Abstract
Non-adherence to antihypertensive drugs is associated with adverse outcomes; however, mediators of this relationship are poorly understood. We examined the association between the ICD-9 code for medical treatment non-adherence (V15.81) assigned prior to initiation of antihypertensive drug therapy and all-cause mortality in a large cohort of incident hypertensive US veterans. A propensity score-matched cohort of 18,822 patients (9,411 patients with and without a V15.81 code) was generated based on variables predictive of the presence of the V15.81 code to assess its independent association with all-cause mortality during 3.8 years of follow-up. We used Cox models before and after adjustment for antihypertensive drug adherence (measured as the proportion of days covered) and for measures of blood pressure to determine if the association of non-adherence with mortality was mediated through consequences of not following prescribed antihypertensive drugs. At baseline, the mean age of patients was 50.0 years, 91.4% were males, and 33.2% were black. The V15.81 code presence was associated with higher all-cause mortality (HR 1.38, 95%CI 1.26–1.52, p<0.001). Adjustment for medication adherence, blood pressure levels, and blood pressure variability during follow up did not alter the association between the V15.81 code and all-cause mortality (HR 1.35, 95%CI 1.20–1.52, p<0.001). In conclusion, assignment of a V15.81 code prior to antihypertensive drug therapy was associated with higher all-cause mortality in incident hypertensive US veterans and can be useful to identify high-risk patients in administrative databases. This association was not mediated by worse adherence to antihypertensive drugs, or differences in follow-up blood pressure.
Keywords: non-adherence, V15.81, all-cause mortality, antihypertensive drugs
Introduction
Arterial hypertension is one of the most common chronic medical conditions and it is linked to reduced life expectancy and increased cardiovascular disease.1, 2 Despite availability of effective antihypertensive drugs shown to improve patient survival and diminish cardiovascular complications,3, 4 the treatment of hypertension remains suboptimal.5, 6 Medical non-adherence is believed to be an important barrier in improving patients’ outcomes in the general hypertensive population.7, 8
Traditionally, medical adherence of large patient cohorts has been evaluated through analysis of electronic pharmacy databases.9 Medication non-adherence inferred from medication refill patterns has been associated with all-cause mortality10 and cardiovascular events.11–13 However, non-adherence with prescribed antihypertensive medications is only one aspect of a patient’s attitude towards healthcare delivery, and patients may display additional features that could be deleterious, such as non-adherence with healthcare appointments, with other medication classes, with recommended vaccinations, and may pursue self-destructive lifestyles such as unhealthy diets or substance abuse. In the case of hypertensive patients it is thus unclear if non-adherence to antihypertensive dugs (AHDs) as assessed from pharmacy dispensation records provides complete prognostic information. Therefore, we examined weather the presence of the V15.81 code for non-adherence with medical treatment from International Classification of Diseases 9th revision, Clinical Modification (ICD-9-CM) can serve as a global measure of non-adherence, and whether it can identify newly diagnosed hypertensives who might be at higher risk for all-cause mortality. The V15.81 code defined “personal history presenting hazards to health and noncompliance with medical treatment” and was intended to describe non-adherence with medications, refusal of medial procedures, non-adherence or inability to follow medical plan or dietary recommendations. To our knowledge, there are no published reports validating the use of V15.81 code. In order to determine if the association of the V15.81 code with mortality is mediated by the effects of non-adherence with AHDs, we also investigated the association between V15.81 and all-cause mortality after adjusting for actual medication adherence to AHDs, and follow-up blood pressure (BP) as higher BP might be expected in non-adherent patients.
