Abstract
Both human and nonhuman primates exhibit a cognitive bias to social threat, but little is known about how this bias develops. We investigated the development of threat bias in free-ranging infant rhesus macaques (Macaca mulatta) at 3 (N = 45) and 9 (N = 46) months of age. Three-month-old infant monkeys did not display bias, but 9-month-olds exhibited increased maintenance of attention to threatening social stimuli (vigilance for threat). To examine whether the social environment affected vigilance for threat, behavioral data on maternal rank and protectiveness were collected across the first 12 weeks of life for infants tested at 9 months. Nine-month-old infants of high-ranking mothers and more protective mothers displayed greater vigilance for threat than infants of lower-ranking and less protective mothers. These results demonstrate that infant social cognition is malleable and shaped by mothers both directly (protectiveness) and indirectly (rank), as maternal characteristics affect infants’ social experiences.
Keywords: Cognitive bias, maternal effects, socio-cognitive development, rhesus macaque, primate
Introduction
The human mind is far from objective or accurate in the processing of information about the world. Rather, humans show many predictable biases in attention, perception, memory, and decision-making (e.g., Kahneman, 2011). Cognitive biases are likely to represent adaptations which aid in survival and reproduction in the environment in which we live (e.g., Kenrick & Griskevicius, 2013; Haselton et al., 2009). For example, it has been shown that monkeys and humans detect more quickly and allocate more attention to snakes than to flowers or other harmless organisms (monkeys: Öhman & Mineka, 2001; humans: LoBue & DeLoache, 2008). As the cost of ignoring a threat may be much greater than the cost of ignoring non-threatening aspects of the environment, both attention to (rapid detection) and vigilance for (maintenance of attention) threat are likely to be adaptive.
Cognitive bias for threat in both attention and vigilance is not limited to natural predators or physical dangers; the social environment is rife with threats as well. Humans demonstrate exceptional attention to and memory for negatively-valenced social stimuli (Anderson, Siegel, Bliss-Moreau & Feldman Barrett, 2011; Kuhbadner, Spitzer & Pekrun, 2011), including a bias for angry or threatening facial expressions (e.g., Fox et al., 2000; Hansen & Hansen, 1988). The notion that a cognitive bias for threat may be universal and adaptive does not imply that this bias is hardwired or that experience is irrelevant to its development. Bias for threatening faces is not present at birth; human newborns display a preference for looking at happy faces (Farroni, Menon, Rigato & Johnson, 2007), and vigilance for threat emerges between 6 and 12 months (e.g., Grossman, Striano & Friederici, 2007; Schupp et al., 2004). Additionally, experience affects its expression; 7-month-old infants of depressed mothers displayed weaker vigilance for fearful expressions than same-age infants raised by non-depressed mothers (de Haan et al., 2004), and maltreated children exhibited greater attention to and vigilance for angry faces and voices than non-maltreated children (Shackman, Shackman & Pollak, 2007). However, how early experience affects the development of bias to threat in non-clinical populations remains under-investigated. Research into experience-dependent aspects of this bias is essential as exacerbated attention to threat and difficulty disengaging from aversive stimuli have been implicated in the etiology of anxiety disorders (Bar-Haim et al., 2007), which often emerge early in life (reviewed in Pine, Cohen, Gurley, Brooke & Ma, 1998).
Similar to the study of bias for threatening non-social stimuli (e.g., snakes), nonhuman primates could contribute to our understanding of the role of experience in the acquisition and expression of biases to social threat. Nonhuman primates, in particular rhesus macaques, are socially and cognitively similar to humans (Frith & Frith, 2007) and are frequently used as a model species for research into the neurobiological mechanisms underpinning cognitive phenomena (e.g., Watson & Platt, 2012), especially the study of anxiety and emotion regulation (Kalin & Shelton, 2003). Rhesus macaques possess several distinct threatening (open-mouth threat), fearful/submissive (fear-grimace), and affiliative (lipsmack) facial expressions (Maestripieri & Wallen, 1997), and adult rhesus display attentional bias to threatening faces (Bethell, Holmes, MacLarnon & Semple, 2012). Whether bias to threat is already present in infancy and the extent to which it is influenced by experience is unknown in nonhuman primates. As the mechanisms underlying the effects of experience on bias to threat remain ambiguous (e.g., Strang, Hanson & Pollak, 2012; Fox et al., 2005), an understanding of how experience affects attention and vigilance to threat in rhesus infants could potentially enhance our understanding of these mechanisms in human and nonhuman primates.
