Abstract
Importance
Current treatments for cystoid macular edema in retinitis pigmentosa are not always effective, may lead to adverse side effects, and may not restore loss of visual acuity. The present research lays the rationale for evaluating whether an iodine supplement could reduce cystoid macular edema in retinitis pigmentosa.
Objective
To determine whether central foveal thickness in the presence of cystoid macular edema is related to dietary iodine intake inferred from urinary iodine concentration in non-smoking adults with retinitis pigmentosa.
Design
Cross-sectional study.
Setting
Institutional referral center.
Participants
Non-smoking adult patients with retinitis pigmentosa (n = 212, ages 18 to 69 years) with a visual acuity ≥ 20/200 in at least one eye.
Main outcome measure
The relationship of log central foveal thickness measured by optical coherence tomography to urinary iodine concentration measured from multiple spot samples and represented as a 3-level classification variable (< 100 μg/L, 100 μg/L - 199 μg/L, and ≥ 200 μg/L), assigning greater weight to patients with more reliable urinary iodine concentration estimates.
Results
Analyses were limited to 199 patients after excluding 11 patients who failed to return urine samples for measuring urinary iodine concentration and 2 outliers for urinary iodine concentration. Thirty-six percent of these patients had cystoid macular edema in one or both eyes. Although log central foveal thickness was inversely related to urinary iodine concentration based on all patients (p = 0.02), regression of log central foveal thickness on urinary iodine concentration separately for patients with and without cystoid macular edema showed a strong inverse significant relationship for the former group (p < 0.001) and no significant relationship for the latter group as tested (p = 0.66). In contrast, we found no significant association between cystoid macular edema prevalence and urinary iodine concentration based on the entire sample as tested.
Conclusions and Relevance
A higher urinary iodine concentration in non-smoking adults with retinitis pigmentosa was significantly associated with less central foveal swelling in eyes with cystoid macular edema. Additional study is required to determine whether an iodine supplement can limit or reduce the extent of cystoid macular edema in patients with retinitis pigmentosa.
Introduction
Based on optical coherence tomography (OCT), cystoid macular edema (CME) occurs as a complication in more than 25% of patients with retinitis pigmentosa (RP) and may be associated with a reduction in visual acuity.1-3 CME in RP ranges from small rare off-center cysts within the inner nuclear layer to multiple large cysts spanning the retinal layers and including the foveal center.3 Accumulation of fluid within the extracellular space could result from a breakdown of retinal pigment epithelial (RPE) tight junctions or diminished retinal capillary endothelial cell adhesion leading to passive leakage of fluid into the retina, or from reduced active transport of fluid out of the retina by an impaired pump.4,5 The RPE is particularly suspect, because dye leakage through the RPE has been observed in RP patients with CME.4,6,7
Treatment with oral or topical carbonic anhydrase inhibitors can increase subretinal fluid resorption and reduce retinal swelling in RP patients with CME.8-11 However, oral carbonic anhydrase inhibitors can lead to systemic side effects that may occasionally include kidney stones or anemia, and both oral and topical carbonic anhydrase inhibitors can lead to a rebound of edema after their termination or with sustained use.11,12 Intravitreal injection of steroids has similarly been found to reduce swelling in these eyes13 but can also be subject to a rebound of edema.14 Moreover, patients with advanced CME treated with carbonic anhydrase inhibitors or steroids can show reductions in swelling without commensurate improvements in visual acuity.5,11,13,14 It is possible that the latter eyes had already suffered permanent photoreceptor damage or cell loss as a result of the edema.15 It would, therefore, be beneficial to search for an alternative, safe, well-tolerated treatment for CME for early intervention to avoid the issue of possible irreversible visual acuity loss.
With a food frequency questionnaire16,17 administered to non-smoking adult RP patients (n = 316), we identified CME by OCT in 12% taking 150-μg potassium iodide (the adult RDA) by multivitamin daily or less often versus 32% not taking this potassium iodide supplement; this difference was statistically significant (MAS, unpublished observation, p = 0.001). Moreover, two studies reported that iodine improved cell-cell adhesion by increasing the transendothelial resistance of leaky human endothelial cell tight junctions18 and the transepithelial resistance of leaky human epithelial breast cancer cells.19 These data raise the possibility that iodine supplementation might impede the development of retinal edema from a leaky RPE.