Methods
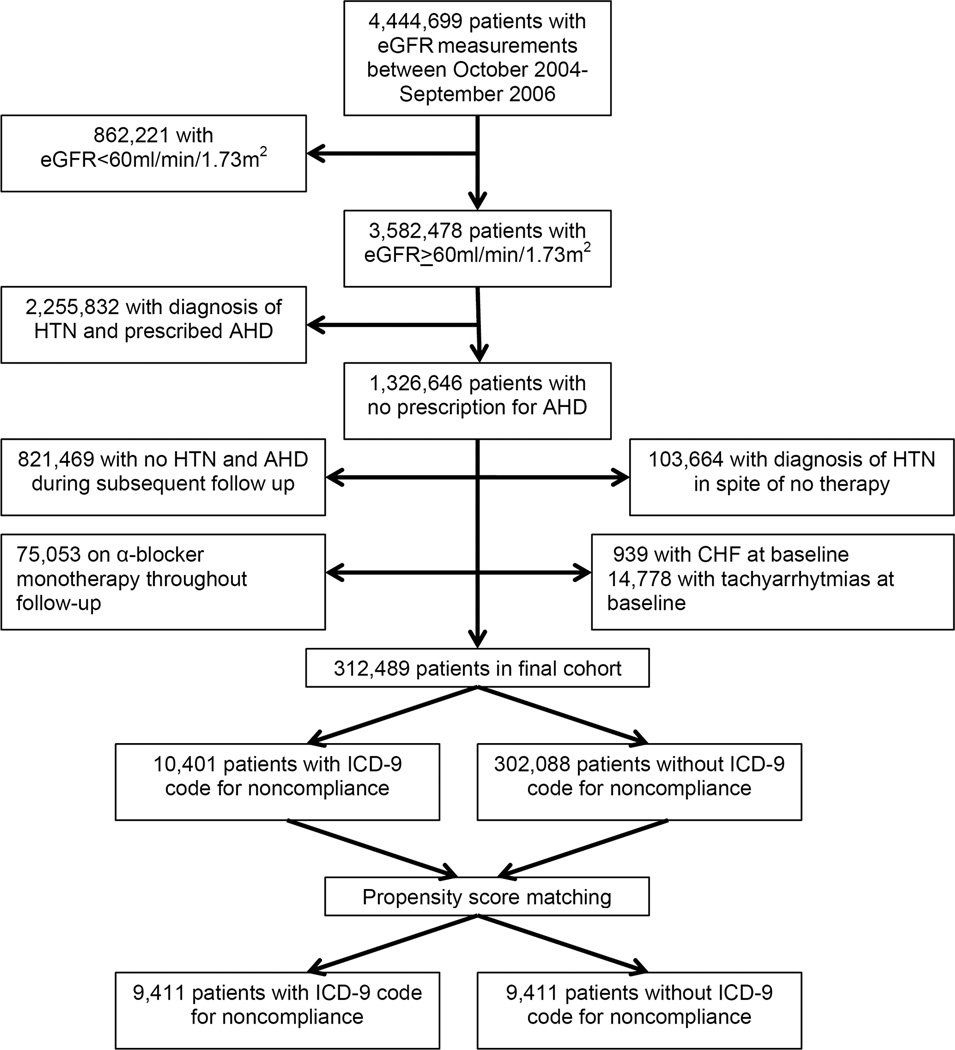
Cohort Definition
The institutional review committees at the Memphis and Long Beach Veterans Affairs Medical Centers approved the study. Our study utilized data from a cohort study examining risk factors in patients with incident CKD (Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study). We used the national Veterans Affairs (VA) Decision Support System National Data Extracts Laboratory Results file to extract data about serum creatinine and identify veterans with normal kidney function on the basis of estimated glomerular filtration rate (eGFR) of >60 ml/min/1.73m2.14 eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation.15 We identified 3,582,478 patients with eGFR>60 ml/min/1.73m2 among a total of 4,444,699 patients with any available eGFR between October 1, 2004 and September 30, 2006. The algorithm for cohort definition is shown in Figure 1. Incident hypertension was defined as the prescription of one or more major AHD classes (α- or β-blockers, calcium channel blockers, thiazide, loop and potassium sparing diuretics, angiotensin converting enzyme inhibitors (ACEi), and angiotensin receptor blockers (ARB)) in the outpatient setting after October 1, 2006, preceded by no prescription of any AHDs during October 1, 2004-September 30, 2006, based on information obtained from VA Pharmacy dispensation records.16 The combined use of all other antihypertensive classes (peripheral vasodilators, other diuretics, carboanhydrase inhibitors and direct renin inhibitors) amounted to only 0.26% of all AHD prescriptions, and hence these were excluded from analyses. We excluded patients on α-blocker monotherapy as we could not ascertain the presence of alternative indications for their use, such as benign prostate hypertrophy. We also excluded patients diagnosed with congestive heart failure (ICD-9-CM codes 428.x) or with tachyarrhytmias (ICD-9-CM codes 427.x). The final cohort included 312,489 patients, among whom we identified 10,401 patients with a V15.81 code prior to initiation of AHDs and 302,088 patients without a V15.81 code. In order to minimize the effect of baseline differences between groups with and without V15.81 on outcomes we generated a propensity score-matched cohort of 18,822 patients (9,411 with and 9,411 without V15.81) for our analysis. In addition, a second propensity score-matched cohort of 40,346 patients was created for a sensitivity analysis, comparing 20,173 patients who were assigned a V15.81 code at baseline or during follow up after the initiation of AHD and 20,173 patients without a V15.81 code.
Figure 1.
Cohort selection flow chart
Socio-demographic characteristics, comorbid conditions and laboratory characteristics were obtained as previously described.17–20 Briefly, information about age, gender, race and BP were obtained through the VA Corporate Data Warehouse (CDW) and from Medicare through the VA-Medicare data merge project.21 Baseline systolic blood pressure (SBP) and diastolic blood pressure (DBP) values were obtained on the date of the first AHD prescription. Longitudinal BP recorded after the date of the first AHD prescription was analyzed as the mean value of all outpatient recordings. The median number of encounters with BP measurements was 17 (interquartile range (IQR) 8–34) overall, with 17 (IQR 8–39) and 13 (IQR 6–24) encounters in patients with and without V15.81, respectively. The median number of BP measurements at each encounter was 1 (IQR 1–2). At the time of this study, the majority of the VA hospitals were utilizing electronic BP monitors.22, 23 Information about comorbidities was collected from the VA Inpatient and Outpatient Medical SAS Datasets20 using ICD-9-CM diagnostic and procedure codes and Current Procedural Terminology codes. Non-adherence was defined as the presence of ICD-9-CM code V15.81 during any inpatient or outpatient encounter preceding the initiation of AHD therapy.