The first year of life has the highest risk of mortality in primates (Gage & Dyke, 1988) and rhesus monkey infants are vulnerable to social threats (e.g., aggression or kidnapping from other group members: Maestripieri, 1993). As higher levels of anxiety early in life may protect newly mobile individuals from dangerous situations (e.g., Blackford & Pine, 2012), it may be hypothesized that a bias for threatening faces should appear early and be well-established by the time infants have become independent from their mothers (LoBue, Rakison & DeLoache, 2010; Vaish, Grossman & Woodward, 2009). Inter-individual variation, however, in the extent to which infants are at risk from others and in exposure to social threats could lead to individual differences in the strength of bias to threatening faces, which in turn might contribute to variation in the development of anxiety disorders (e.g., Bar-Haim et al., 2007).
Variation in risk and early social experience may depend on the infant’s mother. Rhesus mothers directly control their infants’ social experiences through their protective or permissive parenting style, paralleling variation in human parenting styles (Maestripieri, 1999). Infant macaques that are restrained or retrieved by protective mothers spend less time interacting with others and are less likely to come near dangerous conspecifics (Maestripieri, 1995; 2001). The dominance rank of rhesus mothers also influences the infant’s social experience; infants of high-ranking mothers receive more attention from conspecifics but mainly have affiliative interactions, whereas infants of low-ranking mothers may receive less attention from others overall but are more likely to be threatened or attacked (Maestripieri, 2001). Thus, the mother’s parenting style and her dominance rank might affect individual differences in the strength of attention and vigilance to threatening faces. This hypothesis is consistent with previously demonstrated maternal influences on infant exploration, reactivity to novelty, and social behavior, as well as with maternal influences on the development of neurobiological substrates involved in emotion regulation (reviewed in Maestripieri et al., 2009).
Here we present the first data on the development of cognitive bias to threat in rhesus macaque infants and the effects of early experience on expression of this bias. We investigated the presence/absence of cognitive bias for threatening faces in 3- and 9-month-old infants in a free-ranging population of rhesus macaques. Additionally, we investigated whether bias manifests itself through attention capture (attention to threat) or maintenance of attention (vigilance for threat), as variation in early life experience has been shown to affect both components of cognitive bias to threat in different ways (i.e., perception versus processing: Bar-Haim et al., 2007). We predicted that infants should show evidence of both components of bias to threat within the first year of life, in line with evidence from human infants, and that individual differences in the strength of this bias may be accounted for by variation in maternal characteristics and an infant’s opportunities for social experience. Specifically, we tested the prediction that infants whose mothers are low-ranking and/or have a less protective parenting style should display a stronger bias for threatening faces, as they are at higher risk for aggression than other infants.
Materials and Methods
Study site and subjects
This study was undertaken on Cayo Santiago, a 15.2 ha island located 1 km off the east coast of Puerto Rico (Rawlins & Kessler, 1986). The Cayo Santiago colony contains approximately 1000 free-ranging rhesus. Macaques on Cayo Santiago are provisioned with monkey chow and rainwater, but also forage naturally on vegetation. Rhesus macaques are seasonal breeders, with the population on Cayo Santiago currently mating during a 6-month window between February and July, with the majority of births occurring between August and October (Caribbean Primate Research Center census records). Cognitive data were collected on two cohorts of subjects; Cohort 1 consisted of 45 3-month-old infants (mean age 15.0 ± 0.30 weeks of age) in Groups R, S, and KK born during the 2013 birth season, and Cohort 2 consisted of 46 9-month-old infants (mean age 36.8 ± 0.36 weeks of age) in Groups R and S born during the 2011 birth season. Subjects were selected opportunistically (i.e., the first 45 (Cohort 1) or 46 (Cohort 2) infants born in the 2013 or 2011 birth season). Dominance interactions to determine sex and group-specific dominance hierarchies for the mothers of infants and behavioral data across the first 12 weeks of life to examine the effects of early life experience on cognitive bias to threat were collected for Cohort 2 only. All methods were approved by the IACUC at both the University of Puerto Rico and University of Chicago.
Cognitive data collection
All infants were tested for cognitive bias to threat using a looking-time paradigm, a commonly used method in studies of non/pre-verbal populations (Spelke, 1985). Individuals were presented simultaneously with two previously validated stimuli (Bethell et al., 2012), a threatening face stimulus (a photograph of an unfamiliar adult male macaque displaying an open-mouth threat: Figure 1a) and a neutral face stimulus (a photograph of the same male with a neutral, non-emotional expression: Figure 1b). Stimuli were color photographs (11.75 × 8.25 inches) presented on a cardboard apparatus (48.25 × 12 inches), with photographs 38 inches apart. Seven sets of stimuli were available, with one set chosen at random for use for each individual. Stimuli were covered by blinders at the onset of the trial. A trial was initiated by setting up the apparatus fewer than 2.5 meters in front of a subject, who was sitting calmly and apart from the group whenever possible. The cameraperson, standing directly behind the presenter and using a handheld camcorder (Canon FS20), started to record as soon as the apparatus was set up and the presenter captured the attention of the subject by tapping the blinders. Once the subject had gazed at both right and left blinders, the presenter redirected infant gaze to orient below the presenter (in the middle of the two stimuli), and then removed the blinders simultaneously exposing both stimuli. Infant eye gaze was videotaped for five seconds following stimuli exposure, as previous experiments have demonstrated that the majority of looking behavior occurs in the first few seconds following stimulus exposure (E. Bethell, personal communication). The eyes of the presenter and cameraperson were hidden during the trial to avoid cueing the subject. The locations of the stimuli (e.g., threatening on the left, neutral on the right) were counterbalanced across subjects.
