Abstract
Menopausal hormone therapy with estrogen plus progestin or estrogen alone (for women with prior hysterectomy) is still used by millions of women for climacteric symptom management throughout the world. Until 2002, hormone therapy influence on cancer risk and other chronic diseases was determined through observational study reports. Since then, results from the Women's Health Initiative randomized, placebo-controlled hormone therapy trials have substantially changed concepts regarding estrogen plus progestin and estrogen alone influence on the most common cancers in postmenopausal women. In these trials, estrogen plus progestin significantly increased breast cancer incidence and deaths from breast cancer, significantly increased deaths from lung cancer, significantly decreased endometrial cancer, and did not have a clinically significant influence on colorectal cancer. In contrast, estrogen alone use in women with prior hysterectomy significantly reduced breast cancer incidence and deaths from breast cancer without significant influence on colorectal cancer or lung cancer. These complex results are discussed in the context of known potential mediating mechanisms of action involved in interactionwith steroid hormone receptors.
There is concerted interest in the role ofhormone therapy influence on steroid receptor function in mediating the development and progression of many common cancers including those of the breast, endometrium, ovary, colon and rectum, and lung (1, 2, 3, 4). Relevant findings on this issue, with emphasis on randomized clinical trial findings, follows.
Interest in relationships between exogenous hormone use and cancer and other chronic diseases developed following the introduction of conjugated equine estrogen (Premarin®,Pfizer) for climacteric symptom management in the early 1940sin the US (5). Use of estrogen was found to increase endometrial cancers (6, 7),which was subsequently shown to be mitigated by progestin use in women with no prior hysterectomy [8].An increase in breast cancer was seen with both estrogen plus progestin and estrogen alone with the latter requiring a longer duration exposure (9, 10, 11, 12). However, the preponderance of observational studies suggested that the breast cancers associated with hormone therapy use would be largelyhormone receptor positive, be identified at relatively early stage and have a favorable prognosis (13, 14, 15). In addition, most studies found no adverse effect of hormone therapy on breast cancer mortality (16). The cumulative body of observational studies led to concepts regarding the association of hormone therapy with common cancers which were prevalent in 2002 (before the findings from the Women's Health Initiative randomized hormone therapy clinical trials began to be published) (Table 1).
Table 1.
| Hormone Therapy and Common Cancers: Concepts in 2002 | |
| 1 | Estrogen plus progestin increases breast cancer |
| Estrogen alone increases breast cancer but may require longer duration exposure | |
| In addition, the predominance of observation studies suggested that the breast cancer risk would be greater in lean hormone therapy users. Also the breast cancers associated with hormone therapy use would commonly be hormone receptor positive, be diagnosed at lower stage, and have favorable prognosis. | |
| 2 | Hormone therapy decreases colorectal cancer (most reports with estrogen alone) |
| 3 | No influence of hormone therapy on lung cancer |
| 4 | Estrogen alone increases endometrial cancer incidence |
| Estrogen plus progestin does not increase endometrial cancer incidence | |
| Hormone Therapy and Common Cancers: Current Concepts | |
| 1 | Estrogen plus progestin increases breast cancers. Effects may be greater in women initiating therapy closer to menopause |
| Estrogen plus progestin interferes with breast cancer mammographic detection resulting in cancers diagnosed at more advanced stage. | |
| Estrogen plus progestin broadly increases breast cancers across subtypes with influence not limited to estrogen receptor positive cancers. | |
| Estrogen plus progestin increases deaths from breast cancer | |
| Estrogen alone reduces breast cancer incidence and reduces deaths from breast cancer | |
| 2 | Estrogen plus progestin reduces colorectal cancer incidence. However, the cancers are diagnosed at higher stage. Estrogen plus progestin does not reduce colorectal cancer mortality. The lack of influence on colorectal cancer mortality from this rapidly fatal disease strongly suggest delay in diagnosis rather than a clinically meaningful reduction in incidence from estrogen plus progestin use. |
| Estrogen alone does not influence colorectal cancer incidence or colorectal cancer mortality. | |
| 3 | Estrogen plus progestin increases lung cancer mortality |
| Estrogen alone doesn't influence lung cancer incidence or mortality | |
| 4 | Estrogen alone increases endometrial cancer incidence |
| Estrogen plus progestin reduces endometrial cancer incidence | |
In the same time frame, observational studiessuggested benefit from menopausal hormone therapy use on several common, life-threatening chronic diseases of postmenopausal women(17) including coronary heart disease (18), hip fracture (19), and dementia (20). With a demonstrated favorable effect on climacteric symptom management, taken on balance,menopausal hormone therapy with both estrogen plus progestin, for women with no prior hysterectomy and estrogen alone, for women with prior hysterectomy, were favorably viewed in the medical community and their use in clinical practice increased to where about 38% of postmenopausal US women were using these preparations in 1993 (21). The influence of estrogen plus progestin and estrogen alone on clinical outcomeshad never been prospectively evaluated in a randomized clinical trial setting.Against this background, the two Women's Health Initiative(WHI) randomized, perspective, placebo-controlled hormone therapy clinical trials were initiated in 1993.
