Abstract
Background and aims
An early age of alcohol initiation (AAI) is associated with and has been hypothesized to be a cause of alcohol use disorders (AUD) in adulthood. Results from twin studies, however, indicate that AAI is an indicator of risk for AUD. We aimed to test a causal hypothesis vs. a risk indicator hypothesis for the relationship between early AAI and AUD.
Design
A population-based twin study using biometric twin modelling.
Setting
Norway.
Participants
A population-based sample of 1336 Norwegian twins.
Measurements
Lifetime DSM-IV AUDs were assessed by structured clinical interview, and AAI by questionnaire.
Findings
The risk indicator model in which the association between AAI and AUD was explained by common vulnerability was the best fit to the data. The heritability was 37% (95%CI 21%, 53%) for AAI and 62% (95%CI 51%, 73%) for AUD. Genetic risk for AAI accounted for 44% (95%CI 17%, 71%) of the total genetic risk for AUD, and the correlation between genetic factors for AAI and AUD was −0.66 (95%CI −0.87, −0.46). Individual-specific environmental risk for AAI explained only 1% (95%CI 0%, 3%) of the risk for AUD. Shared environmental factors did not influence AUD, but accounted for 25% (95%CI 7%, 35%) of the variance in AAI.
Conclusions
The association between early age of alcohol initiation and alcohol use disorders in later life does not reflect a causal relationship, but is almost entirely due to common genetic risk factors.
Introduction
Individuals who initiate alcohol use at an early age are at increased risk for problem drinking and DSM-IV alcohol use disorders (AUD) (i.e. alcohol abuse or dependence) in adolescence and adulthood (1–14). There is, however, a lack of agreement regarding the interpretation of these findings. One hypothesis is that the association between early age at alcohol initiation (AAI) and AUD is due to a direct causal link, i.e. that AAI is a risk factor for developing AUD (4, 9, 14, 15). However, an alternative hypothesis based on findings from studies using genetically informative data has been proposed, suggesting that the relationship results from common factors that influence both AAI and AUD (16). From a public health point-of-view, the first hypothesis would support preventive interventions for AUD aimed at delaying AAI. By contrast, the second would predict that such an intervention would have no effect given that it would not reduce the underlying factors influencing AUD.
By using genetically informative data like samples from twins, it is possible to estimate the relative contribution of genetic and environmental factors on individual variations in behavior, traits, and disorders (17). It is also possible to test whether the association between two phenotypes is due to a direct causal effect or the result of common underlying genetic or environmental factors (18).
Twin studies have demonstrated that while AAI is influenced by genetic factors, environmental factors shared by family members, and environmental factors specific to each individual (18–21), AUD is more strongly influenced by genetic factors and, to a lesser extent, by environmental factors shared by family members (21, 22). The heritability of DSM-IV AUD, i.e. the proportion of the observed variance attributable to genetic factors, has been estimated as 50–60% (22–28) and greater than 70% when diagnostic misclassification is considered (29). Results from previous studies using different twin methods indicate that the association between AAI and AUD is due to common genetic factors (16, 18, 30–32).
It is important to replicate these findings, which have significant implications for designing preventive strategies. We do that here in an independent sample with a narrower age range (17 years) than those in previous studies (18, 30, 32) (weighted range, 25.2 years) and thereby reduce the risk of cohort effects. Random measurement error, which could deflate estimates of individual-specific environmental factors common to AAI and AUD, will also be considered. While earlier twin studies explored whether there are both common genetic and environmental factors for AAI and AUD, no study to date has applied a model-fitting approach akin to the comorbidity models described by Neale and Kendler (1995) (33, 34). The advantage of the Neale and Kendler comorbidity models is that a comprehensive set of possible reasons for the comorbidity between disorders can be tested. In the current study we specifically want compare the fit of the “Correlated Liabilities” model and the “A causes B” model of the Neale and Kendler comorbidity models (34).
The aim of the current study is to use data from a population-based sample of young adult male and female twins to test whether the association between AAI and AUD is best explained by a causal or risk indicator mechanism.
Methods
Sample
Study participants came from the Norwegian Institute of Public Heath Twin Panel (NIPHTP) (35, 36). The Norwegian Medical Birth Registry, which contains information about all births in Norway since January 1, 1967, was used to identify twins in the general population. In 1998, a questionnaire was sent to all twins born in Norway between 1967 and 1979. Altogether, 12,700 twins received the questionnaire, and 8,045 responded after one reminder. This included 3,334 complete pairs and 1,377 single responders.