Figure 1.
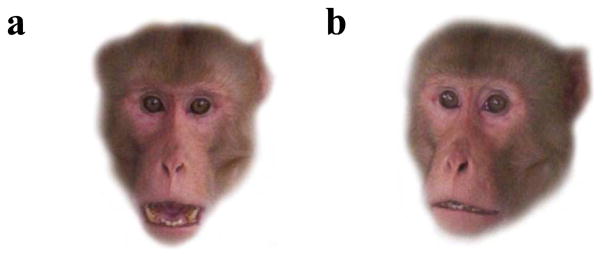
a. Threatening face stimulus; b. Neutral face stimulus.
Cognitive test video coding
Video data were blind coded on a frame-by-frame basis using JWatcher (http://www.jwatcher.ucla.edu) from videos edited in VideoPad Video Editor (http://www.nchsoftware.com/videopad). The number of frames spent looking at the threatening and the neutral stimuli were quantified separately, frame by frame, with a frame rate of 30 frames per second. Each video was coded for four primary variables, the looking times at the: 1.) threatening stimulus during the first second, 2.) neutral stimulus during the first second, 3.) threatening stimulus over all five seconds, and 4.) neutral stimulus over all five seconds. Two additional variables were created to quantify detection (attention to threat) and attention maintenance (vigilance for threat). “Attention to threat” quantified attention capture to threat over and above attention capture by the neutral stimulus, in which the time spent looking at the neutral stimulus was subtracted from the time spent looking at the threatening stimulus for the first second following stimulus exposure. “Vigilance for threat” was obtained by calculating the difference between the looking times at threatening and neutral stimuli over all five seconds. For both variables, a positive value indicated more time looking at the threatening stimulus, and a negative value indicated more time looking at the neutral stimulus, during either the attention capture period (first second only) or the attention maintenance period (all five seconds). All videos were blind coded by an additional coder and inter-observer reliability for direction of gaze was attained at Cohen’s k = 0.82, with any inconsistencies resolved through discussion. Following all video coding, the location of each stimulus was incorporated into the dataset.
Behavioral data collection
Behavioral data on early life experiences were collected from Cohort 2 only. These 46 infants were observed between 7:00 and 14:30, 5 days a week, using continuous focal animal sampling (Altmann, 1974) across the first 12 weeks of life. Infants were observed for two 30-minute periods each week using a handheld videocamera (Kodak PlaySport Z3). Videos were coded using Microsoft Excel with an automatic time-stamp function. The order of focal animal sampling was randomized and behavioral data were converted into mean hourly frequencies (event behaviors) in Microsoft Access for analysis. Our dataset contained 542.5 hours of observational data over the 12-week period (behavioral observation frequency mean and SEM across all infants: 11.8 ± 0.05 hours, range: 10.5–12 hours). Observers only began behavioral data coding once inter-observer reliability reached Cohen’s k = 0.90, as determined by tests of agreement on individual occurrences of maternal interactions (e.g., Caro, Roper, Young & Dank, 1979).
Female dominance ranks are known on Cayo Santiago as they are maternally inherited and recorded in a database maintained by the Caribbean Primate Research Center, but we collected additional data on the outcome of dyadic agonistic interactions between mothers in Cohort 2 to determine group- and sex-specific dominance hierarchies to allow us to control for rank effects in our statistical analyses. These dyadic interaction data were placed into a winner-loser matrix and MatMan was used to generate dominance hierarchies. Following 10,000 iterations, significant linear hierarchies were produced (linearity test using Landau’s linearity index corrected for unknown relationships, Group R: p = 0.03; Group S: p = 0.03), which were combined with matriline data to categorize each adult female as high-, middle-, or low-ranking, for each social group separately. To conduct analyses on the possible effect of rank, infants were placed in rank categories based on their mother’s rank.
To investigate whether maternal style early in life affects the development of bias to threat, we collected behavioral data on maternal protectiveness for Cohort 2 only. Maternal protectiveness was quantified as the summed rate of restraint (i.e., mother prevents infant from breaking contact by holding its arm, leg or tail) and retrieval (i.e., mother retrieves infant from another individual or when infant is alone and more than 1 meter from the mother). Hourly rates of each behavior were calculated for all observations and then averaged across the first 12 weeks for each infant separately to determine a mean frequency of protectiveness for each infant in Cohort 2. Mean maternal protectiveness for each mother-infant dyad was log transformed to minimize the effect of any outliers.