However, taking or not taking an iodine supplement may not be representative of total dietary intake of iodine. Since (a) dietary iodine is absorbed with 92% bioavailability,20 (b) 90% of ingested iodine is excreted in the urine within 24 hours,21 and (c) dietary iodine intake is significantly correlated with urinary excretion of iodine,22,23 urinary iodine is considered to be a good monitor of recent iodine intake. We, therefore, decided to conduct a new observational study to assess whether the degree of CME among non-smoking adults with RP was related to their urinary iodine concentration (UIC).
Methods
Patients
The protocol for this study was approved by the Institutional Review Board of the Massachusetts Eye and Ear Infirmary and conformed to the tenets of the Declaration of Helsinki and to HIPAA regulations. Informed consent was obtained from the patients after explanation of the nature and possible consequences of the study.
We excluded patients with a best-corrected Snellen visual acuity < 20/200 in both eyes, because the presence of maculopathy might confound the appearance of CME. Although we had reported that CME in RP was not significantly related to aphakia/pseudophakia,3 as a precaution we excluded patients who had undergone cataract surgery within the past year. We excluded patients who were currently smoking, since the inverse relationship between CME risk and iodine supplementation in our previous analysis was based on non-smoking patients. We also excluded patients who were taking Levothyroxine (for hypothyroidism) or Amiodarone (for cardiac arrhythmia) and individuals who had received iodinated radiographic contrast within the previous 6 months — because these patients would markedly skew the distribution of iodine intake — and patients with gastrointestinal malabsorption due to prior bowel resection, a history of Crohn's disease, or inflammatory bowel disease.
We enrolled 212 patients with typical RP and a corrected visual acuity of 20/200 or better in at least one eye. Eleven of these patients (5%) failed to send in multiple spot urine samples for measuring UIC following their examination, leaving 201 patients (101 males and 100 females, ages 18 to 69 years) with complete data. Our cohort comprised 41 patients with dominant disease, 151 patients with autosomal recessive or sporadic disease, 6 patients with X-linked disease, and 3 patients with undetermined inheritance.
Dietary iodine estimates
Total dietary intake of iodine was estimated from urine collected at home. Since UIC from spot samples is the recommended indicator for population assessment24 and since the dayto-day variation in UIC is considerable,23 patients were provided with a custom mail-in kit and asked to collect multiple (preferably, 10) spot urine samples over consecutive days for us to derive a better estimate of chronic intake than from single spot urine samples or a single 24-hour urine sample.20,25 Containers included labels for patients to record the date and time of each sample. The cohort provided an average of 9.8 iodine samples/patient (range: 2 to 10 samples/patient) obtained over an average of 6.1 days (median, 4 days).
The urine samples were stored at −80° F prior to iodine analysis. UIC from each spot sample was measured at least twice by a modification of the Benotti method.26 If the initial two measurements were not within 15% of each other, a third or fourth measurement was obtained and the average of all measurements reported. The interassay coefficient of variation for this assay ranges from 2.7% - 7%. The cohort was iodine sufficient by WHO standards,27 with an overall median UIC of 143 μg/L. However, we noted that 55 (27%) of these patients had a median UIC below 100 μg/L — suggesting possible iodine intake below the RDA of 150 μg/day.
The distribution of median UIC was positively skewed (skewness = 9.22), and UIC values were converted to natural logarithms to better approximate a normal distribution. Two patients — with median UIC values of 2,272 μg/L and 3,470 μg/L that reflect dietary intake above the upper tolerable limit for adults of 1,100 μg/day28— were found to be outliers by the extreme studentized deviate test29 and were excluded from further study, leaving 199 patients. The distribution of loge median UIC after removal of the outliers had a skewness of - 0.30.