Data for the present report come from a population-based study of psychiatric disorders in Norwegian twins recruited from the NIPHTP (35, 37–39). Between 1999 and 2004, Axis I and Axis II psychiatric disorders were measured using structured clinical interviews in twins born between 1967 and 1979. Participants were recruited among the 3,153 complete pairs of twins who responded to the questionnaire and agreed to participate in an interview study, and 68 pairs of twins drawn directly from the NIPHTP. Of these 3,221 eligible pairs, 0.8% were unwilling or unable to participate, and in an additional 16.2% of pairs only one twin responded to the interview invitation. Altogether, 2,801 twins were assessed with structured interviews for Axis I and Axis II disorders. Of these, 2,794 responses were valid; 128 respondents were from twin pairs where one or both members had not yet initiated alcohol use. These cases were excluded from further analyses. The final sample consisted of 208 monozygotic (MZ) and 113 dizygotic (DZ) pairs of male twins, 430 MZ and 255 DZ pairs of female twins, 324 opposite-sex twin pairs, and 6 single responders. The mean age of participants was 28.7 (range, 19–36) years. Zygosity was determined by a combination of questionnaire items and genotyping. The misclassification rate was estimated at less than 1.0%, which is unlikely to substantially bias the results (40).
Measurements
To measure AAI, we used an item from the 1998 questionnaire where participants were asked how old they were when they felt influenced by alcohol for the first time.
Life-time DSM-IV AUD were assessed using the Composite International Diagnostic Interview (CIDI), developed by the World Health Organization. The CIDI has a good test-retest and inter-rater reliability (41, 42). We used a Norwegian version of the Munich-CIDI (43). Alcohol dependence was assessed independently of alcohol abuse so a participant could be positive for both. The respondents were asked questions about their alcohol use before entering the AUD module of the CIDI. We used two alcohol use items to model AUD as a problem continuum (44). Binge drinking was defined as consuming five or more units of alcohol on a single occasion. Participants were asked how often this occurred. The binge drinking item measured current binge drinking. The second alcohol use item concerned life-time high alcohol consumption (“Have there been periods of your life when you have consumed too much alcohol”). The response alternatives for this item were: no (0), maybe (1), and yes (2).
Interviewers were mostly psychology students in the final part of their training, and experienced psychiatric nurses. They attended a standardized CIDI training program administered by teachers certified by the World Health Organization, and were closely followed up individually during the whole data collection period. The interviews were largely conducted face-to-face. For practical reasons, 231 interviews (8.3%) were done by telephone. Each twin in a pair was interviewed by a different interviewer.
Approval was received from The Norwegian Data Inspectorate and the Regional Ethical Committee, and written informed consent was obtained from all participants after the study was completely described to them.
Statistical analyses
We tested for sex differences in the mean AAI using an independent sample t-test. To calculate male/female risk ratios and chi-square-based confidence intervals, we used “tables for epidemiologists” in STATA. Risk ratios for each category of the ordinal variables were calculated separately.
We used an item response theory (IRT) model for AUD, allowing life-time DSM-IV alcohol abuse, alcohol dependence (without the alcohol abuse criterion), binge drinking frequency, and self-perceived life-time high alcohol consumption to be indicators. The IRT model is a confirmatory factor analysis parameterized using a logit link function where the indicators are categorical variables and the latent variable has a mean of zero and a variance of unity. AAI was kept on a continuous scale with age as integers. To estimate the root mean square error of approximation (RMSEA) (45) and the confirmatory fit index (CFI) (46) for the IRT-model on AUD, we used the WLSMV-estimator in Mplus 6.11 (47). RMSEA values of 0.06 or less and CFI values of 0.95 or more indicate a close fit to the data (48). We used the latent AUD factor in all the following twin analyses and estimated the biometric twin models using full information maximum likelihood for categorical data.
In the classical twin model, individual differences in risk are assumed to originate from three latent causal sources: additive genetic risk; shared environmental factors, comprising all environmental factors contributing to similarity in twins; and individual-specific factors, comprising all environmental exposures not shared by twins. Because MZ twins share all the same genes and DZ twins share on average 50% of the same genes by descent, additive genetic factors contribute twice as much to phenotypic similarity in MZ twins as in DZ twins. By definition, MZ and DZ twins share all of their common environmental factors and none of their individual-specific environmental factors. Individual-specific environment contributes to make MZ twins truly different. Measurement error makes MZ twins appear different when they are not. Measurement error is therefore confounded with individual-specific environment. In the current study, we modelled AUD as a latent variable. Latent variables are without random measurement error; hence, individual-specific environmental influences on the AUD latent variable provide a more accurate estimate of the true size of individual-specific environmental factors for AUD in the population. The latent AUD variable, and thus individual-specific environmental influences on the latent AUD variable, could still comprise measurement error common to all indicators, such as error associated with occasions of measurement or error associated with the interviewer.
