structures to those already described by Abreu et al. [29].
Many of these salicinoids are dominant or characteristic for other species in the Salicaceae [3]. The acetylated compounds 2′-acetylsalicin, 2′-acetylsalicortin, lasiandrin, and acetyltremulacin co-occur in Salix pentandra and S. lasiandra [35], [47], [48]. HCH-salicortin (salicortin derivative or disalicortin [49]) and HCH-tremulacin both occur in S. myrsinifolia [2], [49]. HCH-salicortin was also isolated from P. fremontii and its F1 hybrids with P. angustifolia [30], and studies with S. sericea found 2′-(E)-cinnamoylsalicortin [31].
Frequencies and distribution of key compounds in SwAsp
Salicinoid profiles readily divided SwAsp genotypes into four chemotypes, based upon the presence or absence of specific moieties. The 2′-cinnamoyl moiety defined the most abundant chemotype with 53 percent representation in SwAsp. In general, the capability to synthesize cinnamoyl salicinoids appears to be mostly either present or absent in a genotype and thus accounts for the strongest division of the population. The ability to add acetyl moieties appeared less channeled compared to the cinnamoyl addition, but curiously acetylcinnamoylsalicortin had somewhat higher clonal repeatabilities in the less stable field environment (Table 3). Across SwAsp chemotypes we found salicortin, tremulacin, 2′-acetylsalicortin, and the 2′-cinnamoylsalicortins as the dominant salicinoids and when present they usually occurred in relatively high amounts.
The distribution of SwAsp chemotypes throughout Sweden did not resemble the clinal structure reported for phenological traits [36], or the north-south clustering that characterizes inducible defense genes [50]. The two most abundant chemotype groups (CN and TL) were evenly distributed among collection sites, whereas the AC and CN-AC chemotypes mainly originated from the central and southern part of the country. After the last ice age, many plants and animals invaded Sweden from both the north east and the south west and later united in central Sweden (along Limes Norrlandicus) where they either formed hybrid zones or distinct subpopulations [51]. Although the postglacial invasion of aspen in Sweden had weak effects on the genetic differentiation of neutral markers [52], it could have had local effects on adaptive traits [40]. Introgression of defense associated genes naturally occurs in hybrid zones [53], [54], and hybrid zones and admixture populations may also promote novel genotypes [30], [32], [33], [53], [54]. Evidence of salicinoid inheritance from hybrid zones includes additive inheritance of HCH-salicortin by F1 hybrids of P. fremontii and P. angustifolia from the P. fremontii parent [30]. Similarly, additive inheritance was found in a P. tremula-alba hybrid zone for HCH-salicortin and 2′-acetylsalicortin, whereas HCH-tremulacin was trangressive at higher concentrations [33]. The geographical distribution of the AC and CN-AC SwAsp chemotypes in central and southern Sweden could consequently mirror fitness properties, recent evolution, or introgression.
Elusive salicinoid biosynthesis
Tsai et al. [16] proposed salicin as the substrate for salicinoid biosynthesis, but studies using labeled compounds could not confirm this suggestion [21] and the biosynthetic route of salicinoids remains poorly understood [15], [16], [21], [22]. Confirming the work by Abreu et al. [29], we found strong correlations between amounts of 2′-acetylsalicin and 2′-acetylsalicortin, as well as associations between cinnamoyl-containing salicinoids. The dynamics of salicinoid pools thus appear to depend on the presence or absence of characteristic chemotype moieties and the relative abundance of common salicinoids vary between chemotypes. For example, the concentration of tremulacin is generally lower in CN chemotype individuals compared to the TL chemotype (Fig. 4). This may reflect competition for salicortin as a substrate for addition of either a cinnamoyl or benzoyl group for synthesis of 2′-cinnamoylsalicortin or tremulacin, respectively.
The composite structure of the salicinoids could further suggest that relatively few enzymes are involved in their biosynthesis. Keeling and Bohlmann [26] and Degenhardt et al. [25] have demonstrated how a few key genes that are differently but consistently expressed result in unique terpenoid profiles in individual conifers, creating high terpenoid diversity at the population level. Although at a lower diversity, the salicinoids of SwAsp may be biosynthesized according to a similar strategy. Association studies relate specific traits to genetic patterns [55], and could potentially be a promising way to get insight into the salicinoid biosynthesis. Thus, rather than relating single compounds to gene sequences, association studies may benefit from grouping the compounds on the basis of presence and absence of specific salicinoid moieties (see also [54]).
