Abstract
Type 2 diabetes is caused by insulin resistance coupled with an inability to produce enough insulin to control blood glucose, and thiazolidinediones (TZDs) are the only current antidiabetic agents that function primarily by increasing insulin sensitivity. However, despite clear benefits in glycemic control, this class of drugs has recently fallen into disuse due to concerns over side effects and adverse events. Here we review the clinical data and attempt to balance the benefits and risks of TZD therapy. We also examine potential mechanisms of action for the beneficial and harmful effects of TZDs, mainly via agonism of the nuclear receptor PPARγ. Based on critical appraisal of both preclinical and clinical studies, we discuss the prospect of harnessing the insulin sensitizing effects of PPARγ for more effective, safe, and potentially personalized treatments of type 2 diabetes.
Keywords: Diabetes, Insulin resistance, Insulin sensitizer, PPARγ, Review, Thiazolidinedione, TZDs
Type 2 diabetes (T2DM) is characterized by insulin resistance and beta cell failure, and thiazolidinedione drugs (TZDs) are the only current antidiabetic agents that function primarily by increasing insulin sensitivity. However, this class of drugs has recently fallen into disuse due to concerns over side effects and adverse events. The rise and fall of TZDs is demonstrated by their use in ambulatory diabetes visits: from 6% in 1997 to 41% in 2005 and down to 16% by 2012 (Turner et al., 2014). Anecdotal evidence suggests an even steeper decline since then, as even diabetes specialists now deploy TZDs sparingly given the proliferation of other treatment options. With this decline in clinical use, research publications on TZDs have also decreased though not to the same extent (Figure 1), reflecting the continuing promise of insulin sensitization to treat and prevent T2DM and cardiometabolic disease. This review summarizes the beneficial and adverse effects of TZDs, focusing on potential mechanisms for each, and highlights recent clinical, basic, and translational studies from the past several years that have sent the field into new and unexpected directions.
Figure 1. The History of TZDs.
The graph shows the annual number of TZD-related publications, with boxes indicating key events in the rise and fall of this drug class. For 2014, publications through May were adjusted to a full year.
TZDs were first reported as insulin-sensitizing drugs in the early 1980s by the pharmaceutical company Takeda (Fujita et al., 1983), but their mechanism remained a mystery until the mid-1990s when they were found to be ligands for the nuclear receptor transcription factor PPARγ (Lehmann et al., 1995). PPARγ is expressed at high levels in adipose tissue, where it functions as a master regulator of adipocyte differentiation, and at much lower levels in other tissues (Tontonoz and Spiegelman, 2008). The simplest model for TZD function involves PPARγ agonism in adipose tissue, but recent studies described below suggest alternatives and additions to this model.
TZDs are potent insulin sensitizers which treat and prevent T2DM
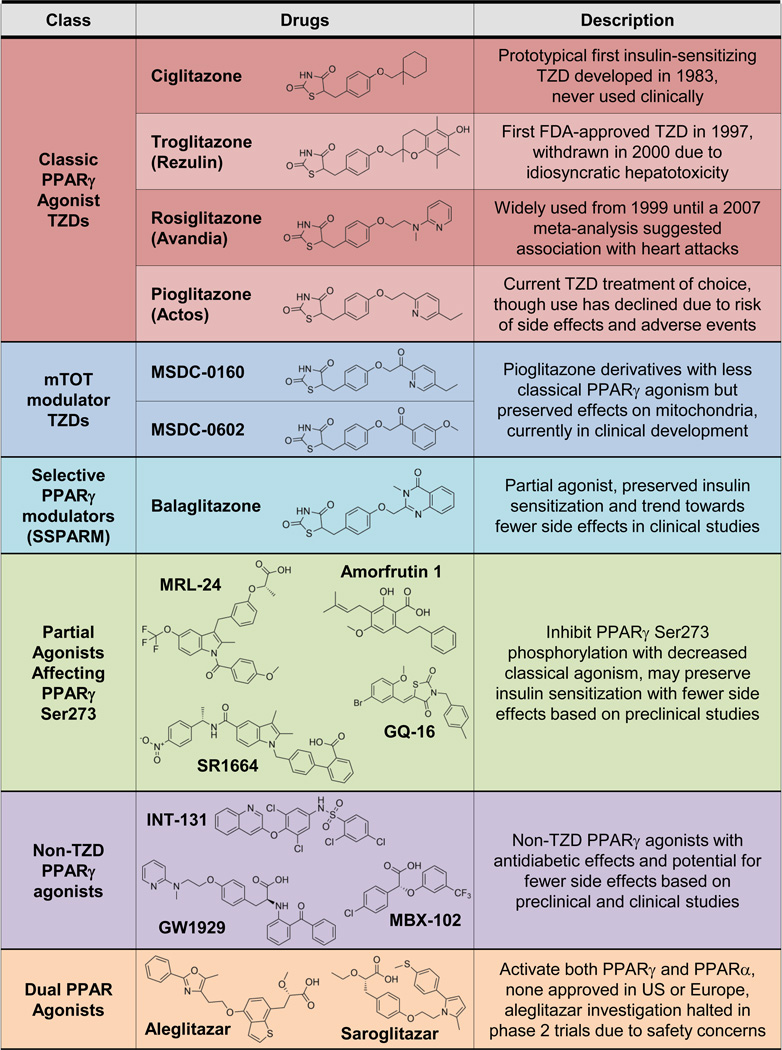
Three TZDs have been FDA-approved for diabetes: troglitazone (Rezulin), rosiglitazone (Avandia), and pioglitazone (Actos) (Figure 2 describes various PPARγ agonist drugs that are discussed in the text). Troglitazone was introduced in 1997 but withdrawn from the market in 2000 due to increased risk of liver failure from fulminant hepatitis (Kohlroser et al., 2000). Some studies suggest the cause is hepatotoxic reactive metabolites of troglitazone (Yokoi, 2010), while others indicate that troglitazone activates the pregnane X receptor in humans but not rodents (Jones et al., 2000). Though the exact mechanism is still uncertain, PPARγ activation is not thought to be involved, and hepatotoxicity is not a TZD class effect but an idiosyncratic effect of troglitazone. Rosiglitazone and pioglitazone were both FDA-approved in 1999, but pioglitazone has become the TZD of choice for reasons described below.
Figure 2. PPARγ Agonist Drugs.
TZDs like pioglitazone and rosiglitazone are potent PPARγ agonists (red), but other weaker or partial agonists share the TZD structure with different side chains (blue). There are additional structurally diverse non-TZD PPARγ agoinsts (green and purple), including dual agonists of PPARα and PPARγ (orange). See text for details.
TZDs lower hemoglobin A1c potently by ~1% as monotherapy in T2DM, where they notably do not cause hypoglycemia like insulin or insulin secretagogues (i.e. sulfonylureas), and they can be used in combination with other antidiabetic agents (reviewed in Yau et al., 2013). The first-line drug metformin is often described as an insulin sensitizer, but its primary effect is suppression of hepatic glucose production while its effects on peripheral insulin sensitivity are quite small, variable across studies, and absent in a meta-analysis (Natali and Ferrannini, 2006). In the same analysis, TZDs have large and consistent effects improving insulin sensitivity. Furthermore, the ADOPT randomized controlled trial (RCT) showed that rosiglitazone provided more durable glycemic control than metformin or a sulfonylurea (Kahn et al., 2006) (Table 1 describes the key clinical studies that are discussed in the text).
Table 1.
Selected key clinical studies of TZDs and related drugs.
| Trial/Publication | Type | Design | Details | Outcome | Result | Comment |
|---|---|---|---|---|---|---|
| ADOPT: A Diabetes Outcome Progression Trial (Kahn et al, 2006) |
RCT | Patients with newly diagnosed T2DM randomized to rosiglitazone, metformin, or glyburide |
4,360 patients, median 4 years |
Time to monotherapy failure |
Only 15% failure at 5 years for rosiglitazone, lower than 21% for metformin and 34% for glyburide |
Supports notion that reducing insulin resistance is beneficial in diabetes pathophysiology |
| ACT NOW: Actos Now for the prevention of diabetes (DeFronzo et al, 2011) |
RCT | Patients with prediabetes randomized to pioglitazone or placebo |
602 patients, median 2.4 years |
Conversion to diabetes |
Pioglitazone decreased conversion to diabetes by 72% |
Similar studies previously showed diabetes prevention by troglitazone and rosiglitazone |
| PIVENS: PIoglitazone or Vitamin E for Nonalcoholic Steatohepatitis (NASH) (Sanyal et al, 2010) |
RCT | Patients with NASH and without diabetes randomized to pioglitazone, vitamin E, or placebo |
247 patients, 96 weeks treatment |
Improvement in histologic features of NASH |
Vitamin E, but not pioglitazone, significantly improved primary histological composite endpoint |
Pioglitazone improved all secondary endpoints: liver fat, inflammation, and serum aminotransferase levels |
| Nissen and Wolski, 2007 | meta-analysis | Data combined by fixed effects model |
42 studies met inclusion criteria |
MI or cardiovascular death |
Rosiglitazone's odds ratio for MI 1.43 (1.03 to 1.98; P=0.03), for death 1.64 (0.98 to 2.74; P=0.06) |
Despite many subsequent studies, the association between rosiglitazone and MI remains controversial (see text for details) |
| RECORD: Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycaemia in Diabetes (Home et al, 2007) |
RCT | Patients with T2DM on metformin or sulfonylurea monotherapy randomized to addition of rosiglitazone |
4,447 patients, mean 5.5 years |
Cardiovascular hospitalization or death |
No significant increase in cardiovascular mortality with rosiglitazone |
Re-adjudicated results (Mahaffey et al, 2013) support original conclusions, though study was likely underpowered |
| BARI 2D: Bypass Angioplasty Revascularization Investigation in type 2 Diabetes (Bach et al, 2013) |
post-hoc analysis |
In original RCT, patients with T2DM and stable coronary disease were randomized to several interventions |
992 on rosiglitazone, mean 4.5 years |
Mortality, MI, stroke | No increase in MI on rosiglitazone, with significant decrease in composite endpoint |
Agrees with RECORD, though trial not originally designed to study effects of rosiglitazone |
| TIDE: Thiazolidinedione Intervention with vitamin D Evaluation (Punthakee et al, 2012) |
RCT | Patients with T2DM randomized to rosiglitazone, pioglitazone, vitamin D, or placebo |
2,553 patients, mean 162 days |
MI, stroke, or cardiovascular death |
Study initially required by FDA but terminated in 2013 when deemed no longer feasible or necessary |
Would have been the only trial comparing rosiglitazone and pioglitazone head-to-head |
| CHICAGO: Carotid Intima-Media Thickness (CIMT) in Atherosclerosis Using Pioglitazone (Mazzone et al, 2006) |
RCT | Patients with T2DM randomized to pioglitazone or glimepiride |
462 patients, mean 7.7 years |
Change from baseline in CIMT |
Pioglitazone slowed progression of CIMT compared to glimepiride |
Evidence that pioglitazone is beneficial in atherosclerosis, slowing plaque progression |
| PERISCOPE: Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation (Nissen et al, 2008) |
RCT | Patients with coronary disease and T2DM randomized to pioglitazone or glimeperide |
543 patients, duration 18 months |
Change from baseline in atheroma |
Coronary atheroma volume decreased on pioglitazone, but progressively increased on glimeperide |
Evidence that pioglitazone is beneficial in atherosclerosis, even causing plaque regression |
| PROactive: PROspective pioglitAzone Clinical Trial In macroVascular Events (Dormandy et al, 2005) |
RCT | Patients with T2DM and evidence of macrovascular disease randomized to pioglitazone or placebo |
5,238 patients, mean 2.9 years |
Composite of mortality, MI, stroke, and leg artery revascularization or amputation |
Pioglitazone did not affect primary composite endpoint (−10%, P=0.09), but reduced predefined secondary endpoint (mortality, MI, and stroke; −16%, P=0.03) |
Pioglitazone is effective in secondary prevention of cardiovascular disease; follow-up analyses showed even more impressive effects in subgroups with prior MI or stroke |
| Colhoun et al, 2012 | database cohort |
Scottish national database of prescriptions, hospitalizations, and deaths |
37,479 patients exposed to TZD |
Hospitalization for hip fracture |
Pioglitazone and rosiglitazone increased risk of hip fracture by 15–20% in men and women |
Prior studies had shown mainly an association of TZDs with distal extremity fractures in women |
| Neumann et al, 2012 | database cohort |
French national databases | 155,535 patients exposed to pioglitazone |
Incident cases of bladder cancer |
Pioglitazone significantly increased bladder cancer by 22%, with dose- and duration-dependent effects |
Other studies support a small but significant increase in bladder cancer on pioglitazone, but not rosiglitazone |
| Colmers et al, 2012b | meta-analysis | 4 RCTs, 7 cohort studies, and 9 nested case control studies were pooled |
data on 2.5 million patients |
Incidence of cancers at various sites |
TZDs confer a small (5–10%) but significant decreased risk of lung, colorectal, and breast cancers |
Compared to bladder cancer, cancers that TZDs may prevent are more common and have greater morbidity and mortality |
| AleCardio: a study with Aleglitazar in patients with a recent acute Coronary syndrome and type 2 diabetes mellitus (Lincoff et al, 2014) |
RCT | Patients with T2DM and hospitalized with acute coronary syndrome were randomized to aleglitazar versus placebo |
7,226 patients, stopped early after median 2 years |
Recurrent heart attack, stroke, or cardiovascular death |
Aleglitazar did not affect the primary endopoint despite having the expected effects on lipids and glucose |
This trial was stopped early due to futility, as this dual PPAR agonist showed no benefit and the suggestion of serious adverse events |
Fourteen key clinical studies are summarized, in the order they are presented in the text. RCT: randomized controlled trial.