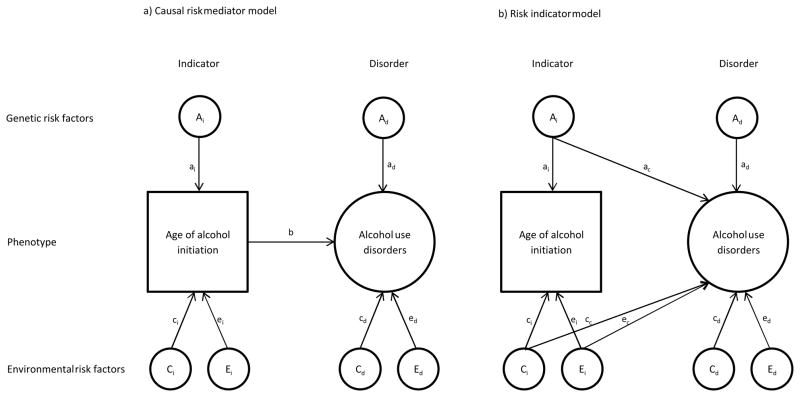
We compared two types of bivariate twin models to test whether AAI influences AUD directly, i.e. as a risk mediator or indirectly, i.e. as a risk indicator for AUD (17). If AAI is directly related to AUD, then the genetic and environmental risk for AUD should be mediated through AAI (Figure 1a, causal model) (17). If AAI is an indicator of genetic or environmental risk for AUD, then the effects should not be mediated, but rather have an effect directly from common genetic and environmental factors for AUD (Figure 1b, risk indicator model). In the first model, there is a direct causal relationship between AAI and AUD as indicated by the arrow (b) in Figure 1a. All covariance between additive genetic, shared environmental, and individual-specific environmental factors for AAI and AUD is mediated through AAI (Figure 1a). In the second model (Figure 1b), AAI and AUD share underlying factors indicated by the arrows ac, cc, and ec. There is no direct relationship between the two, and AAI is therefore an indicator of risk (Figure 1b). While three parameters are used in the risk indicator model to represent the association between the two phenotypes, only one parameter is used in the causal model. According to the principle of parsimony, the causal model should be favored above the risk indicator model if there is no significant difference in fit to the data. We used Akaike’s information criterion, an index of parsimony, calculated as Δχ2-2Δdf (49), to select between the two competing models. To the best of our knowledge, no prior twin studies have directly compared a model specifying a direct relationship between AAI and AUD and a model where the association between AAI and AUD is explained by common underlying genetic and environmental factors.
Figure 1.
Causal risk mediator model (a) and risk indicator model (b) for the association between age of alcohol initiation (AAI) and alcohol use disorders (AUD). Squares denote observed variables (i.e. AAI). Circles denote latent variables. AUD was modelled as a latent variable (not shown). Model (a) is a mediation model, where there is a direct causal relationship between AAI and AUD as indicated by the arrow (b). All covariance between additive genetic, shared environmental, and individual-specific environmental risk factors for AAI and AUD is mediated through AAI. In model (b), AAI and AUD share underlying risk factors indicated by the arrows ac, cc and ec. There is no direct causal relationship between the two, and AAI is therefore an indicator of risk.
We estimated the biometric twin models by full information maximum likelihood using the Mplus statistical software, version 6.11 (47).
Results
AAI did not differ across the sexes, with an average (S.D.) of 16.3 (2.05) years and 16.1 (2.12) years for females and males, respectively. However, men had a significantly higher prevalence of all indicators of AUD, with female/male risk ratios between 1.27 and 4.10 (Table 1). The prevalence of life-time DSM-IV alcohol dependence and abuse was 4.1% and 3.3% for women, and 11.0% and 11.3% for men, respectively. Among men, 8.6% had episodes of binge drinking in which they consumed five or more units of alcohol 1–2 times weekly. The corresponding figure for women was a quarter of that of men (2.1%). While 17.1% of men reported drinking too much at some point in their lives, only 10.7% of women admitted the same.
Table 1.
Descriptives on age of alcohol initiation and indicators of alcohol use disorders in 2666 young adult twins from a population-based twin study.
| Females | Males | Female/male risk ratio (95%CI) | |
|---|---|---|---|
| Age of alcohol initiation, years | 16.3 (2.12)a | 16.1 (2.06)a | - |
| Life-time DSM-IV alcohol dependence | 4.1% (69)b | 11.0% (106)b | 2.71 (2.02 – 3.63) |
| Life-time DSM-IV alcohol abuse | 3.3% (56) | 11.3% (108) | 3.41 (2.49 – 4.66) |
| Binge drinking (5+ units) | |||
| 0–12 times per year | 83.1% (1396) | 66.4% (632) | 0.80 (0.76 – 0.84) |
| 2–4 times per month | 14.8% (249) | 25.0% (238) | 1.69 (1.44 – 1.98) |
| 1–2 times per week or more often | 2.1% (35) | 8.6% (82) | 4.10 (2.88 – 6.37) |
| Self-perceived life-time high alcohol consumption | |||
| No | 80.0% (1346) | 71.2% (676) | 0.89 (0.85 – 0.93) |
| Maybe | 9.3% (157) | 11.8% (112) | 1.26 (1.01 – 1.58) |
| Yes | 10.7% (180) | 17.1% (162) | 1.59 (1.31 – 1.94) |
Mean and SD (all such values).
Percentage and frequency (all such values).
The IRT-model on AUD had a good fit to the data, with a RMSEA of 0.049 (90%CI 0.028 – 0.074) and a CFI of 0.994. The IRT-analysis uncovered moderate to high reliability for the four indicators of AUD (Table 2). The standardized factor loadings were excellent for DSM-IV alcohol dependence and abuse (0.95 and 0.83), indicating that the latent factor is a valid measure of AUD, and moderate for binge drinking and self-perceived life-time alcohol abuse (both 0.65).