The Women's Health Initiative (WHI) Hormone Therapy Trials
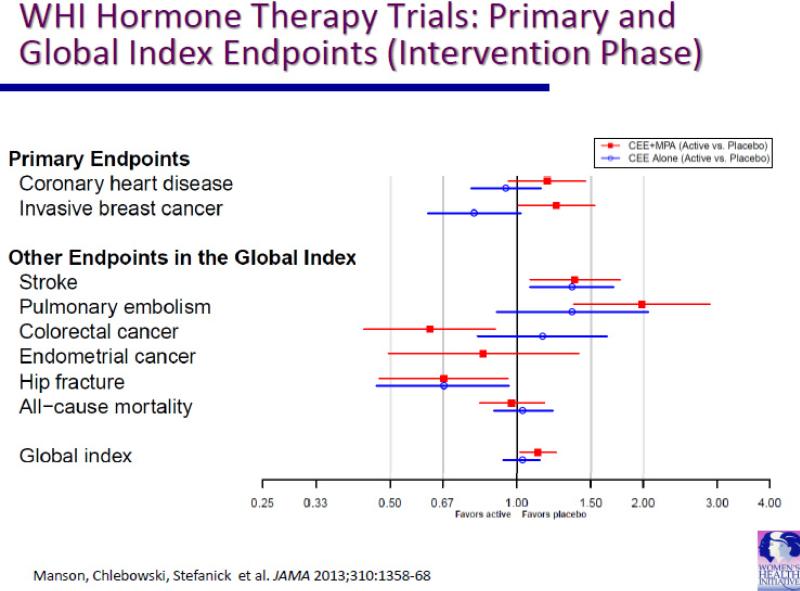
In one WHI hormone therapy trial, estrogen plus progestin, as conjugated equine estrogen and medroxyprogesterone acetate,was evaluated in 16,608 postmenopausal women with an intact uterus (22). In the other WHI hormone therapy trial, estrogen alone, as conjugated equine estrogen, was evaluated in 10,739 postmenopausal women with prior hysterectomy (23). Eligible women were between the ages 50-79 years, and who had a baseline mammogram not suspicious for malignancy, no prior breast cancer or any invasive cancer within 10 years, and provided informed, written consent (24). The primary study outcome monitored for benefit was coronary heart disease and the primary study outcome monitored for harm was invasive breast cancer. Once the monitoring boundary for either benefit or harm was exceeded, the intervention influence on a global index, the time to first event of a series of medical conditions likely to be under hormone therapy influence which included, besides invasive breast cancer and coronary heart disease, stroke, pulmonary embolism, hip fracture, colorectal cancer and endometrial cancer (the latter only in the estrogen plus progestin trial) and all-cause mortality.The findings from both trials, which were published in a series of reports beginning in 2002 (Table 2),have substantially altered concepts regarding hormone therapy use and cancer incidence and outcome. In the estrogen plus progestin trial, intervention was stopped after 5.6 years (median) when an increase in invasive breast cancer was seen and calculation of the global index indicated more chronic disease risks than benefits were associated with estrogen plus progestin use (22). In the estrogen alone trial, intervention was stopped after 7.1 years (median) when an increase in stroke was seen with no overall improvement in chronic disease risk (23). The influence of estrogen plus progestin and estrogen alone on the primary and global index endpoints during the intervention period in both trials are provided in Figure 1.
Table 2.
Cancer Findings from the Women's Health Initiative Hormone Therapy
| Randomized Clinical Trials | |
|---|---|
| 2002 | Estrogen plus progestin significantly increases breast cancer, significantly decreases colorectal cancer and has no effect on endometrial cancer (Rossouw 2002) |
| 2003 | Estrogen plus progestin interfered with breast cancer mammographic detection resulting in diagnostic delay and higher stage cancers (Chlebowski 2003) |
| 2003 | Estrogen plus progestin associated with a non-significant higher ovarian cancer risk (Anderson 2003) |
| 2004 | Estrogen plus progestin significantly decreases colorectal cancers but cancers were diagnosed at higher stage (Chlebowski 2004) |
| 2006 | Estrogen alone with trend for lower breast cancer incidence (Stefanick 2006) |
| 2009 | Estrogen plus progestin significantly increases lung cancer mortality (Chlebowski, Schwartz 2009) |
| 2009 | After stopping estrogen plus progestin, breast cancer incidence rapidly declined while mammography was constant suggesting secular trend in decrease in breast cancer incidence was associated with decrease in hormone therapy use (Chlebowski, Kuller 2009) |
| 2010 | Estrogen plus progestin increases breast cancer incidence broadly across subtypes and significantly increases breast cancer mortality (Chlebowski, Anderson 2010) |
| 2010 | Estrogen alone does not influence lung cancer incidence or mortality (Chlebowski, Anderson, Gass 2010) |
| 2011 | Estrogen alone significantly decreases breast cancer incidence (Anderson 2012) |
| 2012 | Estrogen plus progestin with no influence on colorectal cancer mortality suggesting lower incidence related to diagnostic delay (Simon 2012) |
Figure 1.
CEE = conjugated equine estrogen; MPA = medroxyprogesterone acetate.
Closed squares and lines = hazard ratio (HR) and 95% confidence interval (CI) for CEE + MPA vs placebo from Cox regression models stratified by age and randomization in the WHI dietary modification trial.
Open circles and lines = HR and 95% CI for CEE alone vs placebo for Cox regression models stratified by age and randomization in the WHI dietary modification trial.
