Abstract
Objective
Duchenne and Becker muscular dystrophy (DBMD) are allelic disorders caused by mutations in dystrophin. Adults with DBMD develop life-threatening cardiomyopathy. Inhibition of phosphodiesterase 5 (PDE5) improves cardiac function in mouse models of DBMD. To determine if the PDE5-inhibitor sildenafil benefits human dystrophinopathy, we conducted a randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov, number NCT01168908).
Methods
Adults with DBMD and cardiomyopathy (ejection fraction ≤50%) were randomized to receive sildenafil (20mg three times daily) or placebo for 6 months. All subjects received an additional 6 months of open-label sildenafil. The primary endpoint was change in left ventricular end-systolic volume (LVESV) on cardiac MRI. Secondary cardiac endpoints, skeletal muscle function, and quality of life were also assessed.
Results
An interim analysis (performed after 15 subjects completed the blinded phase) revealed that 29% (4/14) of subjects had a ≥10% increase in LVESV after 6 months of sildenafil compared to 13% (1/8) of subjects receiving placebo. Subjects with LVESV >120ml at baseline were more likely to worsen at 12 months regardless of treatment assignment (p=0.035). Due to the higher number of subjects worsening on sildenafil, the Data and Safety Monitoring Board recommended early termination of the study. There were no statistically significant differences in outcome measures between treatment arms.
Interpretation
Due to the small sample size, comparisons between groups must be interpreted with caution. However, this trial suggests that sildenafil is unlikely to improve cardiac function in adults with DBMD.
Introduction
Duchenne muscular dystrophy is one of the most common fatal genetic disorders of mankind. It is caused by mutations in the dystrophin gene, which lead to progressive weakness and death in early adulthood from respiratory failure and cardiomyopathy.1–3 Becker muscular dystrophy is an allelic disorder caused by dystrophin mutations that allow some dystrophin expression. Patients with Becker muscular dystrophy have a wider phenotypic range than patients with Duchenne, but they also develop progressive muscle weakness and cardiomyopathy.4
Several lines of investigation indicate that loss of cardiac and skeletal muscle function in Duchenne and Becker muscular dystrophy (DBMD) is mediated by reduction in cGMP. In skeletal muscle, dystrophin binds neuronal nitric oxide synthase (nNOS), which catalyzes nitric oxide (NO) production. NO stimulates soluble guanylate cyclase (sGC) to produce cGMP, which in turn stimulates protein kinase G (PKG). In the absence of dystrophin, nNOS no longer localizes to the skeletal muscle membrane, disrupting the NO-sGC-cGMP pathway.5 Although the targets of PKG in DBMD have not yet been elucidated, modulating this pathway through expression of a nNOS transgene has been shown to improve cardiac pathology in mdx mice.6, 7 Increasing cGMP, through transgenic overexpression of sGC or inhibition of its hydrolysis, can also overcome loss of nNOS in mice.8
The nucleotide phosphodiesterases (PDEs) hydrolyze the cyclic nucleotide monophosphates cAMP and cGMP and regulate their downstream signaling. Phosphodiesterase 5 (PDE5) is specific for cGMP (which is particularly important in cardiovascular function and morphology) and may have a role in modifying cardiomyopathy in DBMD.9 PDE5 expression in cardiomyocytes is low at baseline and increases in response to ischemia or pressure overload from heart failure.10, 11 Thus, inhibition of PDE5 and the resultant increase in cGMP have little effect on basal cardiac function, but are protective against myocardial stressors.12 In mouse models, PDE5 inhibition with sildenafil has been shown to prevent decline in cardiac function and reverse cardiac hypertrophy in early and late stages of disease.6, 13–15
PDE5 inhibition may also ameliorate dystrophin-deficient skeletal muscle. Sildenafil has been found to increase diaphragm strength and reduce endomysial fibrosis in mdx mice.16 Impaired blood flow to skeletal muscle in dystrophin- or nNOS-deficient mice can also be rescued by PDE5 inhibition,17 and a recent trial of the PDE5 inhibitor tadalafil showed restoration of normal blood flow to exercising muscles in men with Becker muscular dystrophy.18
Given this evidence of PDE5 inhibition improving pathology in dystrophinopathy, we performed a clinical trial, REVERSE-DBMD (Revatio for heart disease in Duchenne and Becker Muscular Dystrophy), to investigate the safety and efficacy of sildenafil in dystrophic cardiomyopathy.
Methods
Study design and participants
Males with Duchenne muscular dystrophy (defined as absent dystrophin staining on muscle biopsy or a dystrophin mutation predictive of the Duchenne phenotype on genetic testing) were enrolled in a single-center, randomized, double-blind, placebo-controlled trial. Inclusion criteria included: age ≥15 years, cardiac ejection fraction (EF) ≤45%, concurrent use of an ACE inhibitor or angiotensin receptor blocker for ≥3 months without any change in dose, and unchanged beta-blocker or corticosteroid dosing for 3 months. Exclusion criteria included: contraindications to MRI, implantable cardiac devices, frequent cardiac arrhythmia, hereditary retinal disorders, bleeding disorders, a systolic blood pressure ≤85mmHg or lower, stage 4 or 5 renal failure, active tobacco use, and concurrent use of nitrates, alpha-adrenergic receptor blockers, or phosphodiesterase inhibitors. The protocol was approved by the Johns Hopkins Institutional Review Board and an independent Data and Safety Monitoring Board (DSMB).