Data Analysis
Data for 3-month-olds (Cohort 1) were analyzed separately from data for 9-month-old infants (Cohort 2). A General Linear Model (GLM) was used to explore the effect of test parameters (i.e., stimulus set, and whether the threatening or the neutral stimulus was looked at first) on the looking time variables. We found an effect of first stimulus looked at on overall looking time at the threatening and neutral stimulus in 3- and 9-month-olds (see Results, Table 1), and an effect of stimulus set on vigilance for threat in 9-month-olds. Therefore, both variables were included as random factors in subsequent analyses to control for their effects.
Table 1.
Attention capture looking times for threatening and neutral stimuli based on first stimulus looked at in 3- and 9-month-old infants.
| 3-month-olds | 9-month-olds | |||
|---|---|---|---|---|
| Stimulus looked at first | ||||
| Threatening | Neutral | Threatening | Neutral | |
| Attention to threatening stimulus during first second Mean ±SEM (95% CI) | 557.6 ±75.4 (401.2–714.0) | 306.1 ±84.2 (130.5–481.7) | 558.7 ±53.4 (448.5–668.8) | 407.0 ±64.0 (273.5–540.6) |
| Attention to neutral stimulus during first second Mean ±SEM (95% CI) | 357.9 ±78.0 (196.6–519.6) | 634.8 ±83.7 (460.3–809.3) | 350.4 ±49.9 (247.3–453.5) | 492.5 ±67.4 (351.9–633.1) |
To determine whether infants displayed a bias to threat during attention capture (the first second following stimulus exposure) or maintenance (all five seconds following stimulus exposure), looking time data in 3-month-old and 9-month-old infants were analyzed using Wilcoxon Signed-Rank tests (i.e., comparing looking time at the threatening stimulus versus looking time at the neutral stimulus during the first second, and overall).
As any cognitive bias for threat was found in 9-month-old infants only, all subsequent analyses were conducted on Cohort 2 only. A Linear Mixed Model (LMM) was used to determine the effect of infant sex, group membership, and maternal rank on attention to threat and vigilance for threat, while controlling for any effects of test parameters. Only maternal rank was found to have a significant effect on either attention to threat or vigilance for threat, therefore it was also incorporated in the final model as a random factor to control for its effects.
We then used a LMM to analyze the effects of maternal protectiveness, while controlling for maternal rank and test parameters, to assess the effects of early life mother-infant interactions on attention to threat and vigilance for threat. Tests were two-tailed with p < 0.05 considered significant, and all analyses were performed in SPSS 22.0.
Results
Three-month-old infants (Cohort 1) did not display a significant bias for threat, failing to discriminate between the threatening and neutral faces during either attention capture (Wilcoxon Signed-Rank test: Z = 0.529, p = 0.597, r = 0.079) or maintenance (Wilcoxon Signed-Rank test: Z = 0.817, p = 0.414, r = 0.122). Nine-month-old infants (Cohort 2) did not display a significant bias for threat during attention capture (Wilcoxon Signed-Rank test: Z = 1.016, p = 0.310, r = 0.163); however, 9-month-olds did exhibit vigilance for threat, spending significantly more time looking at the threatening face than at the neutral face (Wilcoxon Signed-Rank test: Z = 2.207, p = 0.027, r = 0.325: Figure 2).
Figure 2.
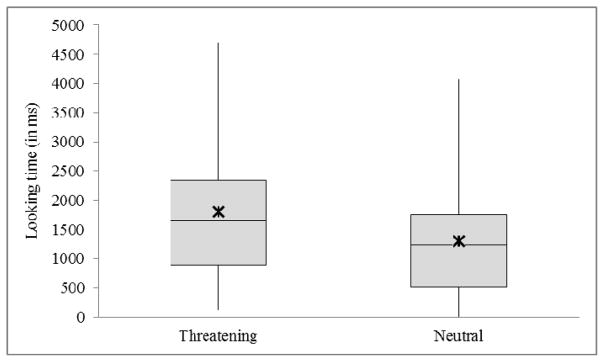
Boxplot of looking time at 9-months of age for each stimulus type in milliseconds (N = 46). Looking time mean ±SEM (95% Confidence Interval) for Threatening stimulus: 1804.5 ±166.4 (1469.8–2139.1) and Neutral stimulus: 1305.8 ±140.8 (1022.2–1589.5). Bars denote range, gray box denotes interquartile range and median, and asterisk denotes mean.