Breast Cancer
With respect to breast cancer, there was a statistically significant increase in invasive breast cancer incidence with combined hormone therapy (25, 26) (HR 1.24 95% CI 1.01-1.54, nominal p = 0.003). Significantly more mammograms with abnormalities, resulting in a significantly increased need for breast biopsies were seen in the combined hormone therapy group and, when formally tested, mammogram and biopsy diagnostic performance was significantly impaired as well (27). In contrast to the preponderance of observational studies (13, 14), the increased risk of breast cancers in the estrogen plus progestin group were not limited to hormone receptor positive cancers, andcancer in that group were diagnosed at more advanced stage (regional/metastatic 25.4% vs 16.0% respectively; p = 0.04). With longer follow-up, deaths from breast cancer were also found to be significantly increased in the estrogen plus progestin group (26).
In the WHI trial, a screening mammogram not suspicious for malignancy was required at entry as was annual mammography (with drug provision not permitted until this ongoing requirement was met). In contrast, older observational studies did not control for mammography and hormone therapy users, under medical supervision, were substantially more likely than non-users to have ongoing mammography (28). Women with regular mammography have cancers which are likely to be hormone receptor positive and be found at earlier stage compared to those seen in unscreened women (29, 30). In the trial, there was a suggestion, supported by findings from observational study cohortanalyses (31, 32, 33, 34), that the combined hormone therapy effect on increasing breast cancer was greater in women initiating therapy closer to menopause.
These findings indicated that estrogen plus progestin stimulated breast cancer growth and hindered breast cancer detection. While examination of the Kaplan-Meier incidence curves suggested a somewhat lower breast cancer incidence in the combined hormone therapy group in the initial 3-4 years of intervention (25, 26), this finding likely represented a delay in breast cancer diagnosis. The effect on diagnostic delay suggests a safe duration for estrogen plus progestin use for breast cancer risk cannot be reliably determined.
Breast cancer findings in the estrogen alone trial were quite different from those in the trial evaluating estrogen plus progestin. In contrast to most observational studies, in the WHI randomized placebo-controlled trial, estrogen alone significantly reduced invasive breast cancer incidence (HR 0.80 95% CI 0.62-1.04),a finding initially of borderline significance (35). After longer follow-up (11.8 years median), a statistically significant, lower incidence of invasive breast cancer was seen in the estrogen alone group (36, 37)whichwas maintained in post-intervention (38).In addition, deaths from breast cancer were also found to be significantly reduced by estrogen alone use (37).
There was suggestion that women beginning estrogen alone closer to menopause (< 5 years)saw less reduction in breast cancer than those initiating use further from menopause (37, 39). Similarly, in the Million women observational study, no increase in breast cancer incidence was seen for estrogen alone users initiating hormone therapy within 12 months of menopause (33).
As the WHI trial of estrogen alone had median intervention duration of 7.1 years and, as not all participants were adherent, the influence of estrogen alone for longer duration of exposure for breast cancer remains an open question. The concept that a longer duration of estrogen alone use is needed to increase breast cancer incidence (12) was more attractive when the concept was that estrogen had less of a proliferative effect on breast epithelium than estrogen plus progestin and hence would require a longer duration of exposure to see a breast cancer effect. However, the finding that estrogen exposure results in a statistically significant decrease in breast cancer incidence and adherence in deaths from breast cancer (37) makes presentation of biological hypotheses regarding an adverse effect of longer duration use challenging.
As conjugated equine estrogen, extracted from pregnant horse's urine, contains a mixture of more than 10 estrogens (40), some of the estrogen have antiestrogenic activity (41) conjugated equine estrogen may uniquely include components which act as selective estrogen receptor modulators, like tamoxifen (42), to lower breast cancer risk. However, in Europe, where hormone therapies are more variable and include formulations with conjugated equine estrogen and with estradiol, breast cancer risk did not differ comparing conjugated equine estrogen with estradiol formulations (43, 44). While overall, estrogen alone users had significantly higher breast cancer risk than non-users, in most cases the increase in risk was, if anything, somewhat higher in conjugated equine estrogen users compared to estradiol alone users (44). With respect to differences in the progestin added to estrogen in combined hormone therapy, substantial preclinical differences are seen (45). However, in the clinic, clear differences have emerged only for progesterone use (46, 47), where combined regimens with this agent have less breast cancer risk (46) but appear to not provide adequate protection against endometrial cancer (47).
While estrogen plus progestin and estrogen alone in the WHI randomized trials have opposite effects on breast cancer incidence, their influence on mammogram density, mammogram detection performance, and the incidence of benign proliferative breast disease are somewhat more congruent (Table 3). In a subset of WHI clinical trial participants, estrogen plus progestin was found to increase mammogram percent density at 2 years by 4.9% (p < 0.001) (48). Similarly, estrogen alone significantly increased mammogram percent density (p < 0.001) but only by 1.7% (49). While estrogen plus progestin increased the frequency of mammograms leading to short interval follow-up and those suspicious or suggestive of malignancy (27), estrogen alone only increased the frequency of mammograms requiring short interval follow-up (50). Finally, both estrogen plus progestin and estrogen alone significantly increased the frequency of benign proliferative breast disease (51, 52) by almost two fold. Thus, it appears that mammogram density change in response to an intervention may not be reliably predict influence on subsequentbreast cancer. While benign breast disease is associated with higher breast cancer risk, the similar influence of combined hormone therapy and estrogen alone on this entity did not predict their influence on invasive breast cancer.