Study treatment and randomization
Screening and enrollment were performed at the Kennedy Krieger Outpatient Center in Baltimore, MD between September 2010 and February 2013. All participants gave written informed consent prior to enrollment. Participants were randomized (with a 1:1 allocation ratio) to either oral sildenafil (20mg 3 times daily) or placebo. The dose of sildenafil was selected based on pulmonary hypertension studies, and dose-escalation was not performed due to concern for systemic vasodilation in a population with normal or low systemic blood pressures.19 A stratified blocked randomization was performed by the Investigational Drug Service (IDS) at the Johns Hopkins Hospital using a computerized pseudorandom number generator. In both treatment arms, the study drug consisted of identical white tablets. Participants and the research staff evaluating them were blinded to treatment allocation. After 6 months of blinded treatment, participants received open-label sildenafil (20mg 3 times daily) for 6 months.
Procedures and follow-up
The primary outcome was left ventricular end systolic volume (LVESV) measured using cardiac MRI. LVESV was selected (as opposed to EF) because proportional worsening of diastolic and systolic LV volumes can occur without producing a change in EF. Myocardial infarction studies have also shown that LVESV is superior to EF in predicting survival, and change in LVESV is a commonly used primary endpoint.20–22 A ≥10% change in LVESV was considered clinically significant based on studies of other cardiac therapies that improve survival.23–25 Cardiac MRI’s were performed at baseline, after 6 months of blinded treatment with sildenafil or placebo, and after completion of the open-label treatment phase (12 months). Images were processed (QMass, Medis, Leiden, The Netherlands) to determine secondary cardiac outcomes, including end-systolic, end-diastolic, and stroke volumes, left ventricular myocardial mass, and EF.26 Cardiac CINE imaging was acquired for functional analysis (Supplemental videos 1–4).27 Delayed contrast-enhanced (DCE) imaging was attempted for all studies, but was frequently limited by insufficient intravenous access or the subject’s inability to tolerate lying supine for the time needed to complete the scan. Enhancement was observed on 2D or 3D DCE short axis images acquired ~10 minutes post-contrast infusion and classified as either present or absent.28, 29 The patterns of enhancement were consistent with the diffuse fibrosis previously described in DBMD.30
Participants were admitted to inpatient research units at the Johns Hopkins Hospital for initiation of the blinded and open-label phases of the study. During hospitalization, the following data were collected: safety laboratory tests (complete blood count, complete metabolic panel, liver function tests, creatine kinase, phosphate, urine studies), quantitative muscle testing (grip and pinch strength obtained using hand-held dynamometry), forced vital capacity (FVC) obtained using bedside spirometry, 2 patient-reported quality of life instruments, Short Form-36v231, 32 (SF36v2) and Individualized Neuromuscular Quality of Life Questionnaire (INQoL)33 and adverse events. Vital signs were obtained before administration of the study drug and every 6 hours during hospitalization. Participants were discharged after the 4th dose and were given a sufficient quantity of the study drug to continue use until the next outpatient visit. Dosing instructions and information about potential side effects were provided by representatives from the IDS.
Follow-up visits were conducted at 1, 2, 3, and 6 months following initiation of the study drug and after crossing to the open-label phase. Safety laboratory tests, physical examination, and evaluation for adverse events and medication changes were performed at each visit. FVC measurements and pinch and grip strength were obtained at 3 and 6-month visits. At each visit, any unused drug and the prescription bottle were collected and a new supply was dispensed.
Study monitoring
Adverse events and protocol deviations were reported to and adjudicated by the Institutional Review Board of the Johns Hopkins Medical Institutes. Outcome data and adverse events were reported every 2 months to a 3-member independent DSMB. The DSMB convened with the principal investigators every 6 months during the trial. The study protocol included plans for an interim analysis after enrollment of half the target study population (15 of 30 participants). The DSMB was asked to recommend termination of the trial if the rate of adverse events was significantly higher in the sildenafil treatment arm.
Amendments to study protocol
The study protocol was amended 8 months after its initiation in response to slow enrollment. Individuals with Becker muscular dystrophy were allowed to enroll, the required EF for enrollment was increased from 45% to 50%, and participants were allowed to have the 1- and 2-month study visits completed locally, either by a primary care physician or a study team member. Twenty months after the initiation of enrollment, the protocol was amended to eliminate the overnight inpatient hospitalizations. By this time, multiple participants had completed the study without adverse events during hospitalization. Admissions were replaced with an extended outpatient clinic visit, during which 2 doses of the study drug were given. The required age of participation for the trial was increased to 18 years in response to an advisory by the U.S. Food and Drug Administration recommending against the use of Revatio (sildenafil) in children (August 30, 2012). No individuals <18 years had been enrolled in the study prior to that date.
