Abstract
Background
Schistosomiasis is a debilitating neglected tropical disease that infects over 200 million people worldwide. To combat this disease, in 2012, the World Health Organization announced a goal of reducing and eliminating transmission of schistosomes. Current control focuses primarily on mass drug administration (MDA). Therefore, we monitored transmission of Schistosoma mansoni via fecal egg counts and genetic markers in a typical school based MDA setting to ascertain the actual impacts of MDA on the targeted schistosome population.
Methods
For 4 years, we followed 67 children enrolled in a MDA program in Kenya. Infection status and egg counts were measured each year prior to treatment. For 15 of these children, for which there was no evidence of acquired resistance, meaning they became re-infected following each treatment, we collected microsatellite genotype data from schistosomes passed in fecal samples as a representation of the force of transmission between drug treatments. We genotyped a total of 4938 parasites from these children, with an average of 329.2 parasites per child for the entire study, and an average of 82.3 parasites per child per annual examination. We compared prevalence, egg counts, and genetic measures including allelic richness, gene diversity (expected heterozygosity), adult worm burdens and effective number of breeders among time points to search for evidence for a change in transmission or schistosome populations during the MDA program.
Findings
We found no evidence of reduced transmission or schistosome population decline over the course of the program. Although prevalence declined in the 67 children as it did in the overall program, reinfection rates were high, and for the 15 children studied in detail, schistosome egg counts and estimated adult worm burdens did not decline between years 1 and 4, and genetic diversity increased over the course of drug treatment.
Interpretation
School based control programs undoubtedly improve the health of individuals; however, our data show that in an endemic area, such a program has had no obvious effect on reducing transmission or of significantly impacting the schistosome population as sampled by the children we studied in depth. Results like these, in combination with other sources of information, suggest more integrated approaches for interrupting transmission and significantly diminishing schistosome populations will be required to achieve sustainable control.
Author Summary
Schistosomiasis is a chronic and debilitating disease. Current control focuses primarily on mass drug administration (MDA). We monitored schistosome transmission via fecal egg counts and genetic markers in a school based MDA setting to learn how the intervention was influencing transmission or the targeted schistosome population. For 4 years, we followed 67 children enrolled in a MDA program in Kenya, and for 15 of these, we repeatedly acquired in depth genetic data regarding the schistosome populations they harbored. Although prevalence declined in the 67 children, we found no evidence of reduced egg counts/worm burdens in those that reacquired infections and genetic diversity of schistosomes increased over the course of treatment. Our data indicate that this school based MDA program had a strong benefit to individual health as fewer children were infected over time. However, this decline does not appear to be due to schistosome population reduction, and may be caused by either acquired resistance or behavioral changes of the children. In conclusion, control programs based on chemotherapy alone or based on only a subset of the population will need to be supplemented with additional approaches if we are to achieve the WHO goal of eliminating human schistosomiasis by 2025.
Introduction
Recently, there has been increased awareness of the massive global health burden of schistosomiasis and other neglected tropical diseases (NTDs). Notably, in 2001, Resolution 19 of the World Health Assembly called for increased drug treatment of NTDs to reach a minimum coverage of 75% of school aged children in endemic areas (WHA 54.19). Thus, the initiation of multiple mass drug administration (MDA) programs was prompted [1]. School aged children have been specifically targeted because they are suspected to harbor the heaviest worm burdens and thus experience a high degree of morbidity from infection. Additionally, school systems provide infrastructure within which such programs can successfully operate, a key consideration for lowering the cost of drug administration.
Traditionally, the primary goal of MDA programs has been to reduce worm burdens in individuals and thus reduce the morbidity caused by NTDs. However, a revised roadmap published by the World Health Organization in 2012 reaches further: toward the elimination of NTD transmission in some regions [2]. To a large part, this goal is being facilitated by a major increase of pharmaceutical donations to expand coverage of MDA. With this goal in mind, it is important to determine if current deworming programs are moving toward elimination by reducing schistosome transmission in endemic regions where MDA programs are ongoing. This knowledge is critical to inform control protocols to achieve the WHO goals.
It is challenging to determine if drug treatment of a focal group of patients is reducing the entire schistosome population in a region (especially so for worms in untreated people) and thus the force of infection. For schistosomes, the force of infection has been defined as the rate of establishment of patent infections. Ideally, one would treat all infected people; however, this is often not logistically possible. Population genetic estimates indicate that schistosome populations are large and widely distributed, not only geographically but also among snails, human hosts of a wide range of ages and reservoir hosts [3]–[6]. Thus, most MDA programs treat a portion of people infected with schistosomiasis, exposing only a portion of the schistosome population to drug treatment while the rest of the population remains in “refugia”, isolated from drug exposure. Because the goal of most deworming programs is to reduce worm burdens in individuals and thus morbidity (rather than elimination or reducing the force of infection), success is typically assessed by comparing infection intensity (worm burden estimated by fecal egg counts) and prevalence of infection in the treated portion of the population from before treatment to some time point afterwards. It is difficult to use these data to assess the reduction of the larger schistosome population for two reasons. First, infection starts in infancy and schistosomes are long lived (5–10 years) [7]–[9], thus pre-mass drug treatment levels of infection reflect long term accumulation of worms and should be higher than recolonization levels after treatment clearance [10]. Second, it has been shown that a large portion of individuals treated for schistosomiasis acquires partial resistance to reinfection that is measurable within 2–3 drug treatments [11]–[15]. Thus, a decrease in schistosome burdens in the treated portion of the population is expected, and this decrease may not reflect an actual decrease in the entire schistosome population or in the force of infection. In fact, it is possible that the force of infection could actually be increasing even when worm burdens of the treated individuals are declining due to acquired resistance in the treated population.
One way to monitor the change in schistosome populations is to monitor those treated individuals who do not acquire resistance to reinfection after repeated treatments (i.e. those that remain susceptible). Such individuals were termed “phenotypically susceptible” by Black et al. 2010 [11] because they failed to produce protective immunity after repeated drug treatment. Assuming comparisons are not made with pre-control baselines, treatment is successful, and prior worm burdens are cleared, the worm populations acquired by patients after treatment can be measured by the number of schistosome eggs released in a fecal sample via the Kato-Katz methodology [16], [17], especially when coupled with genetic data on miracidia from these eggs. Genetic data can provide an additional and powerful perspective because they can be used to estimate worm burdens within an individual ([i.e. number of breeding pairs represented by sibling groups 18]), to detect overall population declines (i.e. population bottlenecks [19], [20]), and measure genetic diversity which is a measure of the parasite's ability to adapt to environmental pressures [21].