Table 3.
Menopausal Hormone Therapy and Breast Cancer Related Outcomes
| Estrogen plus Progestin | Estrogen alone | |
|---|---|---|
| Invasive breast cancer incidence | 1.25 (1.07-1.46)1 (p = 0.044) | 0.71 (0.62-0.95)2 (p = 0.02) |
| Mammogram % Density (2 year) Increase | 4.9% (2.3-7.8)3 (p < 0.001) | 1.7% (0.7-2.7)4 (p < 0.001) |
| Mammogram Performance vs. placebo | ||
| Short interval follow-up | 26% vs 18%5 (p = 0.05) | 30% vs 22%6 (p < 0.001 ) |
| Suspicious or suggestive | 6% vs 3% (p = 0.05) | 6% vs 6% NS |
|
Benign Proliferative Breast Disease (BPBD) (n = 470) |
HR 1.74 (1.35-2.25)7 (p < 0.001) | HR 2.23 (1.71-3.20)8 (p < 0.001) |
Chlebowski, Anderson, Goss, et al. JAMA 2010;304(15):1684-92.
Anderson, Chlebowski, Aragaki, et al. Lancet Oncology 2012;13(5):476-86.
McTiernan, Martin, Peck, et al. J Nat Cancer Inst 2005;97(18):1366-76.
McTiernan, Chlebowski, Martin, et al. J Clin Oncol 2009;27(36):6135-43.
Chlebowski, Anderson, Pettinger, et al. Arch Intern Med 2008;168(4):370-7.
Chlebowski, Anderson, Manson, et al. J Clin Oncol 2010;28(16):2690-7.
Rohan, Negassa, Chlebowski, et al. Cancer Epidemiol Biomarker Prev 2008;17(9):2337-43.
Rohan, Negassa, Chlebowski, et al. J Natl Cancer Inst 2008;100(8):563-71.
Potential Mediating Mechanisms of Action on Hormone Therapy on Breast Cancer
Recently, findings from two placebo-controlled, full scale clinical prevention trials indicated that aromatase inhibitors, which substantially reduce estrogen levels in postmenopausal women, significantly reduced breast cancer incidence (53, 54). Thus, there is randomized clinical trial evidence that estrogen deprivation using aromatase inhibitors (53, 54) and estrogen addition (using conjugated equine estrogen) (37) both reduce breast cancer incidence. These apparently paradoxical results are consistent with findings emerging from preclinical studies which have identified complex, time dependent effects of changes in the estrogen environment on the influence of estrogen on mammary tissue. While estrogen commonly stimulates breast cell proliferation and inhibitsapoptosis, under conditions of estrogen deprivation,gene expression profile changes occur such that estrogen then inducesrather than inhibits apoptosis (40, 55, 56). In addition, estrogen deprivation can also result in increased estrogen receptor sensitivity, such thatrelatively low estrogen levels are sufficient to stimulate the estrogen pathway and accelerate mammary tumor growth (57). Thus, these time dependent, exposure dependent changes in mammary glandular tissue suggest that many breast cancers in postmenopausal women can survive only in limited range of estrogen exposure. Recent analyses from a case-control study within the WHI randomized clinical trial suggest that changes in sex hormones with conjugated equine estrogen (CEE) use may also play a role in mediating the breast cancer reduction seen. In these analyses, a two-fold increase in sex hormone binding globulin (SHBG) was seen with CEE which largely offset the corresponding increase in serum estrogens associated with CEE use (58). The finding from the WHI randomized trial of combined hormone therapy that progestin addition to estrogen significantly increases breast cancer incidence (26) suggests progestin has a determinant role in this complex process.
Other Cancers
During intervention in the estrogen plus progestin trial, no increase in all cancer incidence was seen (22). However, with extended follow-up 3 years post-intervention, an overall 25% increase in malignancies was identified (59), prompting a post-hoc analysis of combined hormone therapy influence on lung cancer. After 7.9 years mean follow-up, 23% more women in the estrogen plus progestin group had lung cancers diagnosed, a finding which was not statistically significant. However, 71% more women died from lung cancer in the combined hormone therapy group (p = 0.01) (60), a result largely attributed to increased risk of deaths from non-small cell lung cancer with no adverse effects on small lung cancer and mortality seen. The absolute risk was especially high in current smokers since where about 1 in 100 would experience an otherwise avoidable death from lung cancerrelated to estrogen plus progestin use for about 5.5 years. In contrast, in the estrogen alone trial, after 7.9 years follow-up, neither lung cancer incidence or lung cancer mortality were influenced by estrogen alone (61). Gender differences in lung cancer incidence and outcome have been previously described (62, 63), as well as estrogen receptor influence on prognosis (64). Currently, strategies targeting estrogen signaling are under development for future lung cancer interventions (65).