Assessment of medication adherence
Adherence to AHD was estimated as the percentage of days a subject had medication available (proportion of days covered, PDC),24 based on medication dispensation records from any VA pharmacy. PDC was calculated as the ratio of the total number of pills and the number of days between the first fill of the medication and the end of the evaluation period. We calculated PDC during the first year after AHD initiation and for the entire follow up period. Results were essentially identical; therefore, for all analyses we used PDC calculated during the first 12 months of AHD therapy. In patients prescribed several AHDs, PDC was calculated as the mean PDC of individual AHDs. Patients were grouped into the following adherence levels: low (PDC<40%), intermediate (PDC 40–79%), and high (PDC >80%).13
Outcome
Data on all-cause mortality was obtained from the VA Vital Status Files (VSF), which contain dates of death or last medical/administrative encounter from all sources in the VA system with sensitivity and specificity of 98.3% and 99.8%, respectively, as compared to the National Death Index as gold standard.25
Statistical Analysis
Descriptive analyses were performed and skewed variables were log-transformed. The start of the follow-up period was the date of initial AHD prescription. Patients were followed until death or were censored at the date of last healthcare or administrative visit, or on July 26, 2013.
The propensity score method was used to account for baseline differences arising from dissimilarities in clinical and demographic characteristics of patients with the V15.81 code. This method allows for the generation of a single variable representing the likelihood of an individual patient having been assigned the V15.81 code based on the presence or absence of defined clinical characteristics in each individual.26 Patients with and without V15.81 can then be matched based on similar propensity scores to secure that its predictors are similar in the two groups, and to eliminate confounding the association of V15.81 with the studied outcome due to baseline differences between the two groups. Variables associated with V15.81 were identified using logistic regression and were used to calculate propensity scores. Stata’s “psmatch2” command suite was used to generate the propensity score-matched cohorts by a 1-to-1 nearest neighbor matching without replacement.
The association of V15.81 with mortality was assessed using the Kaplan-Meier method and Cox regression. The V15.81 code may represent a broad category of non-adherent patient behavior that could include both medication non-adherence and non-adherence with prescribed treatment plans, diets or lifestyle. Therefore, patients with V15.81 may be more likely to have higher rates of AHD non-adherence with subsequent higher chance of differences in follow-up BP and BP variability. We hypothesized that medication non-adherence and follow-up BP could be mediators of V15.81’s effects on all-cause mortality. Hence, we adjusted our models for PDC, mean follow-up systolic and diastolic BP and BP variability (defined as the median absolute deviation (MAD) of systolic and diastolic BP).
Analyses were repeated in the entire cohort by examining crude and adjusted associations of V15.81 with all-cause mortality in Cox models. Models were adjusted for the following confounders based on a priori considerations: age, gender, race-ethnicity, marital status, mean income level, service-connectedness (a measure indicating whether one or more of a patient’s comorbidities were caused by their military service, and resulting in certain privileges such as preferential access to care and lower co-payments), baseline eGFR, BMI, SBP and DBP, and comorbid conditions (diabetes, coronary artery disease-CAD, peripheral artery disease-PVD, chronic lung disease, dementia, liver disease, malignancies, HIV/AIDS and depression).
Statistical analyses were performed using STATA MP Version 12 (STATA Corporation, College Station, TX).
Results
Baseline Characteristics
The mean (SD) age of the full cohort of 312,489 patients was 53.8 (12.6) years, 90.9% were males, and 20.3% were African American. Baseline characteristics of the overall cohort and of patients categorized by the presence or absence of V15.81 (overall and after propensity score matching) are shown in Table 1. Groups with and without V15.81 were significantly different in all baseline characteristics; however, these differences were no longer present after propensity-score matching. Among the 312,489 patients of the full cohort those with V15.81 were younger, more likely to be African-American, and to be unmarried. The V15.81 “+” group also had a higher eGFR and lower SBP, a higher prevalence of diabetes, chronic lung and liver disease and depression, and a lower prevalence of malignancies. Table 2 shows the predictors of V15.81 in a multivariable logistic regression analysis. Younger age, male gender, unmarried status, African American race-ethnicity, and lower income were associated with the presence of V15.81. Additionally, patients with chronic medical conditions such as diabetes, CAD, PVD, chronic lung and liver disease, HIV, and depression were more likely to have a V15.81 code.
Table 1.