Statistical analysis
We estimated that a sample size of 15 subjects per treatment group would be required to detect a mean improvement in LVESV of 10% over 6 months (with 80% power and a 2-sided significance level of 0.05). Statistical analyses were performed by members of the Biostatistics Department at the Johns Hopkins Bloomberg School of Public Health. Only subjects with complete cardiac MRI data for the first 6 months of the trial were included in the analysis. Although a per protocol analysis carries greater risk for selection bias than an intention-to-treat analysis, the variability of the outcome data raised concerns over the appropriateness of using imputation methods to estimate missing data. Furthermore, the subjects excluded from analysis were evenly distributed between treatment arms and 2 were administratively censored, making bias based on treatment assignment less likely. Primary and secondary outcome measures were analyzed using linear regression models adjusting for baseline values and age. Outcome measures that were collected every 3 months (FVC, grip strength, pinch strength) were also analyzed longitudinally using random effects models to account for within-person correlation of measures over time. These outcomes were regressed on measurement time points to assess for temporal trends.
Results
Clinical trial initiation and early termination
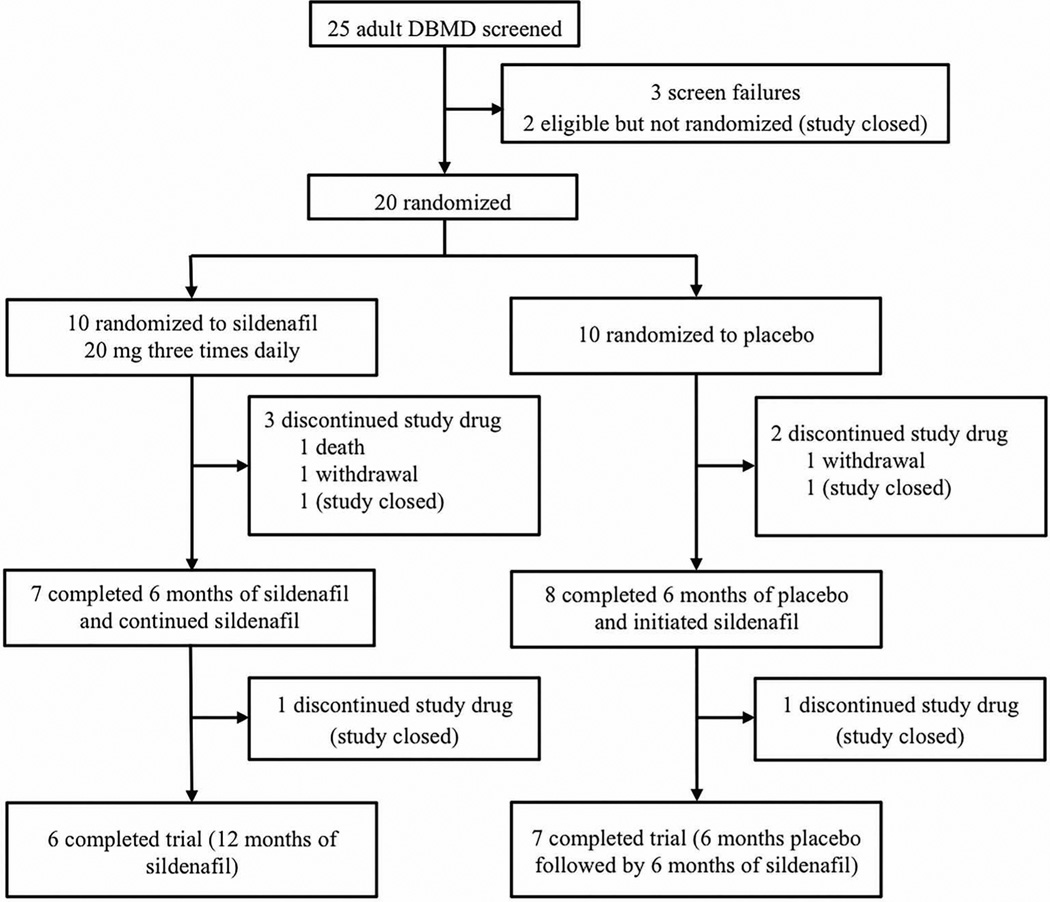
Twenty-five subjects were screened and 20 were randomized to treatment (Figure 1). Three subjects failed screening (2 had EF’s>50% and 1 was unable to participate due to surgery) and 2 subjects were not randomized due to early termination of the trial. One death from heart failure occurred in the sildenafil arm during the first 6 months of treatment. The DSMB determined that this event was unlikely to be related to the study drug. Two subjects withdrew from the trial (1 from each treatment arm) due to the burden of trial participation.
Figure 1.
The number of subjects screened and randomized to each arm is shown in this schematic, followed by the number who discontinued the trial prematurely, completed 6 months of the trial, or completed 12 months of the trial.