Observational studies have consistently found an association with hormone therapy use and lower colorectal cancer incidence (66, 67). With respect to colorectal cancer, in the estrogen plus progestin randomized WHI trial, there were 44% fewer colorectal cancers in the combined hormone therapy group diagnosedduring a mean of 5.6 years intervention (nominal p = 0.003) (68). However, the cancers had more lymph node involvement (59% vs 29%, p = 0.003) and were diagnosed at substantially higher stage (regional/metastatic, 76% vs 49%, respectively, p = 0.004). Now with longer 11.6 years follow-up, there is a non-significant increase indeaths from colorectal cancer in the combined hormone therapy group (37 vs 27 deaths; 0.04% vs 0.03%: HR 1.29 95% CI 0.78-2.11, p = 0.320) (67). These data are not compatible with a clinically meaningful reduction in colorectal cancer since this disease carries a 30% 5 year mortality risk (69). Rather, these findings are suggestive of diagnostic delay.In contrast, in the WHI estrogen alone trial, no influence of estrogen on colorectal cancer incidence or mortality was seen (70).
With respect to endometrial cancer, results are only available from the WHI estrogen plus progestin trial where only women with no prior hysterectomy were entered. In that setting, with longer follow-up, a statistically significant 37% reduction in endometrial cancer incidence has emerged with estrogen plus progestin use (p < 0.04) (38). Observational studies consistently find a substantial increase in endometrial cancer incidence with estrogen alone use (47, 71, 72). Taken together with the WHI results, estrogen alone compared to estrogen plus progestin have completely opposite effects on breast cancer and endometrial cancer incidence and outcome. Namely, estrogen plus progestin reduces endometrial cancer incidence (38) and increases breast cancer incidence (25, 26, 37), while estrogen alone reduces breast cancer incidence and increases endometrial cancer incidence (47, 71). These findings again present an apparent paradox since both malignancies are similarly adversely influenced by higher endogenous estrogen levels. Outcomes for breast cancer, lung cancer, colorectal cancer and endometrial cancer from the WHI hormone therapy trials are summarized in Table 4.
Table 4.
Selected Cancer Outcomes in the Women's Health Initiative Hormone Therapy Randomized Trials
| Cancer category | Estrogen plus progestin trial | Estrogen alone trial | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Invasive breast cancer | ||||
| Incidence | 1.25 (1.07-1.46) | 0.004 | 0.71 (0.62-0.95) | 0.02 |
| Death from breast cancer | 1.96 (1.00-4.04) | 0.049 | 0.37 (0.13-0.91)2 | 0.03 |
| Colorectal cancer | ||||
| Incidence | 0.72 (0.56-0.94) | 0.01 | 1.12 (0.77-1.63) | 0.55 |
| Death from colorectal cancer | 1.29 (0.78-2.11) | 0.32 | 0.99 (0.50-1.96) | 0.99 |
| Lung cancer | ||||
| Incidence | 1.23 (0.92-1.63) | 0.16 | 1.17 (0.81-1.69) | 0.39 |
| Death from lung cancer | 1.71 (1.16-2.52) | 0.01 | 1.07 (0.66-1.72) | 0.79 |
| Endometrial cancer | 0.37 (0.49-0.91) | 0.007 | NA | NA |
1) Hazard ratios (HR) are from Cox proportional hazards regression models, stratified by age, randomization group in the Women's Health Initiative dietary modification trial and prior disease when applicable. CI indicates confidence interval; all analyses start at time of randomization. Intervention duration was 5.6 yrs (mean) in the oestrogen plus progestin trial and 7.1 yrs (mean) in the oestrogen alone trial.
2) Results as published for E plus P: breast cancer (Chlebowski 2010 JAMA), colorectal cancer (Simon 2012), lung cancer (Chlebowski 2009 Lancet), endometrial cancer (Manson 2013), for E alone:breast cancer (Anderson 2012), colorectal cancer (Ritenbaugh 2008), lung cancer (Chlebowski 2010 JNCI).
With respect to ovarian cancer, findings only from the estrogen plus progestin trial have been reported. There, while ovarian cancer incidence was 58% higher in the combined hormone therapy users, the difference did not meet statisticalsignificance (73). These findings could not address the issue raised by cohort studies, namely whether both estrogen alone and estrogen plus progestin, as compared to only estrogen alone, increases ovarian cancer risk (74).
Conclusions
The concepts which have emerged from the WHI two randomized hormone therapy trials with respect to cancer influence are outlined in Figure 1. As seen, they differ substantially from the preponderance of older observational studies reports. Some of the differences between the observational studies and the randomized clinical trials can reasonably be reconciled by consideration of potential confounding factors such as differential mammography use and gap time considerations for breast cancer diagnosis and interference with colorectal cancer diagnosis by combined hormone therapy usebut other differences remained largely unexplained.
With respect to cancer, the balance of benefit and risks seem to be somewhat more favorable for estrogen alone than for estrogen plus progestin, but the pattern of benefits and risks was complex in both trials. The opposite effects of estrogen plus progestin and estrogen alone on incidence of endometrial cancer and breast cancer, two cancers under similar endogenous estrogen influence, represent an interpretive challenge.