Salicinoid profile stability and implications for chemical ecology
Salicinoid composition of individual trees across environments with very different growth histories showed overall high heritabilities, confirming that both salicinoid quality (composition) and quantity (abundance) is likely to be highly channeled in aspen, and thus relatively stable in different environments [29], [56]. Interestingly, we found that the most represented group in SwAsp, the CN chemotype, also showed the largest plasticity of salicinoids in response to environmental differences. This suggests an elevated level of plasticity in cinnamoyl-containing salicinoid expression and a potential fitness advantage. Salicinoid toxicity to herbivores has been attributed to the HCH moiety, even at low concentrations [3], [14], [57]. Most of the newly described salicinoids in this study contain one or two HCH groups, and the high heritability of the HCH containing compounds in field samples supports that they may be emphasized in more challenging environments (Table 3). Lindroth et al. [14] found that tremulacin greatly reduced herbivore survival and performance and suggested that the benzoyl group synergizes the toxic effect of the HCH group. Similarly, the cinnamoyl and acetyl groups may also synergize the effects of the HCH moiety on relevant molecules (2′-cinnamoylsalicortin and 2′-acetylsalicortin).
Given the apparent stability of salicinoid profiles in P. tremula and the clearly defined chemotypes, the SwAsp collection represents an ideal system for the study of chemical-ecological interactions. With almost the entire collection in tissue culture, and with the present robust salicinoid profiling (Material S2), we can propagate our chemotypes to specifically test properties of resistance and tolerance to various kinds of associated herbivores and fungi [13], [45]. In conclusion, we observed a striking division of aspen into four chemotypes, some of which co-occur across latitudes. These chemotypes differ in the dominant moieties and future studies are needed to explore their relative bioactivity, especially including the new dominant compounds (2′-cinnamoylsalicortins and 2′-acetylsalicortin) that are poorly studied. We further suggest that ecological studies, both locally and across latitudes, must take P. tremula's salicinoid diversity into account.
Supporting Information
Salicinoid identification: Exact masses of 55 salicinoid compounds (Table S1), Literature references (List S1), UV spectra of nine new salicinoids found in the P. tremula foliage (Fig. S1 a), and high-resolution MS/MS (Fig. S1 b).
(DOCX)
Average percentages of 19 salicinoids from the foliage of different Populus tremula clones (Clone), grown in two different environments (Evir: GH = greenhouse, Sävar = field) and mg g−1 for field trees. Chemo = chemotype: CN = 2′-cinnamoyl, AC = 2′- acetyl, CN-AC = 2′-cinnamoyl/2′-acetyl, and TL = tremuloides-like. Salicinoids: 1 = salicortin, 2 = tremulacin, 3 = salicin, 4 = tremuloidin, 5 = HCH-salicortin, 6 = HCH-tremulacin, 7 = salicyloylsalicin, 8 = 6′-acetyl-tremulacin, 9 = 2′-(Z)-cinnamoylsalicortin, 10 = 2′-(E)- cinnamoylsalicortin, 11 = cinnamoylsalicin I1, 12 = cinnamoylsalicin I2, 13 = acetylcinnamoylsalicortin I1, 14 = acetylcinnamoylsalicortin I2, 15 = HCH-cinnamoylsalicortin I1, 16 = HCH-cinnamoylsalicortin I2, 17 = 2′-acetylsalicortin, 18 = 2′-acetylsalicin, 19 = lasiandrin (HCH-2'-acetylsalicortin). I1 and I2 = isomers 1 and 2, respectively.
(DOCX)
Acknowledgments
We thank the Carl Trygger Foundation for Scientific Research, the Swedish Foundation for Strategic Research, and the Swedish Research Foundation for funding. The staff of the Swedish Metabolomics Center (www.swedishmetabolomicscentre.se) was instrumental in the completion of chemical analyses for this project.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors thank the Carl Trygger Foundation for Scientific Research, the Swedish Foundation for Strategic Research, and the Swedish Research Foundation for funding. The staff of the Swedish Metabolomics Center (www.swedishmetabolomicscentre.se) was instrumental in the completion of chemical analyses for this project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lindroth RL, Hsia MTS, Scriber JM (1987) Characterization of phenolic glycosides from quaking aspen. Biochem Syst Ecol 15: 677–680. [Google Scholar]
- 2. Haikio E, Makkonen M, Julkunen-Tiitto R, Sitte J, Freiwald V, et al. (2009) Performance and secondary chemistry of two hybrid aspen (Populus tremula L. × Populus tremuloides Michx.) clones in long-term elevated ozone exposure. J Chem Ecol 35: 664–678. [DOI] [PubMed] [Google Scholar]
- 3. Boeckler GA, Gershenzon J, Unsicker SB (2011) Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 72: 1497–1509. [DOI] [PubMed] [Google Scholar]
- 4. Fritz RS, Hochwender CG, Lewkiewicz DA, Bothwell S, Orians CM (2001) Seedling herbivory by slugs in a willow hybrid system: developmental changes in damage, chemical defense, and plant performance. Oecologia 129: 87–97. [DOI] [PubMed] [Google Scholar]
- 5. Albrectsen BR, Gutierrez L, Fritz RS, Fritz RD, Orians CM (2007) Does the differential seedling mortality caused by slugs alter the foliar traits and subsequent susceptibility of hybrid willows to a generalist herbivore? Ecol Entomol 32: 211–220. [Google Scholar]
- 6.Constabel CP, Lindroth RL (2010) The impact of genomics on advances in herbivore defene and secondary metabolim in Populus In: S J, R B, A G, editors. Genetics and Genomics of Populus: Springer Verlag. pp.279–305.