Insulin sensitization also appears to be the mechanism whereby TZDs prevent or delay development of T2DM in individuals with prediabetes. The ACT NOW RCT involved 602 patients with impaired glucose tolerance (IGT), and pioglitazone decreased progression to T2DM by 74% over 2.4 years (DeFronzo et al., 2011). Earlier studies of patients with prediabetes showed that troglitazone (Knowler et al., 2005) or rosiglitazone (DREAM Trial Investigators et al., 2006) similarly decreased progression to diabetes.
TZDs and insulin sensitization: beyond diabetes
Non-alcoholic fatty liver disease (NAFLD) is now the most common chronic liver disease in the US, associated with obesity, insulin resistance, dyslipidemia, and hypertension, as part of the metabolic syndrome (Lomonaco et al., 2013). The mildest form of NAFLD is simple hepatic steatosis, which can progress to nonalcoholic steatohepatitis (NASH), leading to cirrhosis and hepatocellular carcinoma. Metformin acts primarily in liver, yet shows no benefit in NASH (Chalasani et al., 2012). Dysfunctional adipose tissue plays a key role in NAFLD (Lomonaco et al., 2012), so targeting adipose tissue with TZDs is an attractive treatment option. In a small RCT of 55 patients with IGT or T2DM and NASH, pioglitazone was quite effective at decreasing histological liver fat, inflammation, and fibrosis (Belfort et al., 2006). The subsequent PIVENS RCT with 247 nondiabetic patients showed that pioglitazone improved all secondary NASH endpoints (Sanyal et al., 2010). Excellent reviews of NAFLD have been published recently (Lomonaco et al., 2013), and current practice guidelines endorse the use of pioglitazone for biopsy proven NASH (Chalasani et al., 2012). Notably, based on the FLIRT trial, rosiglitazone does not appear as effective as pioglitazone in NASH (Ratziu et al., 2010), and gene expression changes in liver biopsies from this trial even suggested pro-inflammatory changes (Lemoine et al., 2013).
In women with polycystic ovarian syndrome (PCOS), guidelines recommend metformin or TZDs to decrease androgen levels, enhance ovulation, and improve glucose tolerance, though not for first line use in treating hirsutism or infertility (ACOG Committee on Practice Bulletins, 2009). In practice, metformin is commonly used in PCOS while pioglitazone is used sparingly, most likely due to concerns over the side effects of pioglitazone and metformin’s established safety in pregnancy.
Fluid Retention, Edema and Congestive Heart Failure due to TZDs
Water retention due to TZDs was noted early, with about 5% of patients developing peripheral lower extremity edema on TZD monotherapy and even more in combination with other drugs - up to 18% when combined with insulin (Nesto et al., 2004). Excess fluid can also lead to exacerbations of congestive heart failure (CHF), thus TZDs are contraindicated in patients with symptomatic heart failure (New York Heart Association class III or IV). While TZDs clearly increase CHF events, these are normally responsive to diuretic therapy, and TZDs do not appear to increase mortality from CHF (Lago et al., 2007).
Even without clinical signs of edema or heart failure, TZD-treated patients retain water, typically evidenced by hemodilution. Even this is controversial, as some methods to assess total body water indicate hemodilution actually does not account for the decreased hematocrit on pioglitazone (Berria et al., 2007). Nonetheless, mouse models show that the apparent volume expansion requires PPARγ expression in the renal collecting duct (Guan et al., 2005; Zhang et al., 2005), and may involve the epithelial sodium transporter as well as other pathways in the renal tubule (reviewed in Bełtowski et al., 2013). Regarding CHF, direct effects of TZDs on cardiac muscle are possible, as mouse models with either cardiomyocyte-selective overexpression (Son et al., 2007) or deletion (Duan et al., 2005) of PPARγ have both reported cardiac dysfunction. The cardiotoxic effects of high doses of rosiglitazone were recently shown to be largely PPARγ-independent and involve mitochondrial dysfunction (He et al., 2014). In human studies, TZDs are associated with lipid accumulation in cardiomyocytes (Marfella et al., 2009), but pioglitazone showed no effect or even beneficial effects on echocardiographic measures of cardiac function (Horio et al., 2005; Sambanis et al., 2008). TZDs are not generally thought to cause cardiomyopathy directly, but rather to exacerbate heart failure via fluid retention in susceptible patients. Recently, some but not all studies have shown an association between TZD use and diabetic macular edema (Idris et al., 2012). The mechanisms for this association may involve the systemic effects of overall volume expansion as well as local effects in the retina.
Weight gain on TZDs: Both Fluid Retention and Adipose tissue
Many studies of TZDs show a typical reported weight gain of ~5kg over 3–5 years. While there is potential for added weight due to fluid retention, there is clearly also expansion of adipose tissue. Studies differ as to the relative contribution of fluid versus adipose tissue: even on the same dose of pioglitazone 45mg, some measure 75% of the added weight due to water retention (Basu et al., 2006), while others propose 89% due to adipose tissue mass (Berria et al., 2007). Multiple imaging studies consistently show a greater increase in peripheral subcutaneous than visceral fat (reviewed in Bray et al., 2013), and a recent study even found a decrease in visceral fat (Punthakee et al., 2014). Rather than an unwanted effect, this adipose tissue weight gain may be integral to the mechanism of action of TZDs (see below). Indeed, a positive correlation has been reported between the degree of weight gain of rosiglitazone and the improvement in insulin sensitivity (Miyazaki et al., 2005), and between weight gain on pioglitazone and improved cardiovascular outcomes (Doehner et al., 2012).
Cardiovascular disease: different effects of rosiglitazone and pioglitazone?
A widely-cited meta-analysis in 2007 raised concerns that rosiglitazone was associated with a significant 43% increased risk of myocardial infarction (MI), with a 64% increase in cardiovascular mortality that did not reach statistical significance (P=0.06) (Nissen and Wolski, 2007). Increased MI risk was surprising given mouse models showing that rosiglitazone markedly inhibits atherosclerosis (Li et al., 2000). Subsequent meta-analyses also showed increased MI risk, though without increased mortality (Nissen and Wolski, 2010; Singh et al., 2007). Based on such studies, in 2010 the FDA placed a restrictions on rosiglitazone through its risk evaluation and mitigation strategies (REMS) program, requiring patient registration and special pharmacies. Only one RCT, an open-label non-inferiority study called RECORD, was specifically designed to assess cardiovascular outcomes on rosiglitazone compared to metformin and a sulfonylurea. The interim results were published early in 2007 (Home et al., 2007), the final results in 2009 (Home et al., 2009), and these were re-adjudicated by independent investigators at the FDA’s request in 2013 (Mahaffey et al., 2013). None of these analyses showed any increased risk of heart attack or death.
Despite RECORD enrolling 4,447 patients, the authors note that it was still underpowered, as a ~60% risk or ~20% benefit of rosiglitazone could not be excluded. Nonetheless, given this new information, in November 2013 the FDA followed advice of an expert panel and removed restrictions on rosiglitazone (FDA, 2013). This decision was supported by a post hoc analysis of the BARI 2D trial, in which 992 subjects on rosiglitazone had no significant change in MI with a trend toward benefit, along with decreased risk of stroke or the composite cardiovascular endpoint (Bach et al., 2013). Furthermore, a recent reassessment of the observational data linking rosiglitazone and MI found many deficiencies and potential for confounding (Rawson, 2014). After the storm of controversy and bad press, it is unlikely this rosiglitazone will ever be widely used again, as there is no unique benefit for this drug compared to pioglitazone - except perhaps for bladder cancer risk (see below). Furthermore, a similar meta-analysis for pioglitazone showed no excess cardiovascular mortality, and in fact an 18% decrease in cardiovascular endpoints (Lincoff et al., 2007). An large observational cohort of US Medicare patients showed that rosiglitazone has cardiovascular harm compared to pioglitazone (Graham DJ et al., 2010), while a UK cohort study showed that pioglitazone but not rosiglitazone reduced all-cause mortality relative to metformin (Tzoulaki et al., 2009). Based on such findings, pioglitazone essentially became the TZD of choice, and prescribing data from the US and UK clearly show a switch from rosiglitazone to pioglitazone between 2007 and 2009 (Hampp et al., 2014; Leal et al., 2013). A large FDA-required RCT called TIDE was designed to compare rosiglitazone and pioglitazone head-to-head for cardiovascular endpoints (Punthakee et al., 2012), but it has been terminated as the FDA deemed it no longer feasible or necessary after the re-adjudication of RECORD.
The potential difference in MI risk between pioglitazone and rosiglitazone may lie in their distinct effects on lipoproteins, with pioglitazone showing a more favorable effect (triglycerides decrease ~15%, HDL cholesterol increase ~10%, with no effect on LDL or total cholesterol) than rosiglitazone (no effect on triglycerides, HDL cholesterol increase ~10%, but 5–10% increases in LDL and total cholesterol) (Chiquette et al., 2004). This difference in lipid effects may reflect weak PPARα agonism by pioglitazone (Sakamoto et al., 2000). Subsequent RCT data validated the favorable effects of pioglitazone versus rosiglitazone on lipoprotein particle concentration and size (Deeg et al., 2007). These and other markers of cardiovascular risk favor pioglitazone, and two studies directly measuring atherosclerotic plaques in patients with T2DM showed benefits of pioglitazone compared to the sulfonylurea glimepiride. In the CHICAGO study, pioglitazone slowed progression of carotid intima media thickness (CIMT) (Mazzone et al., 2006), while in PERISCOPE pioglitazone actually led to regression in coronary atheroma volume assessed by intravascular ultrasound (Nissen et al., 2008). In the ACT NOW study of patients with prediabetes, pioglitazone likewise decreased progression of CIMT, and this was interestingly independent of effects on glycemia, insulin resistance, lipids, or inflammatory markers (Saremi et al., 2013). Direct beneficial effects of pioglitazone in the vascular wall are proposed, and many basic studies have explored effects of TZDs and PPARγ in vascular smooth muscle cells, macrophages, and endothelial cells (Tontonoz and Spiegelman, 2008).
PROactive was a landmark RCT assessing the effect of pioglitazone on secondary prevention of cardiovascular disease in patients with diabetes, enrolling 5,238 patients for an average of 2.85 years (Dormandy et al., 2005). While pioglitazone resulted in a non-significant 10% reduction in the primary composite endpoint (all-cause mortality, nonfatal MI, acute coronary syndrome, stroke, revascularization of coronary or leg arteries, and amputation above the ankle), there was a significant 16% reduction in the main pre-specified secondary composite endpoint (all-cause mortality, nonfatal MI, and stroke). In effect, the pioglitazone reduced all cardiovascular endpoints except for peripheral vascular revascularization. Post-hoc subgroup analyses showed that those 2,445 patients with previous MI had a significant 28% decrease in recurrent MI on pioglitazone (Erdmann et al., 2007), while those 984 patients with previous strokes had a significant 47% reduction in recurrent stroke (Wilcox et al., 2007). Despite these impressive cardiovascular benefits to pioglitazone, its use has also declined markedly, potentially due to “guilt by association” with rosiglitazone and the description of new risks for fractures and bladder cancer.