Table 2.
Parameter estimates with 95% confidence intervals for the Item Response Theory (IRT) analysis on indicators of alcohol use disorders in a sample of 1336 twin pairs from the general population who have initiated alcohol use.
| Standardized factor loading | Factor loading | Threshold #1 | Threshold #2 | |
|---|---|---|---|---|
| Life-time DSM-IV alcohol dependence | 0.95 (0.91 – 0.99) | 5.24 (3.18 – 7.30) | 6.52 (4.23 – 8.81) | - |
| Life-time DSM-IV alcohol abuse | 0.83 (0.78 – 0.88) | 2.65 (2.14 – 3.16) | 3.96 (3.42 – 4.51) | - |
| Binge drinking: ≥5 units | 0.65 (0.60 – 0.70) | 1.56 (1.35 – 1.77) | 1.12 (0.94 – 1.29) | 3.46 (3.19 – 3.73) |
| Self-perceived life-time alcohol abuse | 0.65 (0.60 – 0.71) | 1.58 (1.35 – 1.77) | 1.06 (0.89 – 1.24) | 2.01 (1.80 – 2.22) |
Mean for men on the AUD risk continuum was fixed to zero, and estimated to be −0.65 (−0.77 to −0.52) for women. The variance of AUD risk continuum was not different across sex, and fixed to unity. The factor loading is the increase in log-odds for the category with an increase on the factor of 1. The first threshold is the log-odds for the category when the factor is at zero. The second threshold is the increase in log-odds beyond threshold 1 for the category when the factor is at zero. The standardized factor loading is the correlation between the latent factor and the observed categorical indicator. All estimates are given with ± standard error. Thresholds for binge drinking were (#1) ‘2–4 times per month’ and (#2) ‘1–2 times per week’ or ‘more often’. For self-perceived life-time alcohol abuse, the thresholds were (#1) ‘maybe’ and (#2) ‘yes’.
In preliminary univariate analyses, we found no qualitative or quantitative sex effects for AAI or AUD. The DZ opposite-sex twin pairs genetic correlation was therefore fixed to 0.5 in the subsequent multivariate models. We proceeded by estimating the additive genetic, shared environmental, and individual-specific environmental parameters as being equal in males and females. Next, in the preliminary univariate analyses, we tested separately for redundancy of the additive genetic, shared environmental, and individual-specific environmental factors for AAI and AUD. The shared environmental factor for AUD was estimated as zero and could be dropped from the model without reducing model fit. The remaining additive genetic, shared environmental, and individual-specific environmental factors all contributed to the model fit.
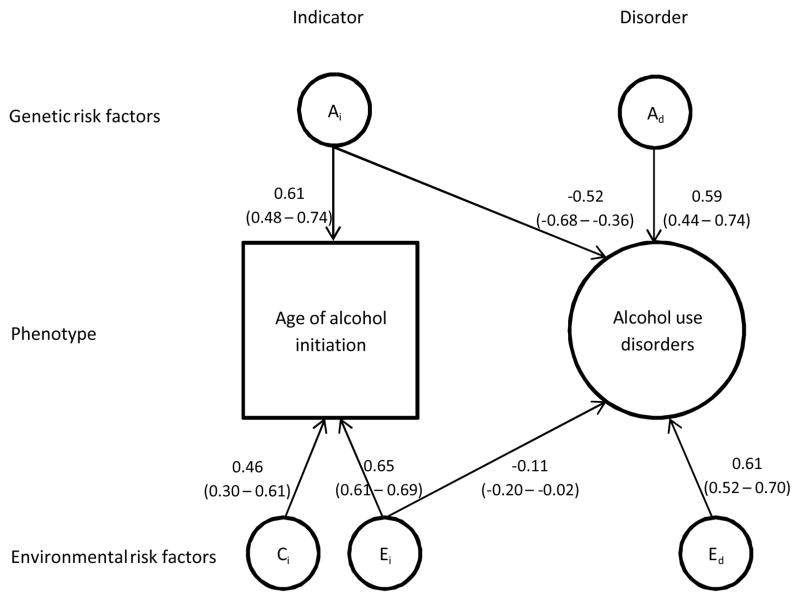
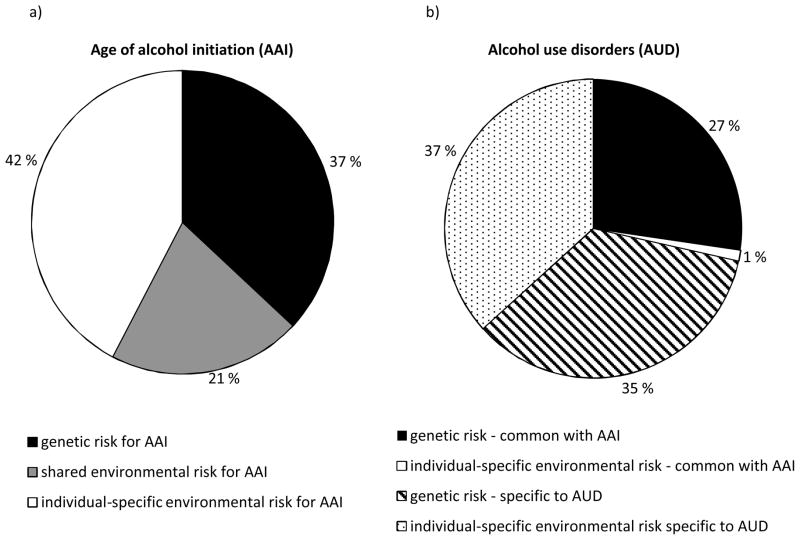
In the bivariate analyses, we first fitted the full bivariate risk indicator model (Table 3; Model #1). The AUD-specific shared environmental factor (cd path) could, as in the preliminary analysis, be dropped from the model (Table 3; Model #2). We then successively tested whether the common additive genetic, shared environmental, and individual-specific environmental paths could be dropped, respectively (Figure 1b, ac, cc, and ec). The common shared environmental path could be eliminated (Table 3; Model #4), but the common additive genetic and individual-specific environmental paths could not be removed without reducing the model fit (Table 3; Models #3 and #5). We tested the causal model (Figure 1a, Table 3; Model #6). This model had an inferior fit compared with the best-fitting risk indicator model (Table 3; Model #4). The parameter estimates