Baseline characteristics of individuals stratified by ICD-9 code for medical treatment non-adherence
| Baseline Characteristics |
Total Cohort | Presence of V15.81 code* |
Absence of V15.81 code* |
Presence of V15.81 code† |
Absence of V15.81 code† |
P value‡ |
|---|---|---|---|---|---|---|
| (N=312, 489) | (N=10,401) | (N= 302,088) | (N=9,411) | (N= 9,411) | ||
| Age, mean (SD), year | 53.8 (12.6) | 50.1 (11.0) | 54.0 (12.7) | 49.9 (10.9) | 50.1 (11.9) | 0.3 |
| Gender, males, n (%) | 284,105 (90.9) | 9,415 (90.5) | 274,690 (90.9) | 8,561 (91.0) | 8,629 (91.7) | 0.08 |
| Race, n (%) | ||||||
| African-American | 9,389 (20.3) | 3,370 (33.3) | 56,019 (19.9) | 3,153 (33.5) | 3,096 (32.9) | 0.4 |
| Non-African | 303,100 (79.7) | 6,764 (66.7) | 225,831 (80.1) | 6,258 (66.5) | 6,315 (67.1) | |
| American | ||||||
| Marital Status, n (%) | ||||||
| Married | 146,441 (48.7) | 3,044 (30.7) | 143,397(49.4) | 2,800 (29.8) | 2,758 (29.3) | 0.5 |
| Non-married | 166,048 (51.3) | 6,861 (69.3) | 147,132 (50.6) | 6,611(70.3) | 6,653 (70.7) | |
| Income, median, $ | 19,851 | 15,489 | 20,009 | 15,275 | 15,308 | 0.7 |
| eGFR, mean (SD), ml/min/1.73m2 |
88.5 (15.8) | 93.4 (16.9) | 88.3 (15.8) | 93.6 (16.9) | 93.3 (16.8) | 0.3 |
| BMI, mean (SD) (kg/m2) | 28.6 (5.4) | 28.0 (5.6) | 28.6 (5.4) | 27.9 (5.6) | 27.9 (5.2) | 0.8 |
| SBP, mean (SD), mmHg | 139.4 (18.7) | 136.6 (19.4) | 139.5 (18.7) | 134.5 (17.8) | 134.2 (17.1) | 0.3 |
| DBP, mean (SD), mmHg | 83.4 (12.8) | 83.5 (13.2) | 83.4 (12.8) | 82.5 (12.3) | 82.6 (12.3) | 0.6 |
| Diabetes, n (%) | 34,383 (11.0) | 2,124 (20.4) | 32,259 (10.7) | 1,920 (20.4) | 1,954 (20.8) | 0.5 |
| CAD, n (%) | 6,678 (2.1) | 209 (2.0) | 6,469 (2.1) | 193 (2.1) | 181 (1.9) | 0.5 |
| Cerebrovascular disease, n (%) |
6,465 (2.1) | 245 (2.4) | 6,220 (2.1) | 224 (2.4) | 218 (2.3) | 0.8 |
| PVD, n (%) | 6,457 (2.1) | 230 (2.2) | 6,227 (2.1) | 219 (2.3) | 444 (2.2) | 0.9 |
| Chronic lung disease, n (%) |
47,462 (15.2) | 1,964 (18.9) | 45,498 (15.1) | 1,785 (19.0) | 1,756 (18.7) | 0.6 |
| Dementia, n (%) | 599 (0.2) | 32 (0.3) | 567 (0.2) | 32 (0.3) | 22 (0.2) | 0.2 |
| Chronic liver disease, n (%) |
2,655 (0.9) | 150 (1.4) | 2,505 (0.8) | 136 (1.5) | 131 (1.4) | 0.8 |
| Malignancies, n (%) | 18,895 (6.1) | 455 (4.4) | 18,440 (6.1) | 419 (4.5) | 429 (4.6) | 0.7 |
| HIV, n (%) | 3,256 (1.0) | 361 (3.5) | 2,895 (1.0) | 326 (3.4) | 306 (3.3) | 0.4 |
| Depression, n (%) | 37,711 (12.1) | 2,971 (28.6) | 34,740 (11.5) | 2,731 (29.0) | 2,736 (29.1) | 0.9 |
Footnotes: SD, standard deviation;
all p values between groups were significant;
propensity score matched cohort stratified by ICD-9 code for non adherence (V15.81),
p-value compares propensity score matched groups with and without ICD-9 code for non adherence; V15.81, ICD-9 code for non adherence; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; CAD, coronary artery disease; PVD, peripheral vascular disease; HIV, human immunodeficiency virus
Table 2.
Odds ratios (95% confidence intervals) of V15.81 code assignment in newly treated hypertensive veterans
| Baseline Characteristics | Odds ratio | 95% CI | P -value |
|---|---|---|---|
| Age (each +10 years) | 0.84 | 0.83–0.86 | <0.001 |
| Gender, female versus male | 0.70 | 0.65–0.75 | <0.001 |
| Race | |||
| African American (vs. non-African-American) | 1.70 | 1.62–1.78 | <0.001 |
| Marital Status | |||
| Unmarried (vs. Married) | 1.65 | 1.57–1.73 | <0.001 |
| Income (for 1 log unit) | 0.88 | 0.87–0.90 | <0.001 |
| eGFR (each +10 ml/min/1.73m2) | 1.05 | 1.03–1.06 | <0.001 |
| BMI (each +10 kg/m2) | 0.80 | 0.77–0.83 | <0.001 |
| SBP (each +10 mmHg) | 0.93 | 0.91–0.94 | <0.001 |
| DBP (each +10 mmHg) | 1.03 | 1.00–1.05 | 0.039 |
| Diabetes (presence versus absence) | 2.35 | 2.25–2.44 | <0.001 |
| Coronary artery disease | 1.23 | 1.06–1.43 | 0.006 |
| Cerebrovascular disease | 1.26 | 1.09–1.45 | 0.001 |
| Peripheral artery disease | 1.17 | 1.02–1.35 | 0.025 |
| Chronic lung disease | 1.32 | 1.25–1.39 | <0.001 |
| Dementia | 2.07 | 1.43–2.99 | <0.001 |
| Chronic liver disease | 1.29 | 1.08–1.54 | 0.005 |
| Any malignancy | 0.93 | 0.83–1.02 | 0.13 |
| HIV | 2.04 | 1.81–2.31 | <0.001 |
| Depression | 2.64 | 2.51–2.77 | <0.001 |
Footnotes: Odds ratios of presence of V15.81 code were calculated from multivariate regression model; eGFR, estimated glomerular filtration rate; BMI, body mas index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HIV, human immunodeficiency virus
Follow up Blood Pressure
Initiation of AHD led to a reduction in both systolic and diastolic BP during follow-up. In the propensity-score matched cohort, mean (SD) SBP was lowered to 129.5 (11.3) mmHg and 130.1 (11.1) mmHg in patients with and without V15.81, respectively (p<0.001). However, the lower SBP in patients with V15.81 was associated with a higher mean (SD) SBP variability, compared to the group without V15.81: 8.3 (3.9) mmHg versus 8.0 (3.7) mmHg, respectively (p<0.001). Similarly, follow-up mean (SD) DBP was lower with higher mean (SD) DBP variability in patients with V15.81. Mean follow-up (SD) DBP and DBP variability were 79.0 (8.0) mmHg and 5.5 (2.4) mmHg in patients with V15.81, and 79.3 (8.3) mmHg and 5.4 (2.4) mmHg in patients without V15.81 (p values of 0.008 and 0.001, respectively).