Public health observations provide support for some of the WHI randomized clinical trial results. As findings from the WHI hormone therapy trials emerged, a substantial and sustained decrease in menopausal hormone therapy use has been seen in the US with about a 66% reduction for estrogen plus progestin use and 33% reduction for estrogen alone use seen (21, 75). In the same period, a substantial decrease in breast cancer incidence was seen in postmenopausal US women, a decline attributed to the decline in hormone therapy (76, 77, 78). A recent review of 40 reports from around the world concluded that the findings provided convincing support for a direct association between decreasing hormone therapy use and a breast cancer incidence decline (79). Most recently, an increase in endometrial cancer incidence has been attributed to the hormone therapy decline in the US (80). Over this same period, no comparable change in colorectal cancer incidence has been reported (81). These findings for breast cancer, endometrial cancer and colorectal cancer provide support for the WHI randomized trial results.
The findings from the WHI randomized hormone therapy clinical trials have changed concepts regarding the complex influences of the two evaluated hormone therapy regimens on cancers and other chronic diseases. In a recent editorial commenting on the most recent update of WHI hormone therapy trials (38), Elizabeth Nabel, MD, from Harvard Medical School, placed the WHI findings in a Public Health context (82). She described the WHI as “a model for publicly funded, rigorous, thorough, and objective clinical trials that have broadly affected human health” and which has “overturned medical dogma regarding the use of menopausal hormone therapy.”
In summary, in the WHI randomized, placebo-controlled hormone therapy trials, estrogen plus progestin significantly increased breast cancer incidence and deaths from breast cancer, increased deaths from lung cancer, significantly decreased endometrial cancer, and did not have a clinically significant influence on colorectal cancer. In contrast, estrogen alone use in women with prior hysterectomy significantly reduced breast cancer incidence and deaths from breast cancer without significant influence on colorectal cancer or lung cancer. Some of the differences seen, compared to observational study results can be explained by potential confounding factors while other differences remain unexplained.
Acknowledgments
We acknowledge the dedicated efforts of investigators and staff at the Women's Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, and the National Heart, Lung and Blood program office (listing available at http://www.whi.org). We also recognize the WHI participants for their extraordinary commitment to the WHI program. The WHI program is funded by the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Women's Health Initiative Investigations
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Roscoe, Shari Ludlum, Dale Burden, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Los Angeles Biomedical Research Institute at the Harbor-UCLA Medical Center, Torrance, CA) Rowan T. Chlebowski; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Conflict of Interest
RTC has received speaker's fees and honorarium from Novartis; honorarium for advisory boards and consulting for Novartis, Pfizer and Amgen. GLA has conflicts to declare.
References
- 1.Zhou W, Slingerland JM. Links between oestrogen receptor activation and proteolysis: relevance to hormone-regulated cancer therapy. Nat Rev Cancer. 2014;14:26–38. doi: 10.1038/nrc3622. [DOI] [PubMed] [Google Scholar]
- 2.Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19(21):8572–8. doi: 10.1158/1078-0432.CCR-13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol. 2014;41(1):5–16. doi: 10.1053/j.seminoncol.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leslie KK, Thiel KW, Reyes HD, Yang S, Zhang Y, Carlson MJ, et al. The estrogen receptor joins other cancer biomarkers as a predictor of outcome. Obstet Gynecol Int. 2013;2013:479541. doi: 10.1155/2013/479541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. American Journal of Medicine. 2005;188:64S–73S. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–7. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- 7.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–71. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]
- 8.Briton BA, Felix AS. Menopausal hormone therapy and risk of endometrial cancer. J Steroid Biochem Mol Biol. 2013 May 13; doi: 10.1016/j.jsbmb.2013.05.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beral V. Breast cancer and hormone replacement therapy in the Million Women Study. Lancet. 2008;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 10.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 11.Colditz GA. Estrogen, estrogen plus progestin therapy, and risk of breast cancer. Clin Cancer Res. 2005;11:909s–17s. [PubMed] [Google Scholar]
- 12.Chen WY, Manson JE, Hankinson SE, Rosner B, Holmes MD, Willett WC, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166(9):1027–32. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 13.Holli K, Isola J, Cuzick J. Low biologic aggressiveness in breast cancer in women using hormone replacement therapy. J Clin Oncol. 1998;16:3115–3120. doi: 10.1200/JCO.1998.16.9.3115. [DOI] [PubMed] [Google Scholar]
- 14.Chen WY. Exogenous and endogenous hormones and breast cancer. Best Pract Res Clin Endocrinol Metab. 2008;22(4):573–585. doi: 10.1016/j.beem.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salagame U, Canfell K, Banks E. An epidemiological overview of the relationship between hormone replacement therapy and breast cancer. Expert Rev Endocrinol Metab. 2011;6:397–409. doi: 10.1586/eem.11.31. [DOI] [PubMed] [Google Scholar]
- 16.Nelson HD, Numphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 17.Greendale GA, Judd HL. The menopause: health implications and clinical management. J Am Geriatr Soc. 1993;41:426–36. doi: 10.1111/j.1532-5415.1993.tb06953.x. [DOI] [PubMed] [Google Scholar]
- 18.Stampfer MJ, Colditz GA, Willet WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurse's Health Study. N Engl J Med. 1991;325:756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 19.Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy I: a 10-year prospective study in the relationship of osteoporosis. Obstet Gynecol. 1979;53(3):277–281. [PubMed] [Google Scholar]