- 7. Bailey JK, Schweitzer JA, Rehill BJ, Irschick DJ, Whitham TG, et al. (2007) Rapid shifts in the chemical composition of aspen forests: An introduced herbivore as an agent of natural selection. Biol Invasions 9: 715–722. [Google Scholar]
- 8. Wooley SC, Walker S, Vernon J, Lindroth RL (2008) Aspen decline, aspen chemistry, and elk herbivory: Are they linked? Society for Range Management 30: 17–21. [Google Scholar]
- 9. Lindroth RL, Scriber JM, Hsia MTS (1986) Differential responses of tiger swallowtail subspecies to secondary metabolites from tulip tree and quaking aspen. Oecologia 70: 13–19. [DOI] [PubMed] [Google Scholar]
- 10. Rank NE (1994) Host-plant effects on larval survival of a salicin-using leaf beetle Chrysomela aeneicollis Schaeffer (Coleoptera, Chrysomelidae). Oecologia 97: 342–353. [DOI] [PubMed] [Google Scholar]
- 11. Orians CM, Huang CH, Wild A, Dorfman KA, Zee P, et al. (1997) Willow hybridization differentially affects preference and performance of herbivorous beetles. Entomol Exp Appl 83: 285–294. [Google Scholar]
- 12. Hjältén J, Niemi L, Wennstrom A, Ericson L, Roininen H, et al. (2007) Variable responses of natural enemies to Salix triandra phenotypes with different secondary chemistry. Oikos 116: 751–758. [Google Scholar]
- 13. Albrectsen BR, Witzell J, Robinson KM, Wulff S, Luquez VMC, et al. (2010) Large scale geographic clines of parasite damage to Populus tremula . L. Ecography 33: 483–493. [Google Scholar]
- 14. Lindroth RL, Scriber JM, Hsia MTS (1988) Chemical ecology of the tiger swallowtail: mediation of host use by phenolic glycosides. Ecology 69: 814–822. [Google Scholar]
- 15. Chen F, Liu CJ, Tschaplinski TJ, Zhao N (2009) Genomics of secondary metabolism in Populus: interactions with biotic and abiotic environments. CRC Crit Rev Plant Sci 28: 375–392. [Google Scholar]
- 16. Tsai CJ, Harding SA, Tschaplinski TJ, Lindroth RL, Yuan YN (2006) Genome-wide analysis of the structural genes regulating defense phenylpropanoid metabolism in Populus . New Phytol 172: 47–62. [DOI] [PubMed] [Google Scholar]
- 17. Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546. [DOI] [PubMed] [Google Scholar]
- 18. Sterky F, Regan S, Karlsson J, Hertzberg M, Rohde A, et al. (1998) Gene discovery in the wood-forming tissues of poplar: Analysis of 5,692 expressed sequence tags. Proc Natl Acad Sci U S A 95: 13330–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, et al. (2003) Proanthocyanidin biosynthesis in plants - Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J Biol Chem 278: 31647–31656. [DOI] [PubMed] [Google Scholar]
- 20. Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins - a final frontier in flavonoid research? New Phytol 165: 9–28. [DOI] [PubMed] [Google Scholar]
- 21. Babst BA, Harding SA, Tsai CJ (2010) Biosynthesis of phenolic glycosides from phenylpropanoid and benzenoid precursors in Populus . J Chem Ecol 36: 286–297. [DOI] [PubMed] [Google Scholar]
- 22. Morse AM, Tschaplinski TJ, DerviniS C, Pijut PM, Schmelz EA, et al. (2007) Salicylate and catechol levels are maintained in nahG transgenic poplar. Phytochemistry 68: 2043–2052. [DOI] [PubMed] [Google Scholar]
- 23. Shelton AL (2000) Variable chemical defences in plants and their effects on herbivore behaviour. Evol Ecol Res 2: 231–249. [Google Scholar]
- 24. Shelton AL (2004) Variation in chemical defences of plants may improve the effectiveness of defence. Evol Ecol Res 6: 709–726. [Google Scholar]
- 25. Degenhardt J, Kollner TG, Gershenzon J (2009) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70: 1621–1637. [DOI] [PubMed] [Google Scholar]