Medication Adherence
In the overall cohort of 312,489 patients, good adherence to AHD during the 1st year of follow up as defined by the proportion of patients with a mean PDC >80% was higher in patients without V15.81 (74.5%), compared to patients with V15.81 (67.5%) (p<0.001). Results were similar in the propensity-score matched cohort: PDC>80% was observed in 67.5% and 71.6% of patients with and without V15.81, respectively (p<0.001). During subsequent follow-up beyond the first year, adherence to AHD declined in both groups but remained higher in patients without V15.81: PDC>80% was seen in 54.2% of patients with V15.81 as compared to 61.0% of patients without V15.81 (p<0.001).
The majority of patients (95.6%) in the propensity-matched cohort were receiving 2 or less AHDs: 77.2% were on a single AHD, 18.4% on 2 AHDs, 3.7% on 3 AHDs, and less than 1% on >4 AHDs. There were no differences in percentages of patients who were receiving 1, 2, and >3 AHD in the groups with and without V15.81 code (p=0.6).
All-cause mortality
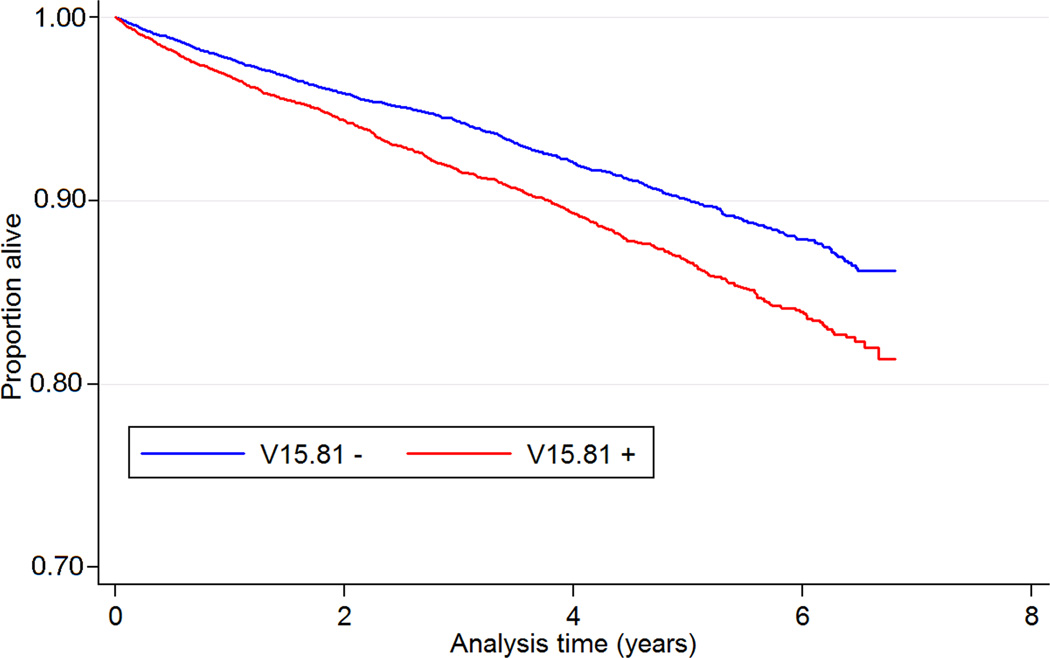
A total of 1,717 patients (9.1%, mortality rate 24.9 [23.8–26.1]/1000 patient-years) died during a median follow up of 3.8 years in the propensity-score matched cohort. There were 937 deaths in the group with V15.81 (10.0%, mortality rate 29.2 [27.4–31.1]/1000 patient-years) and 780 deaths in the group without V15.81 (8.3%, mortality rate 21.2 [19.8–22.8]/1000 patient-years). The presence of V15.81 was associated with 38% higher risk of all-cause mortality (HR 1.38, 95% CI 1.26–1.52, p<0.001). Figure 2 shows Kaplan-Meier curves for all-cause mortality in groups with and without V15.81.
Figure 2.