- 20.McBee WL, Dailey ME, Dugan E, Shumaker SA. Hormone replacement therapy and other potential treatments for dementias. Endocrinol Metab Clin North Am. 1997;26:329–45. doi: 10.1016/s0889-8529(05)70250-4. [DOI] [PubMed] [Google Scholar]
- 21.Hersh AL, Stefanick ML, Stafford RS. National use of menopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 22.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 23.Anderson GL, Limacher M, Assaf R, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 25.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 26.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chlebowski RT, Anderson GL, Pettinger M, Lane D, Langer RD, Gilligan MA, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168:370–377. doi: 10.1001/archinternmed.2007.123. [DOI] [PubMed] [Google Scholar]
- 28.Joffe MM, Byrne C, Colditz GA. Postmenopausal hormone use, screening, and breast cancer: characterization and control of a bias. Epidemiology. 2001;12:429–38. doi: 10.1097/00001648-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Hofvind S, Sorum R, Haldorsen T, Langmark F. Incidence of breast cancer before and after implementation of mammography screening. Tidsskr Nor Laegeforen. 2006;126:2935–8. [PubMed] [Google Scholar]
- 30.Sihto H, Lundin J, Lehtimaki T, Sarlomo-Rikala M, Butzow R, Holli K, et al. Molecular subtypes of breast cancers detected in mammography screening and outside of screening. Clin Cancer Res. 2008;14:4103–4110. doi: 10.1158/1078-0432.CCR-07-5003. [DOI] [PubMed] [Google Scholar]
- 31.Prentice RL, Chlebowski RT, Stefanick ML, Manson JE, Pettinger M, Hendrix SL, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008;167:1207–16. doi: 10.1093/aje/kwn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fournier A, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Estrogen-progestagen menopausal hormone therapy and breast cancer: does delay from menopause onset to treatment initiation influence risks? J Clin Oncol. 2009;27:5138–5143. doi: 10.1200/JCO.2008.21.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103:1–10. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chlebowski RT, Anderson GL. The influence of time from menopause and mammography on hormone therapy-related breast cancer risk assessment. J Natl Cancer Inst. 2011;103:284–5. doi: 10.1093/jnci/djq561. [DOI] [PubMed] [Google Scholar]
- 35.Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, Rodabough RJ, Paskett ED, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295:647–657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 36.Lacroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health risks and benefits 5 years after stopping randomized treatment with conjugated equine estrogens in postmenopausal women with prior hysterectomy. JAMA. 2011;305:1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson GL, Chlebowski RT, Aragaki A, Manson J, Roham T, Kuller L, et al. Oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy extended follow-up of the Women's Health Initiative randomized trial. Lancet Oncology. 2012;13(5):476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manson JA, Chlebowski RT, Stefanick ML, Aragaki AK, Howard B, Rossouw J, et al. The Women's Health Initiative hormone therapy trials: update and overview of health outcomes during the intervention and post-stopping phases. JAMA. 2013;310(3):1–17. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prentice RL, Chlebowski RT, Stefanick ML, Manson JE, Langer RD, Pettinger M, et al. Conjugated equine estrogens and breast cancer risk in the Women's Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167:1407–15. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obiorah I, Jordan VC. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause. 2012;2013;20(4):372–382. doi: 10.1097/GME.0b013e31828865a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howell A, Cuzick J. Oestrogen and breast cancer: results from the WHI trial. Lancet Oncol. 2012;13(5):437–8. doi: 10.1016/S1470-2045(12)70110-9. [DOI] [PubMed] [Google Scholar]
- 42.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast Cancer and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 43.Cordina-Duverger E, Truong T, Anger A, Sanchez M, Arveux P, Kerbrat P, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among post-menopausal women in France. PLos ONE. 2013;8(11):e78016. doi: 10.1371/journal.pone.0078016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, clavel-Chapelon F, et al. Menopausal hormone therapy and breast cancer risk : impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128:144–56. doi: 10.1002/ijc.25314. [DOI] [PubMed] [Google Scholar]
- 45.Santen RJ. Risk of breast cancer with progestins: critical assessment of current data. Steroids. 2003;68:953–64. doi: 10.1016/s0039-128x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- 46.Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N-EPIC cohort study. Breast Cancer Res Treat. 2008;107:103–11. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen NE, Tsilidis KK, Key TJ, Dossus L, Kaaks R, Lund E, et al. Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women. European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172:1394–1403. doi: 10.1093/aje/kwq300. [DOI] [PubMed] [Google Scholar]
- 48.McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED. Estrogen plus progestin use and mammographic density in postmenopausal women: Women's Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–1376. doi: 10.1093/jnci/dji279. et at. [DOI] [PubMed] [Google Scholar]
- 49.McTiernan A, Chlebowski RT, Martin C, Peck JD, Aragaki A, Pisano Ed, et al. Conjugated equine estrogen influence on mammographic density in postmenopausal women in a sub-study of the Women's Health Initiative randomized trial. J Clin Oncol. 2009;27:6135–43. doi: 10.1200/JCO.2008.21.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chlebowski RT, Anderson GL, Manson JE, Pettinger M, Yasmeen S, Lane D, et al. Estrogen alone in postmenopausal women and breast cancer detection by means by mammography and breast biopsy. J Clin Oncol. 2010;28:2690–7. doi: 10.1200/JCO.2009.24.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohan TE, Negassa A, Chlebowski RT, Habel L, McTiernan A, Ginsberg M, et al. Conjugated equine estrogen and risk of benign proliferative breast disease: a randomized controlled trial. J Natl Cancer Inst. 2008;100(8):563–71. doi: 10.1093/jnci/djn075. [DOI] [PubMed] [Google Scholar]