TZDs and Skeletal Fractures
The first large RCT to suggest this association was ADOPT, in which pre- and post-menopausal women (but not men) randomized to rosiglitazone had significantly more fractures (2.74 per 100 patient-years) than those receiving metformin or glyburide (1.54 or 1.29 per 100 patient years) (Kahn et al., 2008). Interestingly, the fractures were not at typical osteoporotic sites of spine and hip, but instead distal fractures of the upper and lower extremities. RCTs of pioglitazone have showed similar risk, and multiple observational studies of both rosiglitazone and pioglitazone have shown heterogeneity but generally supported this association with distal fractures in women but not men (reviewed in Yau et al., 2013). These findings have led to recommendations against using TZDs in those at risk for osteoporosis and fracture, such as postmenopausal women. These concerns were amplified after a recent national database cohort study in Scotland showed in a large population that risk of hip fracture increased in both women and men, risk was cumulative (increased by 18% for each year of TZD exposure), and the 90-day mortality from hip fractures was expectedly high at ~15% with or without TZD (Colhoun et al., 2012). Consistent with this risk, pioglitazone reduces bone mineral density and content in multiple sites in men and women (Bray et al., 2013). Of the potential adverse effects of TZD, fracture risk may be the most convincing reason to curtail long-term TZD use for treatment and prevention of T2DM, particularly if increased hip fractures are validated.
The mechanism for the skeletal effects of TZDs remains uncertain. One model involves PPARγ activation driving mesenchymal precursor cells to adipogenesis rather than osteogenesis, and a recent translational study with human cells from bone marrow biopsies supported aspects of this model (Beck et al., 2013). Also consistent with this model, some mice develop more bone marrow fat on rosiglitazone, though this effect was not observed in humans (Harsløf et al., 2011). The molecular mechanisms for TZD effects on bone are complex and involve both decreased osteoblast function and increased osteoclast function (Grey, 2009). For instance, rosiglitazone was very recently shown to alter expression in cell culture of a novel micro-RNA involved in osteoclastogenesis (Krzeszinski et al., 2014), and to increase the number of circulating osteoclast precursors in postmenopausal women (Rubin et al., 2014). Studies of TZD skeletal effects are also complicated by the effects of diabetes itself on bone, as patients with diabetes have a higher risk of fracture independent of bone density, and poor glycemic control may itself worsen fracture risk (Oei et al., 2013).
Pioglitazone and Bladder Cancer
In pharmacological carcinogenicity studies, pioglitazone increased urothelial bladder cancer in male rats but not females rats or mice (reviewed in Tseng and Tseng, 2012), leading to several studies in humans indicating that pioglitazone increases bladder cancer risk. Adverse event reporting to the FDA first suggested the risk in 2011 (Piccinni et al., 2011). A longitudinal cohort from Kaiser Permanente Northern California (KPNC) of ~200,000 diabetic patients with ~30,000 on pioglitazone showed a non-significant 20% increased risk of bladder cancer overall, but a significant 20% increase in the subgroup with >24 months of drug exposure (Lewis et al., 2011). A French retrospective cohort study of ~1.5 million patients with ~150,000 exposed to pioglitazone used similar methods reached similar conclusions: overall pioglitazone significantly but slightly increased risk by 22%, with dose and duration-dependent further increases up to 75% with cumulative exposure to >28,000 mg (Neumann et al., 2012). Bladder cancer overall was 8 times more common in men in this study, and consequently it was underpowered to find effects in women. Nonetheless, all of the excess risk of pioglitazone was in males, and a lack of effect in females would mirror the apparent sex-selectivity in rats. Analysis of UK general practice databases confirmed increased bladder cancer risk on pioglitazone (Azoulay et al., 2012), though similar population cohorts in Taiwan, Japan, and Korea failed to show this association (reveiwed in Yau et al., 2013). Two meta-analyses have included these studies and others to find risk increases of 22–23%, though both perform detailed assessments and find moderate overall risk-of-bias (Colmers et al., 2012a; Ferwana et al., 2013). Recent efforts have tried to identify and eliminate potential confounding sources of bias in these observational studies (Lewis et al., 2014).
RCTs would be the gold standard to show increased bladder cancer risk, but the relative rarity of bladder cancer has limited these efforts. The PROactive study in 2005 initially reported 14 bladder tumors with pioglitazone versus 6 with placebo (P=0.069), but subsequent elimination of a benign bladder mass from the placebo group gave significant risk at P=0.04 (Hillaire-Buys et al., 2011). However, the recently published 6 year interim analysis showed that this imbalance was likely due to chance, as it did not persist into the follow-up period despite randomized exposure to high cumulative doses of pioglitazone and the long latency to development of bladder cancer (Erdmann et al., 2014).
Rat studies have attempted to elucidate pioglitazone’s mechanism of bladder carcinogenesis. In addition to pioglitazone, a number of dual PPARγ/PPARα agonists (see below) have also shown bladder carcinogenicity in rats (Tseng and Tseng, 2012). Some studies support a “crystalluria hypothesis” with rat-specific formation of urinary solids leading to mucosal irritation and tumors, which is theorized not to occur in mice and humans (Sato et al., 2011; Suzuki et al., 2010). However, other mechanisms are possible, and PPARγ-dependent effects have not been ruled out.
Overall, the weight of current evidence supports a small but real increase in bladder cancer risk with pioglitazone therapy (Faillie et al., 2013), while no study has seen increased risk with rosiglitazone. It is important to note that the absolute risk increase is very small. The French cohort showed that pioglitazone is associated with an increase from 42.8 to 49.4 cases of bladder cancer per 100,000 person-years (Neumann et al., 2012), meaning an individual’s annual risk goes from 0.043% to 0.049%, or a 0.006% increase in absolute risk. Similarly, a number needed to harm calculation showed that over 20,000 patients would need to be treated with pioglitazone to cause one additional case of bladder cancer (Ferwana et al., 2013).
TZD effects on other cancers
Despite the attention to bladder cancer, the effects of TZDs on other cancers remain uncertain, and if anything there may be protective effects. In a population of almost 90,000 veterans with diabetes, TZD use had no significant effect on prostate and colon cancer, but there was a significant 33% decrease in lung cancer (Govindarajan et al., 2007), consistent with pioglitazone’s protective effect in a mouse model of lung cancer (Li et al., 2012). In the study populations that showed increased bladder cancer risk, the KPNC cohort showed no significant effects of pioglitazone on the 10 most common cancers (Ferrara et al., 2011), but in the French cohort pioglitazone and rosiglitazone actually had significant protective effects for several other cancers (breast, colon, lung, and head and neck) (Neumann et al., 2012).
While many individual cohorts showed a neutral effect of TZDs on cancers (for example, Koro et al., 2007), the largest meta-analysis to date with data on 2.5 million people supports the idea that TZDs confer a small (5–10%) but significant decreased risk of lung, colorectal, and breast cancers (Colmers et al., 2012b). Given that these cancers are far more common than bladder cancer, these decreased risks assuage the increased risk of bladder cancer. This risk balancing may be particularly relevant as the great majority of bladder cancers on pioglitazone were non-muscle invasive and managed with transurethral resection (Ferwana et al., 2013), while TZDs could protect against more common cancers with greater morbidity and mortality. There are many proposed mechanisms for the effects of PPARγ and TZDs in various cancers, many involving increased apoptosis, and this extensive literature with much conflicting data has been reviewed recently (Robbins and Nie, 2012).
Balancing Pioglitazone Clinical Risks and Benefits
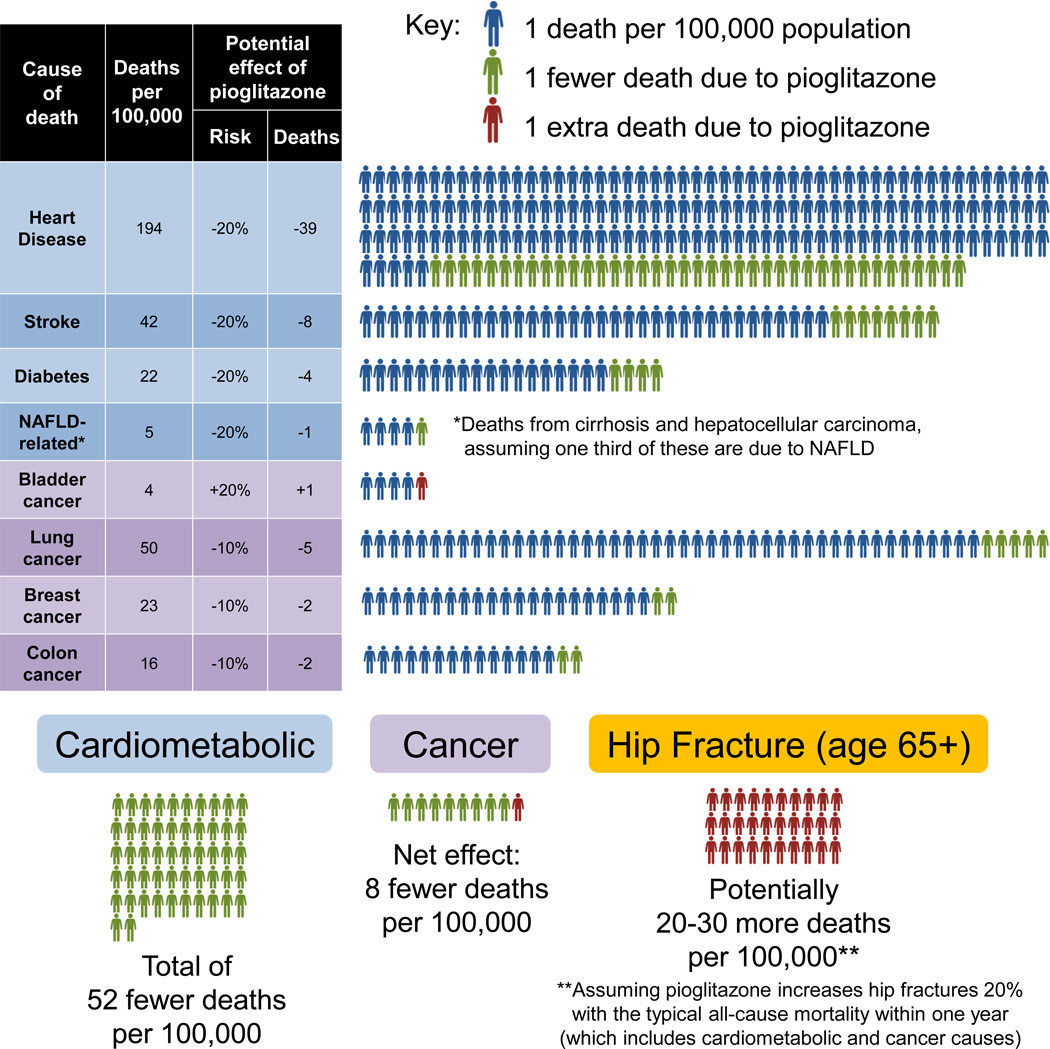
The relative risks and benefits of pioglitazone can be inferred based on the abundance of trial data summarized above and published mortality statistics in the US (Figure 3) (CDC, 2010; National Program of Cancer Registries; Yoon and Yi, 2012). Heart disease, stroke, and diabetes are the first, fourth, and seventh leading causes of death, and based on the PROactive trial and improved glycemic control, pioglitazone might decrease these deaths by ~20% - thus 52 fewer deaths per 100,000. Assuming that one third of cirrhosis and liver cancer (9.4 and 5.6 deaths per 100,000) is attributable to NAFLD, and that pioglitazone might decrease these by ~20%, this means another 1 fewer death per 100,000. This number alone is enough to mitigate the increased risk of bladder cancer: 4.4 deaths per 100,000 increased by ~20% is only 0.9 additional deaths. Given that pioglitazone may protect ~10% against more common cancers, this means ~9 fewer deaths - so pioglitazone would prevent almost 10-fold more cancer deaths than it causes. Hip fracture mortality is more complicated. Based on a landmark analysis of Medicare data, the incidence of hip fractures is 957 and 414 per 100,000 in women and women over 65, with respective 1 year mortalities of 22% and 32% (Brauer et al., 2009), thus an average mortality of 172 per 100,000. If pioglitazone truly increases hip fractures by 20%, then there are ~34 additional deaths per 100,000 people over 65 - similar to the estimate of 21 by Colhoun et al. Thus, fractures due to pioglitazone carry a much greater mortality than bladder cancer. Considering all the numbers above, pioglitazone would prevent ~60 deaths and cause only ~30 per 100,000, favoring benefits over risks by 2-fold. These are crude approximations for many reasons (i.e. the mortality statistics are from the overall population, not those with T2DM), but they are nonetheless informative.
Figure 3. Weighing Mortality Risks and Benefits of Pioglitazone.
Age-adjusted mortality rates for the entire U.S. population were derived from published government statistics for 2010–2011. Potential effects of pioglitazone on mortality from cardiometabolic causes or certain cancers were approximated from published analyses. The mortality from hip fracture is more complex to estimate, as it is not a proximal cause of death but clearly carries mortality risk in the elderly. See text for details.