for this model are shown in Figure 2. The negative cross loadings in Figure 2 mean that the younger the AAI, the greater the risk for AUD. The squared pathway coefficients indicate the proportion of variance accounted for. The heritability of AAI was found to be 34% (0.582). Twenty-five percent of the variance was accounted for by shared environmental and 44% by individual-specific environmental factors (Figure 2). Figure 3a shows the same results in a pie diagram. The total heritability for AUD was 62%. Figure 3b shows that this includes genetic risk specific to AUD (35%) and genetic risk shared in common with AAI (27%). Genetic factors for AAI accounted for 44% of the total genetic risk for AUD. Individual-specific environmental risk for AAI, however, explained only 1% of the risk for AUD (Figure 3b), and accounted for 3% of the total individual-specific environmental influence on AUD. The correlation between the genetic factors for AAI and the genetic factors for AUD (i.e. the genetic correlation) was −0.66. The corresponding individual-specific environmental correlation was −0.18.
Table 3.
Model fitting of bivariate risk indicator and causal models on the association between age of alcohol initiation and alcohol use disorders in 1336 young adult twins sampled from a population-based registry.
| Parameters estimated
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | ai | ci | ei | c | cc | ec | ad | cd | ed | b | Δχ2 | Δdf | p | AIC |
| 1. Risk indicator modela | x | x | x | x | x | x | x | x | x | |||||
| 2. Risk indicator model | x | x | x | x | x | x | x | x | 0.6 | 1 | 0.56 | −1.4 | ||
| 3. Risk indicator model | x | x | x | x | x | x | x | 5.4 | 2 | 0.07 | 1.4 | |||
| 4. Risk indicator model b | x | x | x | x | x | x | x | 1.1 | 2 | 0.58 | −2.9 | |||
| 5. Risk indicator model | x | x | x | x | x | x | x | 6.2 | 2 | 0.05 | 2.2 | |||
| 6. Causal model | x | x | x | x | x | x | 5.0 | 3 | 0.17 | −1.0 | ||||
Risk indicator model # 1 had a −2loglikelihood of 22867.7 and 25 free parameters. Model #1 serves as a reference for fit of the other models.
Best fitting model. A description of the parameters estimated is given in the legend for Figure 2. The parameters estimated correspond to the ones given in Figure 1a and Figure 1b. The a, c, and e denote additive genetic, shared environmental, and individual-specific environmental risk factors, respectively. Subscript i, c, and d denote indicator-specific effects, effects common for indicator and disorder, and effects specific for disorder, respectively. The b denotes a phenotypic association between the indicator and the disorder. Δχ2 is the difference in −2loglikelihood as compared to the reference model. Δdf is the difference in degrees of freedom as compared with the reference model. Akaike’s information criterion (AIC) is calculated as Δχ2-2Δdf.
Figure 2.
The estimated path coefficients with 95% confidence intervals for the best-fitting risk indicator model. All paths denote standardized factor loadings. Squares denote observed variables (i.e. AAI). Circles denote latent variables. AUD was modelled as a latent variable, but for simplicity the results for the measurement part of the model are shown in Table 2.
Figure 3.
Percentage of variance explained in age of alcohol initiation (AAI) and alcohol use disorders (AUD) by genetic, shared environmental, and individual-specific risk factors.