In the first second of testing (attention capture period), both 3-month-old and 9-month-old infants paid more attention to whichever stimulus they happened to look at first, regardless of the nature of the stimulus (3-month-olds: first stimulus and attention to threat: GLM: F1,36 = 4.80, p = 0.035, R2 = 0.11; first stimulus and attention to neutral: GLM: F1,36 = 5.53, p = 0.024, R2 = 0.13; 9-month-olds: first stimulus and attention to threat: GLM: F1,38 = 4.41, p = 0.042, R2 = 0.07; first stimulus and attention to neutral: GLM: F1,38 = 4.37, p = 0.043, R2 = 0.07) (Table 1). In contrast, vigilance for threat across the full 5 seconds was not affected by the first stimulus looked at in either age group (both p > 0.05).
While there was no significant relationship between attention to threat and maternal rank or maternal protectiveness (both p > 0.05), there was a significant effect of social-experiential variables on vigilance for threat in 9-month-old infants. Among these older infants, the offspring of mothers who were more protective (LMM: F1,41.8 = 5.988, p = 0.019, R2 = 0.42: Figure 3) or were higher-ranking (LMM: F2,36.5 = 3.42, p = 0.043, R2 = 0.29: Figure 4) showed heightened vigilance for threat (maternal rank and protectiveness were not significantly correlated; Kruskal-Wallis test, p > 0.05).
Figure 3.
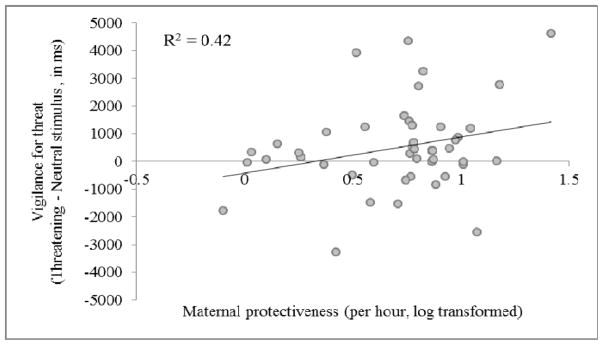
Relationship between vigilance for threat and log transformed frequency of maternal protectiveness (N = 46).
Figure 4.
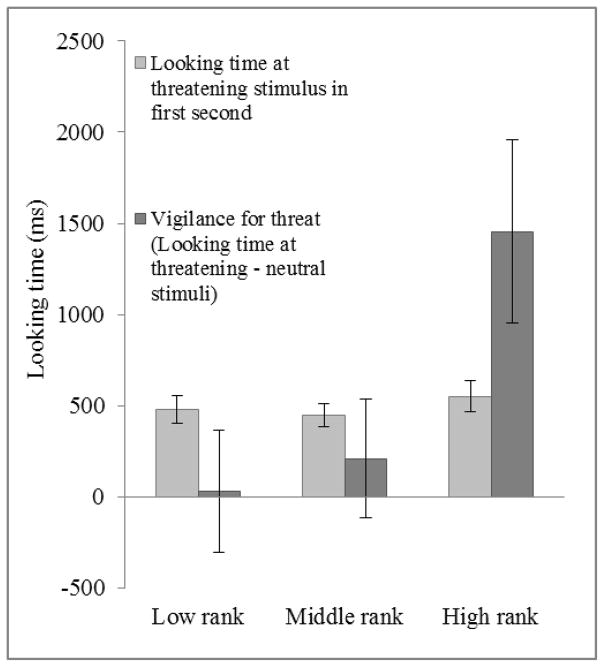
Relationship between looking time at threatening stimulus in the first second, vigilance for threat, and maternal rank at 9-months of age (N = 46). Looking time at threatening stimulus during first second mean ±SEM (95% CI) for infants born to low-ranked females: 480.8 ±78.2 (327.5–634.1); middle-ranked females: 449.8 ±64.7 (323.0–576.6); and high-ranked females: 551.9 ±86.5 (382.4–721.4). Vigilance for threat (Looking time at Threatening stimulus minus looking time at Neutral stimulus) mean ±SEM (95% CI) for infants born to low-ranked females: 28.9 ±335.1 (−627.9–685.7); middle-ranked females: 209.8 ±327.0 (−431.1–850.7); and high-ranked females: 1454.5 ±501.0 (472.5–2436.5).
Taken together, the above results indicate that significant differences in looking behavior among older infants emerged after attention capture, and that social-experiential variables affected the maintenance of attention toward threatening stimuli rather than initial detection of threat. Consistent with this, infants of high-ranked mothers looked significantly more at the threatening stimulus than middle- or low-ranked infants in the four seconds following attention capture (Kruskal-Wallis test: Z = 2.207, p = 0.027, r = 0.325: Figure 5). There was a difference in the expected direction when examining the relationship between maternal protectiveness and post-attention capture looking behavior (looking times at threatening and neutral stimulus after the first second of the test), but it failed to reach significance (p > 0.05).
Figure 5.