Kaplan-Meier survival curves of 9,411 patients with and 9,411 patients without V15.81 code matched by propensity scores
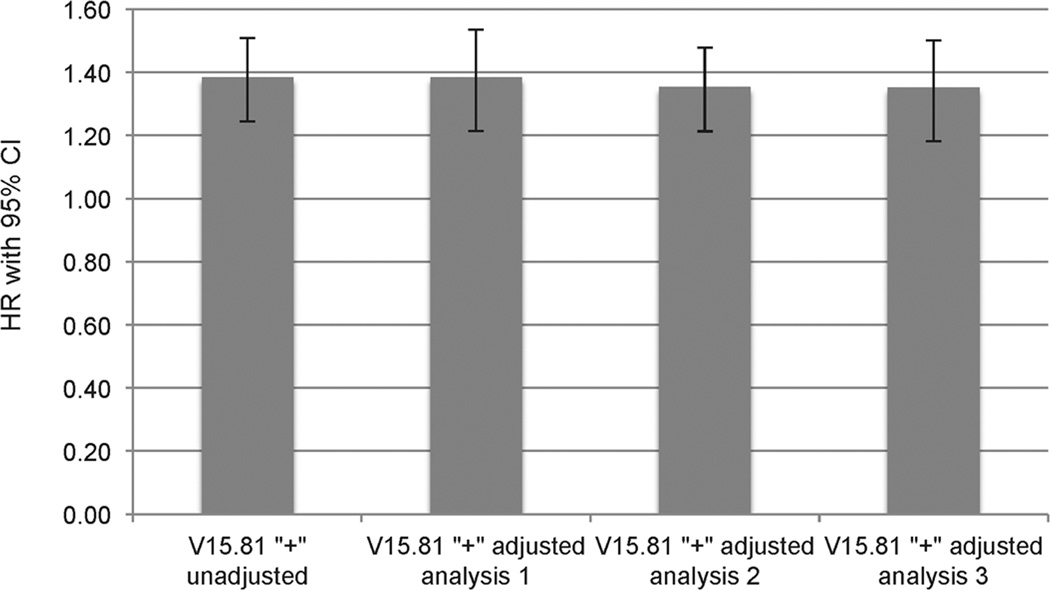
Figure 3 shows the multivariable adjusted hazard ratios for all-cause mortality associated with V15.81 in the propensity-score matched cohort after adjustment for PDC, follow-up SBP and DBP, and corresponding BP variability. The inclusion of PDC, SBP, DBP, and BP variability into the adjusted model did not alter the association between V15.81 and all-cause mortality (adjusted HR 1.35, 95%CI 1.20–1.52, p<0.001).
Figure 3.
Hazard ratios (95% confidence intervals) of all-cause mortality of propensity-score matched group with V15.81 code using unadjusted analysis and various adjusted models
Footnotes: V15.81 group served as a reference group for all analyzes; HR, hazard ratio; CI, confidence interval; adjusted analysis 1, all-cause mortality adjusted for the anti-hypertensive drug adherence assessed by the proportion of days covered (PDC); adjusted analysis 2, all-cause mortality adjusted for the mean follow up systolic and diastolic blood pressure (BP), and mean follow up systolic and diastolic BP variability; adjusted analysis 3, adjusted analysis 2, all-cause mortality adjusted for the anti-hypertensive drug adherence assessed by the proportion of days covered (PDC), the mean follow up systolic and diastolic blood pressure (BP), and mean follow up systolic and diastolic BP variability.
We additionally performed a sensitivity analysis where the association with all-cause mortality was studied in patients who had the V15.81 code assigned not just at baseline, but also during follow-up using a propensity score-matched cohort consisting of 20,173 patients with V15.81 and 20,173 patients without V15.81. Similarly to patients with V15.81 assigned at baseline, the cohort of patients combining V15.81 codes assigned at baseline and during follow up had 21% higher all-cause mortality compared to patients with no V15.81 (HR 1.21, 95%CI 1.14–1.29, p<0.001). However, in contradistinction to the results of our primary analysis, the inclusion of PDC, SBP, DBP and BP variability during follow up into the adjusted model lead to attenuation of the association between V15.81 and all-cause mortality (HR 1.10, 95%CI 0.99–1.15, p=0.09).
Discussion
We examined patient-related demographic and clinical characteristics associated with the presence of the ICD-9-CM code for non-adherence with medical treatment (V15.81), and its effect on all-cause mortality in a large cohort of US veterans with newly treated hypertension. Similarly to previous reports about the impacts of race27, 28 and age29 on adherence, we found that African-Americans and younger patients had a higher prevalence of V15.81. Although female gender has been linked to lower adherence,28, 29 in our study male veterans were more likely to have the V15.81 code. Female veterans are likely to be different from females in the general population. Better life ratings were reported in female veterans as compared with male counterparts;30 and personal optimism, in turn, might be associated with better medical adherence. Depression is a well-described barrier to medical adherence,31 and we similarly observed that concomitant depression was strongly associated with the presence of V15.81 code. In addition to depression, other comorbidities strongly associated with V15.81 were diabetes, dementia and HIV/AIDS, suggesting that patients suffering from conditions predisposing to suboptimal behavior or that carry poor survival are especially prone to non-adherence.
The presence of V15.81 prior to initiation of AHD therapy was independently associated with higher all-cause mortality. Despite being an integral part of successful treatment of chronic medical conditions, measurement of adherence has no accepted gold standard method.32 In general, adherence includes many components, such as adherence to medications, non-pharmacologic aspects such as healthy life style and diet, adherence to medical appointments, and receptiveness to follow medical advice to modify risky behaviors or dangerous habits. In addition, adherence to medical treatment results from a complex interaction of several elements: patient-related (demographic, social, and medical condition-specific factors), provider and healthcare-delivery specific (accessibility, restricted formulary, provider-patient communications, continuity of care), and treatment-specific (cost, complexity of a regimen, adverse effects, lack of immediate effects of therapy).33 Traditionally, studies that evaluated effects of adherence and health-related outcomes in hypertensive patients involved the assessment of adherence via evaluation of AHD-usage behavior through analysis of pharmacy dispensation databases or self-reported adherence.34–36 Because medication adherence is only a single component of adherent behavior, it has limited ability to assess non-pharmacologic components of adherence, or pharmacologic components related to non-AHD type medications. Hence, the presence of V15.81 could be a more inclusive marker of non-adherence.