- 52.Rohan TE, Negassa A, Chlebowski RT, Lasser NL, McTiernan A, Schenken RS, et al. Estrogen plus progestin and risk of benign proliferative breast disease. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2337–43. doi: 10.1158/1055-9965.EPI-08-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goss PE, Ingle JN, Ales-Martinez J, Cheung A, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 54.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2013;383(9922):1041–8. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 55.Jordan VC, Ford LG. Paradoxical clinical effects of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res. 2011;4:633–7. doi: 10.1158/1940-6207.CAPR-11-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunbier AK, Martin LA, Dowsett M. New and translational perspective of oestrogen deprivation in breast cancer. Mol Cell Endocrinol. 2011;340(2):137–141. doi: 10.1016/j.mce.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 57.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura S, et al. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhitbor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95:155–165. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 58.Zhao S, Chlebowski RT, Anderson GL, Kuller LH, Manson JE, Gass M, et al. Sex hormone associations with breast cancer risk and the mediation of randomized trial postmenopausal hormone therapy effects. Breast Cancer Research. 2014;16:R30. doi: 10.1186/bcr3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 60.Chlebowski RT, Schwartz AG, Wakelee H, Anderson GL, Stefanick ML, Manson JE, et al. Estrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomized controlled trial. Lancet. 2009;374:1243–51. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chlebowski RT, Anderson GL, Manson JE, Schwartz AG, Wakelee H, Gass M, et al. Lung cancer among postmenopausal women treated with estrogen alone in the Women's Health Initiative randomized trial. J Natl Cancer Inst. 2010;102:1413–1421. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palomares MR, Sayre JW, Shekar KC, Lillington LM, Chlebowski RT. Gender influence on weight-loss pattern and survival of non-small cell lung carcinoma patients. Cancer. 1996;78:2119–26. doi: 10.1002/(sici)1097-0142(19961115)78:10<2119::aid-cncr12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 63.Harichand-Herdt S, Ramalingam SS. Gender-associated differences in lung cancer: clinical characteristics and treatment outcomes in women. Seminars in Oncol. 2009;36:572–580. doi: 10.1053/j.seminoncol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Olivo-Marston SE, Mechanic LE, Mollerup S, Bowman ED, Remaley AT, Forman MR, et al. Serum estrogen and tumor-positive estrogen receptor-alpha are strong prognostic classifers of non-small-cell lung cancer survival in both men and women. Carcinogenesis. 2010;31:1778–86. doi: 10.1093/carcin/bgq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marquez-Garban DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann NY Acad Sci. 2009;115:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999;106:574–582. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 67.Simon MS, Chlebowski RT, Wactawski-Wende J, Johnson KC, Muskovitz A, Kato I, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30(32):3983–90. doi: 10.1200/JCO.2012.42.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 69.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 70.Ritenbaugh C, Stanford JL, Wu L, Shikany JM, Schoen RE, Stefanick ML, et al. Conjugated equine estrogens and colorectal cancer incidence and survival: The Women's Health Initiative randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2008;17:2609–2618. doi: 10.1158/1055-9965.EPI-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beral V, Bull D, Reeves G, Million Women Study Collaborators Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–51. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 72.Karageorgi S, Hankinson SE, Kraft P, De Vivo I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses’ Health Study cohort 1976-2004. Int J Cancer. 2010;126:208–216. doi: 10.1002/ijc.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures. The Women's Health Initiative randomized trial. JAMA. 2003;290:1739–1748. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- 74.Zbuk K, Sun Q, Cong R, Gu H, Tang N, Yang L, et al. Hormone replacement therapy and ovarian cancer risk: a meta-analysis. Gynecol Oncol. 2008;108(3):641–51. doi: 10.1016/j.ygyno.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000-2009. Menopause. 2011;18:385–392. doi: 10.1097/gme.0b013e3181f43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clarke CA, Glasser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49–e50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 77.Ravdin PM, Cronin KA, Howlander B, Berg CD, Chlebowski RT, Fewer EJ, et al. The decrease in breast cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 78.Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, et al. Breast cancer after estrogen plus progestin use in postmenopausal women. New Eng J Med. 2009;360:573–87. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zbuk K, Anand SS. Declining incidence of breast cancer after decreased use of hormone-replacement therapy: magnitude and time lags in different countries. J Epidemiol Community Health. 2012;66:1–7. doi: 10.1136/jech.2008.083774. [DOI] [PubMed] [Google Scholar]
- 80.Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B. Recent changes in endometrial cancer trends among menopausal-age US women. Cancer Epidemiol. 2013;37(4):374–377. doi: 10.1016/j.canep.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nahum GG, Stanislaw H, Simon JA. Stopping menopausal hormone therapy: If breast cancer really decreased, why did colorectal cancer not increase? Maturitas. 2012;71(4):354–359. doi: 10.1016/j.maturitas.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Nabel EG. The Women's Health Initiative – a victory for women and their health. JAMA. 2013;310(13):1349–1350. doi: 10.1001/jama.2013.278042. [DOI] [PubMed] [Google Scholar]