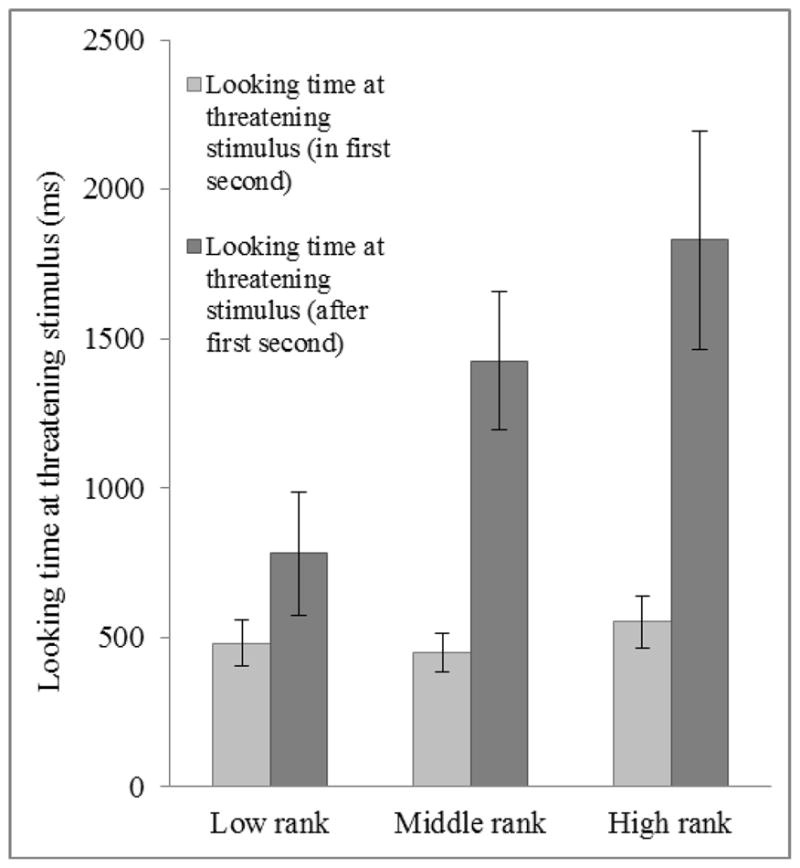
Relationship between looking time at threatening stimulus during the first second and looking time at threatening stimulus during subsequent four seconds, separated by maternal rank at 9-months of age (N = 46). Looking time at threatening stimulus during first second mean ±SEM (95% CI) for infants born to low-ranked females: 480.8 ±78.2 (327.5–634.1); middle-ranked females: 449.8 ±64.7 (323.0–576.6); and high-ranked females: 551.9 ±86.5 (382.4–721.4). Post attention capture (looking time at threatening stimulus during subsequent four seconds) mean ±SEM (95% CI) for infants born to low-ranked females: 779.8 ±206.8 (374.5–1185.1); middle-ranked females: 1424.7 ±232.1 (969.8–1879.6); and high-ranked females: 1830.2 ±364.6 (1150.9–2509.5).
Discussion
Our study documented the early development of cognitive bias to threat for the first time in a nonhuman species, the rhesus macaque. Three-month-old rhesus infants did not show evidence of vigilance for threat but 9-month-old infants did. In older infants, both maternal rank and the frequency of maternal protectiveness in the first 12 weeks of infant life affected the expression of vigilance for threat. Infants raised by high-ranking mothers or by protective mothers displayed stronger vigilance for threat than infants raised by lower ranking mothers or by less protective mothers.
Cognitive bias to threat in 9-month-olds was not the result of preferential attention capture by the threatening stimulus; there was no significant attention to threat in 3- or 9-month-olds, and no significant effect of experience on attention to threat in 9-month-olds. In the first second of testing, both 3- and 9-month-olds paid more attention to the stimulus they happened to look at first, suggesting that infants find it difficult to disengage quickly from social stimuli regardless of its emotional content (e.g., Amir, Elias, Klumpp & Przeworski, 2003).
After the first second, 9-month-old infants of high-ranking or protective mothers continued to look at the threatening stimulus more than the neutral stimulus for the duration of the test, whereas infants of lower ranking or less protective mothers looked at the threatening stimulus less frequently. Both the overall bias for threat and the effects of socio-experiential variables emerged during attention maintenance (vigilance for threat).
These results suggest that both direct (protectiveness) and indirect (rank) maternal regulation of the infant’s social environment are important to the development of cognitive bias to threat, possibly by altering infant exposure to threatening or dangerous interactions. However, these effects are opposite to what we predicted. We predicted that a higher probability of exposure to threats early in life would lead to a stronger bias for threat, but found instead that threat bias was stronger in infants who were less likely to be exposed to threats.