- 26. Keeling CI, Bohlmann J (2006) Diterpene resin acids in conifers. Phytochemistry 67: 2415–2423. [DOI] [PubMed] [Google Scholar]
- 27. Palo RT (1984) Distribution of birch (Betula spp.), willow (Salix spp.), and poplar (Populus spp.) secondary metabolites and their potential role as chemical defense against herbivores. J Chem Ecol 10: 499–520. [DOI] [PubMed] [Google Scholar]
- 28. Lindroth RL, Hwang SY (1996) Clonal variation in foliar chemistry of quaking aspen (Populus tremuloides Michx). Biochem Syst Ecol 24: 357–364. [Google Scholar]
- 29. Abreu IN, Ahnlund M, Moritz T, Albrectsen BR (2011) UHPLC-ESI/TOFMS determination of salicylate-like phenolic glycosides in Populus tremula leaves. J Chem Ecol 37: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rehill B, Clauss A, Wieczorek L, Whitham T, Lindroth R (2005) Foliar phenolic glycosides from Populus fremontii, Populus angustifolia, and their hybrids. Biochem Syst Ecol 33: 125–131. [Google Scholar]
- 31. Orians CM, Fritz RS (1995) Secondary chemistry of hybrid and parental willows: phenolic glycosides and condensed tannins in Salix sericea, S. eriocephala, and their hybrids. J Chem Ecol 21: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 32. Orians CM, Griffiths ME, Roche BM, Fritz RS (2000) Phenolic glycosides and condensed tannins in Salix sericea, S. eriocephala and their F1 hybrids: not all hybrids are created equal. Biochem Syst Ecol 28: 619–632. [DOI] [PubMed] [Google Scholar]
- 33. Caseys C, Glauser G, Stölting KN, Christe C, Albrectsen BR, et al. (2012) Effects of interspecific recombination on functional traits in trees revealed by metabolomics and genotyping-by-resequencing. Plant Ecol Divers 5: 457–471. [Google Scholar]
- 34. Nichols-Orians CM, Clausen TP, Fritz RS, Reichardt PB, Wu JJ (1992) 2'-Cinnamoylsalicortin, a phenolic glycoside from Salix sericea . Phytochemistry 31: 2180–2181. [Google Scholar]
- 35. Ruuhola T, Julkunen-Tiitto R (2003) Trade-off between synthesis of salicylates and growth of micropropagated Salix pentandra . J Chem Ecol 29: 1565–1588. [DOI] [PubMed] [Google Scholar]
- 36. Luquez V, Hall D, Albrectsen BR, Karlsson J, Ingvarsson P, et al. (2008) Natural phenological variation in aspen (Populus tremula): the SwAsp collection. Tree Genet. Genomes 4: 279–292. [Google Scholar]
- 37. Audette RCS, Blunden G, Steele JW, Wong CSC (1966) Thin-layer chromatography of phenolic glycosides and its use as a screening procedure for genus Salix . J Chrom 25: 367–&. [Google Scholar]
- 38. Binns WW, Blunden G, Woods DL (1968) Distribution of leucoanthocyanidins phenolic glycosides and imino-acids in leaves of Salix species. Phytochemistry 7: 1577–1581. [Google Scholar]
- 39. Keefover-Ring K, Carlsson M, Albrectsen BR (2014) 2′-(Z)-Cinnamoylsalicortin: A novel salicinoid isolated from Populus tremula . Phytochem Lett 7: 212–216. [Google Scholar]
- 40. de Carvalho D, Ingvarsson PK, Joseph J, Suter L, Sedivy C, et al. (2010) Admixture facilitates adaptation from standing variation in the European aspen (Populus tremula L.), a widespread forest tree. Mol Ecol 19: 1638–1650. [DOI] [PubMed] [Google Scholar]
- 41. Robinson KM, Ingvarsson PK, Jansson S, Albrectsen BR (2012) Genetic variation in functional traits influences arthropod community composition in aspen (Populus tremula L.). PloS One 7: e37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paunonen R, Julkunen-Tiitto R, Tegelberg R, Rousi M, Heiska S (2009) Salicylate and biomass yield, and leaf phenolics of dark-leaved willow (Salix myrsinifolia Salisb.) clones under different cultivation methods after the second cultivation cycle. Ind Crops Prod 29: 261–268. [Google Scholar]
- 43.Umetrics (2012) SIMCA-P+ v. 12.0. Umeå, Sweden.