Some attempts have been made to assess changes in schistosome populations following drug treatment. Norton et al. [21]. monitored changes in microsatellite populations of Schistosoma mansoni miracidia (n = 20 per child) derived from Tanzanian school children following a single round of treatment and found a significant decline in genetic diversity [21]. They noted this was true even for young children entering the school who had not been treated previously. Following the fate of infections in individual hosts as an important indicator of efficacy of control was also suggested. Using cytochrome c oxidase I as a marker, Betson et al. [22] found substantial genetic diversity of S. mansoni within both children less than six years old and their mothers (they examined 1347 parasites from 35 mothers and 45 children) [22]. They did not observe any change in schistosome genetic diversity before or six months after treatment in samples collected from the same individual or from the same family. The authors noted that even exposure times of 1.5 years were sufficient to result in genetically diverse infections in young children.
Within the context of a typical school-based MDA program in central Kenya targeting S. mansoni for control on an annual basis, we took an approach that emphasized a deeper sampling of miracidia within individual children, and that began with examination of children for four years following treatment. Deeper sampling enables the calculation of novel measurements of worm burdens within a patient and also enables genetic diversity to be more accurately measured because the bias that is introduced by including related parasites in the sample can be reduced (see Steinauer et al. [23]). We used both fecal egg counts and genetic measures to assess changes in phenotypically susceptible children over a 4 year period (2008–2011).
Materials and Methods
Ethical Statement
This protocol was approved by both the Kenya Medical Research Institute (KEMRI) Scientific committee and the KEMRI Ethical committee. Parents/guardians of all children involved provided written informed consent on behalf of all child participants. Informed consent was obtained by first holding a meeting with the parents/guardian to explain the purpose of study followed by a question and answer session. Thereafter, they were requested to sign the written consent forms on behalf of the children.
Sample collection
A school-based schistosomiasis and soil-transmitted helminth (STH) control project in Mwea, Kenya was established in 2004 through a collaboration between the Japan International Cooperation Agency (JICA) and the Kenya Medical Research Institute (KEMRI). Mwea is a large rice growing region in the Kirinyaga County, central Kenya. The Thiba and Nyamindi Rivers that pass through this area serve as source of water for the irrigation schemes, and schistosomiasis transmission is believed to take place primarily from the irrigation canals that supply water to the rice schemes. A pilot study in 2004 measured prevalence of helminths in school aged children followed by MDA (praziquantel and albendazole) [24]. At this time, the human population of the area was estimated to be 125,000 and the prevalence of Schistosoma mansoni in school aged children was 47.4%.
Starting in 2004, the KEMRI-JICA Project administered annual doses of anthelmintics to all school aged children (>40,000) in the region regardless of their infection status. This treatment included a single dose of 40 mg/kg of praziquantel using the tablet dose pole to determine the number of tablets [25] and albendazole in a 400 mg single dose. Prior to the beginning of the program a baseline determination of prevalence and intensity of parasitic infections through examination of stool samples of class three children (age range 5–14 years) was undertaken [26]. Our study recruited the subjects by randomly sampling among the children who had previously tested positive within this cohort. We focused on infected individuals because we were seeking those that were not acquiring resistance to reinfection with repeated drug treatment. Starting in 2008, we followed 67 students previously enrolled in the KEMRI-JICA Project. These students were between 5 and 14 years of age and were from four primary schools: Kirogo (00°39S/37°23E), Nyamindi (00°40 S/37°24E), Mukou (00°40S/37°20E) and Ngurubani (00°41S/37°21E). The schools are less than 8 km apart ([figure in 3]). Prior to each treatment, fecal samples were obtained from three consecutive days and infection was assessed using the Kato Katz methodology [16], [17]. Following each treatment, there was a three month follow-up stool exam for each child, and each child was found to be negative (treatment was successful in every case). For the Kato-Katz procedure, two slides per patient were examined for schistosome eggs.
To assess population level reduction in schistosome transmission, we collected genetic data from the schistosome infrapopulations of 15 of the 67 children. These 15 children were deemed phenotypically susceptible throughout the four year time period because they became reinfected at every time point with large worm burdens (as determined by egg counts) indicating high susceptibility of these children. More of the 67 children also fell into the phenotypically susceptible category, but we only compiled genetic data from the 15 children from whom we were able to collect samples at each sampling period. Miracidia were collected from their fecal samples and used for microsatellite genotyping. Schistosome miracidia were hatched from fecal samples [27] and individuals were genotyped at 12 microsatellite loci [28] and included GenBank accession numbers: AF325695, AF325698, AF202965, AF202966, AF202968, L46951, M85305, R95529, AF202968, AI395184, AI067617 and BF936409.
Data Analysis
We compared reductions in fecal egg counts in the 15 phenotypically susceptible children and in the other 52 children over the 4 time points (annual samples prior to MDA 2008–2011) using a repeated measures ANOVA with Systat 11 (Systat Software, Inc.). Data were natural log transformed to meet the assumptions of parametric statistical tests. Single degree of freedom polynomial contrasts were used to determine the significance of trends in the data over time. The hypothesis was that a reduction in the force of infection of schistosomiasis would be matched with a reduction in fecal egg counts in all children and in the 15 phenotypically susceptible children.
We also compared population genetic parameters across the four time points at two levels: populations within each patient (infrapopulations) ([e.g. 21]) and all patients combined (component population) ([e.g. 10]). We hypothesized that significant reductions in the schistosome population should be accompanied by reductions in all of these genetic parameters over the four year time period.
For the infrapopulation level analysis, we first compared two estimators of the worm burdens within a patient: the number of full sibling families, and the effective number of breeders (Nb). These parameters were compared across all time points using repeated measures ANOVA for each dependent variable. The number of full sibling families is a measure of the number of breeding worms within a patient (worm burden) because the miracidia collected in a fecal sample are offspring of the adult worms inside the patient. The offspring were partitioned into their families based on shared alleles using COLONY v.2.0 [29], [30]. COLONY has been shown to accurately reproduce schistosome families using genotype data at the same microsatellite loci [18]. Nb was estimated using the sibling assignment method implemented in COLONY [31]. Nb is the effective population size Ne measured from a single breeding cohort (miracidia derived from a single patient).