OCT evaluations
CME and central foveal thickness (CFT) were coded for eyes with a visual acuity of 20/200 or better and for which the central macula could be adequately visualized by OCT. We used a Stratus High-Resolution Optical Coherence Tomographer (Model 3000, Carl Zeiss Meditec, Dublin, CA) to assess retinal structure and measure CFT after pupillary dilation3,30 in 192 of the 199 patients. We recorded twelve 6-mm radial scans spaced in 15° intervals (or, less often, six radial scans spaced in 30° intervals). One eye was excluded in each of 23 patients because of a visual acuity < 20/200 (7 cases), a pseudohole (8 cases), or poor imaging (8 cases).
On 33 patients we recorded tomograms with the Zeiss Cirrus HD-OCT (Carl Zeiss Meditec): 7 patients were tested on the Cirrus alone because our Stratus was unavailable, and 26 patients tested with the Stratus, chosen randomly, were also tested with the Cirrus instrument to serve as a calibration subset for quantifying CFT (see below). The Cirrus acquisition protocol covered a 6-mm × 6-mm retinal area with 128 horizontal lines, each consisting of 512 A-scans. The Cirrus files were copied as avi movies to a usb drive and ported to a MacBook Pro notebook (Apple, Cupertino, CA) for off-line identification of CME and analysis of CFT with ImageJ (version 1.46j, http://imagej.nih.gov/ij).
CME was defined as ranging from rare discrete vacuoles as small as 50 μm in height at the level of the inner nuclear layer to multiple vacuoles of more than 400 μm in height that distorted the cytoarchitecture.30 Seventy-two of the 199 patients (36%) had CME in one or both eyes — 48 bilaterally, 18 unilaterally, and 6 in whom the second eye was not categorized due to reduced visual acuity or inadequate imaging.
CFT was measured as the distance between the high-reflectance vitreoretinal interface and the RPE/choriocapillaris complex30 based on the vertical and horizontal B-scans of the Stratus or based on the central B-scan of the Cirrus. Testing a subset of patients on both instruments led to an algorithm for estimating Stratus CFT from Cirrus CFT (r2 = 0.91); this algorithm was used to infer what the Stratus thickness would have been for those 7 patients tested only on the Cirrus, so that the latter patients could be included with the other patients in a group assessment of CFT.
Statistical analyses
The distributions of CFT by eye were positively skewed (skewness = 2.22 OD and 2.18 OS), so CFT values were converted to natural logarithms to better approximate normal distributions. With the eye as the unit of analysis (to utilize the data from both eyes of patients with unilateral CME), we used PROC MIXED of SAS (version 9.3, SAS Institute, Cary, NC) to perform several analyses with loge CFT as the dependent variable. PROC MIXED adjusts for the intra-subject correlation between eyes and permits missing values. In Model 1, median UIC was the independent class variable representing 3 ranges (< 100 μg/L, 100 μg/L to 199 μg/L, or ≥ 200 μg/L) to minimize the effect of any high-leverage values and to allow for a nonlinear relationship between loge CFT and UIC. Model 2 added CME status as a 2-level classification (no CME = 0, CME = 1) and its cross-product with UIC classification as independent variables; the cross-product term allowed us to determine if the effect of UIC classification depended on CME status. With the cohort divided by CME status, Models 3 and 4 used UIC classification as the only independent variable to estimate the relationships for eyes with and without CME, respectively. For Models 1 and 3 we performed linear contrasts to compare CFT differences for specific pairs of UIC ranges, reporting Bonferroni corrections to take into account simultaneous multiple comparisons in identifying significant relationships (p < 0.05).
RP patients have been encouraged to consider taking a supplement of vitamin A palmitate and to eat a diet rich in docosahexaenoic acid (DHA) to slow the course of their disease because of results from three studies.31-33 Based on a food-frequency questionnaire,34 our patients showed variable amounts of vitamin A and DHA in their diets (including supplements) — raising the possibility that one or both of these nutrients might confound any significant relationship between CFT and UIC. To test this, we repeated Model 3 adjusting for tertile (0/1/2) of dietary vitamin A or DHA as a continuous variable. Lastly, we used PROC GENMOD to perform eye-level logistic regression to test whether the likelihood of CME in the entire cohort depended on UIC classification recoded as a continuous variable to test for trend.