There are several non-mutually exclusive explanations for how low infant exposure to threatening situations could result in increased vigilance for threat. First, it is possible that infants who display greater vigilance for threat do so because the threatening stimulus is novel and salient, due to lower exposure to threatening faces during development in high-ranked or protected infants. Second, if these same infants receive less aggression from others their social expectations are positively skewed, subsequent negativity is unexpected, and infants display increased attention to threat (e.g., Range Frequency Theory: Vaish, Grossman & Woodward, 2009). Third, lower-ranked and less protected infants exposed to more threatening stimuli early in life learn to avoid threatening stimuli to diminish the effects of an aversive environment (for evidence in humans, see Stirling, Eley & Clark, 2006).
Finally, although our study was not designed to assess genetic influences on behaviour, it is possible that variation in infant experience and in the expression of bias to threat may have a genetic basis. In rhesus macaques, it has been shown that maternal genotype can affect maternal behavior. For example, abusive mothers tend to carry a different variant of the serotonin linked polymorphic region (5-HTTLPR) allele than non-abusive mothers (McCormack et al., 2009). Infants who possess this same variant often demonstrate impaired emotion regulation and heightened anxiety, but only when exposed to early life adversity (e.g., Spinelli et al., 2012; McCormack et al., 2009). In humans, the serotonin transporter gene polymorphism has been linked to variation in temperament and anxiety (Lakatos et al., 2003), in attentional responses to positive and negative emotional expressions (Pérez-Edgar et al., 2010), and in threat detection (Miu, Vulturar, Chis, & Ungureanu, 2012), which is consistent with the notion that that this polymorphism alters an individual’s sensitivity to his or her environment (e.g., Caspi et al., 2010). Therefore, although our results are consistent with an explanation emphasizing the effects of early experience on the development of vigilance for threat, we cannot rule out the possibility that variation in vigilance for threat is due primarily, or even solely, to genetic factors (e.g., maternal genotype acting via maternal behavior and/or genetic similarities between mothers and infants leads to correlations between maternal characteristics and infant biases in vigilance for threat). Further research on the development of bias to threat in monkeys could address the role of genetic factors (e.g., with data on maternal and infant genotype, or with cross-fostering experiments) as well as further investigate the importance of social experience in the development of this cognitive bias. Further work should also address how variation in the development of cognitive biases affects both typical and pathological cognitive and social development, and whether similar processes and outcomes occur in human and nonhuman primates.
Acknowledgments
We thank Auberi Courchay, Sean Coyne, Keiran Mandalaywala, and Greg Ruber for assistance collecting and coding behavioral and cognitive data, John Addicott for creating our Access database, and the CPRC staff for logistical assistance. We are extremely grateful to James Higham and Emily Bethell for thoughtful discussion of experiments and analyses. This research was supported by NIH grant R01-HD067175 to DM and KJP, and grant number 8 P40 OD012217-25 from the National Center for Research Resources and the Office of Research Infrastructure Programs of the NIH to the CPRC of the University of Puerto Rico. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41:1325–1335. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Anderson E, Siegel EK, Bliss-Moreau E, Feldman Barrett L. The visual impact of gossip. Science. 2011;332:1446–1448. doi: 10.1126/science.1201574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry. 2003;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bethell EJ, Holmes A, MacLarnon A, Semple S. Evidence that emotion mediates social attention in rhesus macaques. Plos One. 2012;7:e44387. doi: 10.1371/journal.pone.0044387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders. Child & Adolescent Psychiatric Clinics of North America. 2012;21:201–252. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro TM, Roper R, Young M, Dank G. Inter-observer reliability. Behaviour. 1979;69:303–315. [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M, Belsky J, Reid V, Volein A, Johnson MH. Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. Journal of Child Psychology and Psychiatry. 2004;45:1209–1218. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- Farroni T, Menon E, Rigato S, Johnson MH. The perception of facial expressions in newborns. European Journal of Developmental Psychology. 2007;4:2–13. doi: 10.1080/17405620601046832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social Cognition in Humans. Current Biology. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: Are angry faces detected more efficiently. Cognition and Emotion. 2000;14:61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage TB, Dyke B. Model life tables for the larger old world monkeys. American Journal of Primatology. 1988;16:305–320. doi: 10.1002/ajp.1350160403. [DOI] [PubMed] [Google Scholar]