Basic Science: How Do TZDs Improve Insulin Sensitivity?
PPARγ is the master regulator of adipose tissue development and function (Tontonoz and Spiegelman, 2008), and is more abundant in adipocytes than in any other cell type (Chawla et al., 1994; Tontonoz et al., 1994). Indeed, TZDs have important effects on adipose biology. TZDs stimulate progenitor stem cells to differentiate in adipocytes (Tang et al., 2011), and they also affect mature adipocytes. The “lipid steal” model proposes that adipose tissue is the metabolically safe place to store fat (Kim et al., 2003; Ye et al., 2004). In obesity, adipocytes become overwhelmed and dysfunctional, resulting in elevated serum free fatty acids and ectopic lipid deposition in liver and muscle, leading to insulin resistance (Samuel et al., 2010). In this model, TZDs improve the function of fat to safely store lipid, resulting in decreased serum free fatty acids, decreased ectopic lipids, and less insulin resistance. While not fully proven, this model explains two otherwise paradoxical observations relating obesity to insulin resistance: (1) TZDs improve insulin sensitivity despite causing weight gain and (2) lack of adequate fat (lipodystrophy) causes extreme insulin resistance (Fiorenza et al., 2011). TZDs are also effective in treating some patients with partial lipodystrophy, including HIV-infected individuals (Hadigan et al., 2004).
Since TZDs affect transcriptional activity of PPARγ, their mechanisms may be elucidated by identifying in an unbiased manner: (1) the transcriptional changes induced by TZDs or (2) the genomic binding sites for PPARγ. Multiple microarray analyses of TZD-regulated gene expression have been performed in cells and animals, and these have revealed many candidate genes involved in lipid and glucose metabolism. The genes activated by TZDs are largely consistent with increased lipid storage capacity in adipocytes, but do not truly explain their efficacy (Moore, 2005). With the recognition of brown-like adipocytes in white adipose tissue (“beige cells”), it has become clear that TZDs increase this signature of browning (Vernochet et al., 2009), perhaps by a mechanisms involving the brown fat transcription factor PRDM16 (Ohno et al., 2012; Qiang et al., 2012), and thus TZDs may also increase fatty acid oxidation and energy expenditure in fat.
Recent advances in DNA sequencing technology have allowed more detailed probing of TZD effects. Chromatin immunoprecipitation followed by massively parallel sequencing (ChIP-seq) has revealed tens of thousands of binding sites for PPARγ throughout the genome, with enrichment near the expected adipocyte metabolic genes (reviewed in Lefterova et al., 2014). In cultured mouse adipocytes, TZD treatment did not induce new PPARγ binding sites and had only small effects on increasing PPARγ occupancy at pre-existing sites (Haakonsson et al., 2013). However, TZDs do have genome-wide effects increasing recruitment of co-activators to PPARγ sites (Haakonsson et al., 2013; Step et al., 2014), thus providing a mechanism for TZD gene activation. Notably, TZD treatment results in repression of similar numbers of genes as are activated, and the mechanism of nuclear receptor ligand-mediated repression is unknown. However, a recent analysis of TZD-repressed transcription at enhancers (eRNAs) has shown that these sites lack PPARγ, such that redistribution of co-activators from these sites to those with strong PPARγ binding may account for repression (Step et al., 2014).
Some targets affected by TZDs and PPARγ are hormones or cytokines secreted by adipose tissue (adipokines), which communicate with other tissues to affect whole body metabolism (Halberg et al., 2008). Serum adiponectin levels correlate with insulin sensitivity and are increased by TZDs (Riera-Guardia and Rothenbacher, 2008). Clinically, in the ACT NOW study, increases in adiponectin levels correlated with improved insulin sensitivity on pioglitazone (Tripathy et al., 2014). Consistent with a causal role of adiponectin in TZD effects, mice lacking adiponectin show decreased response to TZD, but still improve insulin sensitivity (Nawrocki et al., 2006). Conversely, TZD treatment may decrease levels of other adipose-derived signaling molecules which are linked to insulin resistance, such as TNFα, RBP4, and resistin (reviewed in Ahmadian et al., 2013).
Recent reports have also implicated two other secreted proteins in the fibroblast growth factor (FGF) family in PPARγ and TZD effects, though acting locally in fat rather than as hormones. TZDs increase adipose tissue expression of FGF21, which acts in an autocrine or paracrine manner to increase PPARγ transcriptional activity (via suppression of Lys107 SUMOylation, see below), such that diet-induced obese mice lacking FGF21 showed decreased response to TZDs in insulin sensitization, weight gain, and even fluid retention (Dutchak et al., 2012). However, another report found that FGF21 null mice responded normally to TZDs (Adams et al., 2013), so further study will be necessary to resolve this discrepancy. FGF21 may also mediate TZD effects on bone, as FGF21 gain-of-function decreases bone mass like TZDs, and FGF21 loss-of-function actually prevents bone loss due to rosiglitazone (Wei et al., 2012). TZDs also increase expression of FGF1 via PPARγ activation, and mice lacking FGF1 show insulin resistance upon high fat diet and failure to remodel adipose tissue upon withdrawal of this diet (Jonker et al., 2012). While response to TZDs has not been reported in FGF1-null mice, pharmacological administration of FGF1 was recently shown to be insulin-sensitizing in mice (Suh et al., 2014).
TZDs are potent synthetic PPARγ ligands, but the endogenous ligand remains uncertain despite a number of candidates (e.g., certain unsaturated fatty acids, prostaglandins, oxidized lipids, and serotonin metabolites) which generally have much lower affinity for PPARγ and uncertain physiological relevance (Schupp and Lazar, 2010). Assuming that endogenous compounds do modulate PPARγ activity, it is also unknown whether TZDs have potent effects because they are simply stronger agonists or, alternatively, that TZDs have actions that are qualitatively different than those of the endogenous regulators.
What tissue(s) are most important for TZD function?
Over the past 15 years, many mouse models have been reported that elucidate the tissue-specific effects of PPARγ and TZDs (Table 2). PPARγ is by far most abundant in adipose tissue, and adipose tissue is necessary for insulin-sensitizing effects of TZDs (Chao et al., 2000). Furthermore, PPARγ gain-of-function in adipose tissue is sufficient to cause whole body insulin sensitization (Sugii et al., 2009). A study of mice with deletion of PPARγ in fat demonstrated loss of some but not all TZD effects, although these mice had a surprisingly mild lipodystrophic phenotype (He et al., 2003). This differs from a more recent deletion model which described a severe lipoatrophic diabetes, though TZD effects were not examined in this case (Wang et al., 2013).
Table 2.
Tissue-specific effects of TZDs and PPARγ in mouse models.
| Tissue | TZD effect in tissue or cell type |
Tissue-specific PPARγ knockout | Note | |
|---|---|---|---|---|
| Overall effect | TZD effect | |||
|
White Adipose |
-Increased adipogenesis -Increased lipid storage -Increased browning -Increased insulin sensitivity |
Severe lipoatrophy with marked insulin resistance (Wang et al, 2013) |
Not tested, but absent in another model of lipoatrophy (Chao et al, 2000) |
In another knockout model with milder lipodystrophy, the TZD effect was diminished but not lost. (He et al, 2003) |
| Liver | -Decreased hepatic steatosis -Increased insulin sensitivity |
Excess adiposity and whole body insulin resistance (Gavrilova et al, 2003) |
Normal response | Less steatosis is likely via “lipid steal” to adipose, as TZD effect on isolated hepatocytes is lipogenic. |
|
Skeletal Muscle |
-Increased insulin sensitivity -Decreased ectopic lipids (likely indirect effects via “lipid steal” to adipose) |
Excess adiposity and whole body insulin resistance (Norris et al, 2003) |
Normal response | Another knockout model had conflicting results, with no response to TZDs. (Hevener et al, 2003) |
|
Pancreatic beta cells |
-Increased insulin secretion | Altered islet mass but normal glucose homeostasis (Rosen et al, 2003) |
Normal response | Improved beta cell function on TZDs is also due to lower glucose and less insulin demand. |
| Macrophage | -Less M1 pro-inflammatory polarization -More M2 polarization |
Whole body insulin resistance (Hevener et al, 2007) |
Partial response | Macrophages reside in adipose tissue as well as atherosclerotic lesions. |
|
Regulatory T cell (Treg) |
-Increased number of Treg cells in obese visceral fat |
Decreased adipose Treg (Cippolletta et al, 2012) |
No longer significant response |
Effects of TZDs on isolated Treg cells have not yet been reported. |
| Brain | -Increased food intake | Less weight gain on high fat diet (Lu et al 2011) |
Normal but no longer increase food intake |
In brain knockout mice, TZDs restored whole body but not hepatic insulin sensitivity. |
| Kidney | -Fluid retention | No whole body effect reported (Guan et al, 2005) |
No longer retain fluid | Same result in a different knockout model. (Zhang et al, 2005) |
| Bone | -Increased osteoblasts -Decreased osteoclasts -Increased adipocytes |
Not known | Not known | FGF21 deletion eliminates TZDs effects on bone. (Wei et al, 2012) |
|
Cardiac Muscle |
-Cardiac hypertrophy (mice) -Increased lipid storage |
Hypertrophy with normal cardiac function (Duan et al 2005) |
Still induce further cardiac hypertrophy |
There is evidence for PPARγ-independent effects of TZDs on cardiomyocytes. |
|
Vascular Smooth Muscle |
-Reduced atherosclerotic lesions (may also be due to effects on macrophages or endothelial cells) |
Perivascular adipose tissue lost (Chang et al, 2012) |
No longer reduces atheromas (Hamblin et al, 2011) |
Both rosiglitazone and pioglitazone protect against atherosclerosis in mouse models. |
While PPARγ and TZD effects in adipose tissue are best validated, they have been investigated in other tissues and cell types. Many TZD effects are reported in isolated cells or in tissues of a whole organism, and gene targeted “knockout” mice have been generated and studied that lack PPARγ in various tissues.
PPARγ deletion in liver and muscle also causes insulin resistance, though to a much lesser degree than deletion in fat and with variable effects in response to TZDs. Two groups have deleted PPARγ selectively in skeletal muscle, with one reporting resistance to insulin sensitization by TZDs (Hevener et al., 2003) yet the other showing normal response to TZDs (Norris et al., 2003). The reasons for this discrepancy remain unclear. Mice lacking PPARγ in liver respond to TZDs normally unless adipose tissue is also defective (Gavrilova et al., 2003). PPARγ and TZDs have also been implicated in pancreatic beta cells. While TZDs enhance insulin secretion from isolated islets in a PPARγ-dependent manner, mice lacking islet PPARγ have normal glucose homeostasis and response to TZDs (Rosen et al., 2003).
In addition to the classic metabolic tissues (fat, liver, muscle, and endocrine pancreas), metabolic phenotypes have also been found in mice lacking PPARγ in immune cells, particularly those cells resident in adipose tissue and altered in obesity. PPARγ is expressed in macrophages and affects their phenotype, as PPARγ (Odegaard et al., 2007) and TZDs (Bouhlel et al., 2007) lead to alternative M2 polarization, as opposed to classic pro-inflammatory M1 polarization. Rather than a direct effect on macrophages, one study proposes that TZDs affect adipose resident macrophage polarization indirectly by lipid partitioning (Prieur et al., 2011), similar to “lipid steal” between tissues. Nonetheless, mice lacking macrophage PPARγ show whole-body insulin resistance, though this is still improved by rosiglitazone (Hevener et al., 2007).
More recently, it was reported that a subset of regulatory T (Treg) cells express high levels of PPARγ and accumulate in visceral fat of lean mice, but they decrease in diet-induced obesity and increase ~4-fold upon pioglitazone treatment. Remarkably, diet-induced obese mice with PPARγ ablation in Treg cells showed no improvement in glucose tolerance or measures of insulin sensitivity in response to pioglitazone (Cipolletta et al., 2012), though the effects were small in the control group. Beyond macrophages and Treg cells, PPARγ and TZDs have also been proposed to have effects in other immune cells like dendritic cells (Szatmari et al., 2006). It should be noted that while inhibition of atherosclerosis by TZDs in mouse models may involve their anti-inflammatory effects in macrophages (Li et al., 2000), the ability of pioglitazone to decrease atherosclerotic lesions is lost in mice lacking PPARγ in smooth muscle cells (Chang et al., 2012; Hamblin et al., 2011).