We also compared two measures of population genetic diversity: allelic richness and gene diversity (expected heterozygosity). We corrected our datasets for the bias induced by related miracidia in a fecal sample by inferring the family structure present in a sample, and then resampling the dataset to include only one member of each family [18], [32]. Ten resampled datasets were generated and both parameters were calculated using FSTAT 2.9.3 [33]. Parameters were natural log transformed and compared across patients between the first and last sample using repeated measures ANOVA (Systat 11, Systat Software, Inc.). One patient was removed from the analysis due to high family structure and resulting low sample size in the resampled datasets (n = 6 unrelated individuals remaining after correction, thus all individuals sampled fell into one of 6 families).
At the component population level (all patients combined within a year), we used permutation tests on the 10 corrected datasets to determine significant differences in gene diversity and allelic richness between year 1 and year 4 of the MDA program. These tests were performed with FSTAT and 10,000 permutations were used to determine significance.
Results
Table 1 provides a summary of the 67 children investigated, and includes their gender, age at baseline, and pre-treatment egg counts (average eggs per gram of feces determined by Kato-Katz) at each of the four annual treatment periods. Table 2 indicates for the 15 phenotypically susceptible children investigated in depth, for each annual observation, the number of miracidia genotyped (N), the effective number of breeders (Nb), and the schistosome census number (Nc). We genotyped a total of 4938 parasites from these children, with an average of 329.2 parasites per child for the entire study, and an average of 82.3 parasites per child per annual examination.
Table 1. A summary of the demographic characteristics of 67 children investigated including their gender, age at baseline, and pre-treatment egg counts (average eggs per gram of feces determined by Kato-Katz) at each of the four annual treatment periods.
| Sample ID | School | Gender | Age at baseline | Year 1 | Year 2 | Year 3 | Year 4 |
| (EPG) | (EPG) | (EPG) | (EPG) | ||||
| TB2* | Nyamindi | M | 6 | 4320 | 6240 | 624 | 6144 |
| MK10* | Nyamindi | M | 6 | 1008 | 864 | 1656 | 2976 |
| TB9* | Nyamindi | M | 6 | 480 | 2784 | 1656 | 1296 |
| TB4* | Nyamindi | F | 6 | 9600 | 1512 | 2304 | 8448 |
| NG46 | Nyamindi | F | 7 | 48 | 96 | 288 | 0 |
| KB35 | Nyamindi | F | 7 | 72 | 48 | 192 | 96 |
| KB81 | Nyamindi | F | 8 | 72 | 0 | 48 | 0 |
| NG84 | Nyamindi | F | 8 | 69 | 26 | 48 | 3 |
| KR1 | Nyamindi | M | 8 | 72 | 2256 | 96 | 24 |
| NG126 | Nyamindi | M | 8 | 192 | 648 | 0 | 0 |
| NG11 | Nyamindi | F | 9 | 312 | 288 | 0 | 336 |
| NY67 | Nyamindi | F | 9 | 912 | 120 | 0 | 0 |
| NY77 | Nyamindi | M | 11 | 24 | 0 | 0 | 24 |
| TB15* | Kirogo | M | 6 | 2880 | 2304 | 168 | 1560 |
| TB120* | Kirogo | F | 6 | 9600 | 1512 | 96 | 600 |
| TB6* | Kirogo | M | 6 | 2400 | 432 | 216 | 2304 |
| TB132* | Kirogo | F | 6 | 960 | 1008 | 336 | 792 |
| KB18 | Kirogo | M | 7 | 432 | 0 | 168 | 96 |
| NY06 | Kirogo | F | 7 | 72 | 2256 | 768 | 96 |
| NY33 | Kirogo | F | 7 | 24 | 240 | 2256 | 0 |
| MU1 | Kirogo | M | 7 | 2664 | 336 | 336 | 0 |
| KB13 | Kirogo | F | 7 | 2784 | 0 | 336 | 0 |
| NY27 | Kirogo | F | 7 | 48 | 0 | 240 | 24 |
| NG24 | Kirogo | F | 7 | 1032 | 336 | 408 | 120 |
| NY68 | Kirogo | F | 8 | 864 | 120 | 144 | 120 |
| NY60 | Kirogo | F | 8 | 240 | 42 | 120 | 216 |
| KB19 | Kirogo | F | 9 | 0 | 120 | 0 | 0 |
| NG118 | Kirogo | F | 9 | 4992 | 168 | 0 | 24 |
| NY14 | Kirogo | F | 10 | 288 | 48 | 0 | 264 |
| KB18 | Kirogo | M | 11 | 24 | 0 | 0 | 48 |
| TB10* | Ngurubani | F | 6 | 480 | 192 | 336 | 288 |
| TB19* | Ngurubani | M | 6 | 8160 | 7776 | 432 | 768 |
| TB3* | Ngurubani | F | 6 | 1440 | 1344 | 4320 | 6096 |
| NG77 | Ngurubani | F | 7 | 72 | 240 | 432 | 0 |
| NG116 | Ngurubani | F | 8 | 144 | 432 | 0 | 432 |
| NY76 | Ngurubani | M | 8 | 120 | 768 | 0 | 648 |
| NG39 | Ngurubani | F | 8 | 72 | 24 | 48 | 0 |
| NG74 | Ngurubani | F | 8 | 336 | 216 | 26 | 0 |
| NY49 | Ngurubani | F | 9 | 264 | 288 | 0 | 72 |
| KB21 | Ngurubani | M | 9 | 0 | 360 | 0 | 0 |
| NY66 | Ngurubani | M | 10 | 312 | 0 | 0 | 0 |
| NG71 | Ngurubani | F | 10 | 1872 | 0 | 0 | 48 |
| NG110 | Ngurubani | M | 10 | 192 | 48 | 0 | 408 |
| NY44 | Ngurubani | M | 11 | 96 | 0 | 0 | 0 |
| NG109 | Mukou | F | 6 | 72 | 240 | 168 | 1128 |
| TB130* | Mukou | M | 6 | 2400 | 7680 | 192 | 2328 |
| TB14* | Mukou | M | 6 | 2880 | 432 | 264 | 2040 |
| MK4* | Mukou | F | 6 | 1128 | 2352 | 2304 | 3024 |
| MK12* | Mukou | F | 6 | 4320 | 6360 | 2304 | 1320 |
| NG41 | Mukou | M | 7 | 264 | 0 | 168 | 0 |
| NY112 | Mukou | F | 7 | 369 | 0 | 2784 | 48 |
| M51 | Mukou | F | 7 | 24 | 432 | 1008 | 168 |
| NG11 | Mukou | M | 7 | 312 | 192 | 384 | 24 |
| NY39 | Mukou | M | 7 | 1128 | 0 | 432 | 384 |
| NG33 | Mukou | F | 7 | 144 | 360 | 264 | 216 |
| NG45 | Mukou | F | 7 | 384 | 48 | 192 | 240 |
| KB67 | Mukou | M | 8 | 24 | 48 | 24 | 0 |
| KR56 | Mukou | F | 8 | 264 | 0 | 48 | 0 |
| NY15 | Mukou | M | 8 | 72 | 24 | 72 | 144 |
| NY78 | Mukou | F | 8 | 528 | 144 | 72 | 0 |
| NY34 | Mukou | F | 8 | 336 | 72 | 96 | 120 |
| NY35 | Mukou | F | 8 | 864 | 384 | 120 | 672 |
| NG128 | Mukou | F | 9 | 48 | 96 | 0 | 24 |
| NY39 | Mukou | M | 9 | 48 | 288 | 0 | 0 |
| NY45 | Mukou | F | 9 | 48 | 408 | 0 | 0 |
| KR38 | Mukou | M | 10 | 168 | 0 | 0 | 0 |
| NY17 | Mukou | F | 10 | 384 | 0 | 0 | 1872 |
Table 2. Temporal estimates of schistosome burdens of fifteen school aged children enrolled in a mass drug administration program in which they are treated annually.