It is important to consider mechanisms responsible for the worse survival in patients with V15.81 in order to implement appropriate interventions and improve patient outcomes. Pharmacological non-adherence was demonstrated as an important barrier in achieving treatment goals in the general hypertensive population.7, 8 We only included V15.81 codes assigned prior to the initiation of AHDs in our cohort, which was thus not affected by future adherence patterns to AHDs, but such future non-adherence to AHDs could be expected in patients displaying non-adherent behavior in other areas. Therefore, we investigated adherence to AHDs as a possible mediator of increased mortality in patients with V15.81. Indeed, lower adherence to AHDs was present in V15.81+ patients; however, it was insufficient to explain the difference in mortality among patients with V15.81 in adjusted analysis. Difference in follow-up BP control is another possible intermediate that could lead to increased mortality in patients with V15.81. Patients with and without V15.81 at baseline had good overall BP control during follow-up according to recent hypertension guidelines,2, 37 with an absolute difference in treated SBP and DBP of less than 1mmHg between the two groups, but with a larger difference in BP variability. Nevertheless, the association between V15.81 and all-cause mortality was unchanged after adjustment for follow-up BP characteristics. These results suggest that newly diagnosed hypertensive individuals with V15.81 exhibit clinically important non-adherent behavior beyond AHD adherence or BP control. Contrasting these findings, once we considered V15.81 codes that were assigned after initiation of AHD therapy, the association between V15.81 and mortality was attenuated after adjustment for AHD adherence and follow-up BP levels. This highlights the complexity of the interaction between V15.81 and all-cause mortality depending on whether it was assigned before or after the initiation of AHD therapy. Consequently, it is essential to have a global approach to adherence beyond focusing on just BP targets and AHD adherence, and to consider non-pharmacologic components of adherence.
Strength and Limitations
The current study has a large sample size representative of veterans from the entire geographic US. However, several limitations need to be acknowledged. No conclusion about causality can be drawn from this study. Therefore, while the presence of V15.81 was associated with 38% higher all-cause mortality, we cannot conclude that any particular aspect of non-adherence was indeed directly responsible for the worse survival in newly treated hypertensive veterans. We used the propensity-score matching method to minimize the effects of bias arising from baseline differences between cohorts with and without V15.81; however, even this method may not fully address unmeasured confounders38 in an observational study. The assignment of V15.81 code is not standardized, and its sensitivity and specificity are unknown. Because non-adherence is in itself a vaguely defined term that has no gold standard diagnostic test, any measure used to describe it would be inherently uncertain. The study population consisted mainly of male veterans; therefore, the results of the study may not be generalized to female patients. We did not collect information about other clinically relevant outcomes such as cardiovascular events and hospitalizations and we had no information about causes of death; thus we could not test the association of the V15.81 code with such outcomes. Lastly, we did not have information about smoking and alcohol use history that may be associated with medical treatment non-adherence and which could also influence all-cause mortality.
Perspectives
In this large cohort of 312,489 incident hypertensive individuals, the V15.81 code for medical treatment non-adherence was associated with worse survival. Given the importance of non-adherence in causing adverse health outcomes and increased medical costs,39, 40 providers should closely monitor patients who bear a V15.81 code. Further research is needed to identify modifiable patient characteristics that might have led to the assignment of the V15.81 code and to determine which aspects of non-adherence are responsible for the observed adverse association in order to implement interventions that could improve outcomes in hypertensive individuals.
Novelty and Significance.
What is new? This is the first study to investigate the association between ICD-9-CM code for medical non-adherence (V15.81) and all-cause mortality in newly treated hypertensive veterans.
What is relevant? The understanding of modifiable patient’s characteristics leading to medical non-adherence is critical for the development of strategies that could improve outcomes in individuals affected by hypertension.
Summary: Assignment of a V15.81 code prior to AHD therapy was associated with higher all-cause mortality in incident hypertensive US veterans and this association was not mediated by worse adherence to AHD, or differences in follow-up BP.
Acknowledgments
Funding Sources
This study is supported by grant 1R01DK096920 from the NIH to CPK and KKZ, and by resources from the US Department of Veterans Affairs.