Discussion
We found that the association between AAI and AUD could best be accounted for by common underlying genetic factors. This is in accordance with findings from previous twin studies using different approaches (16, 18, 30–32). The specific hypothesis postulating a direct causal effect on AUD was not supported in data from young adult twins from the general population. According to the best fitting risk indicator model, AUD was 62% heritable, and almost half of the total genetic risk for AUD could be explained by genetic factors common to AAI, as reflected in the high genetic correlation (−0.66). These results are similar to those reported by Sartor et al. (18) where the heritability of alcohol dependence was 0.53 and the correlation between genetic factors for first alcohol use and alcohol dependence was 0.59. Agrawal et al. (21) also found the genetic factors for AUD and AAI to be substantially correlated (0.50 to 0.95). Our results indicate that AAI is a good indicator of genetic risk for AUD in young adulthood. Environmental factors for AAI proved to be trivial risk factors for AUD. Specifically, shared environmental factors for AAI did not increase the risk for AUD, and individual-specific environmental factors for AAI explained only 1% of the total risk for AUD.
Common genetic and environmental factors for age of alcohol initiation and alcohol use disorders
Recently, Kendler et al. (50) suggested a comprehensive model for AUD focusing on adolescent alcohol use as a precursor for AUD. In a model comprising a range of genetic/temperamental and familial/social factors, they found that alcohol use between ages 15 and 17 years could best be predicted by peer group deviance, alcohol availability, and concurrent conduct disorder. All the former three predictors at this age are somewhat influenced by shared environmental factors (24, 51, 52), and this fits well with our finding that shared environmental factors are of importance for AAI. In the study by Kendler et al. (50), AUD, on the other hand, was, in addition to adolescent alcohol use, best predicted by genetic risk of alcoholism, early onset anxiety disorder, and conduct disorder between ages 15 to 17 years. In prospective cohort studies, the association between early onset of drinking and AUD disappeared after adjusting for family alcoholism history, parental drinking, and conduct problems (53–55). Seen together with findings from multivariate studies of psychiatric comorbidity (24, 56), this indicates that while the common genetic risk for AAI and AUD is shared with a genetic risk for externalizing disorders, specific genetic and environmental risks for AUD could also partly reflect risk for early onset internalizing disorders. Future longitudinal studies investigating the association between AUD and onset of internalizing disorders in young adulthood would therefore be of interest.
Implications
If early AAI is not directly causally related to AUD in adulthood, public health programs aimed at postponing the onset of alcohol use, although good for several reasons, will not be successful in preventing later development of AUD. However, we found that early AAI is a strong indicator of genetic liability to AUD, which could be relevant for designing preventive programs. We also found that environmental factors for AAI and AUD were more or less distinct. This indicates that interventions only targeting adolescent environmental factors impacting on AAI will not likely succeed in preventing the development of AUD. However, if AAI is a moderator of the effect of genetic factors, as found by Agrawal et al. (21), an early age of the first drink may enhance the expression of genes associated with AUD. This suggests that AAI is a modifiable factor requiring further consideration.
Strengths and Limitations
The present study has three main methodological strengths compared with previous studies. First, it was performed in a large population-based sample using structured interviews for the AUD diagnoses. Second, by using latent variable modelling for AUD, we were able to distinguish true individual-specific environmental factors for AUD from measurement error. Life-time DSM-IV AUD contributed most to the AUD latent variable, and the self-report measures of present binge drinking and self-perceived life-time high alcohol consumption contributed to a lesser degree. Since the IRT-model had a good fit to the data, the residual variance for the two latter self-report items was assumed to reflect random error. A life-time measure of binge drinking, which was not available, could have strengthened the study. Third, unlike previous twin studies, which used either the co-twin control method (30, 32) or a single risk indicator model (Choleskey decomposition) (18), we directly compared both a causal and a risk indicator model, which enabled us to determine which best fit the data.
Our results should, however, be interpreted in the context of four potential methodological limitations. First, our sample consisted of young adult Norwegian twins, and these results may not be generalizable to other populations. Although our results are similar to those found in an Australian population with a wider age range (18), the fact that the young adults in our sample could still develop AUD later on might underestimate familial effects. Second, substantial attrition was observed in this sample from the birth registry through three waves of contact. We report detailed analyses of the predictors of non-response across waves elsewhere (57). Briefly, cooperation was strongly predicted by female sex, monozygosity, and higher educational status. Cooperation was not predicted by symptoms of psychiatric disorders. Females have a lower risk for AUD. Therefore, our total sample might be constrained in variance of risk factors for AUD. Third, although we did not find any indications of sex differences in the genetic and environmental influences on AAI and AUD, some, but not all, previous studies have identified such differences (27, 58). If there were true unidentified sex differences in etiology, such as higher heritability in males, our heritability estimates would be downward biased for males and upward biased for females. Finally, it is important to point out that there are a number of inherent limitations in all twin studies using symptom-based data. Our models deal with latent liability factors rather than measurable factors. The methods that are being used can offer only a crude approximation of the likely true underlying genetic and environmental structure.
Acknowledgments
Supported in part by grant MH-068643 from the National Institutes of Health and the Norwegian Research Council (no. 190509), the Norwegian Foundation for Health and Rehabilitation, the Norwegian Council for Mental Health, the European Commission under the program “Quality of Life and Management of the Living Resources” of the Fifth Framework Program (no. QLG2-CT-2002-01254). These funding agencies played no role in the design and conduct of the study, its data collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
The authors declare no conflicts of interest.