It is more difficult to assess the iodine nutrition of individuals than of the population, because there is a substantial amount of diurnal and day-to-day variation in an individual's dietary iodine intake and urinary iodine excretion23,35 — and previous studies have found a high mean within-patient coefficient of variation (CV) for UIC.35-39 Since our distribution of within-patient CVs had a high mean (59%) and substantial variation (Figure 1), we used the weight option in PROC MIXED and PROC GENMOD to weight all analyses in the Results by the normalized inverse-squared within-patient CV for UIC to assign greater importance to less variable measurements while controlling for mean level.40 This step proved essential to identifying significant relationships between CFT and UIC.
Figure 1.
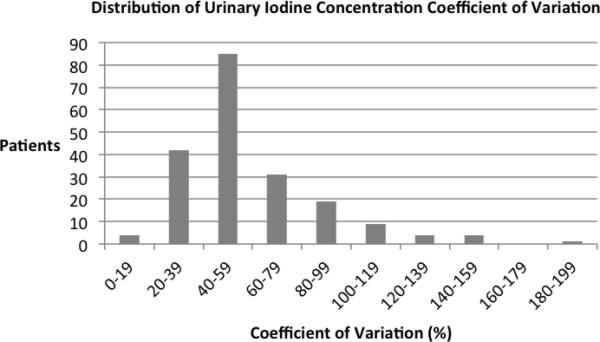
The distribution of the within-subject coefficient of variation for urinary iodine concentration based on multiple spot samples from 199 patients with retinitis pigmentosa.
Results
Loge CFT was inversely related to UIC classification with Model 1 (Table 1, p = 0.02). By linear contrasts, the difference in mean loge CFT (geometric mean difference = 24 μm) for UIC < 100 μg/L versus UIC from 100 - 199 μg/L was borderline significant (p = 0.08), while the corresponding difference (geometric mean difference = 7 μm) for UIC from 100 - 199 μg/L versus UIC ≥ 200 μg/L was not significant (p = 0.78) after Bonferroni corrections — raising the possibility of a nonlinear trend.
Table 1.
Central Foveal Thickness by Median Urinary Iodine Concentration in Patients with Retinitis Pigmentosa
| Model | Variable | Eyes | Loge CFT ± SEMa(Geometric Mean) | P-valueb |
|---|---|---|---|---|
| 1 | Median UICc | 0.02 | ||
| < 100 μg/L | 103 | 5.254 ± 0.052 (191 μm) | ||
| 100 - 199 μg/L | 157 | 5.120 ± 0.039 (167 μm) | ||
| ≥ 200 μg/L | 115 | 5.076 ± 0.034 (160 μm) |
CFT: central foveal thickness. Normal values for CFT with the same instrumentation are 167 ± 5 μm (mean ± sem) based on 22 volunteers (aged 25–56 years).30
By PROC MIXED of SAS with the eye as the unit of analysis. See Methods for additional details.
UIC: urinary iodine concentration. The geometric mean values for these three ranges were 68 μg/L, 139 μg/L, and 263 μg/L, respectively.
However, when we included CME status and its interaction with UIC classification as independent variables (Model 2), we found that the relationship between loge CFT and UIC classification depended on CME status (p_interaction < 0.001) — which necessitated reanalyzing the data after separating the cohort into those with and without CME. For the subset with CME (Model 3), loge CFT was now more strongly related to UIC classification (p < 0.001) than in Model 1, ranging from a geometric mean of 267 μm for median UIC below 100 μg/L to a geometric mean of 172 μm for median UIC of 200 μg/L and greater (Table 2). By linear contrasts, the difference in mean loge CFT (geometric mean difference = 79 μm) for UIC < 100 μg/L versus UIC from 100 - 199 μg/L was significant (p = 0.008), while the corresponding difference (geometric mean difference = 16 μm) for UIC from 100 - 199 μg/L versus UIC ≥ 200 μg/L was not significant (p = 0.76), after applying Bonferroni corrections — consistent with a nonlinear trend. For the subset without CME (Model 4), loge CFT was not significantly related to UIC classification (p = 0.66). Figure 2 illustrates that the decline of geometric mean CFT with increasing UIC among patients with CME appears exponential, asymptotically approaching the CFT values for the patients without CME.