| Year 1 | Year 2 | Year 3 | Year 4 | |||||||||||||
| Patient | n | Nb | CI | Nc | n | Nb | CI | Nc | n | Nb | CI | Nc | n | Nb | CI | Nc |
| 1 | 96 | 172 | 130–233 | 344–1720 | 81 | 20 | 12–38 | 40–200 | 81 | 158 | 117–220 | 316–1580 | 103 | 210 | 160–285 | 420–2100 |
| 2 | 89 | 151 | 114–206 | 302–1510 | 68 | 127 | 92–182 | 254–1270 | 81 | 120 | 89–168 | 240–1200 | 76 | 97 | 72–137 | 194–970 |
| 3 | 73 | 99 | 72–142 | 198–990 | 86 | 149 | 112–203 | 298–1490 | 86 | 52 | 36–77 | 104–520 | 81 | 209 | 149–301 | 418–2090 |
| 4 | 68 | 142 | 103–205 | 284–1420 | 73 | 195 | 142–289 | 390–1950 | 76 | 154 | 108–220 | 308–1540 | 107 | 103 | 75–141 | 206–1030 |
| 5 | 144 | 160 | 125–206 | 320–1600 | 81 | 24 | 14–43 | 48–240 | 103 | 118 | 88–160 | 236–1180 | 63 | 122 | 87–175 | 244–1220 |
| 6 | 128 | 153 | 120–199 | 306–1530 | 150 | 246 | 195–316 | 492–2460 | 139 | 256 | 201–334 | 512–2560 | 78 | 98 | 71–140 | 196–980 |
| 7 | 58 | 81 | 56–118 | 162–810 | 57 | 67 | 46–100 | 134–670 | 83 | 28 | 17–49 | 56–280 | 60 | 107 | 76–160 | 214–1070 |
| 8 | 68 | 130 | 93–186 | 260–1300 | 60 | 136 | 95–200 | 272–1360 | 53 | 172 | 113–298 | 344–1720 | 85 | 117 | 88–161 | 234–1170 |
| 9 | 58 | 75 | 51–112 | 150–750 | 68 | 114 | 83–162 | 228–1140 | 92 | 127 | 94–172 | 254–1270 | 70 | 186 | 135–276 | 372–1860 |
| 10 | 87 | 166 | 125–230 | 332–1660 | 81 | 108 | 78–149 | 216–1080 | 45 | 32 | 20–54 | 64–320 | 74 | 164 | 116–239 | 328–1640 |
| 11 | 57 | 8 | 4–23 | 16–80 | 87 | 247 | 183–346 | 494–2470 | 46 | 53 | 34–83 | 106–530 | 147 | 47 | 32–72 | 94–470 |
| 12 | 74 | 115 | 83–159 | 230–1150 | 86 | 174 | 129–246 | 348–1740 | 45 | 132 | 84–253 | 264–1320 | 118 | 251 | 195–331 | 502–2510 |
| 13 | 73 | 125 | 90–174 | 250–1250 | 105 | 243 | 186–327 | 486–2430 | 90 | 195 | 144–270 | 390–1950 | 87 | 187 | 138–256 | 374–1870 |
| 14 | 55 | 90 | 61–132 | 180–900 | 70 | 179 | 129–278 | 358–1790 | 67 | 123 | 88–178 | 246–1230 | 81 | 80 | 56–115 | 160–800 |
| 15 | 87 | 123 | 92–169 | 246–1230 | 81 | 175 | 128–240 | 350–1750 | 77 | 202 | 148–293 | 404–2020 | 92 | 140 | 104–189 | 280–1400 |
For each patient, the number of miracidia genotyped (n), the estimated effective number of breeders (Nb) and 95% confidence interval (CI) are given. For each patient, the schistosome census size (Nc) is estimated using an Nb/Nc ratio of 0.1 to 0.5.