The authors declare no conflicts of interest. EY had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Reference List
- 1.Clapper RL, Buka SL, Goldfield EC, Lipsitt LP, Tsuang MT. Adolescent problem behaviors as predictors of adult alcohol diagnoses. Int J Addict. 1995;30:507–23. doi: 10.3109/10826089509048741. [DOI] [PubMed] [Google Scholar]
- 2.Fergusson DM, Lynskey MT, Horwood LJ. Childhood exposure to alcohol and adolescent drinking patterns. Addiction. 1994;89:1007–16. doi: 10.1111/j.1360-0443.1994.tb03360.x. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–90. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen W, Skrondal A. Alcohol consumption debut: Predictors and consequences. J Stud Alcohol. 1998;59:32–42. doi: 10.15288/jsa.1998.59.32. [DOI] [PubMed] [Google Scholar]
- 5.Singer MI, Petchers MK. A biracial comparison of adolescent alcohol-use. Am J Drug Alcohol Abuse. 1987;13:461–74. doi: 10.3109/00952998709001528. [DOI] [PubMed] [Google Scholar]
- 6.Labouvie E, Bates ME, Pandina RJ. Age of first use: Its reliability and predictive utility. J Stud Alcohol. 1997;58:638–43. doi: 10.15288/jsa.1997.58.638. [DOI] [PubMed] [Google Scholar]
- 7.Newcomb MD, Bentler PM. Frequency and sequence of drug use: a longitudinal study from early adolescence to young adulthood. J Drug Educ. 1986;16:101–20. doi: 10.2190/1VKR-Y265-182W-EVWT. [DOI] [PubMed] [Google Scholar]
- 8.Tennant FS, Jr, Detels R, Clark V. Some childhood antecedents of drug and alcohol abuse. Am J Epidemiol. 1975;102:377–85. doi: 10.1093/oxfordjournals.aje.a112176. [DOI] [PubMed] [Google Scholar]
- 9.Dewit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: A risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 10.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 11.Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–46. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- 12.Mcgue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–73. [PubMed] [Google Scholar]
- 13.Nelson CB, Wittchen HU. DSMI-V alcohol disorders in a general population sample of adolescents and young adults. Addiction. 1998;93:1065–77. doi: 10.1046/j.1360-0443.1998.937106511.x. [DOI] [PubMed] [Google Scholar]
- 14.Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol–related problems. Br J Addict. 1992;87:1199–204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 15.Buchmann AF, Schmid B, Blomeyer D, Becker K, Treutlein J, Zimmermann US, et al. Impact of age at first drink on vulnerability to alcohol-related problems: Testing the marker hypothesis in a prospective study of young adults. J Psychiatr Res. 2009;43:1205–12. doi: 10.1016/j.jpsychires.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Prescott CA, Kendler KS. Early age at first alcoholic drink. Am J Psychiatry. 2001;158:1530. doi: 10.1176/appi.ajp.158.9.1530-a. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS, Prescott CA. Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. New York, NY: Guilford Press; 2006. [Google Scholar]
- 18.Sartor CE, Lynskey MT, Bucholz KK, Madden PAF, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: evidence of common genetic influences. Addiction. 2009;104:1512–8. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stallings MC, Hewitt JK, Beresford T, Heath AC, Eaves LJ. A twin study of drinking and smoking onset and latencies from first use to regular use. Behav Genet. 1999;29:409–21. doi: 10.1023/a:1021622820644. [DOI] [PubMed] [Google Scholar]
- 20.Sartor CE, Agrawal A, Lynskey MT, Bucholz KK, Heath AC. Genetic and environmental influences on the rate of progression to alcohol dependence in young women. Alcohol Clin Exp Res. 2008;32:632–8. doi: 10.1111/j.1530-0277.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, et al. Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcoholism-Clinical and Experimental Research. 2009;33:2047–56. doi: 10.1111/j.1530-0277.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of swedish male twins, born 1902 to 1949. Arch Gen Psychiatry. 1997;54:178–84. doi: 10.1001/archpsyc.1997.01830140090015. [DOI] [PubMed] [Google Scholar]
- 23.Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–96. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 24.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 25.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–20. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 26.Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- 27.Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of US twins. Alcohol Clin Exp Res. 1999;23:1136–44. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- 28.True WR, Xian H, Scherrer JF, Madden PAF, Bucholz KK, Heath AC, et al. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–61. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 29.Ystrom E, Reichborn-Kjennerud T, Aggen SH, Kendler KS. Alcohol dependence in men: reliability and heritability. Alcohol Clin Exp Res. 2011;35:1716–22. doi: 10.1111/j.1530-0277.2011.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A noncausal association. Alcohol Clin Exp Res. 1999;23:101–7. [PubMed] [Google Scholar]
- 31.Mcgue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–65. [PubMed] [Google Scholar]