The DSMB reviewed adverse events and primary outcome data after 15 subjects completed 6 months of the trial. Among this cohort, 14 received sildenafil for at least 6 months, 8 of whom had received placebo for the first 6 months. The interim analysis showed that 4 of the 14 subjects (29%) experienced a ≥10% increase in LVESV (i.e. worsening cardiac function) during their first 6 months of treatment with sildenafil. Two of these events occurred during the blinded phase, and 2 occurred during the open-label phase. Only 1 of 8 subjects (12.5%) experienced this degree of worsening while on placebo. Due to the higher number of participants experiencing a ≥10% increase in LVESV while taking sildenafil (OR 2.8, 95%CI 0.20–40.10 based on a clustered regression model), the DSMB recommended discontinuation of the trial. Enrollment was halted, and the subjects who remained active in the study were asked to discontinue the study drug and return for an early termination visit.
Baseline characteristics
The baseline characteristics of the 20 subjects randomized to treatment are summarized in Table 1. Despite randomization, the mean LVESV was higher in the sildenafil group (99.8 ± 55.3 mL) than in the placebo group (86.7 ± 31.9 mL), suggesting that baseline cardiac function was worse in the group randomized to sildenafil. This disparity is further evidenced by the larger proportion of subjects using beta blockers, digoxin, and non-invasive positive pressure ventilation in the sildenafil group.
Table 1.
Baseline characteristics of participants in the sildenafil and placebo treatment arms.
| All randomized subjects | Subjects with complete data for 6 months |
|||
|---|---|---|---|---|
| Sildenafil (n=10) |
Placebo (n=10) |
Sildenafil (n=7) |
Placebo (n=8) |
|
| Age (years) | 25.5 (8.2) | 22.6 (4.4) | 26.9 (9.5) | 23.4 (4.7) |
| Diagnosis | ||||
| Duchenne | 9 (90%) | 9 (90%) | 6 (86%) | 7 (88%) |
| Becker | 1 (10%) | 1 (10%) | 1 (14%) | 1 (13%) |
| Ethnic Group | ||||
| Caucasian | 7 (70%) | 8 (80%) | 4 (57%) | 7 (88%) |
| Asian | 1 (10%) | 2 (20%) | 1 (14%) | 1 (13%) |
| African American | 2 (20%) | 0 (0%) | 2 (29%) | 0 (0%) |
| Height (cm) | 160 (13) | 162 (15) | 162 (13) | 160 (16) |
| Weight (kg) | 69 (21.8) | 66.3 (20) | 76.1 (21.6) | 65.0 (18.5) |
| Systolic blood pressure (mmHg) | 110 (17) | 115 (16) | 117 (15) | 116 (15) |
| Diastolic blood pressure (mmHg) | 68 (13) | 69 (10) | 71 (14) | 67 (8) |
| Heart rate (bpm) | 80 (16) | 88 (11) | 85 (15) | 89 (12) |
| Ejection fraction (%) | 40.8 (8.9) | 43.9 (7.7) | 41.8 (9.6) | 45.3 (7.2) |
| Left ventricular end-systolic volume (ml) | 99.8 (55.3) | 86.7 (31.9) | 105.2 (63.8) | 84 (32.9) |
| ACE inhibitor or ARB | 10 (100%) | 10 (100%) | 7 (100%) | 8 (100%) |
| Beta blocker | 7 (70%) | 4 (40%) | 5 (71%) | 3 (38%) |
| Digoxin | 3 (30%) | 1 (10%) | 2 (29%) | 1 (13%) |
| Diuretics | 2 (20%) | 3 (30%) | 1 (14%) | 2 (25%) |
| Mechanical Ventilation | 2 (20%) | 2 (20%) | 1 (14%) | 2 (25%) |
| Non-invasive Ventilation | 6 (60%) | 3 (30%) | 4 (57%) | 1 (13%) |
Data are mean (SD) or n (%).
Adverse events
Patient-reported adverse events, changes in laboratory values, and abnormal vital signs were documented at each study visit. These are summarized in Supplementary Table 1.