Table 2.
Central Foveal Thickness by Urinary Iodine Concentration in Patients with Retinitis Pigmentosa with and without Cystoid Macular Edema
| Model | Variable | Eyes | Loge CFT ± SEMa (Geometric Mean) | P- valueb |
|---|---|---|---|---|
| 3 | Median UICc for Eyes with CMEd | < 0.001 | ||
| < 100 μg/L | 35 | 5.589 ± 0.090 (267 μm) | ||
| 100 - 199 μg/L | 50 | 5.238 ± 0.077 (188 μm) | ||
| ≥ 200 μg/L | 35 | 5.149 ± 0.062 (172 μm) | ||
| 4 | Median UICc for Eyes without CMEd | 0.66 | ||
| < 100 μg/L | 68 | 5.004 ± 0.048 (149 μm) | ||
| 100 - 199 μg/L | 107 | 5.055 ± 0.033 (157 μm) | ||
| ≥ 200 μg/L | 80 | 5.030 ± 0.031 (153 μm) |
CFT: central foveal thickness.
By PROC MIXED of SAS with the eye as the unit of analysis. See Methods for additional details.
UIC: urinary iodine concentration.
CME: cystoid macular edema.
Figure 2.
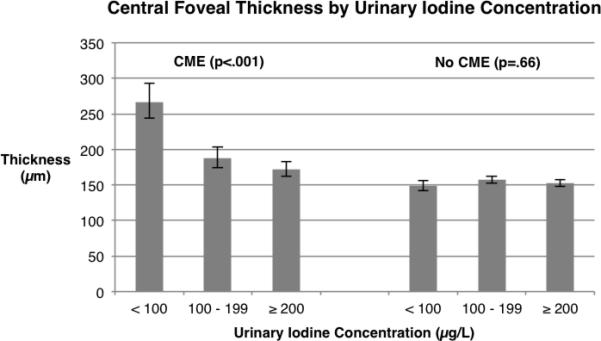
Central foveal thickness by urinary iodine concentration in retinitis pigmentosa based on 120 eyes with CME (Model 3, left) and 255 eyes without CME (Model 4, right). Error bars designate ± 1 standard error. See Methods for details of analyses.
With tertile of vitamin A added to Model 3, loge CFT remained significantly related to UIC (p < 0.001) and was not significantly related to vitamin A (p = 0.79) among eyes with CME. With tertile of DHA, instead, added to Model 3, loge CFT remained significantly related to UIC (p = 0.002) and was not significantly related to DHA (p = 0.30).
We found no significant trend between the likelihood of CME based on the entire cohort versus UIC classification (odds ratio = 1.01, 95% confidence interval = 0.38 - 2.67, p = 0.99).
Discussion
This study found that higher urinary iodine concentration (UIC) was associated with a smaller central foveal thickness (CFT) among a cohort of 199 patients with retinitis pigmentosa (RP), more than a third of whom had cystoid macular edema (CME). However, the relationship was different for the two groups: those with CME showed a strong inverse relationship, while those without CME showed no significant relationship as tested. These two findings taken together suggest that higher UIC in this cohort was specifically associated with a reduced swelling due to CME. Including total vitamin A or DHA intake as a covariate in analyses showed that the relationship of CFT to UIC classification in our patients with CME as tested was not confounded by either of these nutrients.