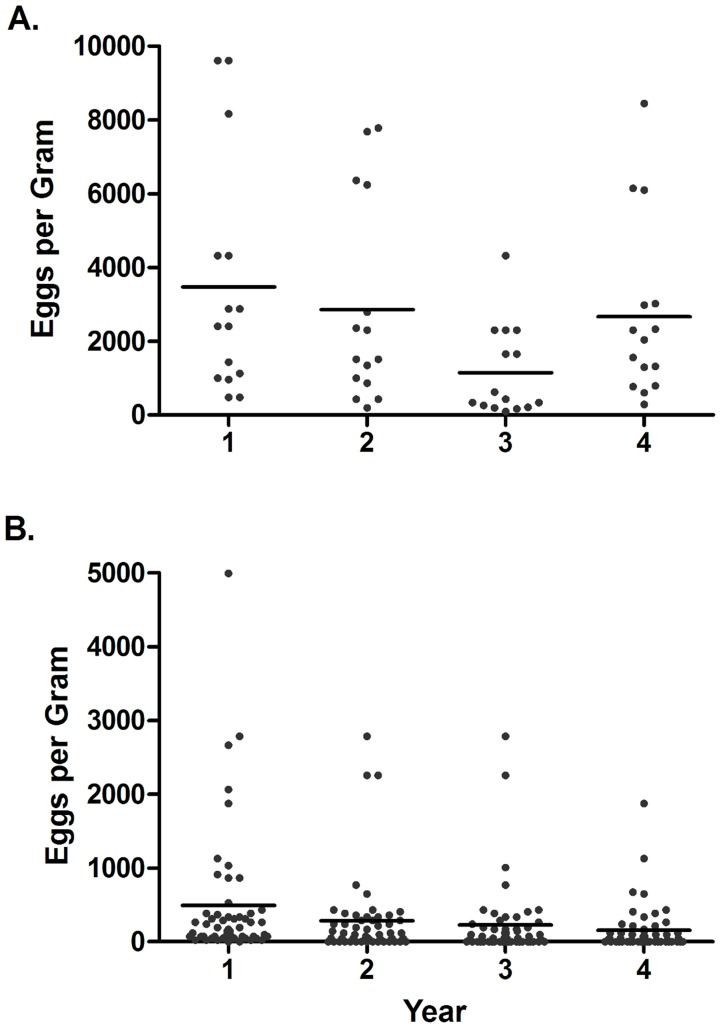
Repeated measures ANOVA of the 15 phenotypically susceptible children indicated that egg counts were significantly different among years (F3,42 = 5·662, P = 0·002) (Fig. 1A). Prevalence of infection in all 67 patients declined significantly from 97% at year one level, to 77.6% in year 2, and 68.7% in years 3 and 4 (Fisher exact test Yr1 v. Yr 4, P = 0·0001) (Fig. 1B). After the first year, three individuals were not found infected in any subsequent years, and three additional children remained negative for the remainder of the study after treatment in the second year. Polynomial contrasts indicated significant second and third degree trends (quadratic and cubic) (F1,14 = 8·012, P = 0·012, F1,14 = 7·014, P = 0·014), but not a significant linear trend (first degree) (F1,14 = 1·183, P = 0·183) (Fig. 2A). The trend was a decline in egg counts through years 1, 2, and 3 and then an incline back to original levels in year 4. Egg counts in the remaining 52 children also changed significantly over time (F3,153 = 9·89, P<0.001) (Fig. 2B) with a significant linear trend (F1,51 = 39·562, P<0.001), but not quadratic or cubic trends (F1,51 = 1·8, P = 0·176; F1,51 = 0·005, P = 0·946). The linear trend was a decline over the four years. This change appeared to be driven by an increase in uninfected individuals (egg counts of 0) rather than a decrease in egg counts of infected individuals, as indicated by the decline in prevalence) because a one-way ANOVA using only values from infected individuals indicated no significant differences between years (F3,145 = 1·019, P = 0·604). Thus, fewer children were infected; however, those that became reinfected did not have significantly lower burdens.
Figure 1. Mean number of schistosome eggs per gram of feces in patients for four years during annual mass praziquantel administration to school aged children.
A. 15 patients deemed “phenotypically susceptible” to schistosomiasis during MDA from which genetic samples were collected. Differences were due to a decrease in prevalence rather than a reduction in egg counts in infected individuals B. 52 randomly sampled children. Note the difference in scale of the Y-axis of both figures as egg burdens were much higher in the phenotypically susceptible group.
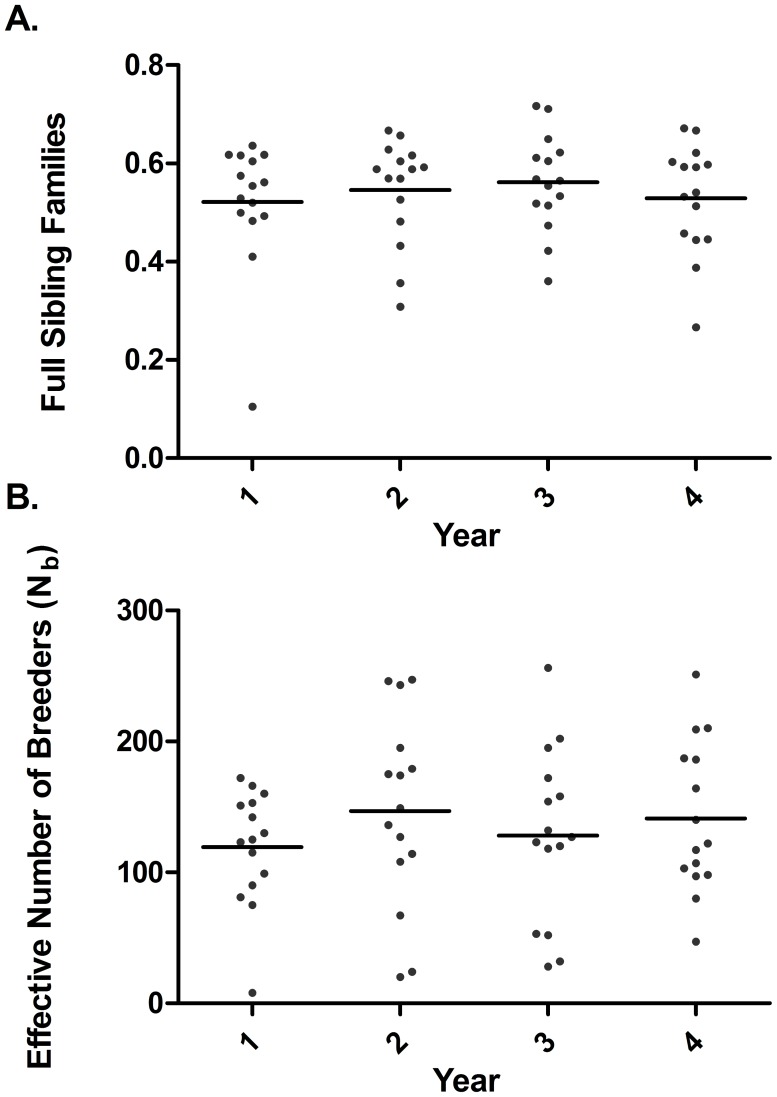
Figure 2. Mean changes in genetic estimators of schistosome worm burdens collected from humans during four years of an annual mass treatment program.
Lines indicate means. A. number of full sibling families standardized according to sample size. B. effective number of breeders as estimated using the sibling assignment method.