Cardiac outcomes
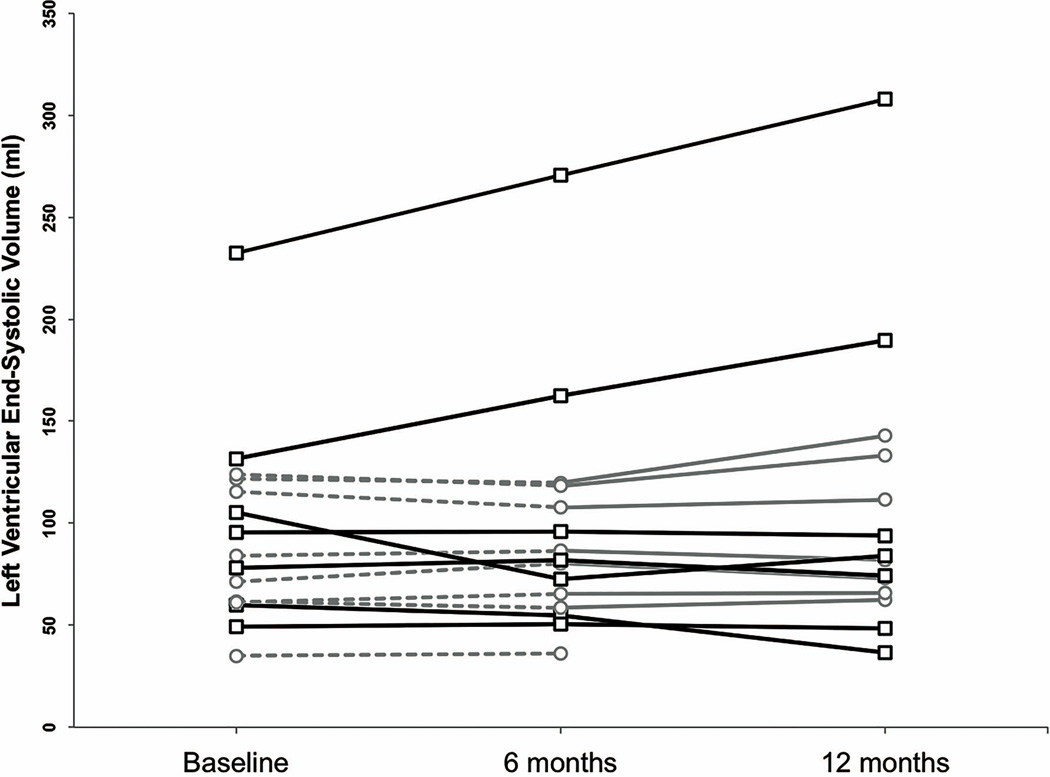
Cardiac function data for participants completing the 6-month blinded study phase are summarized in Table 2 (and Supplementary Table 2). As expected with small sample sizes, standard deviations were large and differences between treatment arms were not statistically significant. This finding holds when regression analyses were performed adjusting for both baseline LVESV and age. Subjects taking placebo experienced an average decrease in LVESV of 0.19ml while subjects taking sildenafil experienced a 5.20ml increase in LVESV (p=0.38). Figure 2 illustrates the changes in mean LVESV for each treatment arm. The mean LVESV increased during all periods in which subjects received sildenafil. However, individual line plots (Figure 3) show that these increases are largely due to severe worsening in a few individuals. Among all participants, those with a LVESV ≥120 ml at baseline were more likely to worsen over 12 months, regardless of treatment (p=0.035, Fisher’s exact test).
Table 2.
Primary and secondary endpoints
Mean (SD) baseline, 6-month, and 6-month minus baseline cardiovascular measurements are listed by treatment arm. The treatment effect represents the differences in 6-month minus baseline measurements between groups (i.e. differences of the differences) adjusted for baseline values and age.
| Placebo (n = 8) | Sildenafil (n = 7) | Treatment effect |
p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6- month |
Paired change |
Baseline | 6- month |
Paired change |
[95% CI] | |||
| Primary Outcome | |||||||||
| LV End-systolic Volume | 84.28 (32.92) | 84.09 (30.02) | −0.19 (5.56) | 107.49 (61.66) | 112.69 (79.02) | 5.20 (23.61) | 6.99 [−0.81, 23.77] | 0.379 | |
| Secondary Outcomes | |||||||||
| LV mass | 96.96 (32.67) | 95.91 (26.88) | −1.05 (12.69) | 107.51 (30.09) | 104.11 (29.78) | −3.40 (7.03) | 0.12 [−12.00, 12.23] | 0.983 | |
| LV End-diastolic volume | 152.14 (44.47) | 152.37 (46.19) | 0.24 (8.78) | 178.94 (75.92) | 184.48 (87.36) | 5.54 (18.85) | 6.98 [−8.21, 22.18] | 0.333 | |
| Stroke volume | 67.85 (13.27) | 68.28 (18.88) | 0.43 (11.46) | 71.46 (14.96) | 71.80 (12.17) | 0.34 (9.75) | −0.37 [−14.07, 13.33] | 0.953 | |
| Ejection fraction | 46.14 (7.04) | 45.64 (6.82) | −0.50 (5.24) | 42.64 (8.60) | 43.25 (12.82) | 0.61 (7.00) | −0.60 [−7.98, 6.80] | 0.864 | |
| Forced Vital Capacity1 | 2.32 (1.74) | 2.13 (1.60) | −0.19 (0.21) | 1.70 (0.97) | 1.57 (0.99) | −0.13 (0.09) | 0.01 [0.17, 0.20] | 0.863 | |
| Grasp2 | 8.06 (5.90) | 8.23 (6.21) | 0.17 (1.25) | 8.99 (9.39) | 9.36 (8.78) | 0.37 (0.90) | 0.48 [−0.69, 1.66] | 0.384 | |
| Pinch3 | 3.13 (2.14) | 3.25 (2.27) | 0.12 (0.45) | 2.00 (1.21) | 1.93 (1.11) | −0.06 (0.30) | −0.18 [0.73, 0.37] | 0.478 | |
7 placebo, 7 treatment
8 placebo, 7 treatment
7 placebo, 6 treatment
Figure 2.