In contrast, an analysis of the risk of CME by UIC classification based on the entire cohort showed no significant relationship as tested. This suggests that UIC classification had no bearing on the initiation of CME irrespective of its extent, and we should look in other directions to predict who will develop cysts. For example, the likelihood of cyst formation in RP may, in part, be under genetic regulation, because CME has been reported to be rarest in patients with X-linked disease.3,41
Iodine has been shown to promote tight junctions between epithelial cells18,19 which could help to block the passage of fluid across the retinal pigment epithelium (RPE) into the neural retina. It is well known from laboratory studies in mammals that sodium iodate and potassium iodate have an affinity for the RPE and in sufficient concentrations will damage it. But at a low concentration injected intravenously in albino and pigmented rabbits sodium iodate acutely and reversibly enhanced the c-wave, which is a physiological measure of RPE integrity.42 So, perhaps higher physiological levels of iodine could promote RPE integrity in RP patients with intraretinal cysts. Iodine might serve a similar role also in patients with CME secondary to uveitis, who are thought to have lost integrity of the outer blood-retina barrier and RPE pump,43 or in patients with CME secondary to diabetic retinopathy, given that dysfunction of the outer blood-retina barrier has been observed in diabetic patients.44-47
One strength of this study was our use of multiple spot urine samples with which to estimate total dietary iodine intake; the patients contributed an average of nearly 10 samples each, obtained over an average of 6 days. This was preferable to attempting to infer total dietary iodine intake from a food frequency questionnaire, which is subject to self-reporting errors. A second strength was to use the eye as the unit of analysis while taking into account the correlation between fellow eyes, so that patients with unilateral CME — who comprised 25% of our patients with CME — could have data from both their eyes included to increase our power in defining the relationships between CFT and UIC.
Although the observed effect of higher dietary iodine was clinically significant in our patients with CME — amounting to a 95-μm average reduction in CFT, it must be borne in mind that this was an observational study and, as such, was not designed to prove that higher dietary iodine intake limited the extent of CME. In particular, we do not know that supplementation with potassium iodide, for example, will reduce central foveal swelling once it has already occurred and, therefore, are not at this time recommending that RP patients with CME augment their dietary intake of iodine. Instead, these data provide a rationale for considering a prospective randomized trial among RP patients to see if iodine supplementation, relative to control, can safely limit or reduce the extent of pre-existing CME.
Acknowledgements
Authors Contributions: conception and design of the study (MAS, ENP, ELB); acquisition of data (MAS, ENP, SH, ELB); analysis and interpretation of data (MAS, BR); drafting or revising the manuscript (MAS, ENP, BR, ELB); and final approval of the manuscript (MAS, ENP, SH, CWD, LH, BR, ELB).
Funding and Support: National Eye Institute Grant EY019767, the Massachusetts Lions Eye Research Fund, Inc., and a Center Grant from the Foundation Fighting Blindness, Columbia, MD. None of these sponsors influenced the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Contributions by Authors: conception and design of the study (MAS, ENP, ELB); acquisition of data (MAS, ENP, SH, ELB); analysis and interpretation of data (MAS, BR); drafting or revising the manuscript (MAS, ENP, BR, ELB); and final approval of the manuscript (MAS, ENP, SH, CWD, LH, BR, ELB).
Conflict of Interest Disclosures: None
MAS (corresponding author) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Potential Conflicts of Interest and Financial Disclosures: No potential conflicts of interest. ENP was an expert witness for DuPont regarding development of hypothyroidism following exposure to radioactive iodine from the Hanford Nuclear reservation and is consultant to Health Canada regarding thyroidal effects of drinking water nitrate concentrations.
References