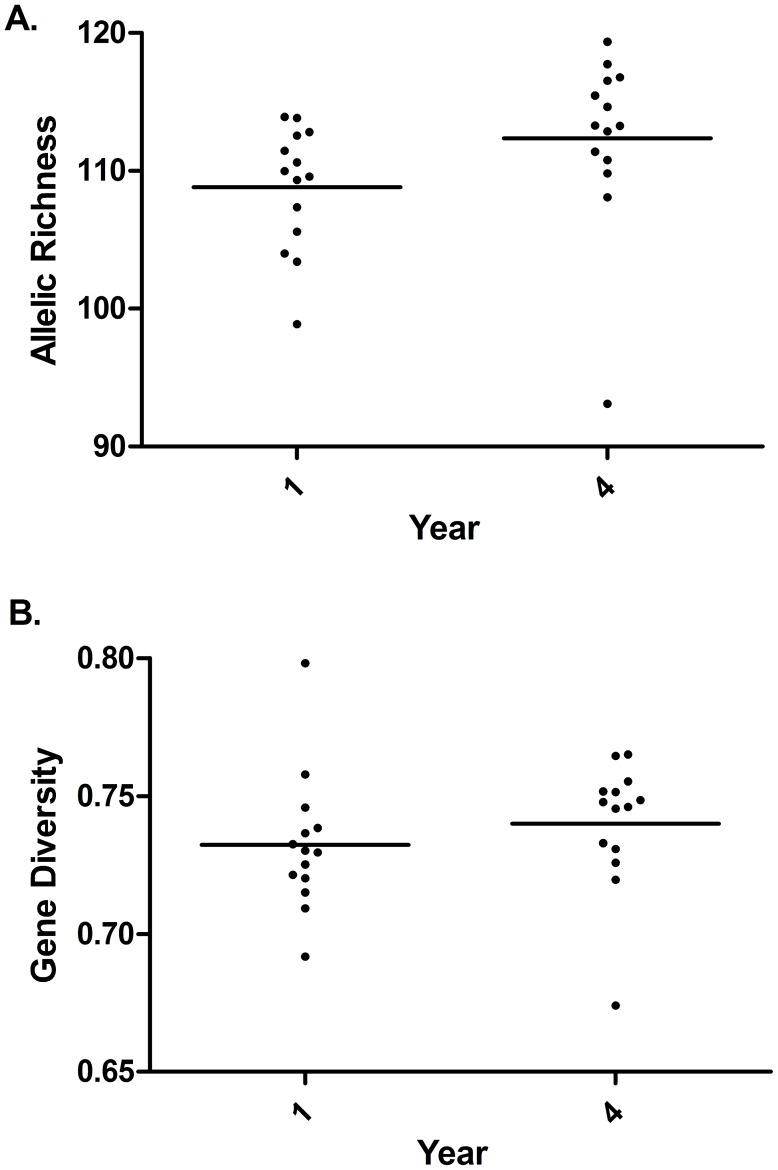
The number of full sibling families and effective number of breeders did not show statistically significant change over time (full sibling families: F3,42 = 0·397, P = 0·756; Nb: 0·757, P = 0·757) (Figure 3). Allelic richness and gene diversity significantly increased over time (allelic richness: F1,126 = 416·2, P<0.0001; gene diversity: F1,126 = 123·5; P<0.0001). Significant interactions were detected between patient and time for both parameters (allelic richness: F13,126 = 159·5; P<0.0001; gene diversity: F13,126 = 159·5; P<0.0001) indicating that for some patients, these values decreased over time.
Figure 3. Mean changes in genetic diversity of schistosomes collected from humans before and after four years of an annual mass treatment program.
Lines indicate means. Data are corrected for sibling structure. A. allelic richness B. gene diversity.
Discussion
The findings of our study highlight the ability of schistosomes to remain stubbornly entrenched in endemic areas despite school based MDA. Over a period of four years, we found a reduction in the number of children infected with schistosomiasis, a primary goal of the JICA/KEMRI program. This program has undoubtedly reduced or prevented severe morbidity caused by schistosome infection in children. However, we have found no evidence of an overall reduction of schistosome transmission in the region either by monitoring worm burdens via either egg counts or using genetic parameters to monitor population changes. We saw changes in egg counts in our 15 phenotypically susceptible patients over time, but this change was a decline in years 1–3, but one that was followed by an increase back to initial levels in year 4. Also, in the other 52 children, although some did not become reinfected at some of the sampling times which is certainly a desired outcome of control, we saw no change in the egg burdens over the four years of study for these individuals when they did reacquire infections. Furthermore, among our 15 phenotypically susceptible children, we detected an increase in genetic diversity over the course of treatment, which would not be expected had a population bottleneck or significant decline actually occurred due to treatment. Increased diversity is unlikely to be explained by selection due to the MDA program because these microsatellite markers are presumably neutral with regard to drug resistance or immune evasion. The mechanism driving the increase in genetic diversity is unknown and could be due to population increase (more individuals means more chance of novel mutations), or immigration from other surrounding schistosome populations (outbreeding). Although annual treatments of all school aged children in the Mwea region has been successful in reducing infection in children, it appears to have had no effect on the overall transmission of schistosomes in the region. This observation is consistent with that of Kihara et al. [26] who observed that following each round of treatment, prevalence increased, albeit not to baseline levels. Note that our sampling did not include a baseline measure.
Interestingly, annual prevalence in the 67 children was seen to decline over time, but this decline was not matched in the egg counts of infected individuals. Following MDA, prevalence is predicted to rapidly rise to pre-control levels, while mean worm burden rises much more slowly due to the highly non-linear relationship between prevalence and intensity (large changes in intensity result in very small changes in prevalence) [34]. This pattern may be reflecting acquired resistance after repeated drug treatment or behavioral changes as the children age and due to education. Another explanation is a lack of reliability of the Kato Katz technique to accurately measure worm burdens [18], [35], [36]. Understanding the reasons behind these changes is important for successful monitoring and also for successful modeling of MDA strategies and should be further investigated.
Our findings differ from recent studies that detected a reduction in genetic diversity (allelic richness and gene diversity) of schistosomes collected from fecal samples of children enrolled in a school based treatment program near Lake Victoria in Tanzania [10], [21]. In these studies, a reduction in allelic richness and gene diversity (heterozygosity) was found between baseline infection levels and a single year following treatment. The differences between these studies and ours may indicate important differences in local epidemiology, MDA programs, or monitoring protocols. For instance, sampling schemes differed greatly between our studies. We sampled over four years rather than between baseline and year 1 which gives a longer perspective on population changes.