Mean LVESV (± SE) over time for groups randomized to sildenafil (black line with squares) and placebo (gray line with circles). The dashed line signifies the period that subjects were taking placebo.
Figure 3.
Individual LVESV measurements for subjects randomized to sildenafil (black lines with squares) and placebo (gray lines with circles) treatment groups over time. The dashed lines signify the periods that subjects were taking placebo.
Myocardial fibrosis was assessed using DCE MRI, which was completed in 17 of 25 (68%) patients at baseline, 11 of 16 (69%) at 6 months, and 6 of 14 patients (42%) at 12 months. These scans were assessed qualitatively for the presence or absence of enhancement (signifying fibrosis), which was identified in 15 (88%) baseline scans, 11 (100%) 6-month scans, and 6 (100%) 12-month scans. If enhancement was observed at any time point for a given subject, it was present on all subsequent scans for that subject. This is consistent with prior observations that cardiac fibrosis does not resolve in DBMD once it presents.30 Only one subject with enhancement present at 6 months did not have enhancement at baseline (this subject was randomized to placebo during this period).
Non-cardiac secondary outcomes
Secondary outcome measures were selected to evaluate the effects of sildenafil on skeletal muscle. No statistically significant differences in FVC, grip strength, and pinch strength were found when comparing treatment groups (Table 2). These findings hold when adjusting for baseline values and age. Longitudinal data analyses were performed using mixed effects models on data from subjects treated with sildenafil for 6 months. These analyses show a statistically significant decrease in mean FVC of 0.06 liters over 6 months (95% CI [−0.130, −0.013], p=0.011). There was an overall decline in grip strength (−0.13 lbs., 95% CI = [−0.424, 0.164], p=0.378) and pinch strength (−0.07 lbs., 95% CI = [−0.235, 0.098], p=0.420); however these findings were not statistically significant. Analyses of the SF-36v2 and INQoL did not show statistically significant differences between the treatment groups in any of the reported domains of function or quality of life when adjusted for baseline values and age.
Futility analysis
Our interim analysis showed that the 7 subjects randomized to sildenafil experienced a 1.1% mean increase in LVESV. If the study enrolled 8 more subjects into the sildenafil group as planned, these subjects would have had to experience a mean decrease in LVESV of 20% to produce the clinically significant improvement in LVESV that the trial was powered to detect. Only 1 of 7 patients (14.3%) randomized to sildenafil experienced this degree of improvement during the first 6 months of treatment. If this proportion represented the true probability of experiencing a reduction in LVESV of this magnitude, the probability of this occurring in the next 8 subjects would be essentially zero (1.75×10−7). Changes in LVESV in the placebo arm were small; only 1 subject experienced a change of ≥10%. Had 7 more subjects been randomized to placebo, their mean change in LVESV would likely have remained stable as well. These calculations suggest that had the trial had not been stopped for safety concerns, the probability of seeing the anticipated decrease in LVESV of 10% (even with full enrollment) would be small.
Discussion
Cardiac muscle involvement is nearly universal in adults with Duchenne muscular dystrophy, and individuals with Becker muscular dystrophy often develop severe cardiomyopathy, even when skeletal muscle function is relatively preserved.34, 35 It is therefore a priority in the muscular dystrophy community to discover and optimize therapies for cardiomyopathy associated with dystrophinopathy. The REVERSE-DBMD trial is the first to target cardiomyopathy in adult DBMD. Unfortunately, the beneficial effects of sildenafil seen in pre-clinical studies were not confirmed in this human trial. Instead of demonstrating a reduction in LVESV, sildenafil-treated subjects showed a higher incidence of increased LVESV compared to the placebo group. Subsequent analysis did not identify a statistically significant treatment effect (partly due to small sample sizes). Nevertheless, extrapolation of the available data makes it unlikely that significant benefit would have been observed had the trial been fully enrolled.
This study adds to a field of conflicting data on the potential therapeutic role of PDE5 inhibitors in heart failure. While smaller trials have shown improvement in exercise capacity and patient-reported quality of life in heart failure patients taking sildenafil,36, 37 the RELAX trial did not show benefit in individuals with heart failure and preserved ejection fraction.38 Our data are also consistent with results of a recent trial of sildenafil in Becker muscular dystrophy conducted by Witting et al., (see page x, this issue) in which no effect on blood flow, hand grip, or heart function was found. This study further identified reduced levels of PDE5 expression in the skeletal muscle of patients with Becker muscular dystrophy compared to healthy controls, which could impact the effectiveness of PDE5 inhibition in treating dystrophin-deficient muscle. The lack of improvement in cardiac function may also be due to insufficient substrate for cGMP production in subjects with severe cardiomyopathy.39 In this case, modulation of the pathway by other approaches, such as direct activation of guanylate cyclase, may be more effective. It is also possible that a higher dose of sildenafil or a different treatment regimen could achieve a therapeutic effect in this population, and pharmacokinetic monitoring of the effective drug concentration in future trials would be informative in this regard.