- 1.Adackapara CA, Sunness JS, Dibernardo CW, Melia BM, Dagnelie G. Prevalence of cystoid macular edema and stability in OCT retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina. 2008;28(1):103–110. doi: 10.1097/IAE.0b013e31809862aa. [DOI] [PubMed] [Google Scholar]
- 2.Hajali M, Fishman GA, Anderson RJ. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br J Ophthalmol. 2008;92(8):1065–1068. doi: 10.1136/bjo.2008.138560. [DOI] [PubMed] [Google Scholar]
- 3.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. Visual acuity is related to parafoveal retinal thickness in patients with retinitis pigmentosa and macular cysts. Invest Ophthalmol Vis Sci. 2008;49(10):4568–4572. doi: 10.1167/iovs.08-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106(9):1190–1195. doi: 10.1001/archopht.1988.01060140350030. [DOI] [PubMed] [Google Scholar]
- 5.Moldow B, Sander B, Larsen M, et al. The effect of acetazolamide on passive and active transport of fluorescein across the blood-retina barrier in retinitis pigmentosa complicated by macular oedema. Graefe's Arch Clin exp Ophthalmol. 1998;236(12):881–889. doi: 10.1007/s004170050175. [DOI] [PubMed] [Google Scholar]
- 6.Spalton DJ, Bird AC, Cleary PE. Retinitis pigmentosa and retinal oedema. Br J Ophthalmol. 1978;62(3):174–182. doi: 10.1136/bjo.62.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newsome DA. Retinal fluorescein leakage in retinitis pigmentosa. Am J Ophthalmol. 1986;101(3):354–360. doi: 10.1016/0002-9394(86)90831-7. [DOI] [PubMed] [Google Scholar]
- 8.Fishman GA, Gilbert LD, Fiscella RG, Kimura AE, Jampol LM. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol. 1989;107(10):1445–1452. doi: 10.1001/archopht.1989.01070020519031. [DOI] [PubMed] [Google Scholar]
- 9.Orzalesi N, Pierrottet C, Porta A, Aschero M. Long-term treatment of retinitis pigmentosa with acetazolamide. A pilot study. Graefes Arch Clin Exp Ophthalmol. 1993;231(5):254–256. doi: 10.1007/BF00919100. [DOI] [PubMed] [Google Scholar]
- 10.Chen JC, Fitzke FW, Bird AC. Long-term effect of acetazolamide in a patient with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1990;31(9):1914–1918. [PubMed] [Google Scholar]
- 11.Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006;141(5):850–858. doi: 10.1016/j.ajo.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Apushkin MA, Fishman GA, Grover S, Janowicz MJ. Rebound of cystoid macular edema with continued use of acetazolamide in patients with retinitis pigmentosa. Retina. 2007;27(8):1112–1118. doi: 10.1097/IAE.0b013e31805f6b79. [DOI] [PubMed] [Google Scholar]
- 13.Ozdemir H, Karacorlu M, Karacorlu S. Intravitreal triamcinolone acetonide for treatment of cystoid macular oedema in patients with retinitis pigmentosa. Acta Ophthalmol Scand. 2005;83(2):248–251. doi: 10.1111/j.1600-0420.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 14.Scorolli L, Morara M, Meduri A, et al. Treatment of cystoid macular edema in retinitis pigmentosa with intravitreal triamcinolone. Arch Ophthalmol. 2007;125(6):759–764. doi: 10.1001/archopht.125.6.759. [DOI] [PubMed] [Google Scholar]
- 15.Chung H, Hwang J-U, Kim J-G, Yoon Y. Optical coherence tomography in the diagnosis and monitoring of cystoid macular edema in patients with retinitis pigmentosa. Retina. 2006;26(8):922–927. doi: 10.1097/01.iae.0000250008.83779.23. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 17.Shai I, Rosner BA, Shahar DR, et al. Dietary evaluation and attenuation of relative risk: multiple comparisons between blood and urinary biomarkers, food frequency, and 24-hour recall questionnaires: the DEARR study. J Nutr. 2005;135(3):573–579. doi: 10.1093/jn/135.3.573. [DOI] [PubMed] [Google Scholar]
- 18.Martin TA, Das T, Mansel RE, Jiang WG. Synergistic regulation of endothelial tight junctions by antioxidant (Se) and polyunsaturated lipid (GLA) via claudin-5 modulation. J Cell Biochem. 2006;98(5):1308–1319. doi: 10.1002/jcb.20860. [DOI] [PubMed] [Google Scholar]
- 19.Martin TA, Das T, Mansel RE, Jiang WG. Enhanced tight junction function in human breast cancer cells by antioxidant, selenium and polyunsaturated lipid. J Cell Biochem. 2007;101(1):155–166. doi: 10.1002/jcb.21162. [DOI] [PubMed] [Google Scholar]
- 20.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011;141(11):2049–2054. doi: 10.3945/jn.111.144071. [DOI] [PubMed] [Google Scholar]
- 21.Nath SK, Moinier B, Thuillier F, Rongier M, Desjeux JF. Urinary excretion of iodide and fluoride from supplemented food grade salt. Int J Vitam Nutr Res. 1992;62(1):66–72. [PubMed] [Google Scholar]