Our modest sample size of patients did not appear to limit statistical power to detect deviations from the null hypothesis of no change among years as we were able to reject the null hypothesis in many of our analyses. The patterns we found did not fit the hypothesized decline over time. Also, in a previous study of schistosome genetic diversity, resampling simulations indicated that data from only 10 hosts are enough to achieve sufficient statistical power to detect differences in genetic diversity [10]. Here we note that caution may be required in extrapolating our results for these15 children to the schistosome population in the entire Mwea scheme area. However, these children come from different schools within the scheme which varied in their baseline prevalence and level of transmission. From our results, we are confident that large declines in the schistosome population, such as those nearing elimination, were not occurring in the Mwea population. Due to costs, a tradeoff between in depth sampling, number of individuals, and number of years to sample is inevitable. By choosing an increased depth of sampling over four years, we were able to look at longer term patterns of change, use novel measures of population change (Nb and FSF), and more accurately measure genetic diversity after correcting for bias that is present when collecting miracidia from a patient.
Our findings coincide with recent modeling results that indicate deworming programs targeted solely at school aged children are likely to be limited in terms of their impact on community-wide parasite transmission even at high levels of drug efficacy (95%) and coverage of school aged children (85%) because of the relatively small portion of the parasite population exposed to treatment [37]. Increasing the demographic that is included in the treatment program is likely to reduce parasite transmission; however, it must also be noted that increasing the proportion of parasites that are exposed to drug treatment is also predicted to increase the selection pressure on drug resistance [38]. This is a particular concern for schistosomiasis given that there is only one drug effective against all schistosomes and that schistosomes with reduced susceptibility to praziquantel have been reported from Kenya [39]. Together, these findings underscore the need to include alternative control approaches [34], [40]. In general, in our view, control efforts should take cognizance of the specific local environmental and epidemiological circumstances, try to use the inherent biodiversity present to limit transmission, and use an integrated multi-pronged approach as a more sustainable way forward.
In summary, after four years of school based MDA, we did not detect a reduction in schistosome population. Our data set is unique in that it combines both egg counts and novel genetic parameters to measure population changes. Also, our study follows a portion of the population that has remained phenotypically susceptible to monitor changes in the schistosome population over a four year period with annual school-based mass treatments. This design allows changes to be monitored without interference of acquired immunity and accumulation of worms over time, two road blocks to measuring the efficacy of school-based treatment programs on a community wide scale.
Supporting Information
STROBE Checklist.
(DOC)
Acknowledgments
We thank the children and teachers of Nyamindi, Kirogo, Ngurubani and Mukou primary schools in Mwea for their participation in the study and cooperation, Mr. Joseph Njoroge of the Division of Vector Borne Disease (DVBD), Mwea and staff for providing field lab space and assisting with sample collection, and Dr. Charles Mwandawiro, Ruth Gakio and other staff of the Eastern and Southern Africa Centre of International Parasite Control (ESACIPAC) for facilitating access to their field sites and the children participating in their schistosomiasis control program. Additionally, we would like to thank Dr. Mark Christie for assistance with infrapopulation simulations. We are equally grateful to Mr. George Rosenberg and the staff of the Molecular Biology Facility in the Department of Biology, University of New Mexico (UNM) for help with genotyping, and highly appreciate the assistance of Mr. Stephen Kamau of KEMRI's Centre for Biotechnology Research and Development in this work. This paper is published with the approval of the Director of KEMRI.
Funding Statement
This study was supported by NIH grants R01 TW008127, R01 AI101438 and P20RR18754 and a Gates Grand Challenges Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, et al. (2009) The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology 136: 1719–1730. [DOI] [PubMed] [Google Scholar]
- 2.WHO (2012) Accelerating work to overcome the global impact of neglected tropical diseases—A roadmap for implementation. Geneva: World Health Organization. Geneva: World Health Organization. [Google Scholar]
- 3. Agola EL, Steinauer ML, Mburu DN, Mungai BN, Mwangi IN, et al. (2009) Genetic diversity and population structure of Schistosoma mansoni within human infra-populations in Mwea, central Kenya assessed by microsatellite markers. Acta Tropica 111: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steinauer ML, Hanelt B, Agola LE, Mkoji GM, Loker ES (2009) Genetic structure of Schistosoma mansoni in western Kenya: the effects of geography and host sharing. International Journal for Parasitology 39: 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agola LE, Mburu DN, DeJong RJ, Mungai BN, Muluvi GM, et al. (2006) Microsatellite typing reveals strong genetic structure of Schistosoma mansoni from localities in Kenya. Infection Genetics and Evolution 6: 484–490. [DOI] [PubMed] [Google Scholar]
- 6. Gower CM, Gouvras AN, Lamberton PHL, Deol A, Shrivastava J, et al. (2013) Population genetic structure of Schistosoma mansoni and Schistosoma haematobium from across six sub-Saharan African countries: Implications for epidemiology, evolution and control. Acta Tropica 10.1016/j.actatropica.2012.09014 [DOI] [PubMed] [Google Scholar]
- 7. Garba A, Barkire N, Djibo A, Lamine MS, Sofo B, et al. (2010) Schistosomiasis in infants and preschool-aged children: Infection in a single Schistosoma haematobium and a mixed S. haematobium-S. mansoni foci of Niger. Acta Tropica 115: 212–219. [DOI] [PubMed] [Google Scholar]
- 8. Fulford AJC, Butterworth AE, Ouma JH, Sturrock RF (1995) A statistical approach to schistosome population dynamics and estimation of the life span of Schistosoma mansoni in man. Parasitology 110: 307–316. [DOI] [PubMed] [Google Scholar]
- 9. Odogwu SE, Ramamurthy NK, Kabatereine NB, Kazibwe F, Tukahebwa E, et al. (2006) Schistosoma mansoni in infants (aged <3 years) along the Ugandan shoreline of Lake Victoria. Annals of Tropical Medicine and Parasitology 100: 315–326. [DOI] [PubMed] [Google Scholar]