Another possible explanation for the observed results relates to the fact that sildenafil inhibits PDE1 in addition to PDE5. PDE1 is less selective for its enzymatic targets and hydrolyzes cAMP as well as cGMP, which may produce off-target side effects. Tadalafil is a PDEi with greater selectivity for PDE5 (versus PDE1c) than sildenafil.40 Small trials have shown that tadalafil improves blood flow to exercising muscle in Becker muscular dystrophy, and a large clinical trial has begun enrollment (NCT01865084) to study the potential benefit of tadalafil in skeletal muscle in Duchenne.18 Given the results of the REVERSE-DBMD trial, it will be important to monitor cardiac function in these subjects.
Baseline LVESV was predictive of worsening cardiac function in this trial. The 4 subjects who worsened by ≥10% had baseline LVESV’s between 121.69 and 232.38ml, and the single subject who died (at age 26) had a baseline LVESV of 127.51ml. The subjects with the 2 highest baseline LVESV’s also experienced the largest increases in LVESV (>50 mls). These individuals may represent a sub-population of DBMD that exhibits comparatively severe cardiomyopathy and responds adversely to PDE5 inhibition. It is also possible that the disease had progressed beyond the aid of intervention in these individuals. In future trials in the DBMD population, stratification by baseline LVESV may be an important trial design consideration.
Supplementary Material
Acknowledgements
The authors thank the participants and their families for participating in this trial. They also wish to acknowledge the contribution of Michael Munchel, BSN, and the F. M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute. This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH.
Grant support
Funding for this trial was received from Action Duchenne, Charley's Fund, Cure Duchenne, Hope for Javier, Nash Avery Foundation, and Zubin's Wish. Further support for participant travel expenses was provided by Ryan’s Quest. Sildenafil and matched placebo were provided by Pfizer, Inc. through an Investigator-Initiated Research award.
Role of the funding source
The sponsors proposed the idea for this study and initiated discussions to plan the trial with KRW and DPJ. The sponsors received regular reports prepared by the investigators for the DSMB. The study design, protocol development, trial implementation, data collection and analysis, decision to publish, and preparation of the manuscript were conducted independently of the sponsors.
Conflicts of Interest
DPJ has received consulting fees from Pfizer Inc., Array BioPharma, and Onyx Pharmaceuticals. KRW is a consultant for Sanofi-Aventis Group, Bristol Myers Squibb Co., Pfizer Inc., and Sarepta Therapeutics and has received honoraria for speaking from Athena Diagnostics. DAK has a patent (20090062313) issued.
Footnotes
Contributors
DPJ and KRW designed the study and were the principal study investigators. DAK provided scientific advice. DGL, DH, WRT, SDR, GB, GT, KHS, and ACL collected data. ACL designed the imaging protocol and directed image analysis. BH and RET did the statistical analysis. DGL, KRW and DPJ drafted the manuscript. All authors interpreted data, critically reviewed the report, and approved the final version of the report.
References
- 1.Eagle M, Bourke J, Bullock R, et al. Managing Duchenne muscular dystrophy--the additive effect of spinal surgery and home nocturnal nventilation in improving survival. Neuromuscular Disorders. 2007;17(6):470–475. doi: 10.1016/j.nmd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Moxley RT, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in Natural History of Duchenne Muscular Dystrophy With Long-term Corticosteroid Treatment: Implications for Management. Journal of Child Neurology. 2010;25(9):1116–1129. doi: 10.1177/0883073810371004. [DOI] [PubMed] [Google Scholar]
- 3.Wagner KR, Lechtzin N, Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochim Biophys Acta. 2007;1772(2):229–237. doi: 10.1016/j.bbadis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Magri F, Govoni A, D’Angelo M, Bo R, Ghezzi S, Sandra G, et al. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J Neurol. 2011;258(9):1610–1623. doi: 10.1007/s00415-011-5979-z. [DOI] [PubMed] [Google Scholar]
- 5.Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82(5):743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 6.Lai Y, Zhao J, Yue Y, Wasala NB, Duan D. Partial restoration of cardiac function with DeltaPDZ nNOS in aged mdx model of Duchenne cardiomyopathy. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Human Molecular Genetics. 2005;14(14):1921–1933. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- 8.Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, et al. Sildenafil and cardiomyocytes-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA. 2008;105(19):7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judge D, Kass D, Thompson WR, Wagner K. Pathophysiology and Therapy of Cardiac Dysfunction in Duchenne Muscular Dystrophy. Am J Cardiovasc Drugs. 2011;11(5):287–294. doi: 10.2165/11594070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, et al. Oxidative Stress Regulates Left Ventricular PDE5 Expression in the Failing Heart. Circulation. 2010;121(13):1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, et al. Ventricular Phosphodiesterase-5 Expression Is Increased in Patients With Advanced Heart Failure and Contributes to Adverse Ventricular Remodeling After Myocardial Infarction in Mice. Circulation. 2009;119(3):408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis SH, Busch JL, Corbin JD. cGMP-Dependent Protein Kinases and cGMP Phosphodiesterases in Nitric Oxide and cGMP Action. Pharmacological Reviews. 2010;62(3):525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil Inhibits β-Adrenergic–Stimulated Cardiac Contractility in Humans. Circulation. 2005;112(17):2642–2649. doi: 10.1161/CIRCULATIONAHA.105.540500. [DOI] [PubMed] [Google Scholar]