- 32.Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, et al. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med. 2006;36:109–18. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- 33.Rhee SH, Hewitt JK, Lessem JM, Stallings MC, Corley RP, Neale MC. The validity of the Neale and Kendler model-fitting approach in examining the etiology of comorbidity. Behav Genet. 2004;34:251–65. doi: 10.1023/B:BEGE.0000017871.87431.2a. [DOI] [PubMed] [Google Scholar]
- 34.Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. Am J Hum Genet. 1995;57:935. [PMC free article] [PubMed] [Google Scholar]
- 35.Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health twin panel: A description of the sample and program of research. Twin Res. 2002;5:415–23. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 36.Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health twin program of research: An update. Twin Res Hum Genet. 2006;9:858–64. doi: 10.1375/183242706779462886. [DOI] [PubMed] [Google Scholar]
- 37.Kendler KS, Aggen SH, Tambs K, Reichborn-Kjennerud T. Illicit psychoactive substance use, abuse and dependence in a population-based sample of Norwegian twins. Psychol Med. 2006;36:955–62. doi: 10.1017/S0033291706007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichborn-Kjennerud T, Czajkowski N, Torgersen S, Neale MC, Orstavik RE, Tambs K, et al. The relationship between avoidant personality disorder and social phobia: A population-based twin study. Am J Psychiatry. 2007;164:1722–8. doi: 10.1176/appi.ajp.2007.06101764. [DOI] [PubMed] [Google Scholar]
- 39.Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Røysamb E, Neale MC, et al. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. 2008;38:1617–25. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- 40.Neale MC. A finite mixture distribution model for data collected from twins. Twin Res Hum Genet. 2003;6:235–9. doi: 10.1375/136905203765693898. [DOI] [PubMed] [Google Scholar]
- 41.Wittchen HU, Lachner G, Wunderlich U, Pfister H. Test-retest reliability of the computerized DSM-IV version of the Munich Composite International Diagnostic Interview (M-CIDI) Soc Psychiatry Psychiatr Epidemiol. 1998;33:568–78. doi: 10.1007/s001270050095. [DOI] [PubMed] [Google Scholar]
- 42.Wittchen HU. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 43.DIA-X-Interviews: Manual für Screening-Verfahren und Interview; Interviewheft Längsschnittuntersuchung (DIA-X-Lifetime); Ergänzungsheft (DIA-X Lifetime); Interviewheft Querschnittuntersuchung (DIA-X-12 Monate); Ergänzungsheft (DIA-X-12 Monate); PC-Programm zur Durchführung des Interviews (Längs-und Querschnittuntersuchung); Auswertungsprogramm [computer program] Frankfurt: Swets & Zeitlinger; 1997. [Google Scholar]
- 44.Krueger RF, Nichol PE, Hicks BM, Markon KE, Patrick CJ, Iacono WG, et al. Using latent trait modeling to conceptualize an alcohol problems continuum. Psychological Assessment. 2004;16:107–19. doi: 10.1037/1040-3590.16.2.107. [DOI] [PubMed] [Google Scholar]
- 45.Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behav Res. 1990;25:173–80. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- 46.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–46. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 47.Muthén LK, Muthén BO. Mplus User’s Guide. 6. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 48.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- 49.Akaike H. Factor-Analysis and Aic. Psychometrika. 1987;52:317–32. [Google Scholar]
- 50.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for alcohol use disorders in men. Twin Res Hum Genet. 2011;14:1–15. doi: 10.1375/twin.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gillespie NA, Kendler KS, Prescott CA, Aggen SH, Gardner CO, Jacobson K, et al. Longitudinal modeling of genetic and environmental influences on self-reported availability of psychoactive substances: alcohol, cigarettes, marijuana, cocaine and stimulants. Psychol Med. 2007;37:947–59. doi: 10.1017/S0033291707009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world: A developmental twin study of peer-group deviance. Arch Gen Psychiatry. 2007;64:958–65. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warner LA, White HR. Longitudinal effects of age at onset and first drinking situations on problem drinking. Subst Use Misuse. 2003;38:1983–2016. doi: 10.1081/ja-120025123. [DOI] [PubMed] [Google Scholar]
- 54.King KM, Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. Journal of Studies on Alcohol and Drugs. 2007;68:256–65. doi: 10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- 55.Rossow I, Kuntsche E. Early onset of drinking and risk of heavy drinking in young adulthood – 13-year prospective study. Alcohol Clin Exp Res. 2013;37:E297–E304. doi: 10.1111/j.1530-0277.2012.01924.x. [DOI] [PubMed] [Google Scholar]
- 56.Cerda M, Sagdeo A, Johnson J, Galea S. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J Affect Disord. 2010;126:14–38. doi: 10.1016/j.jad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tambs K, Rønning T, Prescott CA, Kendler KS, Reichborn-Kjennerud T, Torgersen S, et al. The Norwegian Institute of Public Health twin study of mental health: Examining recruitment and attrition bias. Twin Res Hum Genet. 2009;12:158–68. doi: 10.1375/twin.12.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Arch Gen Psychiatry. 2000;57:803–11. doi: 10.1001/archpsyc.57.8.803. [DOI] [PubMed] [Google Scholar]