- 22.Kim JY, Moon SJ, Kim KR, Sohn CY, Oh JJ. Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J. 1998;39(4):355–362. doi: 10.3349/ymj.1998.39.4.355. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53(5):401–407. doi: 10.1038/sj.ejcn.1600762. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30(4):376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 25.Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008;99(4):813–818. doi: 10.1017/S0007114507842292. [DOI] [PubMed] [Google Scholar]
- 26.Benotti J, Benotti N, Pino S, Gardyna H. Determination of total iodine in urine, stool, diets and tissue. Clin Chem. 1965;11(10):932–936. [PubMed] [Google Scholar]
- 27.World Health Organization United Nations Children's Fund, International Council for the Control of Iodine Deficiency Disorders . Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. WHO; Geneva: 2007. [Google Scholar]
- 28.Food and Nutrition Board, Institute of Medicine 2006 Dietary reference intakes. National Academy Press; Washington, DC: p. 320. [Google Scholar]
- 29.Rosner B. Fundamentals of Biostatistics. 5th ed. Duxbury Press; Boston, MA: 2000. [Google Scholar]
- 30.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2005;46(9):3349–3354. doi: 10.1167/iovs.04-1383. [DOI] [PubMed] [Google Scholar]
- 31.Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(6):761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 32.Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Moser A, Brockhurst RJ, Hayes KC, Johnson CA, Anderson EJ, Gaudio AR, Willett WC, Schaefer EJ. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: subgroup analyses. Arch Ophthalmol. 2004;122(9):1306–1314. doi: 10.1001/archopht.122.9.1306. [DOI] [PubMed] [Google Scholar]
- 33.Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Willett WC. ω-3 intake and visual acuity in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2012;130(6):707–711. doi: 10.1001/archophthalmol.2011.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 35.Als C, Helbling A, Peter K, Haldimann M, Zimmerli B, Gerber H. Urinary iodine concentration follows a circadian rhythm: a study with 3023 spot urine samples in adults and children. J Clin Endocrinol Metab. 2000;85(4):1367–1369. doi: 10.1210/jcem.85.4.6496. [DOI] [PubMed] [Google Scholar]
- 36.Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008;99(4):813–818. doi: 10.1017/S0007114507842292. [DOI] [PubMed] [Google Scholar]
- 37.Busnardo B, Nacamulli D, Zambonin L, Mian C, Piccolo M, Girelli ME. Restricted intraindividual urinary iodine concentration variability in nonfasting subjects. Eur J Clin Nutr. 2006;60(3):421–425. doi: 10.1038/sj.ejcn.1602334. [DOI] [PubMed] [Google Scholar]
- 38.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011;141(11):2049–2054. doi: 10.3945/jn.111.144071. [DOI] [PubMed] [Google Scholar]
- 39.Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol. 2002;9(6):1235–1239. doi: 10.1128/CDLI.9.6.1235-1239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chinn S. The assessment of methods of measurement. Statistics in Medicine. 1990;9(4):351–362. doi: 10.1002/sim.4780090402. [DOI] [PubMed] [Google Scholar]
- 41.Kuchle M, Nguyen NX, Martus P, et al. Aqueous flare in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 1998;236(6):426–433. doi: 10.1007/s004170050101. [DOI] [PubMed] [Google Scholar]
- 42.Nao-i N, Kim SY, Honda Y. Paradoxical enhancement of the ERG c-wave by a small dose of sodium iodate. Acta Ophthalmol (Copenh) 1986;64(2):206–211. doi: 10.1111/j.1755-3768.1986.tb06901.x. [DOI] [PubMed] [Google Scholar]
- 43.de Smet MD, Okada AA. Cystoid macular edema in uveitis. Dev Ophthalmol. 2010;47:136–47. doi: 10.1159/000320077. [DOI] [PubMed] [Google Scholar]
- 44.Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol. 1989;134(2):231–235. [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger D, Fink-Cohen S, Gaton DD, Priel E, Yassur Y. Non-retinovascular leakage in diabetic maculopathy. Br J Ophthalmol. 1995;79(8):728–731. doi: 10.1136/bjo.79.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol. 1999;97(3-4):217–228. doi: 10.1023/a:1002136712070. [DOI] [PubMed] [Google Scholar]
- 47.Xu H-Z, Le Y-Z. Significance of outer blood–retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52(5):2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]