- 14.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 15.Adamo CM, Dai D-F, Percival JM, Minami E, Willis MS, Patrucco E, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107(44):19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. The Journal of Pathology. 2012;228(1):77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456(7221):511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, et al. Tadalafil alleviates muscle ischemia in patients with Becker Muscular Dystrophy. Sci Transl Med. 2012;28(162):162ra55. doi: 10.1126/scitranslmed.3004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 20.White HD, Norris RM, Brown MA, Brandt PW, Witlock RM, Wild CJ. Left ventricular endsystolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 21.Mathiasen AB, Jorgensen E, Qayyum AA, Haack-Sorensen M, Ekblond A, Kastrup J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial) American heart journal. 2012;164(3):285–291. doi: 10.1016/j.ahj.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 23.Ghio S, Magrini G, Serio A, Klersy C, Fucilli A, Ronaszèki A, et al. Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the SENIORS echocardiographic substudy. European Heart Journal. 2006;27(5):562–568. doi: 10.1093/eurheartj/ehi735. [DOI] [PubMed] [Google Scholar]
- 24.Konstam M, Rousseau M, Kronenberg M, Udleson J, Melin J, Stewart D, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patienst with heart faiulre. SOLVD Investigators. Circulation. 1992;86(2):431–438. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 25.St John Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained Reverse Left Ventricular Structural Remodeling With Cardiac Resynchronization at One Year Is a Function of Etiology: Quantitative Doppler Echocardiographic Evidence From the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Circulation. 2006;113(2):266–272. doi: 10.1161/CIRCULATIONAHA.104.520817. [DOI] [PubMed] [Google Scholar]
- 26.Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JAC, et al. Physiological basis of myocardiacl contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902–1910. doi: 10.1161/01.cir.92.7.1902. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson DJ, Edelman RR. Cineangiography of the heart in a single breath hold with a segmented turboFLASH sequence. Radiology. 1991;178(2):357–360. doi: 10.1148/radiology.178.2.1987592. [DOI] [PubMed] [Google Scholar]
- 28.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2002;47(2):372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Schar M, Kozerke S, Harouni A, Sena-Weltin V, Zviman MM, et al. Independent respiratory navigators for improved 3D PSIR imaging of myocardial infarctions. Journal of Cardiovascular Magnetic Resonance. 2011;13(Suppl 1):P18. [Google Scholar]
- 30.Hor KN, Taylor MD, Al-Khalidi HR, Cripe LH, Raman SV, Jefferies JL, et al. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:107. doi: 10.1186/1532-429X-15-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 32.Ware JEJ, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. In: SF-36v2 Health Survey: Administration guide for clinical trial investigators. Incorporated Q, editor. Lincoln, RI: 2008. [Google Scholar]
- 33.Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL) Neurology. 2007;68(13):1051–1057. doi: 10.1212/01.wnl.0000257819.47628.41. [DOI] [PubMed] [Google Scholar]
- 34.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. International journal of cardiology. 1990;26(3):271–277. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 35.Melacini P, Fanin M, Danieli GA, Villanova C, Martinello F, Miorin M, et al. Myocardial Involvement Is Very Frequent Among Patients Affected With Subclinical Becker's Muscular Dystrophy. Circulation. 1996;94(12):3168–3175. doi: 10.1161/01.cir.94.12.3168. [DOI] [PubMed] [Google Scholar]
- 36.Giannetta E, Isidori AM, Galea N, Carbone I, Mandosi E, Vizza CD, et al. Chronic Inhibition of cGMP Phosphodiesterase 5A Improves Diabetic Cardiomyopathy: A Randomized, Controlled Clinical Trial Using Magnetic Resonance Imaging With Myocardial Tagging. Circulation. 2012;125(19):2323–2333. doi: 10.1161/CIRCULATIONAHA.111.063412. [DOI] [PubMed] [Google Scholar]
- 37.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 Inhibition With Sildenafil Improves Left Ventricular Diastolic Function, Cardiac Geometry, and Clinical Status in Patients With Stable Systolic Heart Failure: Results of a 1-Year, Prospective, Randomized, Placebo-Controlled Study. Circulation: Heart Failure. 2011;4(1):8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 38.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309(12):1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kass DA. Cardiac role of cyclic-GMP hydrolyzing phosphodieterase type 5: from experimetnal models to clinical trials. Current Heart Failure Reports. 2012;9(3):192–199. doi: 10.1007/s11897-012-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright PJ. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. J Clin Pract. 2006;60(8):967–975. doi: 10.1111/j.1742-1241.2006.01049.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.