Two recent rodent studies support a role for PPARγ and TZDs in the central nervous system (Lu et al., 2011; Ryan et al., 2011). Both showed that rosiglitazone increases food intake and weight gain, and this was also seen with administration at low doses into the third cerebral ventricle to avoid systemic effects (Ryan et al., 2011). The hyperphagic effects of oral rosiglitazone were lost upon blocking brain PPARγ activity (in mice by neuron-specific PPARγ knockout or in rats by CNS-treatment with a PPARγ antagonist or siRNA). Furthermore, excess brain PPARγ activity resulted in weight gain, while attenuation of brain PPARγ activity reduced weight gain on a high fat diet. Rosiglitazone still improved whole body (but not hepatic) insulin sensitivity in mice lacking PPARγ in the brain (Lu et al., 2011) indicating that CNS effects may account for some but not all the metabolic effects of TZDs. PPARγ mRNA and immunoreactive protein can be detected in certain brain regions (Sarruf et al., 2009), but it remains uncertain whether functional levels of PPARγ protein are present in neurons, and if so which genes are TZD-regulated.
Taken together, it is remarkable that the insulin-sensitizing effects of TZDs dissipate not only when PPARγ is removed in adipose tissue, but also in several different non-adipose tissues. The evidence for adipose tissue is very robust, validated in multiple independent studies with complementary lines of evidence. The effects in other tissues, however, are typically quite small and based on one or two studies, without independent validation. Future studies are necessary to determine conclusively which of these effects are most important, which are additive, and whether they are coordinated in the context of the whole organism.
Targeting PPARγ through post-translational modifications
The now classic model of nuclear receptor function posits that ligand binding causes a conformational change, resulting in release of co-repressors and recruitment of co-activators, thus transcriptional activation of target genes (Lehrke and Lazar, 2005). Ligands are thus often defined by “classical agonism” in in vitro assays of transcriptional activation or nuclear receptor association with co-regulatory peptides (for example, Hughes et al., 2014). However, challenges to this model have emerged, as TZDs have other effects on PPARγ, including post-translational modifications.
The two best-studied phosphorylation sites on PPARγ are Ser112 and Ser273 (numbering is for the PPARγ2 isoform). Other phosphorylation sites have been proposed (Ser46 and Ser51) which may affect PPARγ subcellular localization (von Knethen et al., 2010), but these have not been studied to the same extent. Phosphorylation of PPARγ at Ser112 is inhibitory, decreasing affinity for TZDs (Hu et al., 1996; Shao et al., 1998). In mice, mutation of Ser112 to Ala mimicked the effect of TZDs, with preserved insulin sensitivity on high fat diet despite similar weight gain to controls (Rangwala et al., 2003). Ser112 phosphorylation is thought to occur via growth-factor stimulated MAP kinases like MEK1, and several phosphatases have been proposed, most recently PPM1B (Tasdelen et al., 2013). Paradoxically, it has also been reported that phosphorylation of Ser112 by the kinase cdk9 stimulates rather than represses PPARγ activity (Iankova et al., 2006). Notably, no reports have indicated that TZDs affect Ser112 phosphorylation. In contrast, the more recently discovered phosphorylation at Ser273 by Cdk5 is blocked by TZDs (Choi et al., 2010). Interestingly, mutation of Ser273 to Ala did not affect overall activity of PPARγ, but led to selective activation of a subset of PPARγ target genes including adiponectin, suggesting that phosphorylation normally suppresses expression of these beneficial genes - and that TZDs activate them. Furthermore, even partial agonists with weak classical agonism of PPARγ (like MRL-24) could inhibit Ser273 phosphorylation similar to full agonists like rosiglitazone. This has led to the hope that drugs decreasing Ser273 phosphorylation of PPARγ with minimal agonist activity might confer the benefits of TZDs without the adverse events. Indeed, two compounds called SR1664 (Choi et al., 2011) and GQ-16 (Amato et al., 2012) are reported to confer equal insulin sensitization to rosiglitazone in mice, yet remarkably without weight gain or edema. Similarly, natural legume-derived compounds called amorfrutins are also weak PPARγ ligands that inhibit Ser273 phosphorylation and have surprisingly potent insulin-sensitizing effects (Weidner et al., 2012). Ser273 phosphorylation was also reduced in mice with adipose tissue deletion of the co-repressor NCoR, another model in which increased PPARγ activity mimics TZD treatment (Li et al., 2011).
PPARγ can also be covalently attached to ubiquitin or small ubiquitin-like modifier (SUMO) proteins. Like the nearby Ser112 phosphorylation, SUMOlyation of PPARγ at Lys107 also represses its transcriptional activity (Floyd and Stephens, 2004; Ohshima et al., 2004; Yamashita et al., 2004), and TZDs are not reported to affect this. In contrast, TZD are reported to induce SUMOlyation at Lys395, which is central to the “transrepression” model whereby PPARγ in macrophages stabilizes co-repressors at inflammatory gene promoters (Pascual et al., 2005). TZDs also cause the ubiquitination and degradation of PPARγ (Hauser et al., 2000), and the ubiquitin ligase Siah2 has been implicated (Kilroy et al., 2012). Ubiquitination occurs in the ligand binding domain though the exact site is unknown. While proteasomal degradation of PPARγ would clearly decrease transcriptional activation, there is also evidence that PPARγ ubiquitination is necessary for its activity (Kilroy et al., 2009).
Most recently, glycosylation and acetylation of PPARγ have been reported. Glycosylation of PPARγ (O-GlcNAc at Thr84) was shown in cultured mouse adipocytes, and this decreased basal and TZD-stimulated reporter activity (Ji et al., 2012), though it was not reported whether TZDs affected glycosylation. Rosiglitazone was shown to decrease acetylation of overexpressed PPARγ at Lys268 and Lys293, while other acetylation sites (Lys98, Lys107, and Lys218) were not affected by TZD (Qiang et al., 2012). These authors propose a model whereby ligand-mediated PPARγ interaction with the deacetylase SirT1 results in deacetylation and alterations in the PPARγ gene activation profile favoring a beige adipocyte phenotype. Another recent study suggests a conflicting model based on an observation that pioglitazone instead increases acetylation of endogenous PPARγ in cultured adipocytes (Jiang et al., 2014).
Given that many modifications of PPARγ are in close proximity based on its crystal structure, there are potential interactions among these phosphorylated, SUMOlyated, ubiquitinated, glycosylated, and acetylated residues. However, it must be noted most studies of PPARγ post-translational modifications have been performed in cells with limited independent validation. Only Ser112 has been shown to affect insulin sensitivity in a whole animal model (Rangwala et al., 2003). While other modifications are potentially attractive targets for new drug development, enthusiasm should be tempered until there is rigorous in vivo validation of their relevance in physiology and disease.
Non-PPARγ targets of TZDs?
TZDs have rapid non-transcriptional effects that appear to be independent of PPARγ. One such effect is activation of AMP-kinase (LeBrasseur et al., 2006), which would be predicted to have insulin-sensitizing effects (Hardie, 2014). TZDs have also been reported to bind mitochondrial membranes (Feinstein et al., 2005), and the pyruvate carriers MCP1 and MCP2 have recently been strongly implicated as the mitochondrial targets of TZDs (mTOTs) (Colca et al., 2013a; Divakaruni et al., 2013). MSDC-0160 (aka PNU-91325) is considered the prototype mTOT modulator, and though it is described as “PPARγ-sparing,” it is important to note that it is a TZD which still activates PPARγ in reporter assays, though ~20-fold less potently than pioglitazone (Bolten et al., 2007). A phase 2 randomized clinical trial of MSDC-0160 was recently reported, and doses of 100–150mg gave similar HbA1c lowering as 45mg of pioglitazone at 12 weeks (Colca et al., 2013b). Notably, however, the typical signs of PPARγ agonism were still present at these doses: there was significant fluid retention (based on hemoglobin decrease), weight gain, and elevations in adiponectin compared to placebo, though each was only ~50% as much as pioglitazone. MSDC-0160 is currently being developed for neurodegenerative conditions rather than T2DM, but the similar drug MSDC-0602 was an effective insulin sensitizer in rodents (Chen et al., 2012) and in an unpublished phase 2 trial for diabetes (NCT01280695). Given that both compounds are pioglitazone derivatives with some PPARγ activation (Figure 2), a better test of mTOT as a drug target would require a non-TZD mTOT inhibitor completely devoid of PPARγ agonism.
It has even been suggested that beneficial effects of TZDs on insulin sensitivity stem primarily from the mitochondrial pathway, while PPARγ mediates undesired effects (Colca et al., 2013b). However, PPARγ is clearly implicated in insulin sensitivity by strong and unbiased evidence from human genetics. Rare families with mutations in the ligand binding domain of PPARγ show autosomal dominant inheritance of a syndrome of lipodystrophy and insulin resistance (Barroso et al., 1999). Furthermore, common polymorphisms in the PPARG gene locus are associated with risk of type 2 diabetes in genome-wide association studies (Gouda et al., 2010). The causative risk allele was long thought to be a coding Pro12Ala polymorphism in PPARγ2, however a recent study has indicated that the true causal polymorphism lies upstream of PPARγ2 and affects its gene regulation (Claussnitzer et al., 2014). Regardless, PPARγ is clearly associated with insulin sensitivity and diabetes, so it would be very surprising if PPARγ agonism was only incidental to the antidiabetic effects of its potent TZD ligands. Another strong argument favoring PPARγ as the target of the insulin sensitizing effects of TZDs is the observation that several PPARγ agonists lack the TZD structure, and thus presumably the non-PPARγ effects of TZDs, yet are potent insulin sensitizers (Figure 2, see below).
More new drug development: Selective, partial, and dual agonists
There has long been hope that, analogous to selective estrogen receptor modifiers (SERMs), selective PPARγ modulators (SPARMs, either TZD or non-TZD) with reduced or partial agonist activity may retain glucose-lowering benefits with decreased risk of adverse effects (Rangwala and Lazar, 2002). Such drugs that affect mTOTs or PPARγ Ser273 phosphorylation are described above. The TZD balaglitazone has partial agonist activity with fewer side effects in rat models (Henriksen et al., 2009), and a human trial showed similar glycemic efficacy to pioglitazone with trends towards fewer sides effects (Henriksen et al., 2011). However, in this trial, balaglitazone at 10mg or 20mg clearly caused fluid retention, edema, and increased body fat compared to placebo, and only the lower dose - which was less effective at lowering glucose - showed differences in these side effects compared to pioglitazone 45mg. Bone loss was not significant in any group, though a trend towards decrease was present for pioglitazone. The diarysulfonamide non-TZD class of PPARγ partial agonists, which includes the drug INT-131, is structurally distinct from TZDs with different binding properties (Bajare et al., 2012). In mouse models, INT-131 failed to cause volume expansion and actually increased bone density, and a recent 24-week trial of 362 patients with T2DM showed equal glucose lowering efficacy to 45mg pioglitazone with evidence for less fluid retention (DePaoli et al., 2014). MBX-102 and GW1929 are two other structurally distinct non-TZD agonists with promising results in animal and cell models (Brown et al., 1999; Gregoire et al., 2009). However, despite this promise, further development of SSPARMs appears to have mostly halted.
Dual agonists of PPARγ and the related nuclear receptor PPARα also hold promise for treating insulin resistance and dyslipidemia in metabolic syndrome, as they may combine the beneficial effects of TZDs and fibrates (hypolipidemic PPARα agonists) with fewer side effects. The dual agonist saroglitazar is approved in India, and a phase 3 study of 302 patients with diabetic dyslipidemia despite statin therapy showed that addition of saroglitazar improved triglycerides and fasting glucose (Jani et al., 2014). AleCardio was a large international multicenter phase 3 trial of 7,226 patients with T2DM hospitalized for acute cononary syndrome, randomized to the dual agonist aleglitazar versus placebo (Lincoff et al., 2014). While aleglitazar had the expected effects on glycemia and lipoproteins, the trial was stopped early at a median of 2 years due to futility, as the primary endpoint of recurrent heart attack, stroke, or cardiovascular death was not altered, yet there was evidence for increased serious adverse events. Smaller trials of other dual agonists (aleglitazar and tesaglitazar) have also been unsuccessful. The failure of the large AleCardio RCT may spell the end of drug development for dual PPAR agonists and unfortunately even dampens enthusiasm for developing new drugs that target PPARγ.