- 44.SAS Institute (2003) SAS version 9.1. Cary, N.C.: SAS Institute.
- 45.R Development Core Team (2013) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 46. Lindroth RL, Hsia MTS, Scriber JM (1987) Seasonal patterns in the phytochemistry of three Populus species. Biochem Syst Ecol 15: 681–686. [Google Scholar]
- 47. Reichardt PB, Merken HM, Clausen TP, Wu JJ (1992) Phenolic glycosides from Salix lasiandra . J Nat Prod 55: 970–973. [Google Scholar]
- 48. Ruuhola T, Julkunen-Tiitto R, Vainiotalo P (2003) In vitro degradation of willow salicylates. J Chem Ecol 29: 1083–1097. [DOI] [PubMed] [Google Scholar]
- 49. Tegelberg R, Julkunen-Tiitto R (2001) Quantitative changes in secondary metabolites of dark-leaved willow (Salix myrsinifolia) exposed to enhanced ultraviolet-B radiation. Physiol Plant 113: 541–547. [Google Scholar]
- 50. Bernhardsson C, Ingvarsson PK (2012) Geographical structure and adaptive population differentiation in herbivore defence genes in European aspen (Populus tremula L., Salicaceae). Mol Ecol 21: 2197–2207. [DOI] [PubMed] [Google Scholar]
- 51. Hewitt GM (1999) Post-glacial re-colonization of European biota. Biol J Linnean Soc 68: 87–112. [Google Scholar]
- 52. Hall D, Luquez V, Garcia VM, St Onge KR, Jansson S, et al. (2007) Adaptive population differentiation in phenology across a latitudinal gradient in European Aspen (Populus tremula, L.): A comparison of neutral markers, candidate genes and phenotypic traits. Evolution 61: 2849–2860. [DOI] [PubMed] [Google Scholar]
- 53. Arnold ML (1992) Natural hybridization as an evolutionary process. Annu Rev Ecol Syst 23: 237–261. [Google Scholar]
- 54. Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM (1996) Role of gene interactions in hybrid speciation: Evidence from ancient and experimental hybrids. Science 272: 741–745. [DOI] [PubMed] [Google Scholar]
- 55. Ingvarsson PK, Street NR (2011) Association genetics of complex traits in plants. New Phytol 189: 909–922. [DOI] [PubMed] [Google Scholar]
- 56. Lindroth RL, Hwang SY, Osier TL (1999) Phytochemical variation in quaking aspen: Effects on gypsy moth susceptibility to nuclear polyhedrosis virus. J Chem Ecol 25: 1331–1341. [Google Scholar]
- 57. Reichardt PB, Bryant JP, Mattes BR, Clausen TP, Chapin FS, et al. (1990) Winter chemical defense of Alaskan balsam poplar against snowshoe hares. J Chem Ecol 16: 1941–1959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Salicinoid identification: Exact masses of 55 salicinoid compounds (Table S1), Literature references (List S1), UV spectra of nine new salicinoids found in the P. tremula foliage (Fig. S1 a), and high-resolution MS/MS (Fig. S1 b).
(DOCX)
Average percentages of 19 salicinoids from the foliage of different Populus tremula clones (Clone), grown in two different environments (Evir: GH = greenhouse, Sävar = field) and mg g−1 for field trees. Chemo = chemotype: CN = 2′-cinnamoyl, AC = 2′- acetyl, CN-AC = 2′-cinnamoyl/2′-acetyl, and TL = tremuloides-like. Salicinoids: 1 = salicortin, 2 = tremulacin, 3 = salicin, 4 = tremuloidin, 5 = HCH-salicortin, 6 = HCH-tremulacin, 7 = salicyloylsalicin, 8 = 6′-acetyl-tremulacin, 9 = 2′-(Z)-cinnamoylsalicortin, 10 = 2′-(E)- cinnamoylsalicortin, 11 = cinnamoylsalicin I1, 12 = cinnamoylsalicin I2, 13 = acetylcinnamoylsalicortin I1, 14 = acetylcinnamoylsalicortin I2, 15 = HCH-cinnamoylsalicortin I1, 16 = HCH-cinnamoylsalicortin I2, 17 = 2′-acetylsalicortin, 18 = 2′-acetylsalicin, 19 = lasiandrin (HCH-2'-acetylsalicortin). I1 and I2 = isomers 1 and 2, respectively.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.