- Grossman T, Striano T, Friederici AD. Developmental changes in infant’s processing of happy and angry facial expressions: A neurobehavioral study. Brain and Cognition. 2007;64:30–41. doi: 10.1016/j.bandc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Hansen RD. Finding the face in the crowd: An anger superiority effect. Attitudes and Social Cognition. 1988;54:917–924. doi: 10.1037//0022-3514.54.6.917. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Bryant GA, Wilke A, Frederick DA, Galperin A, Frankenhuis WE, Moore T. Adaptive rationality: An evolutionary perspective on cognitive bias. Social Cognition. 2009;27:733–763. [Google Scholar]
- Kahneman D. Thinking, Fast and Slow. New York, NY: Farrar, Strauss, Giroux; 2011. [Google Scholar]
- Kalin N, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Annals of the New York Academy of Sciences. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kenrick DT, Griskevicius V. The Rational Animal: How evolution made us smarter than we think. New York, NY: Basic Books; 2013. [Google Scholar]
- Kuhbandner C, Spitzer B, Pekrun R. Read-out of emotional information from iconic memory: The longevity of threatening stimuli. Psychological Science. 2011;22:695–700. doi: 10.1177/0956797611406445. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Birkas E, Ronai Z, Kovacs E, Ney K, Toth I, Sasvari-Szekely M, Gervai J. Association of D4 dopamine receptor gene and serotonin transporter promoter polymorphisms with infants’ response to novelty. Molecular Psychiatry. 2003;8:90–97. doi: 10.1038/sj.mp.4001212. [DOI] [PubMed] [Google Scholar]
- LoBue V, DeLoache JS. Detecting the snake in the grass: Attention to fear-relevant stimuli by adults and young children. Psychological Science. 2008;19:284–289. doi: 10.1111/j.1467-9280.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- LoBue V, Rakison DH, DeLoache JS. Threat perception across the life span: Evidence for multiple converging pathways. Current Directions in Psychological Science. 2010;19:375–379. [Google Scholar]
- Maestripieri D. Infant kidnapping among group-living rhesus macaques: Why don’t mothers rescue their infants? Primates. 1993;34:211–216. [Google Scholar]
- Maestripieri D. Assessment of danger to themselves and their infants by rhesus macaque (Macaca mulatta) mothers. Journal of Comparative Psychology. 1995;109:416–420. doi: 10.1037/0735-7036.109.4.416. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. The biology of human parenting: Insights from nonhuman primates. Neuroscience and Biobehavioral Reviews. 1999;23:411–422. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Intraspecific variability in parenting styles of rhesus macaques (Macaca mulatta): The role of the social environment. Ethology. 2001;107:237–248. [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother-infant interactions in free-ranging rhesus macaques: Relationships between physiological and behavioral variables. Physiology and Behavior. 2009;96:613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Wallen K. Affiliative and submissive communication in rhesus macaques. Primates. 1997;38:127–138. [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miu AC, Vulturar R, Chis A, Ungureanu L. Attentional biases to threat and serotonin transporter gene promoter (5-HTLPR) polymorphisms: Evidence from a probe discrimination task with endogenous cues. Translational Neuroscience. 2012;3:160–166. [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott JM, Gorodetsky E, Hodgkinson CA, Goldman D, Ernst M, Pine DS, Fox NA. Variations in the serotonin-transporter genes are associated with attention bias patterns to positive and negative emotion faces. Biological Psychology. 2010;83:269–271. doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. The Cayo Santiago macaques: history, behavior, and biology. Albany, NY: State University of New York Press; 1986. [Google Scholar]
- Schupp HT, Junghöfer M, Oehmann A, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: An ERP analysis. Emotion. 2004;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Shackman JE, Shackman AJ, Pollak SD. Physical abuse amplifies attention to threat and increases anxiety in children. Emotion. 2007;7:838–852. doi: 10.1037/1528-3542.7.4.838. [DOI] [PubMed] [Google Scholar]
- Spelke ES. Preferential-looking methods as tools for the study of cognition in infancy. In: Gottlieb G, Krasnegor NA, editors. Measurement of audition and vision in the first year of postnatal life: A methodological overview. Westport, CT: Ablex Publishing; 1985. pp. 323–363. [Google Scholar]
- Spinelli S, Schwandt ML, Lindell SG, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS. The serotonin transporter gene linked polymorphic region is associated with the behavioral response to repeated stress exposure in infant rhesus macaques. Develompental Psychopathology. 2012;24:157–165. doi: 10.1017/S0954579411000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Hanson JL, Pollak SD. The importance of biological methods in linking social experience with social and emotional development. Monographs of the Society for Research in Child Development. 2012;77:61–66. [Google Scholar]
- Stirling LJ, Eley TC, Clark DM. Preliminary evidence for an association between social anxiety symptoms and avoidance of negative faces in school-age children. Journal of Clinical Child & Adolescent Psychology. 2006;35:431–439. doi: 10.1207/s15374424jccp3503_9. [DOI] [PubMed] [Google Scholar]
- Vaish A, Grossman T, Woodward A. Not all emotions are created equal: The negativity bias in social-emotional development. Psychological Bulletin. 2008;134:383–403. doi: 10.1037/0033-2909.134.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Platt ML. Of mice and monkeys: using non-human primate models to bridge mouse- and human-based investigations of autism spectrum disorders. Journal of Neurodevelopmental Disorders. 2012;4:21. doi: 10.1186/1866-1955-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]