Why does it matter? The case for reducing insulin levels
Diabetes continues to increase in prevalence, affecting 29 million people in the United States (9.3%) in 2012, up 3 million from 2010 (CDC, 2014). Insulin resistance is the sine qua non of T2DM, and hence only therapies that improve insulin sensitivity address the basic pathophysiology of this condition (DeFronzo, 2004). Furthermore, there is a school of thought that the increased concentrations of insulin that are prevalent in T2DM are a major contributor to the co-morbidities, particularly macrovascular disease (Després et al., 1996). This would explain why even insulin resistant patients without diabetes are prone to the same vascular complications (Facchini et al., 2001), and thus it is of great concern that one third of Americans (86 million) have pre-diabetes (CDC, 2014). Reaven has suggested that hyperinsulinemia is an etiologic component of other dysmetabolic parameters associated with metabolic syndrome and cardiovascular risk, including hypertension, low HDL, and hypertriglyceridemia (Reaven, 1988). Even further, while hyperinsulinemia certainly results from obesity and insulin resistance, Ludwig and Friedman have promoted the model whereby insulin positively feeds back to cause overeating and adiposity in a vicious cycle (Ludwig and Friedman, 2014). Indeed, a mouse model with decreased insulin gene dosage shows remarkable resistance to diet-induced hyperinsulinemia and weight gain (Mehran et al., 2012).
Even beyond cardiometabolic disease, hyperinsulinemia is also thought to be central to the elevated cancer risk associated with diabetes and obesity (Gallagher and LeRoith, 2013). However, many therapies for T2DM focus on overcoming insulin resistance by increasing insulin levels, either by stimulating secretion of endogenous insulin (e.g., glucagon-like peptide 1 [GLP-1] receptor agonists, DPP4 inhibitors, sulfonylureas) or by providing insulin exogenously. A very recent retrospective cohort study of VA patients (Roumie CL et al., 2014) supports the idea that high insulin levels are harmful. In patients with diabetes started on metformin from 2001–2008, those for whom insulin was added as the second agent had a significant 44% higher all-cause mortality (mainly due to cancer) versus those adding a sulfonylurea. Note that patients receiving any other anti-diabetic medications, including TZDs, were excluded from the study, and thus the likely effect of TZDs to reduce insulin requirements was not evaluated. Another very recent industry-funded retrospective cohort study did compare TZD to insulin and found that patients started on pioglitazone from 2000–2010 had a remarkably significant 67% lower all-cause mortality than those started on insulin (Yang et al., 2014), though this study was unable to adjust for glycemic control.
Since hyperinsulinemia is a plausible contributor to the comorbidities of type 2 diabetes, it is prudent to develop novel therapies that address this underlying problem. Treatment of obesity could address this, and while bariatric surgery is effective, clinical experience with lifestyle medication and pharmacotherapy for obesity has been disappointing to date, particularly in terms of weight regain (Hainer et al., 2008). Novel therapeutics directed at activating brown or beige adipocytes have promise for treating obesity as well as diabetes, but none are currently available for clinical use. Since TZDs are the most potent known insulin sensitizers, by definition patients on TZDs will require lower levels of endogenous and exogenous insulin to maintain euglycemia. Indeed, patients using insulin who are started on TZDs typically reduce their insulin dose or even discontinue insulin injections (Yau et al., 2013). Therefore, understanding how TZDs work and effectively harnessing the underlying mechanisms with fewer side-effects would be a welcome advance in the arsenal of antidiabetic drugs (Kahn and McGraw, 2010).
There is great hope that recent studies highlighted here may translate to new drug development. There is promise of selective, partial, and dual agonism, as well as specifically targeting post-translational modifications of PPARγ. In addition to the canonical PPARγ ligand binding domain, recent studies have identified an alternate site is occupied potently by several non-TZD ligands but not TZDs, adding additional complexity to agonist pharmacology (Hughes et al., 2014). Furthermore, rather than PPARγ per se, novel drugs may also achieve insulin sensitization by targeting other TZD-related systems such as AMP kinase, mitochondrial transporters, or FGFs. Tissue selectivity is also a key issue. For instance, a PPARγ agonist that failed to reach the brain, kidneys, or skeleton might be expected to eliminate side effects of weight gain, fluid retention, and bone loss, respectively. The future may involve targeting small molecule drugs to relevant tissues - such as TZDs to adipose tissue - by conjugation with selective peptides, as estrogen has been conjugated to GLP-1 to target tissues that express the GLP-1 receptor and treat metabolic syndrome in mice (Finan et al., 2012). Another way to achieve tissue selectivity might be an orally administered PPARγ agonist that is inactivated by first pass liver metabolism, thus having selective effects on visceral fat without reaching other tissues. Targeting of TZDs selectively to immune cells, both in adipose tissue and the vascular wall, may likewise be beneficial.
Another way forward would be to identify those patients most likely to benefit from TZDs or novel insulin sensitizers with minimal risks. It has been noted that about a quarter of patients with T2DM are “non-responders” who do not improve insulin sensitivity on TZDs, while an equal number have large responses (Sears et al., 2009). The umbrella of T2DM encompasses many heterogeneous phenotypes (Gale, 2013), and further investigation may identify a distinct subset of patients in which TZDs may be most effective. Genetic predispositions, such as polymorphisms affecting PPARγ genomic occupancy, may also modulate response to the insulin-sensitizing or harmful effects of TZDs. Together, new drug development and more personalized pharmacotherapy may fulfill the promise of insulin sensitization in T2DM.
Conclusions
Despite the benefits of insulin sensitization by TZDs, this once widely-used class of drugs has fallen into disrepute and disuse. The goal of diabetes therapy is not only glucose lowering, but also protection from its comorbidities and, in this aspect, TZDs carry benefits and risks that must be weighed, as for any pharmacological treatment. In the case of TZDs, public attention has been focused more on the potential harms of these drugs than on their benefits. For instance, the meta-analysis showing cardiovascular risk for rosiglitazone had drastic effects on policy and prescribing, while other meta-analyses showing beneficial effects - like cancer protection by TZDs - are largely dismissed. Even randomized studies consistently indicating cardiovascular benefits of pioglitazone are dismissed for various reasons (i.e. PROactive did not meet the primary endpoint, PERISCOPE looked at a surrogate endpoint, etc). Bladder cancer risk based on observational studies is now widely cited as a reason to abandon pioglitazone, yet the absolute risk increase is extremely small, and fracture risk - with better clinical and mechanistic evidence and likely greater harm - is a more convincing reason to reconsider this drug.
The data may warrant a more balanced view weighing the potential risks and benefits for TZDs, particularly pioglitazone, which might be used more often in selected patients. For instance, there may be a role in younger patients with prediabetes or early T2DM, when insulin sensitization may do the most to reduce hyperinsulinemia and preserve beta-cell function - but fracture risk is much less than in the elderly. This is consistent with the current evidence-based trend to individualize T2DM management, as early glycemic control may have long-term cardiovascular benefits via “metabolic memory,” yet tight glycemic control in older patients with long-standing diabetes does not carry these benefits and may even cause harm (American Diabetes Association, 2014).
Whatever the balance between benefits and risks of current TZD therapies, the promise of insulin sensitization for treatment of metabolic syndrome and diabetes should not be abandoned. Basic mechanistic studies continue to unravel the complex biology underlying the beneficial and adverse effects of TZDs, and better understanding of their salient as well as their harmful effects has great potential to pave the way for the next generation of therapeutics.
Acknowledgements
Work in the Lazar lab on PPARγ is supported by NIH R01 DK49780 and the JPB Foundation. RES is also supported by NIH K08 DK094968.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACOG Committee on Practice Bulletins--Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet. Gynecol. 2009;114:936–949. doi: 10.1097/AOG.0b013e3181bd12cb. [DOI] [PubMed] [Google Scholar]
- Adams AC, Coskun T, Cheng CC, O Farrell LS, Dubois SL, Kharitonenkov A. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Mol. Metab. 2013;2:205–214. doi: 10.1016/j.molmet.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato AA, Rajagopalan S, Lin JZ, Carvalho BM, Figueira ACM, Lu J, Ayers SD, Mottin M, Silveira RL, Souza PCT, et al. GQ-16, a novel peroxisome proliferator-activated receptor γ (PPARγ) ligand, promotes insulin sensitization without weight gain. J. Biol. Chem. 2012;287:28169–28179. doi: 10.1074/jbc.M111.332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes--2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- Azoulay L, Yin H, Filion KB, Assayag J, Majdan A, Pollak MN, Suissa S. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ. 2012;344:e3645–e3645. doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach RG, Brooks MM, Lombardero M, Genuth S, Donner TW, Garber A, Kennedy L, Monrad ES, Pop-Busui R, Kelsey SF, et al. Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2013;128:785–794. doi: 10.1161/CIRCULATIONAHA.112.000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajare S, Anthony J, Nair A, Marita R, Damre A, Patel D, Rao C, Sivaramakrishnan H, Deka N. Synthesis of N-(5-chloro-6-(quinolin-3-yloxy)pyridin-3-yl)benzenesulfonamide derivatives as non-TZD peroxisome proliferator-activated receptor γ (PPARγ) agonist. Eur. J. Med. Chem. 2012;58:355–360. doi: 10.1016/j.ejmech.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Basu A, Jensen MD, McCann F, Mukhopadhyay D, Joyner MJ, Rizza RA. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care. 2006;29:510–514. doi: 10.2337/diacare.29.03.06.dc05-2004. [DOI] [PubMed] [Google Scholar]
- Beck GR, Jr, Khazai NB, Bouloux GF, Camalier CE, Lin Y, Garneys LM, Siqueira J, Peng L, Pasquel F, Umpierrez D, et al. The effects of thiazolidinediones on human bone marrow stromal cell differentiation in vitro and in thiazolidinedione-treated patients with type 2 diabetes. Transl. Res. J. Lab. Clin. Med. 2013;161:145–155. doi: 10.1016/j.trsl.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- Bełtowski J, Rachańczyk J, Włodarczyk M. Thiazolidinedione-induced fluid retention: recent insights into the molecular mechanisms. PPAR Res. 2013;2013:628628. doi: 10.1155/2013/628628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berria R, Glass L, Mahankali A, Miyazaki Y, Monroy A, De Filippis E, Cusi K, Cersosimo E, Defronzo RA, Gastaldelli A. Reduction in hematocrit and hemoglobin following pioglitazone treatment is not hemodilutional in Type II diabetes mellitus. Clin. Pharmacol. Ther. 2007;82:275–281. doi: 10.1038/sj.clpt.6100146. [DOI] [PubMed] [Google Scholar]
- Bolten CW, Blanner PM, McDonald WG, Staten NR, Mazzarella RA, Arhancet GB, Meier MF, Weiss DJ, Sullivan PM, Hromockyj AE, et al. Insulin sensitizing pharmacology of thiazolidinediones correlates with mitochondrial gene expression rather than activation of PPAR gamma. Gene Regul. Syst. Biol. 2007;1:73–82. [PMC free article] [PubMed] [Google Scholar]
- Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, et al. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA J. Am. Med. Assoc. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Smith SR, Banerji MA, Tripathy D, Clement SC, Buchanan TA, Henry RR, Kitabchi AE, Mudaliar S, Musi N, et al. Effect of pioglitazone on body composition and bone density in subjects with prediabetes in the ACT NOW trial. Diabetes Obes. Metab. 2013;15:931–937. doi: 10.1111/dom.12099. [DOI] [PubMed] [Google Scholar]
- Brown KK, Henke BR, Blanchard SG, Cobb JE, Mook R, Kaldor I, Kliewer SA, Lehmann JM, Lenhard JM, Harrington WW, et al. A novel N-aryl tyrosine activator of peroxisome proliferator-activated receptor-gamma reverses the diabetic phenotype of the Zucker diabetic fatty rat. Diabetes. 1999;48:1415–1424. doi: 10.2337/diabetes.48.7.1415. [DOI] [PubMed] [Google Scholar]
- CDC. FASTSTATS - Deaths and Mortality. 2010 [Google Scholar]