Footnotes
Disclosures
CPK is an employee of the Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part, except for abstract submission to the 46th Annual American Society of Nephrology Conference, November 11–14, 2014, Philadelphia, PA.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association. Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr., Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA : the journal of the American Medical Association. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3.fficers A, Coordinators for the ACRGTA, Lipid-Lowering Treatment to Prevent Heart Attack T. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat) JAMA : the journal of the American Medical Association. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 4.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: A network meta-analysis. JAMA : the journal of the American Medical Association. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 5.Yoon PW, Gillespie CD, George MG, Wall HK, Centers for Disease C, Prevention Control of hypertension among adults--national health and nutrition examination survey, united states, 2005–2008. MMWR. Morbidity and mortality weekly report. 2012;(61 Suppl):19–25. [PubMed] [Google Scholar]
- 6.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among united states adults with hypertension: The national health and nutrition examination survey, 2001 to 2010. Circulation. 2012;126:2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 7.Elliott WJ. Improving outcomes in hypertensive patients: Focus on adherence and persistence with antihypertensive therapy. Journal of clinical hypertension. 2009;11:376–382. doi: 10.1111/j.1751-7176.2009.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke TA, Sturkenboom MC, Lu SE, Wentworth CE, Lin Y, Rhoads GG. Discontinuation of antihypertensive drugs among newly diagnosed hypertensive patients in uk general practice. Journal of hypertension. 2006;24:1193–1200. doi: 10.1097/01.hjh.0000226211.95936.f5. [DOI] [PubMed] [Google Scholar]
- 9.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: Its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 10.Esposti LD, Saragoni S, Benemei S, Batacchi P, Geppetti P, Di Bari M, Marchionni N, Sturani A, Buda S, Esposti ED. Adherence to antihypertensive medications and health outcomes among newly treated hypertensive patients. ClinicoEconomics and outcomes research : CEOR. 2011;3:47–54. doi: 10.2147/CEOR.S15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 12.Breekveldt-Postma NS, Penning-van Beest FJ, Siiskonen SJ, Falvey H, Vincze G, Klungel OH, Herings RM. The effect of discontinuation of antihypertensives on the risk of acute myocardial infarction and stroke. Current medical research and opinion. 2008;24:121–127. doi: 10.1185/030079908x253843. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA : the journal of the American Medical Association. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 14.Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Annals of internal medicine. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Us department of veterans affairs, va information resource center (virec) Virec research user guide: Vha pharmacy prescription data. 2nd. Hines, IL: VIReC; 2008. [Google Scholar]
- 17.Molnar MZ, Kalantar-Zadeh K, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Quarles DL, Kovesdy CP. Ace inhibitor and angiotensin receptor blocker use and mortality in patients with chronic kidney disease. Journal of the American College of Cardiology. 2014;63:650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K. Outcomes associated with microalbuminuria: Effect modification by chronic kidney disease. Journal of the American College of Cardiology. 2013;61:1626–1633. doi: 10.1016/j.jacc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virec research user guide; vha medical sas inpatient datasets fy2006–2007. Hines, IL: U.S. Department of Veterans Affairs. VA Information Resource Center; 2007. [Google Scholar]
- 21.Us department of veterans affairs va information resource center data quality update: Race [Google Scholar]
- 22.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: Where and how many measures? Annals of internal medicine. 2011;154:781–788. doi: 10.7326/0003-4819-154-12-201106210-00005. W-289-790. [DOI] [PubMed] [Google Scholar]
- 23.Nord J, Stults B, Rose R, Underwood AE, Williams T, West G, Huhtala T, Milne CK. Optimizing office blood pressure measurement at a vamc. Fed Pract. 2012;29:35–39. [Google Scholar]
- 24.Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, Solomon DH. Measuring concurrent adherence to multiple related medications. The American journal of managed care. 2009;15:457–464. [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold NSM, Maynard C, Hynes DM. Virec technical report 2: Va-ndi mortality data merge project. In: Center vir, editor. Hines, IL: 2006. [Google Scholar]
- 26.D’Agostino RB., Jr. Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 27.Gerber BS, Cho YI, Arozullah AM, Lee SY. Racial differences in medication adherence: A cross-sectional study of medicare enrollees. The American journal of geriatric pharmacotherapy. 2010;8:136–145. doi: 10.1016/j.amjopharm.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewey J, Shrank WH, Bowry AD, Kilabuk E, Brennan TA, Choudhry NK. Gender and racial disparities in adherence to statin therapy: A meta-analysis. American heart journal. 2013;165:665–678. doi: 10.1016/j.ahj.2013.02.011. 678 e661. [DOI] [PubMed] [Google Scholar]
- 29.Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10-year persistence with antihypertensive drugs. Journal of hypertension. 2005;23:2101–2107. doi: 10.1097/01.hjh.0000187261.40190.2e. [DOI] [PubMed] [Google Scholar]
- 30.Gallup healthways well-being index. GALLUP. 2012 [Google Scholar]
- 31.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of internal medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 32.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. Journal of clinical epidemiology. 2001;(54 Suppl 1):S57–60. doi: 10.1016/s0895-4356(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 33.Organization WH. Adherence to long-term therapies -evidence for action. 2003 [Google Scholar]
- 34.Turner BJ, Hecht FM. Improving on a coin toss to predict patient adherence to medications. Annals of internal medicine. 2001;134:1004–1006. doi: 10.7326/0003-4819-134-10-200105150-00015. [DOI] [PubMed] [Google Scholar]
- 35.Vitolins MZ, Rand CS, Rapp SR, Ribisl PM, Sevick MA. Measuring adherence to behavioral and medical interventions. Controlled clinical trials. 2000;21:188S–194S. doi: 10.1016/s0197-2456(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 36.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. Journal of clinical epidemiology. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 37.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Task Force M. 2013 esh/esc guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of hypertension (esh) and of the european society of cardiology (esc) Journal of hypertension. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 38.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate behavioral research. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgson TA, Cai L. Medical care expenditures for hypertension, its complications, and its comorbidities. Medical care. 2001;39:599–615. doi: 10.1097/00005650-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Medical care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]