- 10. French MD, Churcher TS, Basáñez MG, Norton AJ, Lwambo NJ, et al. (2012) Reductions in genetic diversity of Schistosoma mansoni populations under chemotherapeutic pressure: The effect of sampling approach and parasite population definition. Acta Tropica 10.1016/j.actatropica.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 11. Black CL, Mwinzi PNM, Muok EMO, Abudho B, Fitzsimmons CM, et al. (2010) Influence of exposure history on the immunology and development of resistance to human schistosomiasis mansoni. Plos Neglected Tropical Diseases 4 10.1371/journal.pntd.0000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mwinzi PNM, Ganley-Leal L, Black CL, Secor WE, Karanja DMS, et al. (2009) Circulating CD23(+) B Cell Subset Correlates with the Development of Resistance to Schistosoma mansoni Reinfection in Occupationally Exposed Adults Who Have Undergone Multiple Treatments. Journal of Infectious Diseases 199: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karanja DMS, Hightower AW, Colley DG, Mwinzi PNM, Galil K, et al. (2002) Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet 360: 592–596. [DOI] [PubMed] [Google Scholar]
- 14. Black CL, Muok EMO, Mwinzi PNM, Carter JM, Karanja DMS, et al. (2010) Increases in Levels of Schistosome-Specific Immunoglobulin E and CD23(+) B Cells in a Cohort of Kenyan Children Undergoing Repeated Treatment and Reinfection with Schistosoma mansoni. Journal of Infectious Diseases 202: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mutapi F, Ndhlovu PD, Hagan P, Spicer JT, Mduluza T, et al. (1998) Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. Journal of Infectious Diseases 178: 289–293. [DOI] [PubMed] [Google Scholar]
- 16. Kato K, Miura M (1954) Comparative examinations. Japanese Journal of Parasitology 3: 35. [Google Scholar]
- 17. Katz N, Chaves A, Pellegrino J (1972) A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 14: 397–400. [PubMed] [Google Scholar]
- 18. Steinauer ML, Christie MR, Blouin MS, Agola LE, Mwangi IN, et al. (2013) Non-invasive sampling of schistosome parasites from humans requires correcting for family structure. PLoS Neglected Tropical Diseases 7: e2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29: 1–10. [DOI] [PubMed] [Google Scholar]
- 20. Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144: 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Norton AJ, Gower CM, Lamberton PHL, Webster BL, Lwambo NJS, et al. (2010) Genetic consequences of mass human chemotherapy for Schistosoma mansoni: Population structure pre- and post-praziquantel treatment in Tanzania. American Journal of Tropical Medicine and Hygiene 83: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richardson DJ, Abdo A (2011) Postcyclic transmission of Leptorhynchoides thecatus and Neoechinorhynchus cylindratus (Acanthocephala) to Largemouth Bass (Micropterus salmoides). Comparative Parasitology 78: 233–235. [Google Scholar]
- 23. Steinauer ML, Christie MR, Blouin MS, Agola LE, Mwangi IN, et al. (2013) Non-Invasive Sampling of Schistosomes from Humans Requires Correcting for Family Structure. Plos Neglected Tropical Diseases 7 10.1371/journal.pntd.0002456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kihara JH, Muhoho N, Njomo D, Mwobobia IK, Josyline K, et al. (2007) Drug efficacy of praziquantel and albendazole in school children in Mwea Division, Central Province, Kenya. Acta Tropica 102: 165–171. [DOI] [PubMed] [Google Scholar]
- 25. Montresor A, Engels D, Chitsulo L, Bundy DA, Brooker S, et al. (2001) Development and validation of a ‘tablet pole’ for the administration of praziquantel in sub-Saharan Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 95: 542–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kihara JH, Muhoho ND, Mwobobia I, French MD, Churcher TS, et al. (2012) A four-year follow-up of school children after mass-treatment for schistosomiasis and soil transmitted helminths in Mwea, Central Kenya. African Journal of Health Sciences 23: 278–291. [Google Scholar]
- 27. Hanelt B, Steinauer ML, Mwangi IN, Maina GM, Agola LE, et al. (2009) A new approach to characterize populations of Schistosoma mansoni from humans: development and assessment of microsatellite analysis of pooled miracidia. Tropical Medicine & International Health 14: 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinauer ML, Agola LE, Mwangi IN, Mkoji GM, Loker ES (2008) Molecular epidemiology of Schistosoma mansoni: a robust, high-throughput method to assess multiple microsatellite markers from individual miracidia. Infection Genetics and Evolution 8: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Santure AW (2009) Parentage and sibship inference from multilocus genotype data under polygamy. Genetics 181: 1579–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones OR, Wang JL (2010) COLONY: a program for parentage and sibship inference from multilocus genotype data. Molecular Ecology Resources 10: 551–555. [DOI] [PubMed] [Google Scholar]
- 31. Wang JL (2009) A new method for estimating effective population sizes from a single sample of multilocus genotypes. Molecular Ecology 18: 2148–2164. [DOI] [PubMed] [Google Scholar]
- 32. DeGiorgio M, Rosenberg NA (2009) An unbiased estimator of gene diversity in samples containing related individuals. Molecular Biology and Evolution 26: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm.
- 34. Anderson R, Hollingsworth TD, Truscott J, Brooker S (2012) Optimisation of mass chemotherapy to control soil-transmitted helminth infection. Lancet 379: 289–290. [DOI] [PubMed] [Google Scholar]
- 35. Kongs A, Marks G, Verle P, Van der Stuyft P (2001) The unreliability of the Kato-Katz technique limits its usefulness for evaluating Schistosoma mansoni infections. Tropical Medicine & International Health 6: 163–169. [DOI] [PubMed] [Google Scholar]
- 36. Zhang YY, Luo JP, Liu YM, Wang QZ, Chen JH, et al. (2009) Evaluation of Kato-Katz examination method in three areas with low-level endemicity of schistosomiasis japonica in China: A Bayesian modeling approach. Acta Tropica 112: 16–22. [DOI] [PubMed] [Google Scholar]
- 37. Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD (2013) How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Neglected Tropical Diseases 7 10.1371/journal.pntd.0002027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Wyk JA (2001) Refugia: Overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. Onderstepoort Journal of Veterinary Research 68: 55–67. [PubMed] [Google Scholar]
- 39. Melman SD, Steinauer ML, Cunningham C, Salter-Kubatko L, Mwangi IN, et al. (2009) Reduced susceptibility of Kenyan Schistosoma mansoni to praziquantel following repeated exposures: origin, measurement, and likelihood of persistence. PLoS Neglected Tropical Diseases 3: e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuenté L-AT, et al. (In Press) Time to set the agenda for schistosomiasis elimination. Acta Tropica 10.1371/journal.pntd.0002474 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE Checklist.
(DOC)