- CDC. More than 29 million Americans have diabetes; 1 in 4 doesn’t know. Press Release, CDC Online Newsroom; 2014. [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatol. Baltim. Md. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Marcus-Samuels B, Mason MM, Moitra J, Vinson C, Arioglu E, Gavrilova O, Reitman ML. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest. 2000;106:1221–1228. doi: 10.1172/JCI11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- Chen Z, Vigueira PA, Chambers KT, Hall AM, Mitra MS, Qi N, McDonald WG, Colca JR, Kletzien RF, Finck BN. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor γ-sparing thiazolidinedione. J. Biol. Chem. 2012;287:23537–23548. doi: 10.1074/jbc.M112.363960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquette E, Ramirez G, Defronzo R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch. Intern. Med. 2004;164:2097–2104. doi: 10.1001/archinte.164.19.2097. [DOI] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussnitzer M, Dankel SN, Klocke B, Grallert H, Glunk V, Berulava T, Lee H, Oskolkov N, Fadista J, Ehlers K, et al. Leveraging cross-species transcription factor binding site patterns: from diabetes risk loci to disease mechanisms. Cell. 2014;156:343–358. doi: 10.1016/j.cell.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, McDonald WG, Cavey GS, Cole SL, Holewa DD, Brightwell-Conrad AS, Wolfe CL, Wheeler JS, Coulter KR, Kilkuskie PM, et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PloS One. 2013a;8:e61551. doi: 10.1371/journal.pone.0061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, VanderLugt JT, Adams WJ, Shashlo A, McDonald WG, Liang J, Zhou R, Orloff DG. Clinical proof-of-concept study with MSDC-0160, a prototype mTOT-modulating insulin sensitizer. Clin. Pharmacol. Ther. 2013b;93:352–359. doi: 10.1038/clpt.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colhoun HM, Livingstone SJ, Looker HC, Morris AD, Wild SH, Lindsay RS, Reed C, Donnan PT, Guthrie B, Leese GP, et al. Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose-lowering drugs. Diabetologia. 2012;55:2929–2937. doi: 10.1007/s00125-012-2668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ Can. Med. Assoc. J. J. Assoc. Medicale Can. 2012a;184:E675–E683. doi: 10.1503/cmaj.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2012b;38:475–484. doi: 10.1016/j.diabet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Deeg MA, Buse JB, Goldberg RB, Kendall DM, Zagar AJ, Jacober SJ, Khan MA, Perez AT, Tan MH GLAI Study Investigators. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30:2458–2464. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med. Clin. North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. ix. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- DePaoli AM, Higgins LS, Henry RR, Mantzoros C, Dunn FL. Can a Selective PPARγ Modulator Improve Glycemic Control in Patients With Type 2 Diabetes With Fewer Side Effects Compared With Pioglitazone? Diabetes Care DC_132480. 2014 doi: 10.2337/dc13-2480. [DOI] [PubMed] [Google Scholar]
- Després JP, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, Anker SD. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int. J. Cardiol. 2012;162:20–26. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJA, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ. Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast Growth Factor-21 Regulates PPARγ Activity and the Antidiabetic Actions of Thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM PROactive Investigators. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J. Am. Coll. Cardiol. 2007;49:1772–1780. doi: 10.1016/j.jacc.2006.12.048. [DOI] [PubMed] [Google Scholar]
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn A-R, Perez A. Observational follow-up of the PROactive study: a 6-year update. Diabetes Obes. Metab. 2014;16:63–74. doi: 10.1111/dom.12180. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J. Clin. Endocrinol. Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- Faillie J-L, Petit P, Montastruc J-L, Hillaire-Buys D. Scientific Evidence and Controversies About Pioglitazone and Bladder Cancer: Which Lessons Can Be Drawn? Drug Saf. Int. J. Med. Toxicol. Drug Exp. 2013 doi: 10.1007/s40264-013-0086-y. [DOI] [PubMed] [Google Scholar]
- FDA. Press Announcements - FDA requires removal of certain restrictions on the diabetes drug Avandia. 2013 [Google Scholar]
- Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem. Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Ferrara A, Lewis JD, Quesenberry CP, Jr, Peng T, Strom BL, Van Den Eeden SK, Ehrlich SF, Habel LA. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011;34:923–929. doi: 10.2337/dc10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwana M, Firwana B, Hasan R, Al-Mallah MH, Kim S, Montori VM, Murad MH. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet. Med. 2013;30:1026–1032. doi: 10.1111/dme.12144. [DOI] [PubMed] [Google Scholar]
- Finan B, Yang B, Ottaway N, Stemmer K, Müller TD, Yi C-X, Habegger K, Schriever SC, García-Cáceres C, Kabra DG, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat. Rev. Endocrinol. 2011;7:137–150. doi: 10.1038/nrendo.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd ZE, Stephens JM. Control of peroxisome proliferator-activated receptor gamma2 stability and activity by SUMOylation. Obes. Res. 2004;12:921–928. doi: 10.1038/oby.2004.112. [DOI] [PubMed] [Google Scholar]
- Fujita T, Sugiyama Y, Taketomi S, Sohda T, Kawamatsu Y, Iwatsuka H, Suzuoki Z. Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(1-methylcyclohexylmethoxy)benzyl]-thiazolidine-2,4-dione (ADD-3878, U-63,287, ciglitazone), a new antidiabetic agent. Diabetes. 1983;32:804–810. doi: 10.2337/diab.32.9.804. [DOI] [PubMed] [Google Scholar]
- Gale EAM. Is type 2 diabetes a category error? Lancet. 2013;381:1956–1957. doi: 10.1016/S0140-6736(12)62207-7. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, LeRoith D. Epidemiology and Molecular Mechanisms Tying Obesity, Diabetes, and the Metabolic Syndrome With Cancer. Diabetes Care. 2013;36:S233–S239. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- Gouda HN, Sagoo GS, Harding A-H, Yates J, Sandhu MS, Higgins JPT. The association between the peroxisome proliferator-activated receptor-gamma2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am. J. Epidemiol. 2010;171:645–655. doi: 10.1093/aje/kwp450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25:1476–1481. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. RIsk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Zhang F, Clarke HJ, Gustafson TA, Sears DD, Favelyukis S, Lenhard J, Rentzeperis D, Clemens LE, Mu Y, et al. MBX-102/JNJ39659100, a novel peroxisome proliferator-activated receptor-ligand with weak transactivation activity retains antidiabetic properties in the absence of weight gain and edema. Mol. Endocrinol. Baltim. Md. 2009;23:975–988. doi: 10.1210/me.2008-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey A. Thiazolidinedione-induced skeletal fragility--mechanisms and implications. Diabetes Obes. Metab. 2009;11:275–284. doi: 10.1111/j.1463-1326.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat. Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- Haakonsson AK, Stahl Madsen M, Nielsen R, Sandelin A, Mandrup S. Acute genome-wide effects of rosiglitazone on PPARγ transcriptional networks in adipocytes. Mol. Endocrinol. Baltim. Md. 2013;27:1536–1549. doi: 10.1210/me.2013-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized, controlled trial. Ann. Intern. Med. 2004;140:786–794. doi: 10.7326/0003-4819-140-10-200405180-00008. [DOI] [PubMed] [Google Scholar]
- Hainer V, Toplak H, Mitrakou A. Treatment modalities of obesity: what fits whom? Diabetes Care. 2008;31(Suppl 2):S269–S277. doi: 10.2337/dc08-s265. [DOI] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North Am. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. x–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M, Chang L, Zhang H, Yang K, Zhang J, Chen YE. Vascular smooth muscle cell peroxisome proliferator-activated receptor-γ mediates pioglitazone-reduced vascular lesion formation. Arterioscler. Thromb. Vasc. Biol. 2011;31:352–359. doi: 10.1161/ATVBAHA.110.219006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003–2012. Diabetes Care. 2014;37:1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014 doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsløf T, Wamberg L, Møller L, Stødkilde-Jørgensen H, Ringgaard S, Pedersen SB, Langdahl BL. Rosiglitazone decreases bone mass and bone marrow fat. J. Clin. Endocrinol. Metab. 2011;96:1541–1548. doi: 10.1210/jc.2010-2077. [DOI] [PubMed] [Google Scholar]
- Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J. Biol. Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- He H, Tao H, Xiong H, Duan SZ, McGowan FX, Jr, Mortensen RM, Balschi JA. Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor γ-independent mitochondrial oxidative stress in mouse hearts. Toxicol. Sci. Off. J. Soc. Toxicol. 2014;138:468–481. doi: 10.1093/toxsci/kfu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen K, Byrjalsen I, Nielsen RH, Madsen AN, Larsen LK, Christiansen C, Beck-Nielsen H, Karsdal MA. A comparison of glycemic control, water retention, and musculoskeletal effects of balaglitazone and pioglitazone in diet-induced obese rats. Eur. J. Pharmacol. 2009;616:340–345. doi: 10.1016/j.ejphar.2009.06.051. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Byrjalsen I, Qvist P, Beck-Nielsen H, Hansen G, Riis BJ, Perrild H, Svendsen OL, Gram J, Karsdal MA, et al. Efficacy and safety of the PPARγ partial agonist balaglitazone compared with pioglitazone and placebo: a phase III, randomized, parallel-group study in patients with type 2 diabetes on stable insulin therapy. Diabetes Metab. Res. Rev. 2011;27:392–401. doi: 10.1002/dmrr.1187. [DOI] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Olefsky JM, Reichart D, Nguyen MTA, Bandyopadyhay G, Leung H-Y, Watt MJ, Benner C, Febbraio MA, Nguyen A-K, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J. Clin. Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillaire-Buys D, Faillie J-L, Montastruc J-L. Pioglitazone and bladder cancer. The Lancet. 2011;378:1543–1544. doi: 10.1016/S0140-6736(11)61662-0. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes--an interim analysis. N. Engl. J. Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- Horio T, Suzuki M, Suzuki K, Takamisawa I, Hiuge A, Kamide K, Takiuchi S, Iwashima Y, Kihara S, Funahashi T, et al. Pioglitazone improves left ventricular diastolic function in patients with essential hypertension. Am. J. Hypertens. 2005;18:949–957. doi: 10.1016/j.amjhyper.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Hughes TS, Giri PK, de Vera IMS, Marciano DP, Kuruvilla DS, Shin Y, Blayo A-L, Kamenecka TM, Burris TP, Griffin PR, et al. An alternate binding site for PPARγ ligands. Nat. Commun. 2014;5:3571. doi: 10.1038/ncomms4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iankova I, Petersen RK, Annicotte J-S, Chavey C, Hansen JB, Kratchmarova I, Sarruf D, Benkirane M, Kristiansen K, Fajas L. Peroxisome proliferator-activated receptor gamma recruits the positive transcription elongation factor b complex to activate transcription and promote adipogenesis. Mol. Endocrinol. Baltim. Md. 2006;20:1494–1505. doi: 10.1210/me.2005-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch. Intern. Med. 2012;172:1005–1011. doi: 10.1001/archinternmed.2012.1938. [DOI] [PubMed] [Google Scholar]
- Jani RH, Pai V, Jha P, Jariwala G, Mukhopadhyay S, Bhansali A, Joshi S. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI) Diabetes Technol. Ther. 2014;16:63–71. doi: 10.1089/dia.2013.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S, Park SY, Roth J, Kim HS, Cho JW. O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochem. Biophys. Res. Commun. 2012;417:1158–1163. doi: 10.1016/j.bbrc.2011.12.086. [DOI] [PubMed] [Google Scholar]
- Jiang X, Ye X, Guo W, Lu H, Gao Z. Inhibition of HDAC3 promotes ligand-independent PPARgamma activation by protein acetylation. J. Mol. Endocrinol. 2014 doi: 10.1530/JME-14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol. Endocrinol. Baltim. Md. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin Y-Q, Phillips CT, et al. A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, McGraw TE. Rosiglitazone, PPARγ, and type 2 diabetes. N. Engl. J. Med. 2010;363:2667–2669. doi: 10.1056/NEJMcibr1012075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, et al. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT) Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- Kilroy G, Kirk-Ballard H, Carter LE, Floyd ZE. The ubiquitin ligase Siah2 regulates PPARγ activity in adipocytes. Endocrinology. 2012;153:1206–1218. doi: 10.1210/en.2011-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilroy GE, Zhang X, Floyd ZE. PPAR-gamma AF-2 domain functions as a component of a ubiquitin-dependent degradation signal. Obes. Silver Spring Md. 2009;17:665–673. doi: 10.1038/oby.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim H-J, Yu C, Chen Y, Qu X, et al. Differential Effects of Rosiglitazone on Skeletal Muscle and Liver Insulin Resistance in A-ZIP/F-1 Fatless Mice. Diabetes. 2003;52:1311–1318. doi: 10.2337/diabetes.52.6.1311. [DOI] [PubMed] [Google Scholar]
- Von Knethen A, Tzieply N, Jennewein C, Brüne B. Casein-kinase-II-dependent phosphorylation of PPARgamma provokes CRM1-mediated shuttling of PPARgamma from the nucleus to the cytosol. J. Cell Sci. 2010;123:192–201. doi: 10.1242/jcs.055475. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, Fowler SE, Nathan DM, Kahn SE Diabetes Prevention Program Research Group. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am. J. Gastroenterol. 2000;95:272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol. Drug Saf. 2007;16:485–492. doi: 10.1002/pds.1352. [DOI] [PubMed] [Google Scholar]
- Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang T-C, Xie X-J, He L, Mangala LS, Lopez-Berestein G, et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. advance online publication. 20142014 doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- Leal I, Romio SA, Schuemie M, Oteri A, Sturkenboom M, Trifirò G. Prescribing pattern of glucose lowering drugs in the United Kingdom in the last decade: a focus on the effects of safety warnings about rosiglitazone. Br. J. Clin. Pharmacol. 2013;75:861–868. doi: 10.1111/j.1365-2125.2012.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrasseur NK, Kelly M, Tsao T-S, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am. J. Physiol. Endocrinol. Metab. 2006;291:E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. TEM. 2014;25:293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The Many Faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lemoine M, Serfaty L, Cervera P, Capeau J, Ratziu V. Hepatic molecular effects of rosiglitazone in human non-alcoholic steatohepatitis suggest long-term pro-inflammatory damage. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2013 doi: 10.1111/hepr.12244. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Jr, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Habel L, Quesenberry C, Mamtani R, Peng T, Bilker WB, Hedderson M, Nessel L, Vaughn DJ, Strom BL, et al. Proteinuria testing among patients with diabetes mellitus is associated with bladder cancer diagnosis: potential for unmeasured confounding in studies of pioglitazone and bladder cancer. Pharmacoepidemiol. Drug Saf. 2014 doi: 10.1002/pds.3619. [DOI] [PubMed] [Google Scholar]
- Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M-Y, Kong AWY, Yuan H, Ma LT, Hsin MKY, Wan IYP, Underwood MJ, Chen GG. Pioglitazone prevents smoking carcinogen-induced lung tumor development in mice. Curr. Cancer Drug Targets. 2012;12:597–606. doi: 10.2174/156800912801784848. [DOI] [PubMed] [Google Scholar]
- Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, et al. Adipocyte NCoR knockout decreases PPARγ phosphorylation and enhances PPARγ activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA J. Am. Med. Assoc. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Tardif J-C, Schwartz GG, Nicholls SJ, Rydén L, Neal B, Malmberg K, Wedel H, Buse JB, Henry RR, et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA J. Am. Med. Assoc. 2014;311:1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatol. Baltim. Md. 2012;55:1389–1397. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs. 2013;73:1–14. doi: 10.1007/s40265-012-0004-0. [DOI] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat. Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA J. Am. Med. Assoc. 2014;311:2167–2168. doi: 10.1001/jama.2014.4133. [DOI] [PubMed] [Google Scholar]
- Mahaffey KW, Hafley G, Dickerson S, Burns S, Tourt-Uhlig S, White J, Newby LK, Komajda M, McMurray J, Bigelow R, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am. Heart J. 2013;166:240–249. doi: 10.1016/j.ahj.2013.05.004. e1. [DOI] [PubMed] [Google Scholar]
- Marfella R, Portoghese M, Ferraraccio F, Siniscalchi M, Babieri M, Di Filippo C, D’Amico M, Rossi F, Paolisso G. Thiazolidinediones may contribute to the intramyocardial lipid accumulation in diabetic myocardium: effects on cardiac function. Heart Br. Card. Soc. 2009;95:1020–1022. doi: 10.1136/hrt.2009.165969. [DOI] [PubMed] [Google Scholar]
- Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D’Agostino RB, Perez A, Provost J-C, Haffner SM. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA J. Am. Med. Assoc. 2006;296:2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu K-Y, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, et al. Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. Cell Metab. 2012;16:723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Filippis ED, Bajaj M, Wajcberg E, Glass L, Triplitt C, Cersosimo E, Mandarino LJ, Defronzo RA. Predictors of improved glycaemic control with rosiglitazone therapy in type 2 diabetic patients: a practical approach for the primary care physician. Br. J. Diabetes Vasc. Dis. 2005;5:28–35. [Google Scholar]
- Moore DD. “No, really, how do they work?”. Genes Dev. 2005;19:413–414. doi: 10.1101/gad.1294105. [DOI] [PubMed] [Google Scholar]
- Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49:434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- National Program of Cancer Registries United States Cancer Statistics (USCS), 2006–2010 [Google Scholar]
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Winter ML, Porte D, Semenkovich CF, Smith S, et al. Thiazolidinedione Use, Fluid Retention, and Congestive Heart Failure A consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27:256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953–1962. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch. Intern. Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochellière R, Staniloae CS, Mavromatis K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA J. Am. Med. Assoc. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei L, Zillikens MC, Dehghan A, Buitendijk GHS, Castaño-Betancourt MC, Estrada K, Stolk L, Oei EHG, van Meurs JBJ, Janssen JAMJL, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013;36:1619–1628. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Koga H, Shimotohno K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J. Biol. Chem. 2004;279:29551–29557. doi: 10.1074/jbc.M403866200. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369–1371. doi: 10.2337/dc10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur X, Mok CYL, Velagapudi VR, Núñez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O’Rahilly S, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punthakee Z, Bosch J, Dagenais G, Diaz R, Holman R, Probstfield JL, Ramachandran A, Riddle MC, Rydén LE, Zinman B, et al. Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE) randomised controlled trial. Diabetologia. 2012;55:36–45. doi: 10.1007/s00125-011-2357-4. [DOI] [PubMed] [Google Scholar]
- Punthakee Z, Alméras N, Després J-P, Dagenais GR, Anand SS, Hunt DL, Sharma AM, Jung H, Yusuf S, Gerstein HC. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance: a sub-study of the DREAM trial. Diabet. Med. J. Br. Diabet. Assoc. 2014 doi: 10.1111/dme.12512. [DOI] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of Pparγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala SM, Lazar MA. The Dawn of the SPPARMs? Sci. Signal. 2002;2002:pe9. doi: 10.1126/stke.2002.121.pe9. [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Rhoades B, Shapiro JS, Rich AS, Kim JK, Shulman GI, Kaestner KH, Lazar MA. Genetic Modulation of PPARγ Phosphorylation Regulates Insulin Sensitivity. Dev. Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T LIDO Study Group. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatol. Baltim. Md. 2010;51:445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- Rawson NSB. Review of the quality of observational studies of the association between rosiglitazone and acute myocardial infarction. J. Popul. Ther. Clin. Pharmacol. J. Thérapeutique Popul; Pharamcologie Clin. 2014;21:e214–e232. [PubMed] [Google Scholar]
- Reaven GM. Role of Insulin Resistance in Human Disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Riera-Guardia N, Rothenbacher D. The effect of thiazolidinediones on adiponectin serum level: a meta-analysis. Diabetes Obes. Metab. 2008;10:367–375. doi: 10.1111/j.1463-1326.2007.00755.x. [DOI] [PubMed] [Google Scholar]
- Robbins GT, Nie D. PPAR gamma, bioactive lipids, and cancer progression. Front. Biosci. Landmark Ed. 2012;17:1816–1834. doi: 10.2741/4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu C-H, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, et al. Targeted Elimination of Peroxisome Proliferator-Activated Receptor γ in β Cells Leads to Abnormalities in Islet Mass without Compromising Glucose Homeostasis. Mol. Cell. Biol. 2003;23:7222–7229. doi: 10.1128/MCB.23.20.7222-7229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumie CL, Greevy RA, Grijalva CG, et al. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA. 2014;311:2288–2296. doi: 10.1001/jama.2014.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M, Manavalan J, Agarwal S, McMahon D, Nino A, Fitzpatrick LA, Bilezikian J. Effects of Rosiglitazone vs Metformin on Circulating Osteoclast and Osteogenic Precursor Cells in Postmenopausal Women with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2014 doi: 10.1210/jc.2013-3666. jc20133666. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat. Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto J, Kimura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, Sawada H. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem. Biophys. Res. Commun. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- Sambanis C, Tziomalos K, Kountana E, Kakavas N, Zografou I, Balaska A, Koulas G, Karagiannis A, Zamboulis C. Effect of pioglitazone on heart function and N-terminal pro-brain natriuretic peptide levels of patients with type 2 diabetes. Acta Diabetol. 2008;45:23–30. doi: 10.1007/s00592-007-0014-7. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. The Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saremi A, Schwenke DC, Buchanan TA, Hodis HN, Mack WJ, Banerji M, Bray GA, Clement SC, Henry RR, Kitabchi AE, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2013;33:393–399. doi: 10.1161/ATVBAHA.112.300346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Awasaki Y, Kandori H, Tanakamaru Z-Y, Nagai H, Baron D, Yamamoto M. Suppressive effects of acid-forming diet against the tumorigenic potential of pioglitazone hydrochloride in the urinary bladder of male rats. Toxicol. Appl. Pharmacol. 2011;251:234–244. doi: 10.1016/j.taap.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Schupp M, Lazar MA. Endogenous Ligands for Nuclear Receptors: Digging Deeper. J. Biol. Chem. 2010;285:40409–40415. doi: 10.1074/jbc.R110.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, Chapman J, Subramaniam S. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18745–18750. doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-γ. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA J. Am. Med. Assoc. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- Son N-H, Park T-S, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang L-S, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Step SE, Lim H-W, Marinis JM, Prokesch A, Steger DJ, You S-H, Won K-J, Lazar MA. Anti-diabetic rosiglitazone remodels the adipocyte transcriptome by redistributing transcription to PPARγ-driven enhancers. Genes Dev. 2014;28:1018–1028. doi: 10.1101/gad.237628.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, Barish GD, Hong S-H, Castro GL, Yin Y-Q, Nelson MC, et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22504–22509. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O, Huang Z, Liu W, Yoshihara E, van Dijk TH, et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014 doi: 10.1038/nature13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Arnold LL, Pennington KL, Kakiuchi-Kiyota S, Wei M, Wanibuchi H, Cohen SM. Effects of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on the urine and urothelium of the rat. Toxicol. Sci. Off. J. Soc. Toxicol. 2010;113:349–357. doi: 10.1093/toxsci/kfp256. [DOI] [PubMed] [Google Scholar]
- Szatmari I, Rajnavolgyi E, Nagy L. PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann. N. Y. Acad. Sci. 2006;1088:207–218. doi: 10.1196/annals.1366.013. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Seo J, Jo A-Y, Graff JM. Thiazolidinediones Regulate Adipose Lineage Dynamics. Cell Metab. 2011;14:116–122. doi: 10.1016/j.cmet.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdelen I, van Beekum O, Gorbenko O, Fleskens V, van den Broek NJF, Koppen A, Hamers N, Berger R, Coffer PJ, Brenkman AB, et al. The serine/threonine phosphatase PPM1B (PP2Cβ) selectively modulates PPARγ activity. Biochem. J. 2013;451:45–53. doi: 10.1042/BJ20121113. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tseng C-H, Tseng F-H. Peroxisome proliferator-activated receptor agonists and bladder cancer: lessons from animal studies. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2012;30:368–402. doi: 10.1080/10590501.2012.735519. [DOI] [PubMed] [Google Scholar]
- Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC. Ambulatory treatment of type 2 diabetes in the U.S., 1997–2012. Diabetes Care. 2014;37:985–992. doi: 10.2337/dc13-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol. Cell. Biol. 2009;29:4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc. Natl. Acad. Sci. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, de Groot JC, Prasad A, Freiwald A, Quedenau C, Kliem M, Witzke A, Kodelja V, Han C-T, Giegold S, et al. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. U. S. A. 2012;109:7257–7262. doi: 10.1073/pnas.1116971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox R, Bousser M-G, Betteridge DJ, Schernthaner G, Pirags V, Kupfer S, Dormandy J PROactive Investigators. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke J. Cereb. Circ. 2007;38:865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Yamaguchi T, Shimizu M, Nakata N, Hirose F, Osumi T. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells Devoted Mol. Cell. Mech. 2004;9:1017–1029. doi: 10.1111/j.1365-2443.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Vallarino C, Bron M, Perez A, Liang H, Joseph G, Yu S. A comparison of all-cause mortality with pioglitazone and insulin in type 2 diabetes: an expanded analysis from a retrospective cohort study. Curr. Med. Res. Opin. 2014:1–25. doi: 10.1185/03007995.2014.941054. [DOI] [PubMed] [Google Scholar]
- Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr. Diab. Rep. 2013;13:329–341. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- Ye J-M, Dzamko N, Cleasby ME, Hegarty BD, Furler SM, Cooney GJ, Kraegen EW. Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia. 2004;47:1306–1313. doi: 10.1007/s00125-004-1436-1. [DOI] [PubMed] [Google Scholar]
- Yokoi T. Troglitazone. Handb. Exp. Pharmacol. 2010:419–435. doi: 10.1007/978-3-642-00663-0_14. [DOI] [PubMed] [Google Scholar]
- Yoon Y-H, Yi H. Liver Cirrhosis Mortality in the United States. 2012:1970–2009. [Google Scholar]
- Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor γ blocks thiazolidinedione-induced fluid retention. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9406–9411. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]